Synthesis and Characterization of Li2MgGeO4:Ho3+
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
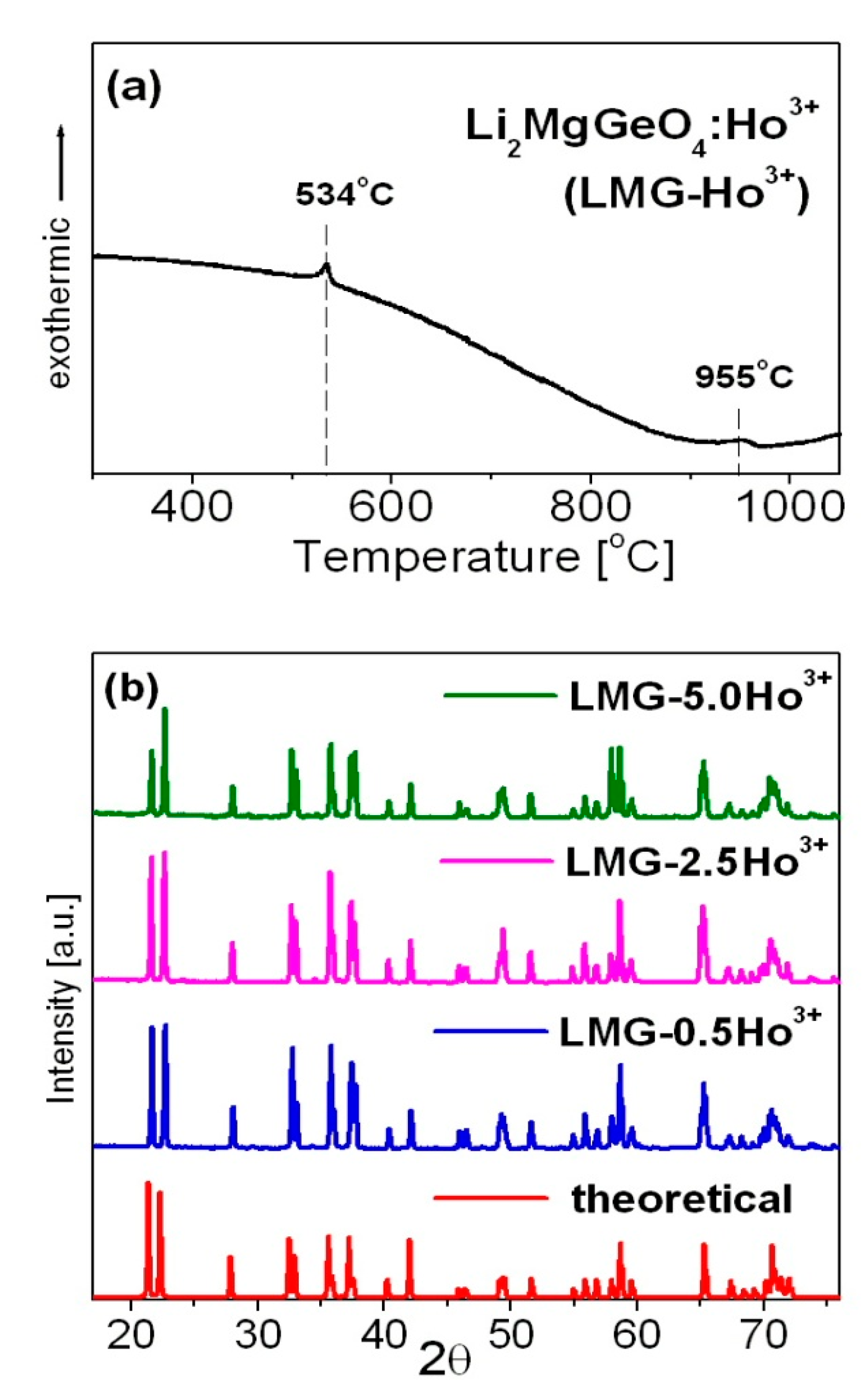
3.1. Synthesis
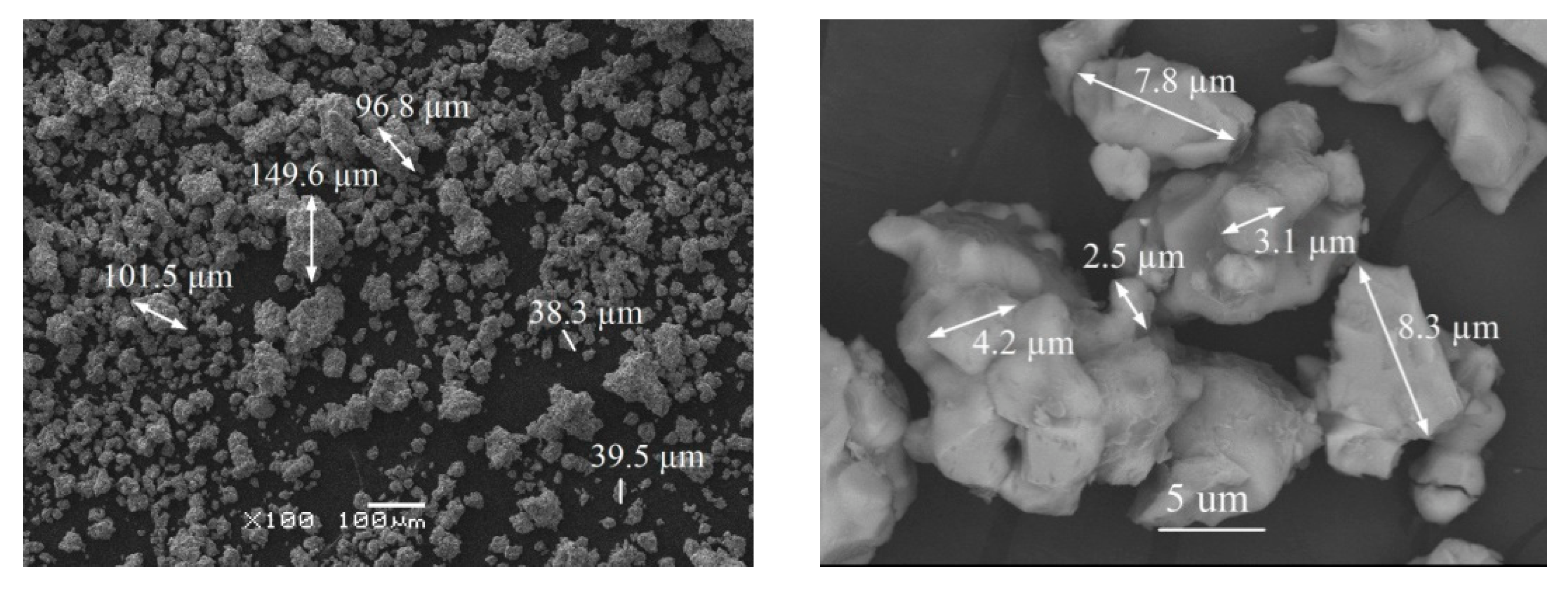
3.2. Characterization
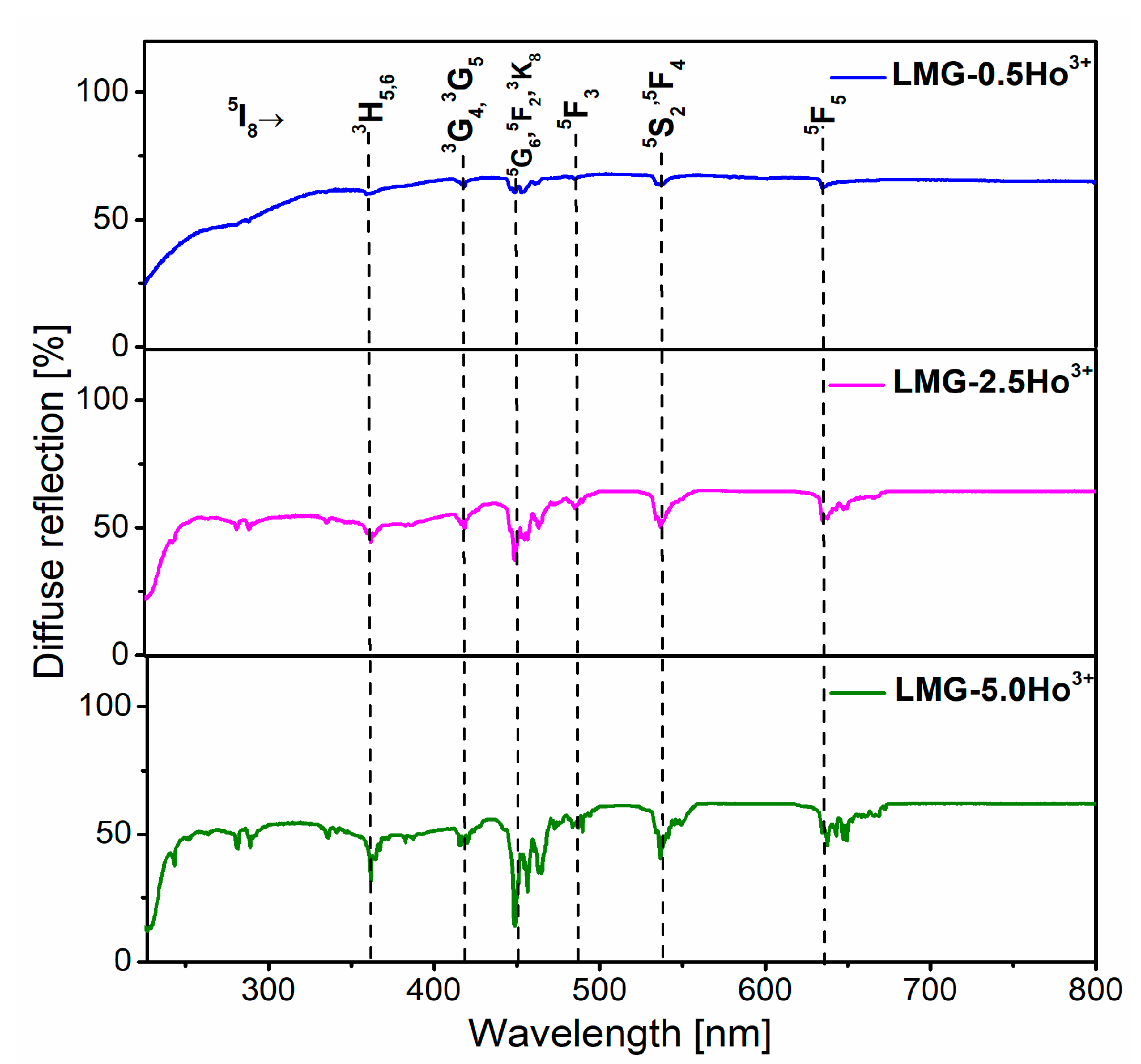
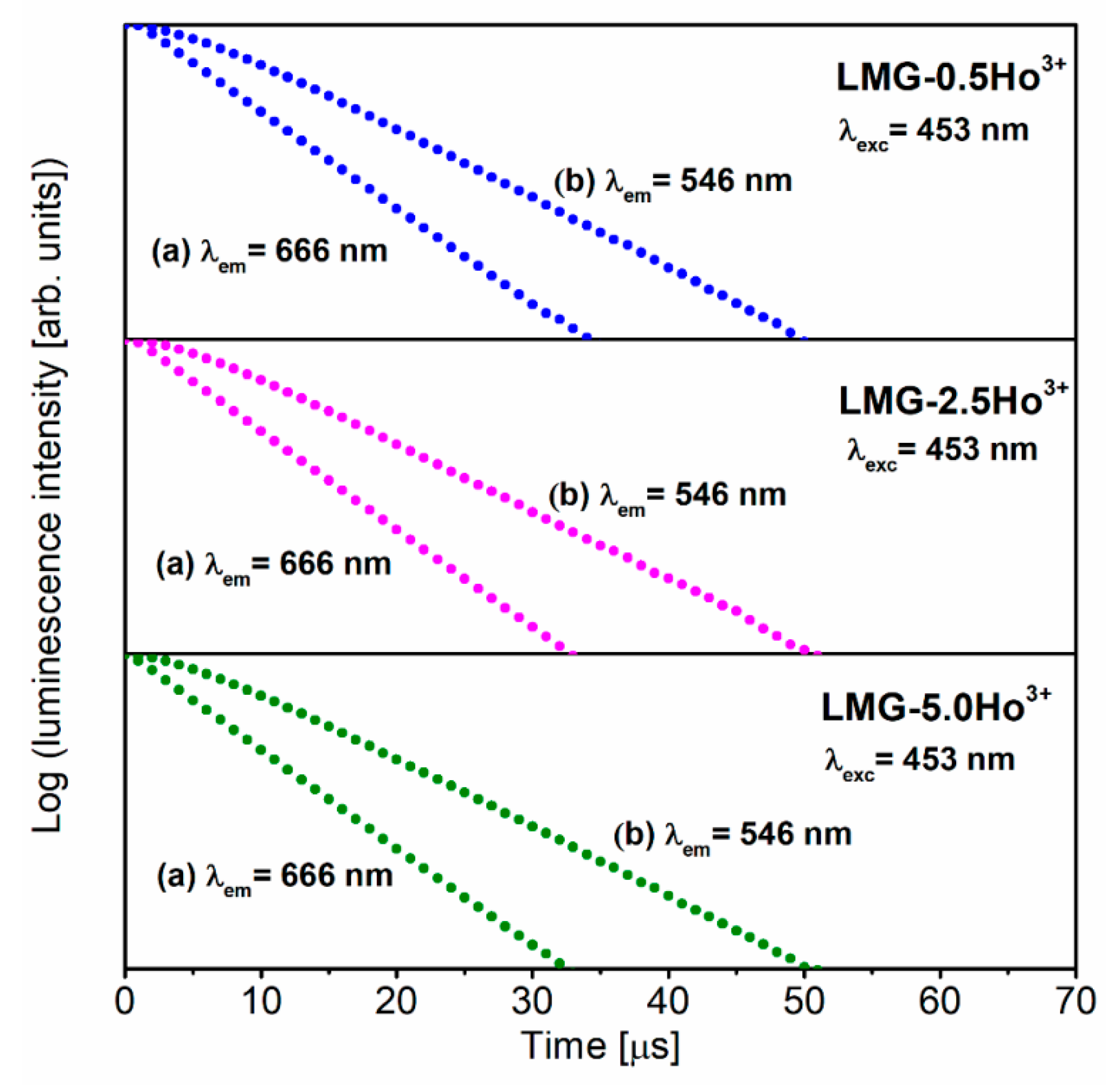
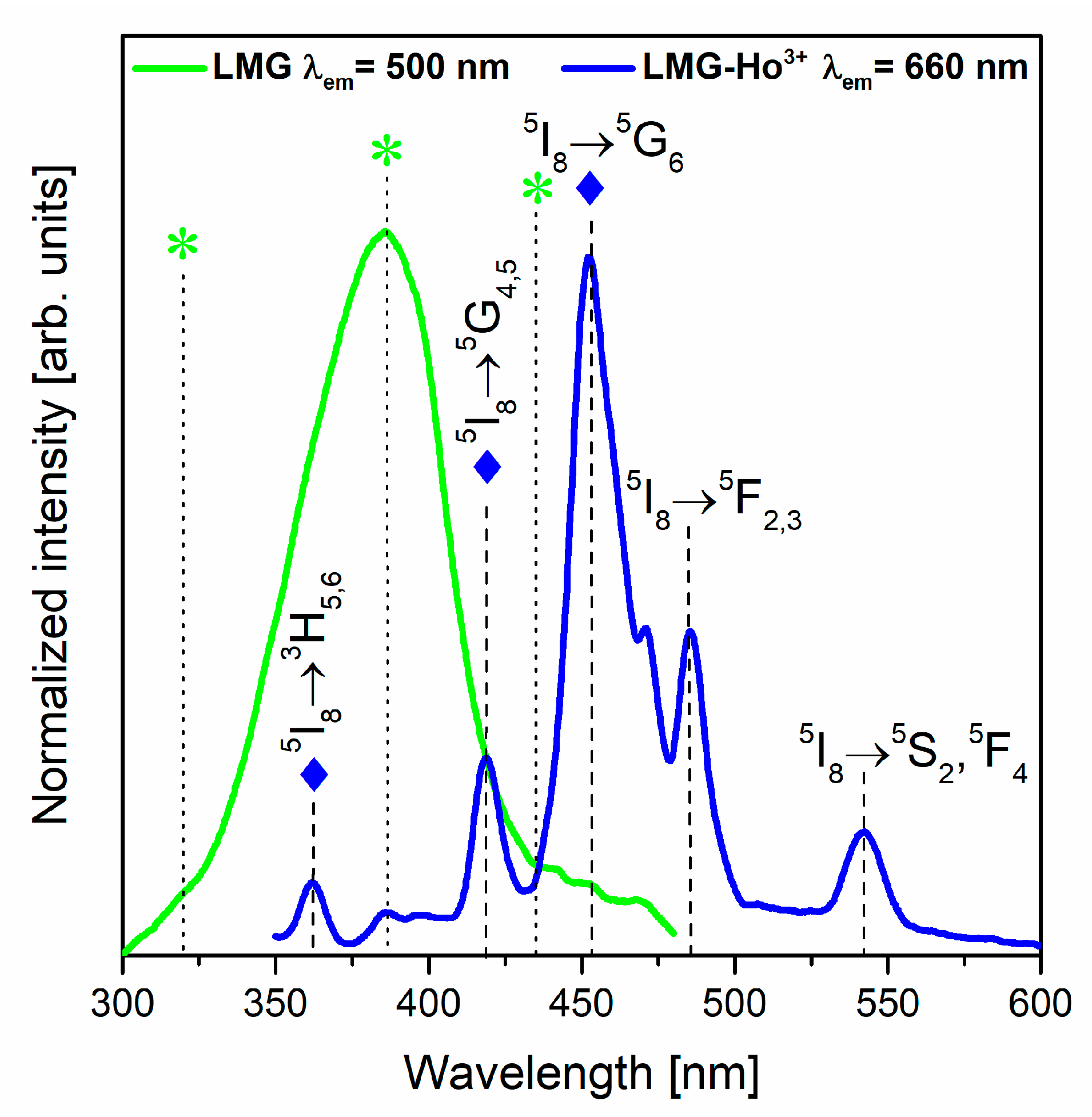
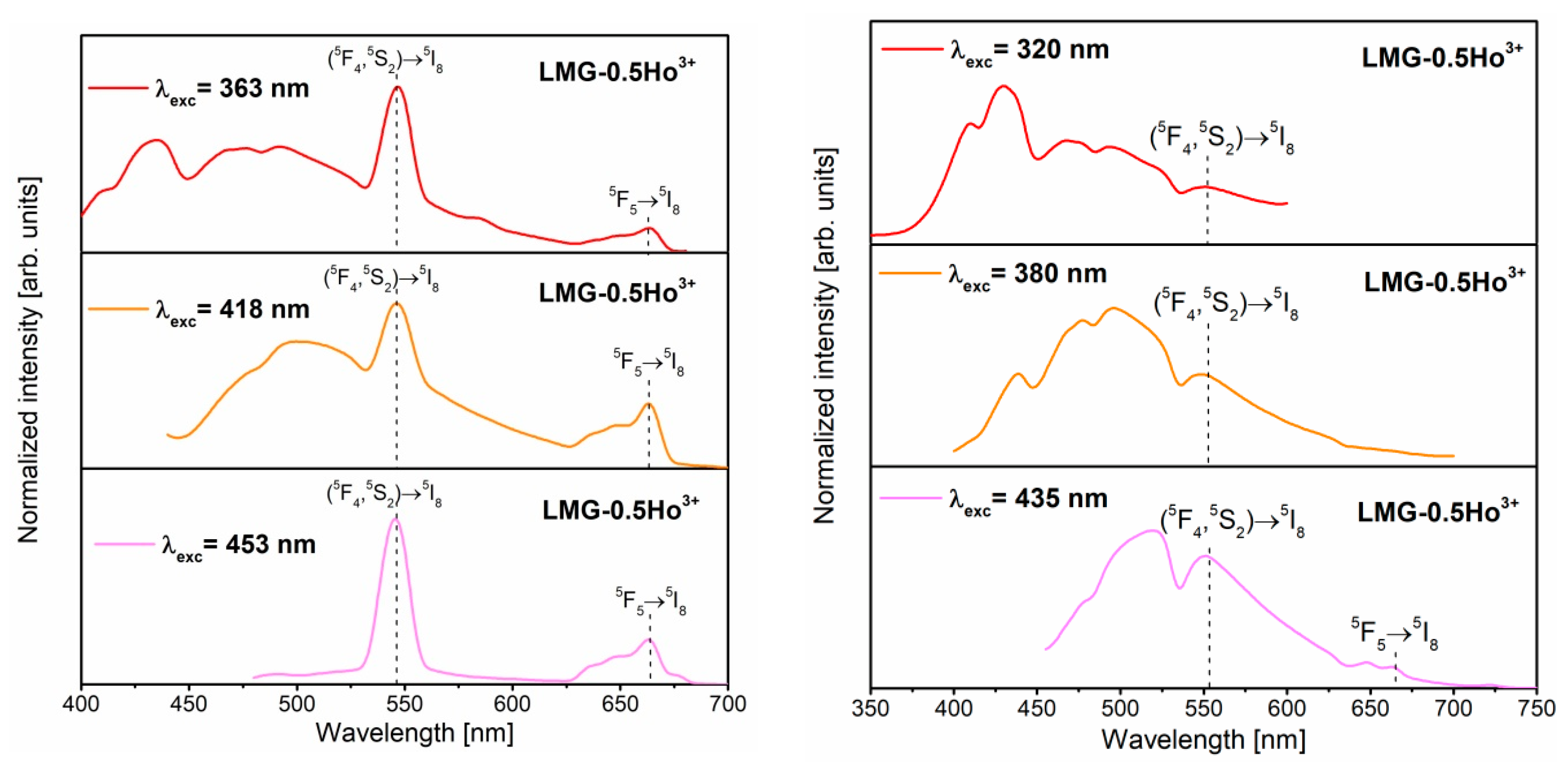
3.3. Luminescence Properties under Different Excitation Wavelengths
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, C.S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. The modulated structure and frequency upconversion properties of CaLa2(MoO4)4:Ho3+/Yb3+ phosphors prepared by microwave synthesis. Phys. Chem. Chem. Phys. 2015, 17, 19278–19287. [Google Scholar] [CrossRef] [PubMed]
- Sukhachev, A.L.; Malakhovskii, A.V.; Aleksandrovsky, A.S.; Gudim, I.A.; Temerov, V.L. Spectroscopic properties of HoFe3(BO3)4, NdFe3(BO3)4 and Ho0.75Nd0.25Fe3(BO3)4 single crystals. Opt. Mater. 2018, 83, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Wang, N.; Jiang, X.; Aleksandrovsky, A.S.; Krylov, A.S.; Oreshonkov, A.S.; Sedykh, A.E.; Volkova, S.S.; et al. Negative thermal expansion in one-dimension of a new double sulfate AgHo(SO4)2 with isolated SO4 tetrahedra. J. Mater. Sci. Technol. 2021, 76, 111–121. [Google Scholar] [CrossRef]
- Lim, C.-S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. Structural and Spectroscopic Effects of Li+ Substitution for Na+ in LixNa1-xCaGd0.5Ho0.05Yb0.45(MoO4)3 Scheelite-Type Upconversion Phosphors. Molecules 2021, 26, 7357. [Google Scholar] [CrossRef]
- Srinivasa Rao, C.; Upendra Kumar, K.; Babu, P.; Jayasankar, C.K. Optical properties of Ho3+ ions in lead phosphate glasses. Opt. Mater. 2012, 35, 102–107. [Google Scholar] [CrossRef]
