Investigation of Potential Use of Soybean Protein Isolate–Chinese Bayberry Tannin Extract Cross-Linked Films in Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
3. Characterization
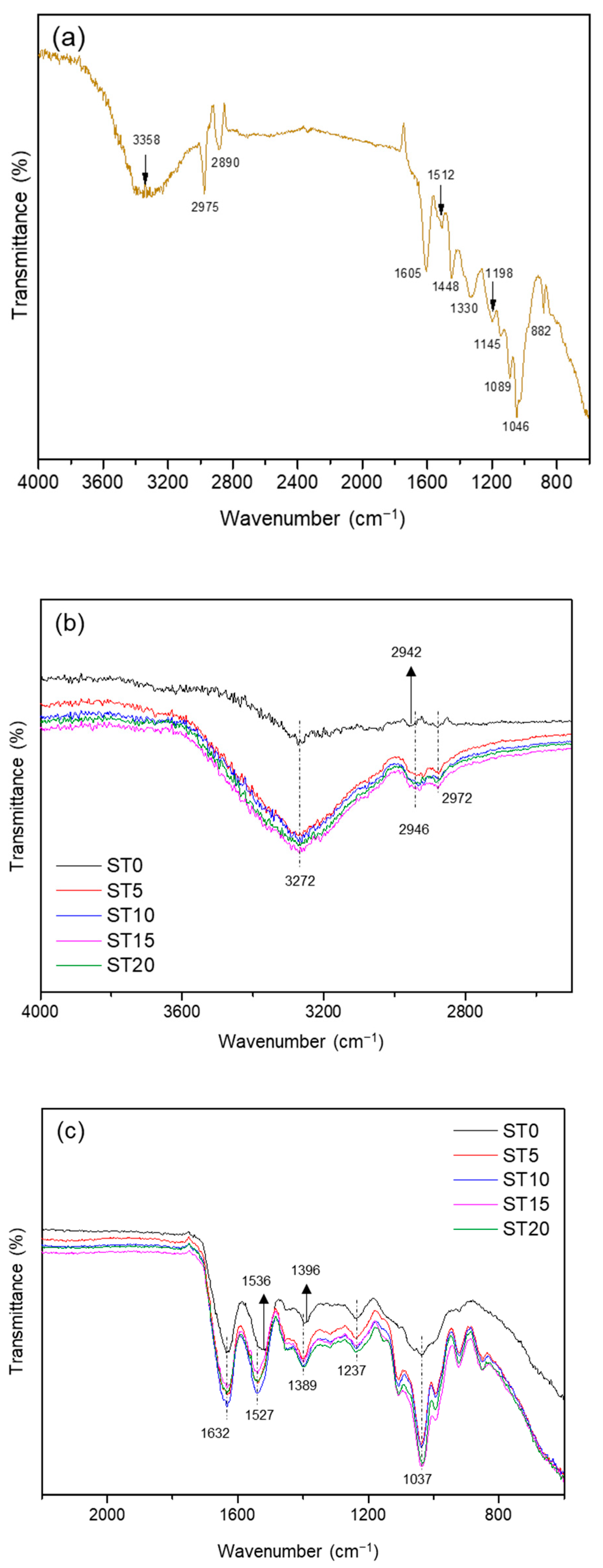
3.1. Fourier Transform Infrared (FTIR)
3.2. Tensile Strength
3.3. Opacity
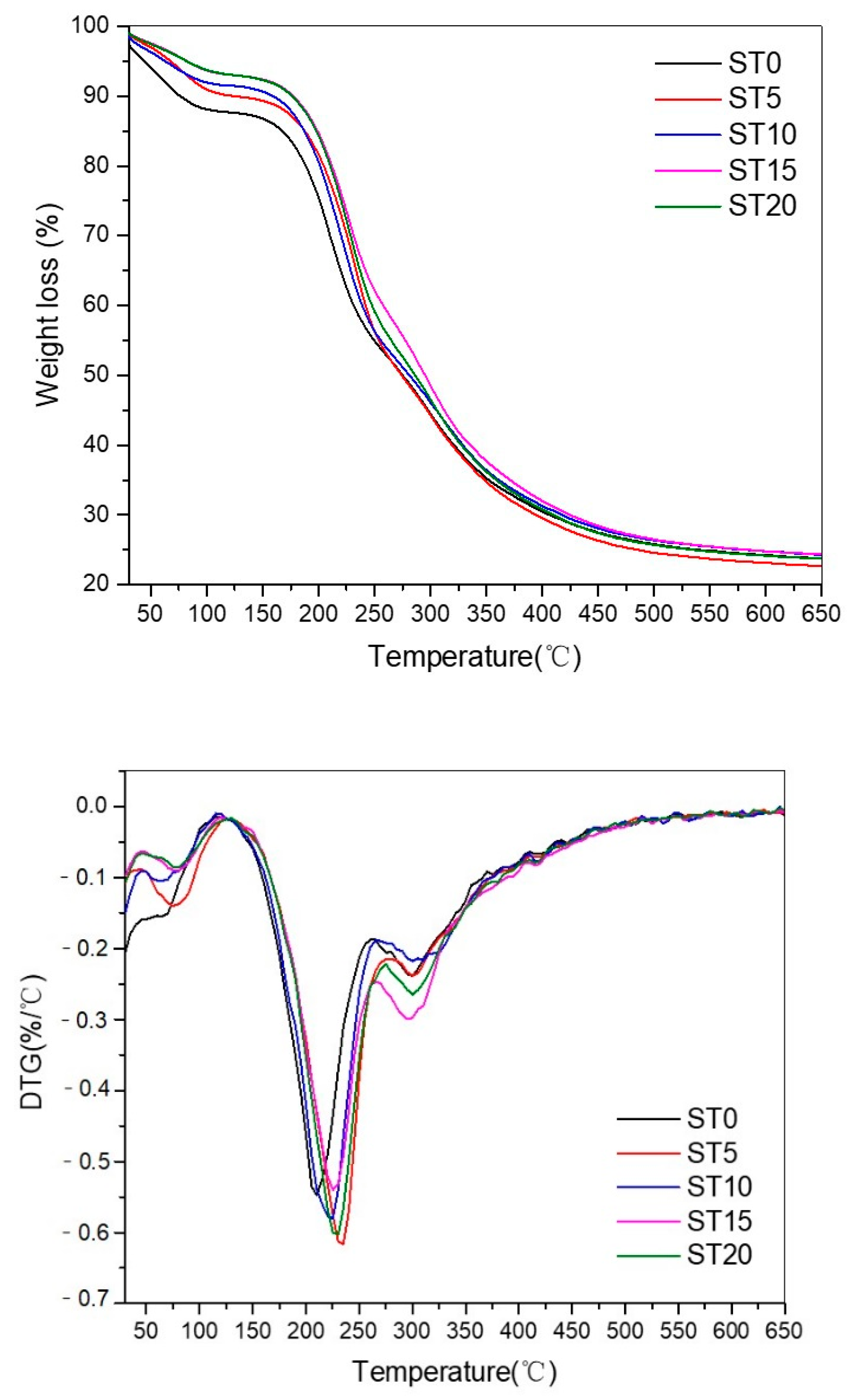
3.4. Thermal Gravimetric Analysis
3.5. Morphology
3.6. Permeability to Water Vapor (WVP)
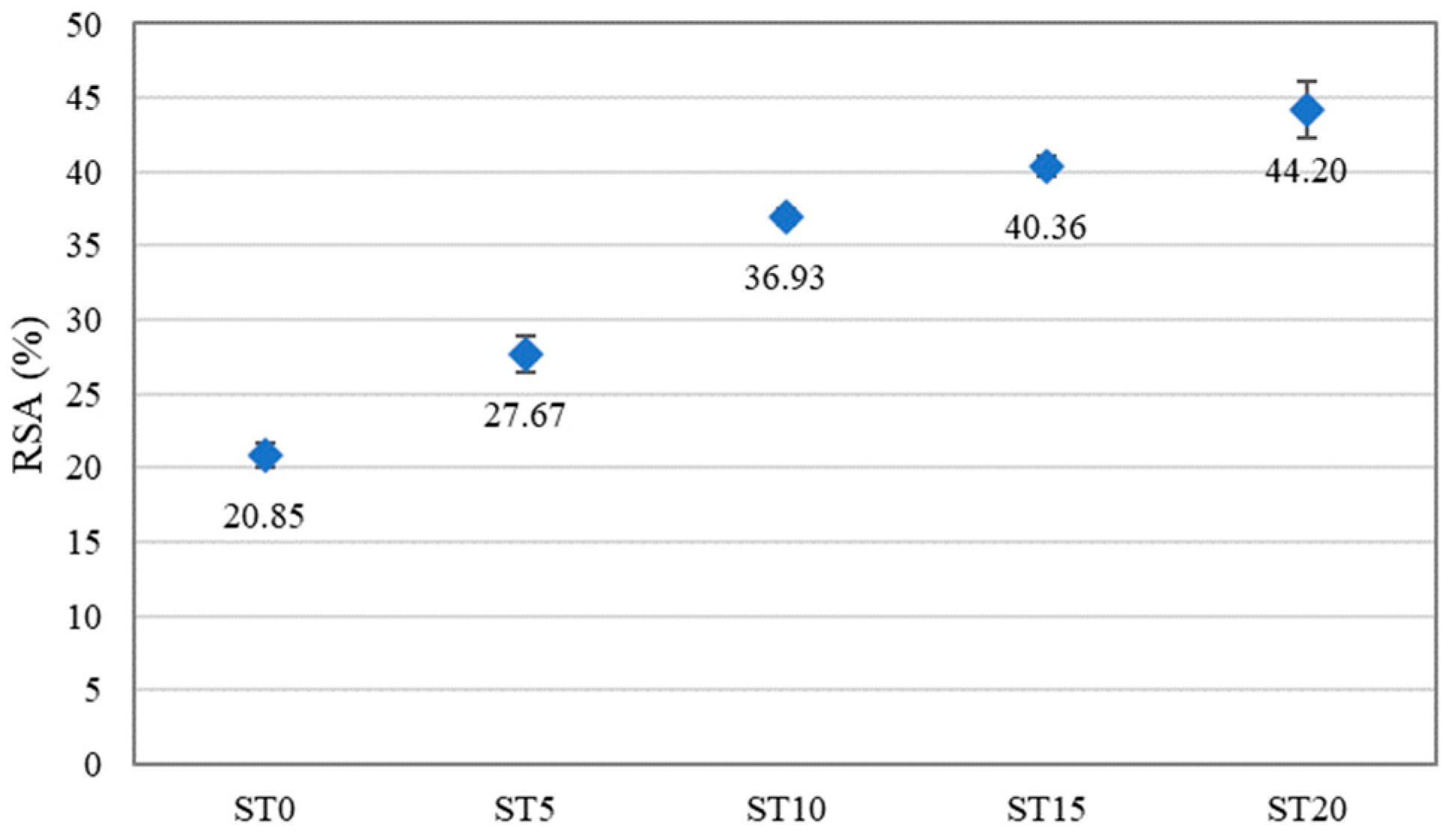
3.7. Antioxidant Activity
4. Results and Discussion
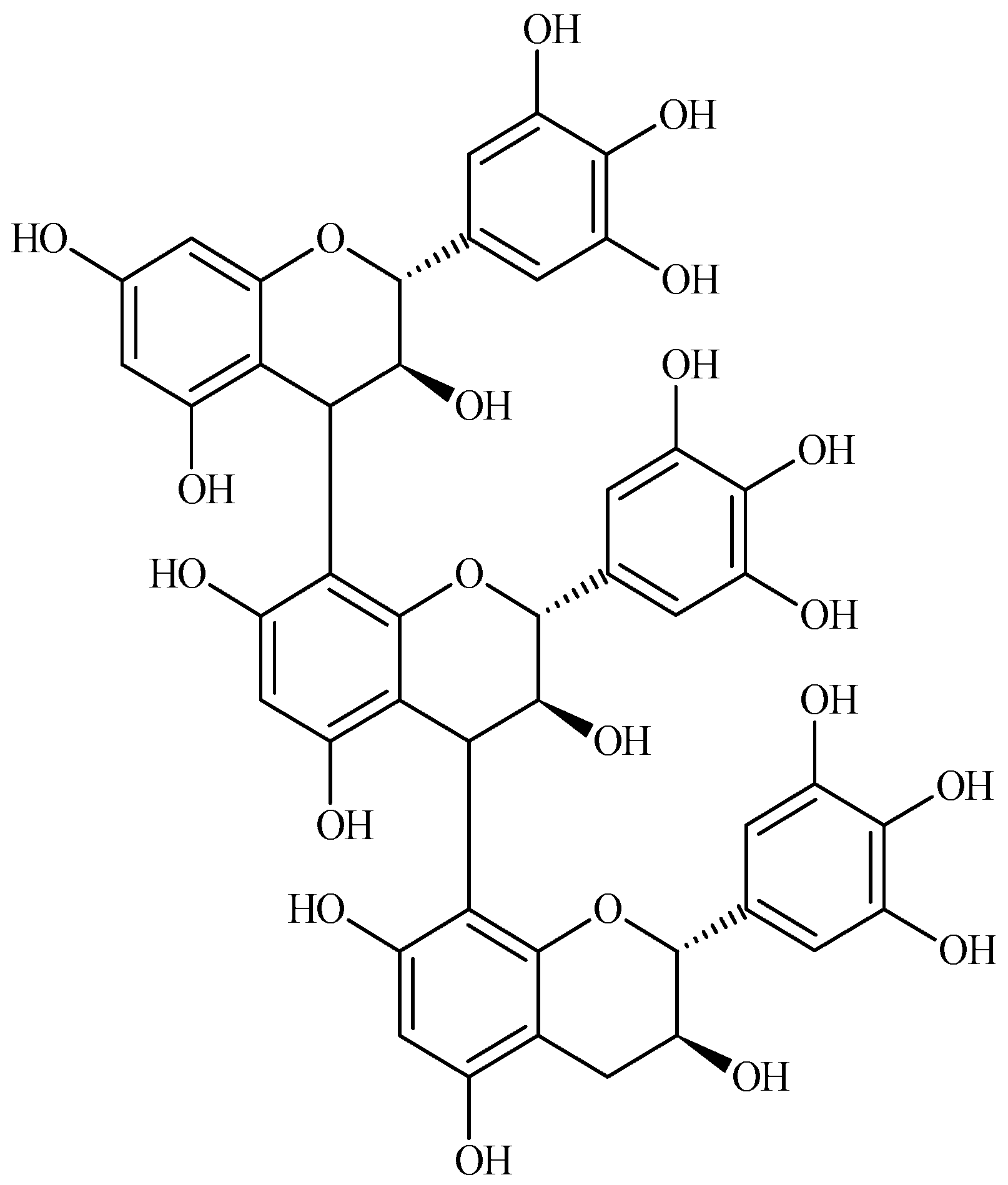
4.1. The Reaction Mechanism of SPI-BT Films
4.2. Strength Evaluation
4.3. Physical Appearance, Opacity and Morphology
4.4. Thermal Stability
4.5. Water Vapor Permeability (MVP)
4.6. Antioxidant Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Matthews, C.; Moran, F.; Jaiswal, A.K. A Review on European Union’s Strategy for Plastics in a Circular Economy and Its Impact on Food Safety. J. Clean. Prod. 2021, 283, 125263. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic Waste Release Caused by COVID-19 and Its Fate in the Global Ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Strumia, M.C.; Alvarez Igarzabal, C.I. Cross-Linked Soy Protein as Material for Biodegradable Films: Synthesis, Characterization and Biodegradation. J. Food Eng. 2011, 106, 331–338. [Google Scholar] [CrossRef]
- Song, F.; Tang, D.-L.; Wang, X.-L.; Wang, Y.-Z. Biodegradable Soy Protein Isolate-Based Materials: A Review. Biomacromolecules 2011, 12, 3369–3380. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Carvajal-Millan, E.; López-Ahumada, G.A.; Castro-Enriquez, D.D.; Barreras-Urbina, C.G.; Rodríguez-Felix, F. Prolamins from Cereal By-Products: Classification, Extraction, Characterization and Its Applications in Micro- and Nanofabrication. Trends Food Sci. Technol. 2019, 90, 111–132. [Google Scholar] [CrossRef]
