A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Surgical Procedure
2.3. Evaluation of Grafted Volume
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- A peri-implant dehiscence defect was successfully reconstructed by GBR with double-layering of allograft and ABBM, which were covered by a preformed ultrafine titanium mesh and an absorbable collagen membrane;
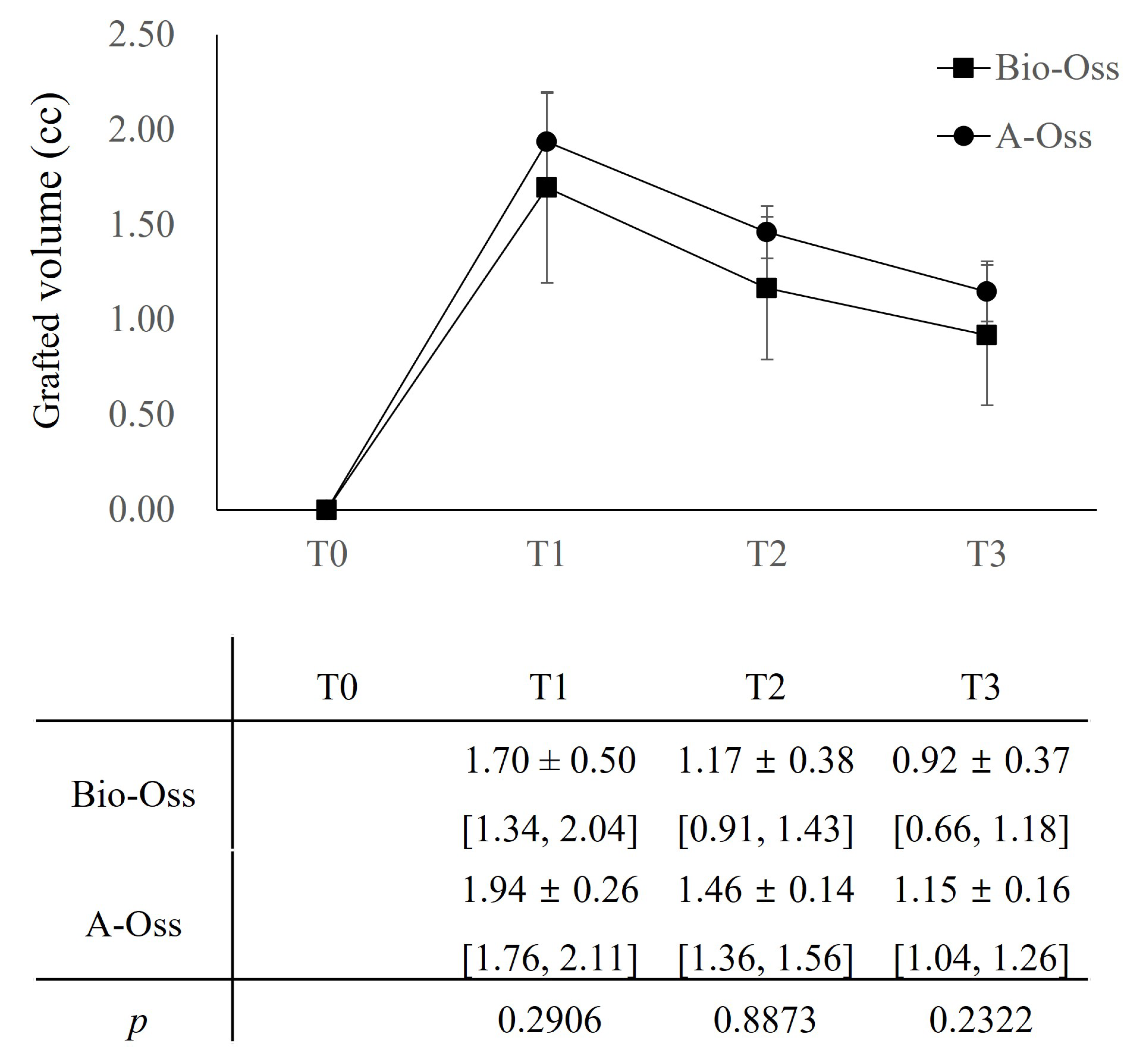
- Despite the volume maintenance effect of ABBMs, approximately 27.8% of grafted volume resorption was noted at T2, and there was no significant difference between the Bio-Oss and A-Oss groups, even in the quality of the regenerated bone;
- The grafted volume loss (approximately 43.2%) continued up to T3 and did not differ between the Bio-Oss and A-Oss groups.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDP s) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.; Caplanis, N.; Lozada, J.L. Simultaneous vertical guided bone regeneration and guided tissue regeneration in the posterior maxilla using recombinant human platelet-derived growth factor: A case report. J. Oral Implantol. 2009, 35, 251–256. [Google Scholar] [CrossRef]
- Adeyemo, W.; Reuther, T.; Bloch, W.; Korkmaz, Y.; Fischer, J.H.; Zöller, J.E.; Kuebler, A.C. Healing of onlay mandibular bone grafts covered with collagen membrane or bovine bone substitutes: A microscopical and immunohistochemical study in the sheep. Int. J. Oral Maxillofac. Surg. 2008, 37, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S.; Kaleem, A. Use of sintered xenograft over allograft for ridge augmentation: Technique note. J. Oral Maxillofac. Surg. 2014, 72, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Chappuis, V.; Kuchler, U.; Bornstein, M.; Wittneben, J.; Buser, R.; Cavusoglu, Y.; Belser, U.C. Long-term stability of early implant placement with contour augmentation. J. Dent. Res. 2013, 92, 176S–182S. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Bosshardt, D.D.; Gruber, R.; Buser, D. Long-term stability of contour augmentation in the esthetic zone: Histologic and histomorphometric evaluation of 12 human biopsies 14 to 80 months after augmentation. J. Periodontol. 2014, 85, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Hwang, K.G.; Jun, S.H.; Tallarico, M.; Kwon, A.M.; Park, C.J. Radiologic comparative analysis between saline and platelet-rich fibrin filling after hydraulic transcrestal sinus lifting without adjunctive bone graft: A randomized controlled trial. Clin. Oral Implant. Res. 2020, 31, 1087–1093. [Google Scholar] [CrossRef]
- Nevins, M.; Nevins, M.L.; Kim, S.-W.; Schupbach, P.; Kim, D.M. The use of mucograft collagen matrix to augment the zone of keratinized tissue around teeth: A pilot study. Int. J. Periodontics Restor. Dent. 2011, 31, 367–373. [Google Scholar]