- Jayachandra Prasad, T.; Neelima, G.; Ravi, N.; Kiran, N.; Nallabala, N.K.R.; Kummara, V.K.; Suresh, K.; Gadige, P. Optical and spectroscopic properties of Ho3+-doped fluorophosphate glasses for visible lighting applications. Mater. Res. Bull. 2020, 124, 110753. [Google Scholar] [CrossRef]
- Ravi Prakash, M.; Neelima, G.; Kummara, V.K.; Ravic, N.; Dwaraka Viswanath, C.S.; Subba Rao, T.; Mahaboob Jilani, S. Holmium doped bismuth-germanate glasses for green lighting applications: A spectroscopic study. Opt. Mater. 2019, 94, 436–443. [Google Scholar] [CrossRef]
- Pisarska, J.; Kuwik, M.; Górny, A.; Kochanowicz, M.; Zmojda, J.; Dorosz, J.; Dorosz, D.; Sitarz, M.; Pisarski, W.A. Holmium doped barium gallo-germanate glasses for near-infrared luminescence at 2000 nm. J. Lumin. 2019, 215, 116625. [Google Scholar] [CrossRef]
- Devaraja, C.; Jagadeesha Gowda, G.V.; Eraiah, B.; Keshavamurthy, K. Optical properties of bismuth tellurite glasses doped with holmium oxide. Ceram. Int. 2021, 47, 7602–7607. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Guan, D.-X. Photoluminescence associated with the site occupations of Ho3+ ions in BaTiO3. Sci. Rep. 2017, 7, 6125. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Cheng, H.; Xu, X.; Chen, H. Preparation and characterization of transparent magneto-optical Ho2O3 ceramics. J. Am. Ceram. Soc. 2019, 102, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Bai, X.; Feng, J.; Huang, L.; Li, G.; Chen, Y. Influences of holmium substitution on the phase structure and piezoelectric properties of BiFeO3-BaTiO3-based ceramics. J. Alloys Compd. 2022, 918, 165582. [Google Scholar] [CrossRef]
- Tsotetsi, D.; Dhlamini, M.; Mbule, P. Sol-gel synthesis and characterization of Ho3+ doped TiO2 nanoparticles: Evaluation of absorption efficiency and electrical conductivity for possible application in perovskite solar cells. Opt. Mater. 2022, 130, 112569. [Google Scholar] [CrossRef]