- Gu, L.; Wang, M.; Zhou, J. Effects of Protein Interactions on Properties and Microstructure of Zein–Gliadin Composite Films. J. Food Eng. 2013, 119, 288–298. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-Polysaccharide Nanoparticles as Matrices for Antioxidant Compounds: A Strategy for Prevention of Chronic Degenerative Diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef] [PubMed]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Alvarez Igarzabal, C.I. Casein Films Crosslinked by Tannic Acid for Food Packaging Applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Rodríguez-Félix, D.E.; Plascencia-Jatomea, M.; Rascón-Chu, A.; López-Ahumada, G.A.; Ruiz-Cruz, S.; Barreras-Urbina, C.G.; Rodríguez-Félix, F. Porous Wheat Gluten Microparticles Obtained by Electrospray: Preparation and Characterization. Adv. Polym. Technol. 2018, 37, 2314–2324. [Google Scholar] [CrossRef]
- Lara, B.R.B.; Araújo, A.C.M.A.; Dias, M.V.; Guimarães, M.; Santos, T.A.; Ferreira, L.F.; Borges, S.V. Morphological, Mechanical and Physical Properties of New Whey Protein Isolate/Polyvinyl Alcohol Blends for Food Flexible Packaging. Food Packag. Shelf Life 2019, 19, 16–23. [Google Scholar] [CrossRef]
- Girard, A.L.; Teferra, T.; Awika, J.M. Effects of Condensed vs Hydrolysable Tannins on Gluten Film Strength and Stability. Food Hydrocoll. 2019, 89, 36–43. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Effects of Edible Plant Polyphenols on Gluten Protein Functionality and Potential Applications of Polyphenol-Gluten Interactions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2164–2199. [Google Scholar] [CrossRef]