- Lee, J.S.; Shin, H.K.; Yun, J.H.; Cho, K.S. Randomized clinical trial of maxillary sinus grafting using deproteinized porcine and bovine bone mineral. Clin. Implant. Dent. Relat. Res. 2017, 19, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Susin, C.; Lee, J.; Fiorini, T.; Koo, K.T.; Schüpbach, P.; Angst, P.D.; Finger Stadler, A.; Wikesjö, U.M. Screening of candidate biomaterials for alveolar augmentation using a critical-size rat calvaria defect model. J. Clin. Periodontol. 2018, 45, 884–893. [Google Scholar] [CrossRef]
- Amara, H.B.; Lee, J.-W.; Kim, J.-J.; Kang, Y.-M.; Kang, E.-J.; Koo, K.-T. Influence of rhBMP-2 on Guided Bone Regeneration for Placement and Functional Loading of Dental Implants: A Radiographic and Histologic Study in Dogs. Int. J. Oral Maxillofac. Implant. 2017, 32, e265–e276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.S.; Kim, H.S.; Um, S.H.; Rhee, S.H. Preparation of a novel anorganic bovine bone xenograft with enhanced bioactivity and osteoconductivity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Kang, T.; Fien, M.J. Titanium mesh as an alternative to a membrane for ridge augmentation. J. Oral Maxillofac. Surg. 2012, 70, 803–810. [Google Scholar] [CrossRef]
- Louis, P.J.; Gutta, R.; Said-Al-Naief, N.; Bartolucci, A.A. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J. Oral Maxillofac. Surg. 2008, 66, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Jung, G.-U.; Jeon, J.-Y.; Hwang, K.-G.; Park, C.-J. Preliminary evaluation of a three-dimensional, customized, and preformed titanium mesh in peri-implant alveolar bone regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 181. [Google Scholar] [CrossRef]
- Lee, S.-H.; Moon, J.-H.; Jeong, C.-M.; Bae, E.-B.; Park, C.-E.; Jeon, G.-R.; Lee, J.-J.; Jeon, Y.-C.; Huh, J.-B. The mechanical properties and biometrical effect of 3D preformed titanium membrane for guided bone regeneration on alveolar bone defect. BioMed Res. Int. 2017, 2017, 7102123. [Google Scholar] [CrossRef] [Green Version]
- Tallarico, M.; Ceruso, F.M.; Muzzi, L.; Meloni, S.M.; Kim, Y.-J.; Gargari, M.; Martinolli, M. Effect of simultaneous immediate implant placement and guided bone reconstruction with ultra-fine titanium mesh membranes on radiographic and clinical parameters after 18 months of loading. Materials 2019, 12, 1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265. [Google Scholar]
- Liyanage, L.; Lee, N.J.; Cook, T.; Herrmann, H.C.; Jagasia, D.; Litt, H.; Han, Y. The impact of gender on cardiovascular system calcification in very elderly patients with severe aortic stenosis. Int. J. Cardiovasc. Imaging 2016, 32, 173–179. [Google Scholar] [CrossRef]