- Wu, X.; Lin, J.; Chen, P.; Liu, C.; Lin, M.; Cong Lin, C.; Luo, L.; Zheng, X. Ho3+-doped (K,Na)NbO3-based multifunctional transparent ceramics with superior optical temperature sensing performance. J. Am. Ceram. Soc. 2019, 102, 1249–1258. [Google Scholar] [CrossRef]
- Lin, J.; Lu, Q.; Xu, K.; Wu, X.; Lin, C.; Lin, T.; Chen, C.; Luo, L. Outstanding optical temperature sensitivity and dual-mode temperature-dependent photoluminescence in Ho3+-doped (K,Na)NbO3-SrTiO3 transparent ceramics. J. Am. Chem. Soc. 2019, 102, 4710–4720. [Google Scholar] [CrossRef]
- Li, J.; Chai, X.; Wang, X.; Xu, C.-N.; Gu, Y.; Zhao, H.; Yao, X. Large electrostrain and high optical temperature sensitivity in BaTiO3-(Na0.5Ho0.5)TiO3 multifunctional ferroelectric ceramics. Dalton Trans. 2016, 45, 11733–11741. [Google Scholar] [CrossRef]
- Yun, X.; Zhou, J.; Zhu, Y.; Li, X.; Xu, D. Up-conversion luminescence and optical temperature sensing properties of Ho3+-doped double-tungstate LiYb(WO4)2 phosphors. J. Mater. Sci. Mater. Electron. 2021, 32, 17990–18001. [Google Scholar] [CrossRef]
- Wu, J.; Ma, C.; Fan, Y.; Sun, B.; Sheng, Y.; Zhai, Z.; Lu, H.; Lv, X. Upconversion luminescence and non-contact optical temperature sensing in Ho3+-doped 0.94Na0.5Bi0.5TiO3-0.06BaTiO3 lead-free ceramics. Opt. Mater. 2022, 127, 112239. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Zhang, Y.; Li, Y.; Yao, X. Optical temperature sensing of up-conversion luminescent materials: Fundamentals and progress. J. Alloys Compd. 2020, 817, 152691. [Google Scholar] [CrossRef]
- Ye, Y.; Tang, Z.; Ji, Z.; Xiao, H.; Liu, Y.; Qin, Y.; Liang, L.; Qi, J.; Lu, T. Fabrication and luminescent properties of holmium doped Y2Zr2O7 transparent ceramics as new type laser material. Opt. Mater. 2021, 121, 111643. [Google Scholar] [CrossRef]