- Rani, P.; Yu, X.; Liu, H.; Li, K.; He, Y.; Tian, H.; Kumar, R. Material, Antibacterial and Anticancer Properties of Natural Polyphenols Incorporated Soy Protein Isolate: A Review. Eur. Polym. J. 2021, 152, 110494. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W. Characterization of Emulsion Films Prepared from Soy Protein Isolate at Different Preheating Temperatures. J. Food Eng. 2021, 309, 110697. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Gennadios, A.; Weller, C.L.; Carole, C.; Hanna, M.A. Soy Protein Isolate–Dialdehyde Starch Films. Ind. Crops Prod. 1998, 8, 195–203. [Google Scholar] [CrossRef]
- Liu, K.; Xu, X.; Liu, H.; Liu, Z.; Zhao, K.; Ma, Y.; Zhang, K. Mechanical Properties and Water Sensitivity of Soybean Protein Isolate Film Improved by Incorporation of Sodium Caseinate and Transglutaminase. Prog. Org. Coat. 2021, 153, 106154. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Liu, H.-F.; Fu, M.; Zhao, X.-H. Structure and Property Changes of Soybean Protein Isolates Resulted from the Glycation and Cross-Linking by Transglutaminase and a Degraded Chitosan. CyTA-J. Food 2016, 14, 138–144. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Tártara, L.I.; Palma, S.D.; Alvarez Igarzabal, C.I. Crosslinked Soy Protein Films and Their Application as Ophthalmic Drug Delivery System. Mater. Sci. Eng. C 2015, 51, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Molino, S.; Casanova, N.A.; Rufián Henares, J.Á.; Fernandez Miyakawa, M.E. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–Polyphenol Conjugates: Antioxidant Property, Functionalities and Their Applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Nie, X.; Gong, Y.; Wang, N.; Meng, X. Preparation and Characterization of Edible Myofibrillar Protein-Based Film Incorporated with Grape Seed Procyanidins and Green Tea Polyphenol. LWT-Food Sci. Technol. 2015, 64, 1042–1046. [Google Scholar] [CrossRef]