- Lodewick, T.M.; Arnoldussen, C.W.; Lahaye, M.J.; van Mierlo, K.M.; Neumann, U.P.; Beets-Tan, R.G.; Dejong, C.H.; van Dam, R.M. Fast and accurate liver volumetry prior to hepatectomy. HPB 2016, 18, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, K.-M.; Lee, J.-K.; Choi, S.-H.; Kim, Y.-K.; Kim, B.-J.; Lee, J.-H. Maxillary sinus augmentation with calcium phosphate double-coated anorganic bovine bone: Comparative multicenter randomized clinical trial with histological and radiographic evaluation. Implant. Dent. 2019, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ohe, J.Y.; Kim, G.T.; Lee, J.W.; Al Nawas, B.; Jung, J.; Kwon, Y.D. Volume stability of hydroxyapatite and β-tricalcium phosphate biphasic bone graft material in maxillary sinus floor elevation: A radiographic study using 3D cone beam computed tomography. Clin. Oral Implant. Res. 2016, 27, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Wui, H.; Jung, R.E.; Hämmerle, C.H.; Benic, G.I. Influence of blinded wound closure on the volume stability of different GBR materials: An in vitro cone-beam computed tomographic examination. Clin. Oral Implant. Res. 2016, 27, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Benic, G.I.; Valmaseda-Castellón, E.; Hämmerle, C.H.; Jung, R.E. Influence of wound closure on the volume stability of particulate and non-particulate GBR materials: An in vitro cone-beam computed tomographic examination. Part II. Clin. Oral Implant. Res. 2017, 28, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, M.; Maghaireh, H.; Grusovin, M.G.; Ziounas, I.; Worthington, H.V. Soft tissue management for dental implants: What are the most effective techniques? A Cochrane systematic review. Eur. J. Oral. Implant. 2012, 5, 221–238. [Google Scholar]
- Jemt, T. Implant failures and age at the time of surgery: A retrospective study on implant treatment in 2915 partially edentulous jaws. Clin. Implant. Dent. Relat. Res. 2019, 21, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implant. 2009, 24, 218–236. [Google Scholar]
- Scheyer, E.T.; Heard, R.; Janakievski, J.; Mandelaris, G.; Nevins, M.L.; Pickering, S.R.; Richardson, C.R.; Pope, B.; Toback, G.; Velásquez, D.; et al. A randomized, controlled, multicentre clinical trial of post-extraction alveolar ridge preservation. J. Clin. Periodontol. 2016, 43, 1188–1199. [Google Scholar] [CrossRef]
- Wang, H.-L.; Misch, C.; Neiva, R.F. “Sandwich” bone augmentation technique: Rationale and report of pilot cases. Int. J. Periodontics Restor. Dent. 2004, 24, 232–245. [Google Scholar]
- Sarnachiaro, G.O.; Chu, S.J.; Sarnachiaro, E.; Gotta, S.L.; Tarnow, D.P. Immediate implant placement into extraction sockets with labial plate dehiscence defects: A clinical case series. Clin. Implant. Dent. Relat. Res. 2016, 18, 821–829. [Google Scholar] [CrossRef]