- Safronova, N.A.; Yavetskiy, R.P.; Kryzhanovska, O.S.; Dobrotvorska, M.V.; Balabanov, A.E.; Vorona, I.O.; Tolmachev, A.V.; Baumer, V.N.; Matolínova, I.; Kosyanov, D.Y.; et al. A novel IR-transparent Ho3+:Y2O3–MgO nanocomposite ceramics for potential laser applications. Ceram. Int. 2021, 47, 1399–1406. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Y.; Chen, L.; Ju, G.; Wu, H.; Mu, Z.; He, M.; Xue, F. Luminescent properties of a green long persistent phosphor Li2MgGeO4:Mn2+. Opt. Mater. Exp. 2016, 6, 929–937. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Jiang, T.; Li, L.; Cheng, L.-X.; Zhang, J.-C. Short-Term Non-Decaying Mechanoluminescence in Li2MgGeO4:Mn2+. Materials 2020, 13, 1410. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Zheng, Z.; Wu, L.; Kong, Y.; Zhang, Y.; Xu, J. Construction of a novel mechanoluminescent phosphor Li2MgGeO4:xMn2+ by defect control. Dalton Trans. 2021, 50, 8803–8810. [Google Scholar] [CrossRef]
- Huang, S.; Li, G. Photoluminescence properties of Li2SrGeO4:RE3+ (RE = Ce/Tb/Dy) phosphors and enhanced luminescence through energy transfer between Ce3+ and Tb3+/Dy3+. Opt. Mater. 2014, 36, 1555–1560. [Google Scholar] [CrossRef]
- Fang, W.; Ao, L.; Tang, Y.; Li, J.; Xiang, H.; Zhang, Z.; Du, Q.; Fang, L. Phase evolution and microwave dielectric properties of the Li2(1+x)ZnGe3O8 spinel oxides. J. Mater. Sci. Mater. Electron. 2020, 31, 13496–13502. [Google Scholar] [CrossRef]
- Li, C.; Yin, C.; Chen, J.; Xiang, H.; Tang, Y.; Fang, L. Crystal structure and dielectric properties of germanate melilites Ba2MGe2O7 (M = Mg and Zn) with low permittivity. J. Eur. Ceram. Soc. 2018, 38, 5246–5251. [Google Scholar] [CrossRef]
- Chen, C.X.; Wu, S.P.; Fan, Y.X. Synthesis and microwave dielectric properties of B2O3-doped Mg2GeO4 ceramics. J. Alloys Compd. 2013, 578, 153–156. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, Y.; Li, J.; Fang, W.; Ma, J.; Yang, A.; Liu, L.; Fang, L. Crystal structure, Raman spectra and microwave dielectric properties of novel low-temperature cofired ceramic Li4GeO4. J. Alloys Compd. 2021, 867, 159059. [Google Scholar] [CrossRef]