- Theivendran, S.; Hettiarachchy, N.S.; Johnson, M.G. Inhibition of Listeria Monocytogenes by Nisin Combined with Grape Seed Extract or Green Tea Extract in Soy Protein Film Coated on Turkey Frankfurters. J. Food Sci. 2006, 71, M39–M44. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, H.; Zhang, W.; Zhang, S.; Li, J. Improvement of Interfacial Interactions Using Natural Polyphenol-Inspired Tannic Acid-Coated Nanoclay Enhancement of Soy Protein Isolate Biofilms. Appl. Surf. Sci. 2017, 401, 271–282. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Mechanical Properties of Gelatin Films Cross-Linked, Respectively, by Ferulic Acid and Tannin Acid. Food Hydrocoll. 2007, 21, 575–584. [Google Scholar] [CrossRef]
- Prodpran, T.; Benjakul, S.; Phatcharat, S. Effect of Phenolic Compounds on Protein Cross-Linking and Properties of Film from Fish Myofibrillar Protein. Int. J. Biol. Macromol. 2012, 51, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xia, N.; Xu, L.; Zhang, H.; Zhang, H.; Chi, Y.; Zhang, Y.; Li, L.; Li, H. Preparation, Characterization and Application of SPI-Based Blend Film with Antioxidant Activity. Food Packag. Shelf Life 2021, 27, 100614. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L. Developing a Bio-Based Packaging Film from Soya by-Products Incorporated with Valonea Tannin. J. Clean. Prod. 2017, 143, 624–633. [Google Scholar] [CrossRef]
- Pena-Rodriguez, C.; Martucci, J.F.; Neira, L.M.; Arbelaiz, A.; Eceiza, A.; Ruseckaite, R.A. Functional Properties and in Vitro Antioxidant and Antibacterial Effectiveness of Pigskin Gelatin Films Incorporated with Hydrolysable Chestnut Tannin. Food Sci. Technol. Int. 2015, 21, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, D.; Ma, Q.; Wang, L. Physical and Antioxidant Properties of Flexible Soy Protein Isolate Films by Incorporating Chestnut (Castanea Mollissima) Bur Extracts. LWT-Food Sci. Technol. 2016, 71, 33–39. [Google Scholar] [CrossRef]