- Meloni, S.M.; Jovanovic, S.A.; Urban, I.; Baldoni, E.; Pisano, M.; Tallarico, M. Horizontal ridge augmentation using GBR with a native collagen membrane and 1:1 ratio of particulate xenograft and autologous bone: A 3-year after final loading prospective clinical study. Clin. Implant. Dent. Relat. Res. 2019, 21, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. Histological and histomorphometric analysis of bone tissue after guided bone regeneration with non-resorbable membranes vs resorbable membranes and titanium mesh. Clin. Implant. Dent. Relat. Res. 2019, 21, 693–701. [Google Scholar] [CrossRef]
- Proussaefs, P.; Lozada, J. Use of titanium mesh for staged localized alveolar ridge augmentation: Clinical and histologic-histomorphometric evaluation. J. Oral Implantol. 2006, 32, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Sagheb, K.; Schiegnitz, E.; Moergel, M.; Walter, C.; Al-Nawas, B.; Wagner, W. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int. J. Implant. Dent. 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| ABBMs | Patient | Site (FDI) | Implant Body (mm) | Defect Size (mm) | Grafted Volume (cc) | Probing Depth (mm) | Titanium Mesh | Collagen Membrane | Sequelae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (M:F) | Age (Year) | Width | Length | Width | Length | T0 | T1 | T2 | T3 | C:H | Type 1:2 | OG:OM | Class I:II | ||||
| Bio-Oss group | 1 | F | 63 | 36 | 5 | 7 | 6 | 3 | 1.14 | 0.91 | 0.80 | 3.5 | H | 2 | M | ||
| 2 | Dropped | ||||||||||||||||
| 3 | M | 56 | 16 | 4.5 | 8.5 | 5 | 4.5 | 1.53 | 1.11 | 0.89 | 2 | H | 1 | G | |||
| 4 | M | 60 | 23 | 4 | 8.5 | 6.5 | 4.5 | 1.91 | 1.40 | 1.10 | 3 | C | 2 | G | I | ||
| 5 | F | 54 | 42 | 4 | 8.5 | 4 | 4 | 0.00 | 1.16 | 0.83 | 0.60 | 2.5 | H | 1 | M | ||
| 6 | F | 55 | 11 | 4 | 10 | 4 | 4 | 2.41 | 1.32 | 0.85 | 3 | H | 1 | M | |||
| 7 | M | 51 | 16 | 4.5 | 8.5 | 4.5 | 3 | 1.33 | 0.91 | 0.67 | 2 | H | 1 | G | |||
| 8 | F | 28 | 12 | 4 | 8.5 | 5.5. | 3 | 2.37 | 1.94 | 1.75 | 2 | C | 2 | M | |||
| 9 | F | 63 | 46 | 5 | 7 | 7 | 4 | 1.72 | 0.91 | 0.70 | 4 | C | 2 | M | II | ||
| A-Oss group | 1 | M | 30 | 46 | 5 | 10 | 5 | 3 | 1.53 | 1.29 | 1.17 | 3 | H | 1 | G | ||
| 2 | F | 54 | 16 | 4.5 | 8.5 | 6.5 | 5 | 2.04 | 1.51 | 1.20 | 3.5 | H | 2 | G | |||
| 3 | M | 50 | 42 | 4 | 8.5 | 6 | 3.5 | 1.98 | 1.67 | 1.25 | 2 | C | 2 | M | |||
| 4 | Dropped | ||||||||||||||||
| 5 | M | 53 | 35 | 4 | 10 | 6 | 6 | 0.00 | 1.73 | 1.39 | 1.18 | 2 | H | 2 | M | ||
| 6 | F | 64 | 15 | 4 | 8.5 | 6 | 7 | 2.12 | 1.60 | 1.37 | 2 | H | 2 | G | |||
| 7 | F | 65 | 25 | 4 | 10 | 6.5 | 2.5 | 1.73 | 1.29 | 1.18 | 1.5 | C | 2 | M | |||