- Koseva, I.; Nikolov, V.; Petrova, N.; Tzvetkov, P.; Marychev, M. Thermal behavior of germanates with olivine structure. Thermochim. Acta 2016, 646, 1–7. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; He, S.; Chen, X.; Wu, Y.; Zhou, H. Microstructural evolution, dielectric performance, and densification behavior of novel NaAGeO4 (A = Sm, Y) ceramics. Ceram. Int. 2022, 48, 16386–16390. [Google Scholar] [CrossRef]
- Li, C.; Xiang, H.; Xu, M.; Tang, Y.; Fang, L. Li2AGeO4 (A = Zn, Mg): Two novel low-permittivity microwave dielectric ceramics with olivine structure. J. Eur. Ceram. Soc. 2018, 38, 1524–1528. [Google Scholar] [CrossRef]
- Shang, M.; Li, G.; Yang, D.; Kang, X.; Peng, C.; Lin, J. Luminescence properties of Mn2+-doped Li2ZnGeO4 as an efficient green phosphor for field-emission displays with high color purity. Dalton Trans. 2012, 41, 8861–8868. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, S.; Hu, Y. Enhanced red-emitting phosphor Na2Ca3Si2O8:Eu3+ by charge compensation. J. Mater. Sci. Mater. Electron. 2017, 28, 5262–5269. [Google Scholar] [CrossRef]
- Puchalska, M.; Watras, A. A clear effect of charge compensation through Na+ co-doping on luminescent properties of new CaGa4O7:Nd3+. J. Alloys Compd. 2016, 688, 253–260. [Google Scholar] [CrossRef]
- Puchalska, M.; Zych, E. The effect of charge compensation by means of Na+ ions on the luminescence behavior of Sm3+-doped CaAl4O7 phosphor. J. Lumin. 2012, 132, 826–831. [Google Scholar] [CrossRef]
- Nagabhushana, H.; Sunitha, D.V.; Sharma, S.C.; Daruka Prasad, B.; Nagabhushana, B.M.; Chakradhar, R.P.S. Enhanced luminescence by monovalent alkali metal ions in Sr2SiO4:Eu3+ nanophosphor prepared by low temperature solution combustion method. J. Alloys Compd. 2014, 595, 192–199. [Google Scholar] [CrossRef]