- Moccia, F.; Piscitelli, A.; Giovando, S.; Giardina, P.; Panzella, L.; d’Ischia, M.; Napolitano, A. Hydrolyzable vs. Condensed Wood Tannins for Bio-Based Antioxidant Coatings: Superior Properties of Quebracho Tannins. Antioxidants 2020, 9, 804. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Chapter 8-Tannins: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 179–199. ISBN 978-0-08-045316-3. [Google Scholar]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Shen, X.; Tao, W.; Mao, G.; Wu, W.; Zhou, S.; Ye, X.; Pan, H. Preparation of a Novel Emulsifier by Self-Assembling of Proanthocyanidins from Chinese Bayberry (Myrica Rubra Sieb. et Zucc.) Leaves with Gelatin. Food Chem. 2020, 319, 126570. [Google Scholar] [CrossRef]
- Fu, Y.; Qiao, L.; Cao, Y.; Zhou, X.; Liu, Y.; Ye, X. Structural Elucidation and Antioxidant Activities of Proanthocyanidins from Chinese Bayberry (Myrica Rubra Sieb. et Zucc.) Leaves. PLoS ONE 2014, 9, e96162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Huang, H.; Xu, C.; Li, X.; Chen, K. Biological Activities of Extracts from Chinese Bayberry (Myrica Rubra Sieb. et Zucc.): A Review. Plant Foods Hum. Nutr. 2013, 68, 97–106. [Google Scholar] [CrossRef]
- Liao, J.; Li, J.; Wang, H.; Zhu, Y.; Essawy, H.; Du, G.; Zhou, X. Development of Antioxidant Packaging Film Based on Chinese Bayberry Tannin Extract and Polyvinyl Alcohol. J. Renew. Mater. 2022, 10, 19–31. [Google Scholar] [CrossRef]
- Liao, J.; Li, J.; Yang, F.; Zhu, Y.; Wang, H.; Du, G.; Essawy, H.; Zhou, X. Assisted Compatibility, and Balanced Regulation of the Mechanical, Thermal, and Antioxidant Activity of Polyvinyl Alcohol-Chinese Bayberry Tannin Extract Films Using Different Di-Aldehydes as Cross-Linkers. J. Renew. Mater. 2022, 10, 359–372. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. “Green” Bio-Thermoset Resins Derived from Soy Protein Isolate and Condensed Tannins. Ind. Crops Prod. 2017, 108, 363–370. [Google Scholar] [CrossRef]