| 8 | F | 72 | 21 | 4 | 8.5 | 4 | 5.5 | 2.03 | 1.42 | 0.87 | 3 | H | 1 | M | |||
| 9 | M | 50 | 33 | 4 | 8.5 | 6 | 4 | 2.33 | 1.51 | 0.97 | 4 | C | 2 | M | I | ||
| Variables | Bio-Oss | A-Oss | p-Value | |
|---|---|---|---|---|
| Sex | (Male) | 3 (37.5) | 4 (50.0) | 0.6143 |
| (Female) | 5 (62.5) | 4 (50.0) | ||
| Age | (Years) | 53.8 ± 10.8 | 54.8 ± 12.2 | 0.9258 |
| Surgical site | (Maxilla) | 5 (62.5) | 4 (50.0) | 0.6143 |
| (Mandible) | 3 (37.5) | 4 (50.0) | ||
| (Anterior) | 4 (50.0) | 3 (37.5) | 0.6143 | |
| (Posterior) | 4 (50.0) | 5 (62.5) | ||
| Defect | (Width) | 5.3 ± 1.1 | 5.8 ± 0.8 | 0.1542 |
| (Height) | 3.8 ± 0.6 | 4.6 ± 1.5 | 0.0892 | |
| Implant (mm) | (Width) | 4.4 ± 0.4 | 4.2 ± 0.4 | 0.0850 |
| (Length) | 8.3 ± 0.9 | 9.1 ± 0.7 | 0.0050 * | |
| Probing depth (mm) | (mm) | 2.8 ± 0.7 | 2.6 ± 0.8 | 0.5001 |
| Titanium mesh | (Cover cap) | 3 (37.5) | 4 (50.0) | 0.6143 |
| (Healing cap) | 5 (62.5) | 4 (50.0) | ||
| Type I (Buccal only) | 4 (50.0) | 2 (25.0) | 0.2369 | |
| Type II (Bucco-proximal) | 4 (50.0) | 6 (75.0) | ||
| Sequelae | (Yes) | 2 (25.0) | 1 (12.5) | 0.4103 |
| Estimates | T Value | p-Value | |
|---|---|---|---|
| T2—T3 | −0.2244 | −5.02 | 0.0007 * |
| T2—T1 | −2.3194 | −15.62 | <0.0001 * |
| T3—T1 | −2.0950 | −16.87 | <0.0001 * |
| Effects | Estimates | T Value | p-Value | |
|---|---|---|---|---|
| Group | −0.2112 | −1.12 | 0.2906 | |
| Time | (T2) | −2.4125 | −11.49 | <0.0001 *** |
| (T3) | −2.2475 | −12.80 | <0.0001 *** | |
| Group × Time † | (T2) | 0.1863 | 0.63 | 0.5460 |
| (T3) | 0.3050 | 1.23 | 0.2505 | |
| Age | −0.0045 | −4.17 | 0.0024 ** | |
| Sequelae | (Yes) | −0.1184 | −2.79 | 0.0210 * |
| Defect | (Width) | 0.0796 | 4.58 | 0.0013 ** |
| Surgical site | (Posterior) | −0.0624 | −2.17 | 0.0583 |
| (Maxilla) | 0.0684 | 2.77 | 0.0218 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Jun, S.H.; Tallarico, M.; Park, J.-B.; Park, D.-H.; Hwang, K.-G.; Park, C.-J. A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials 2022, 15, 5294. https://doi.org/10.3390/ma15155294
Lim J, Jun SH, Tallarico M, Park J-B, Park D-H, Hwang K-G, Park C-J. A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials. 2022; 15(15):5294. https://doi.org/10.3390/ma15155294
Chicago/Turabian StyleLim, JaeHyung, Sang Ho Jun, Marco Tallarico, Jun-Beom Park, Dae-Ho Park, Kyung-Gyun Hwang, and Chang-Joo Park. 2022. "A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes" Materials 15, no. 15: 5294. https://doi.org/10.3390/ma15155294
APA StyleLim, J., Jun, S. H., Tallarico, M., Park, J.-B., Park, D.-H., Hwang, K.-G., & Park, C.-J. (2022). A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials, 15(15), 5294. https://doi.org/10.3390/ma15155294