- Tang, W.; Guo, Q.; Su, K.; Liu, H.; Zhang, Y.; Mei, L.; Liao, L. Structure and photoluminescence properties of Dy3+ doped phosphor with whitlockite structure. Materials 2022, 15, 2177. [Google Scholar] [CrossRef]
- Praveena, R.; Sravani Sameera, V.; Babu, P.; Basavapoornima, C.; Jayasankar, C.K. Photoluminescence properties of Ho3+/Tm3+-doped YAGG nanocrystalline powders. Opt. Mater. 2017, 72, 666–672. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Chimitova, O.D.; Krylov, A.S.; Molokeev, M.S.; Bazarov, B.G.; Bazarova, J.G.; Xia, Z. Synthesis and spectroscopic properties of multiferroic β’-Tb2(MoO4)3. Opt. Mater. 2014, 36, 1631–1635. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Chimitova, O.D.; Diao, C.-P.; Gavrilova, T.A.; Kesler, V.G.; Molokeev, M.S.; Krylov, A.S.; Bazarov, B.G.; Bazarova, J.G.; et al. Electronic structure of β-RbSm(MoO4)2 and chemical bonding in molybdates. Dalton Trans. 2015, 44, 1805–1815. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Sedykh, A.E.; Khritokhin, N.A.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Shestakov, N.P.; Adichtchev, S.V.; Pugachev, A.M.; et al. Exploration of the Crystal Structure and Thermal and Spectroscopic Properties of Monoclinic Praseodymium Sulfate Pr2(SO4)3. Molecules 2022, 27, 3966. [Google Scholar] [CrossRef]
- Tran, L.T.N.; Massella, D.; Zur, L.; Chiasera, A.; Varas, S.; Armellini, C.; Righini, G.C.; Lukowiak, A.; Zonta, D.; Ferrari, M. SiO2-SnO2:Er3+ Glass-Ceramic Monoliths. Appl. Sci. 2018, 8, 1335. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Rodríguez, L.; Balda, R.; Fernández, J.; Durán, A.; Pascual, M.J. Role of Eu2+ and Dy3+ Concentration in the Persistent Luminescence of Sr2MgSi2O7 Glass-Ceramics. Materials 2022, 15, 3068. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Goryczka, T.; Pisarska, J.; Pisarski, W.A. Structural and Photoluminescence Investigations of Tb3+/Eu3+ Co-Doped Silicate Sol-Gel Glass-Ceramics Containing CaF2 Nanocrystals. Materials 2021, 14, 754. [Google Scholar] [CrossRef]