- Teng, B.; Jian, X.; Gao, Y.; Chen, W. Comparison of Polyflavonoids in Bayberry Tanning Effluent and Commercial Bayberry Tannin: Prerequisite Information for Vegetable Tanning Effluent Recycling. J. Clean. Prod. 2016, 112, 972–979. [Google Scholar] [CrossRef]
- Wen, H.; Hsu, Y.-I.; Asoh, T.-A.; Uyama, H. Antioxidant Activity and Physical Properties of PH-Sensitive Biocomposite Using Poly(Vinyl Alcohol) Incorporated with Green Tea Extract. Polym. Degrad. Stab. 2020, 178, 109215. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Y.; Li, Y. Development of Tea Extracts and Chitosan Composite Films for Active Packaging Materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. A High-Performance Bio-Adhesive Derived from Soy Protein Isolate and Condensed Tannins. RSC Adv. 2017, 7, 21226–21233. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier Transform Infrared (FTIR) Spectroscopy in the Characterization of Tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, Y.; Sun, K.; Chang, C.; Liu, W. Effects of Covalent Interactions and Gel Characteristics on Soy Protein-Tannic Acid Conjugates Prepared under Alkaline Conditions. Food Hydrocoll. 2021, 112, 106293. [Google Scholar] [CrossRef]
- Kang, H.; Wang, Z.; Zhang, W.; Li, J.; Zhang, S. Physico-Chemical Properties Improvement of Soy Protein Isolate Films through Caffeic Acid Incorporation and Tri-Functional Aziridine Hybridization. Food Hydrocoll. 2016, 61, 923–932. [Google Scholar] [CrossRef]
- Han, J.; Shin, S.-H.; Park, K.-M.; Kim, K.M. Characterization of Physical, Mechanical, and Antioxidant Properties of Soy Protein-Based Bioplastic Films Containing Carboxymethylcellulose and Catechin. Food Sci. Biotechnol. 2015, 24, 939–945. [Google Scholar] [CrossRef]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of Cross-Linked Soy Protein Isolate-Based Environmentally-Friendly Films Enhanced by PTGE and PAM. Ind. Crops Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- Nie, X.; Zhao, L.; Wang, N.; Meng, X. Phenolics-Protein Interaction Involved in Silver Carp Myofibrilliar Protein Films with Hydrolysable and Condensed Tannins. LWT-Food Sci. Technol. 2017, 81, 258–264. [Google Scholar] [CrossRef]



| TS (MPa) | EB (%) | Optical Appearance | Opacity | |
|---|---|---|---|---|
| ST0 | 0.94 ± 0.06 | 247.69 ± 45.24 |  | 2.35 ± 0.15 |
| ST5 | 2.09 ± 0.13 | 76.35 ± 12.74 |  | 4.46 ± 0.02 |
| ST10 | 2.20 ± 0.47 | 240.51 ± 71.88 |  | 4.19 ± 0.05 |
| ST15 | 2.01 ± 0.07 | 56.65 ± 7.65 |  | 5.10 ± 0.10 |
| ST20 | 2.04 ± 0.22 | 229.95 ± 41.52 |  | 5.37 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Deng, S.; Essawy, H.; Bao, X.; Wang, H.; Du, G.; Zhou, X. Investigation of Potential Use of Soybean Protein Isolate–Chinese Bayberry Tannin Extract Cross-Linked Films in Packaging Applications. Materials 2022, 15, 5260. https://doi.org/10.3390/ma15155260
Liao J, Deng S, Essawy H, Bao X, Wang H, Du G, Zhou X. Investigation of Potential Use of Soybean Protein Isolate–Chinese Bayberry Tannin Extract Cross-Linked Films in Packaging Applications. Materials. 2022; 15(15):5260. https://doi.org/10.3390/ma15155260
Chicago/Turabian StyleLiao, Jingjing, Shuangqi Deng, Hisham Essawy, Xiaoyan Bao, Hongyan Wang, Guanben Du, and Xiaojian Zhou. 2022. "Investigation of Potential Use of Soybean Protein Isolate–Chinese Bayberry Tannin Extract Cross-Linked Films in Packaging Applications" Materials 15, no. 15: 5260. https://doi.org/10.3390/ma15155260
APA StyleLiao, J., Deng, S., Essawy, H., Bao, X., Wang, H., Du, G., & Zhou, X. (2022). Investigation of Potential Use of Soybean Protein Isolate–Chinese Bayberry Tannin Extract Cross-Linked Films in Packaging Applications. Materials, 15(15), 5260. https://doi.org/10.3390/ma15155260