- Pirri, A.; Maksimov, R.N.; Li, J.; Vannini, M.; Toci, G. Achievements and Future Perspectives of Trivalent Thulium-Ion-Doped Mixed Sesquioxide Ceramics for Laser Applications. Materials 2022, 15, 2084. [Google Scholar] [CrossRef]
- Miniajluk-Gaweł, N.; Bondzior, B.; Lemanski, K.; Vu, T.H.Q.; Stefańska, D.; Boulesteix, R.; Dereń, P.J. Effect of Ceramic Formation on the Emission of Eu3+ and Nd3+ Ions in Double Perovskites. Materials 2021, 14, 5996. [Google Scholar] [CrossRef]
- Siva Sesha Reddy, A.; Purnachand, N.; Kostrzewa, M.; Brik, M.G.; Venkatramaiah, N.; Ravi Kumar, V.; Veeraiah, N. The role of gold metallix particles on improving green and NIR emissions of Ho3+ ions in non-conventional SeO2 based glass ceramics. J. Non-Cryst. Solid 2022, 576, 121240. [Google Scholar] [CrossRef]
- Singh, V.; Lakshminarayana, G.; Wagh, A.; Singh, N. Luminescence features of green-emitting CaLa4Si3O13:Ho3+ phosphors. Optik 2020, 207, 164284. [Google Scholar] [CrossRef]
- Li, W.; Hao, J.; Fu, P.; Du, J.; Li, P.; Li, H.; Li, W.; Yue, Z. High-temperature and long-term stability of Ho-doped potassium sodium niobate-based multifunctional ceramics. Ceram. Int. 2021, 47, 13391–13401. [Google Scholar] [CrossRef]
- Raman, T.R.; Vijayalakshmi, R.P.; Ratnakaram, Y.C. Effect of Ho3+ ion concentration on structure and spectroscopic properties of LiPbB5O9:Ho3+ phosphor. J. Mol. Struct. 2021, 1243, 130759. [Google Scholar] [CrossRef]
- Georgescu, S.; Stefan, A.; Toma, O.; Voiculescu, A.-M. Judd-Ofelt analysis of Ho3+ doped in ceramic CaSc2O4. J. Lumin. 2015, 162, 174–179. [Google Scholar] [CrossRef]
- Kaczkan, M.; Malinowski, M. Optical Transitions and Excited State Absorption Cross Sections of SrLaGaO4 Doped with Ho3+ Ions. Materials 2021, 14, 3831. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L. Intensive green light emission from MgO nanobelts. J. Phys. Chem. Lett. 2002, 363, 293–297. [Google Scholar] [CrossRef]
- Janet, C.M.; Viswanathan, B.; Viswanath, R.P.; Varadarajan, T.K. Characterization and photoluminescence properties of MgO microtubes synthesized from hydromagnesite flowers. J. Phys. Chem. C 2007, 111, 10267–10272. [Google Scholar] [CrossRef]
- Uchino, T.; Okutsu, D. Broadband laser emission from color centers inside MgO microcrystals. Phys. Rev. Lett. 2008, 101, 117401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Boubeta, C.; Martinez, A.; Hernandez, S.; Pellegrino, P.; Antony, A.; Bertomeu, J.; Balcells, L.; Konstantinovic, Z.; Martinez, B. Blue luminescence at room temperature in defective MgO films. Solid State Commun. 2011, 151, 751–753. [Google Scholar] [CrossRef] [Green Version]

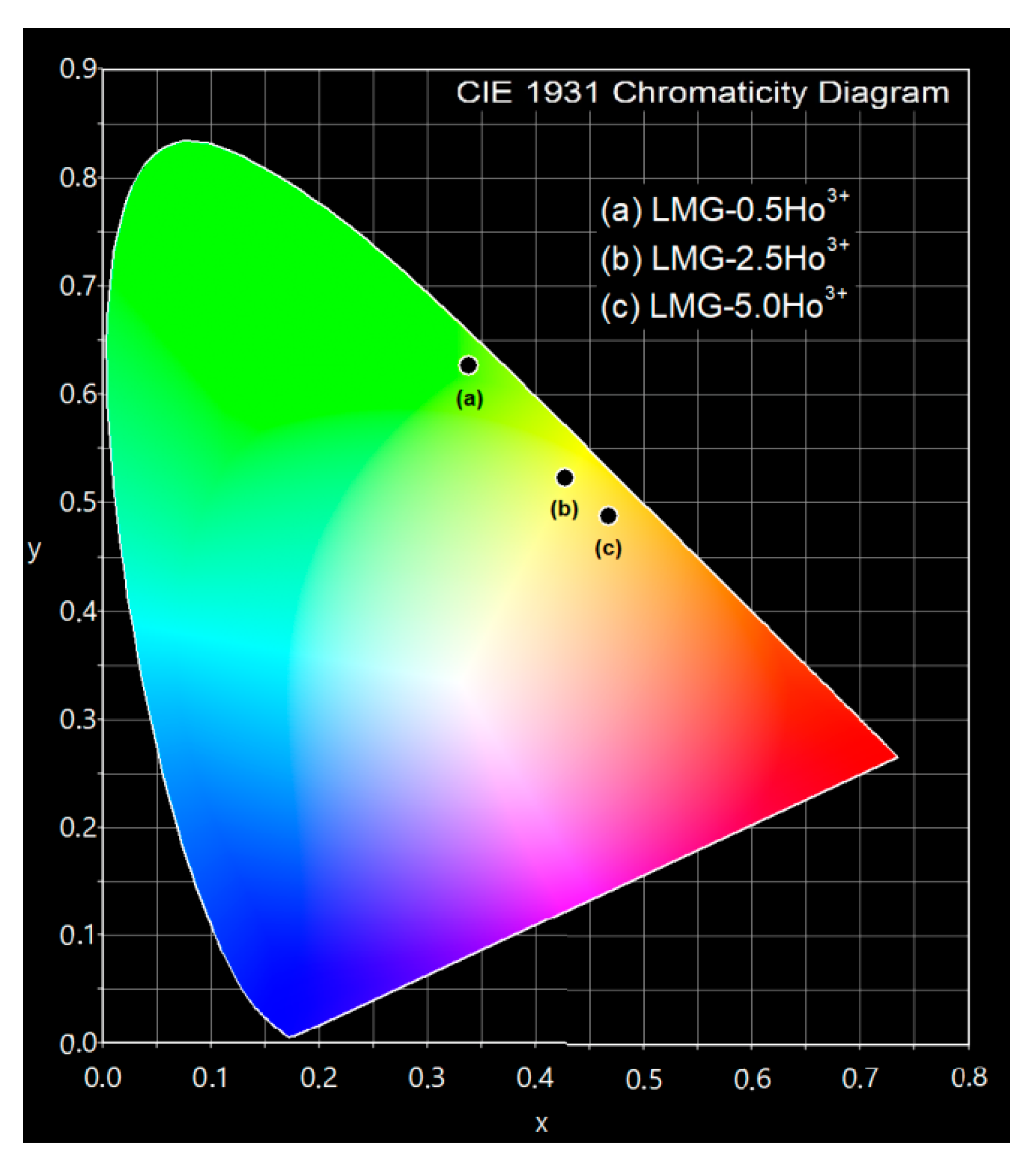
| Ceramic Code | CIE Coordinates | |
|---|---|---|
| x | y | |
| LMG-0.5Ho3+ LMG-2.5Ho3+ LMG-5.0Ho3+ | 0.338 0.427 0.468 | 0.627 0.523 0.488 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarska-Adam, N.; Kuwik, M.; Pietrasik, E.; Pisarski, W.A.; Goryczka, T.; Macalik, B.; Pisarska, J. Synthesis and Characterization of Li2MgGeO4:Ho3+. Materials 2022, 15, 5263. https://doi.org/10.3390/ma15155263
Bednarska-Adam N, Kuwik M, Pietrasik E, Pisarski WA, Goryczka T, Macalik B, Pisarska J. Synthesis and Characterization of Li2MgGeO4:Ho3+. Materials. 2022; 15(15):5263. https://doi.org/10.3390/ma15155263
Chicago/Turabian StyleBednarska-Adam, Nikola, Marta Kuwik, Ewa Pietrasik, Wojciech A. Pisarski, Tomasz Goryczka, Bogusław Macalik, and Joanna Pisarska. 2022. "Synthesis and Characterization of Li2MgGeO4:Ho3+" Materials 15, no. 15: 5263. https://doi.org/10.3390/ma15155263
APA StyleBednarska-Adam, N., Kuwik, M., Pietrasik, E., Pisarski, W. A., Goryczka, T., Macalik, B., & Pisarska, J. (2022). Synthesis and Characterization of Li2MgGeO4:Ho3+. Materials, 15(15), 5263. https://doi.org/10.3390/ma15155263







