A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Surgical Procedure
2.3. Evaluation of Grafted Volume
2.4. Statistical Analysis
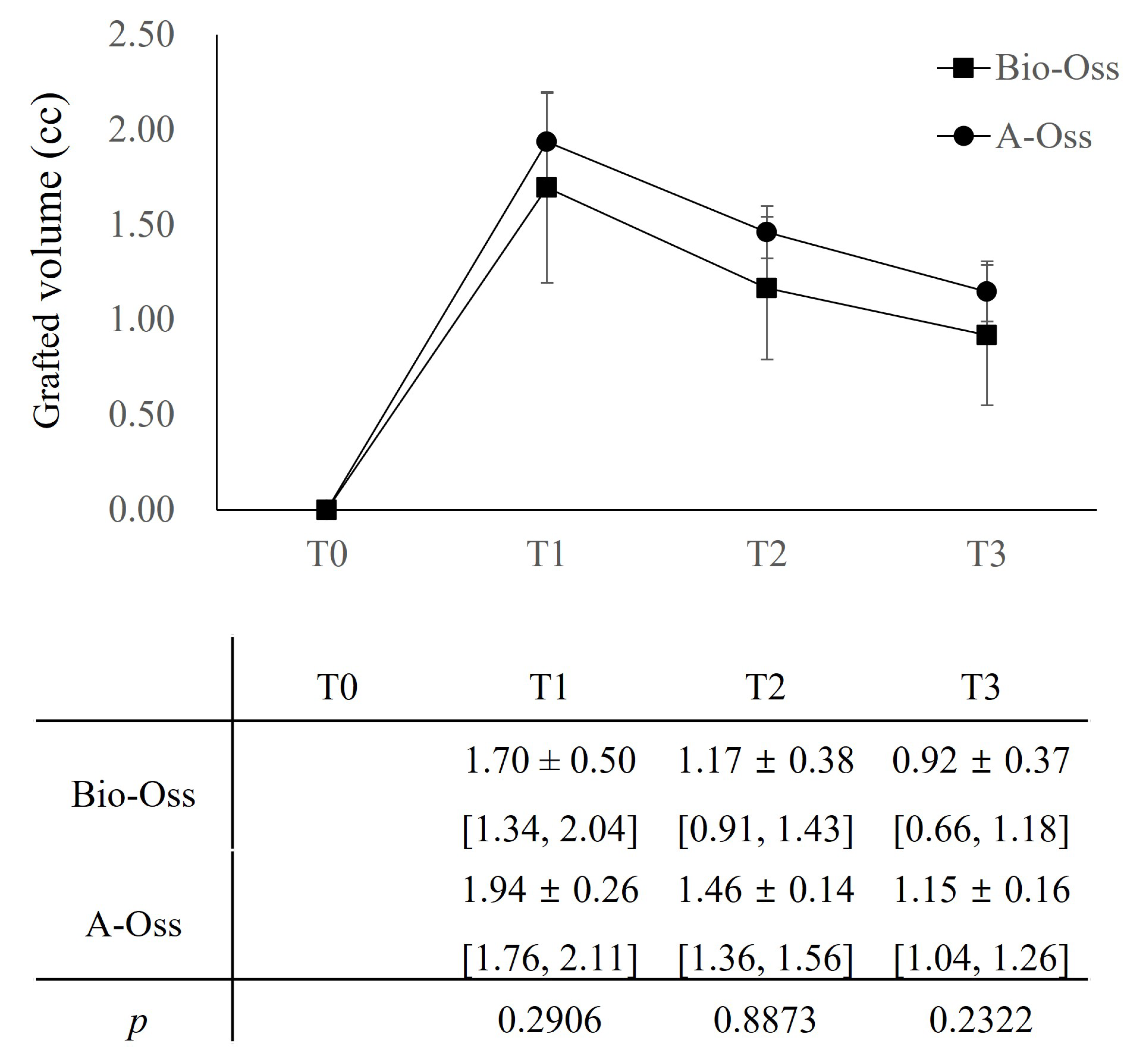
3. Results
4. Discussion
5. Conclusions
- A peri-implant dehiscence defect was successfully reconstructed by GBR with double-layering of allograft and ABBM, which were covered by a preformed ultrafine titanium mesh and an absorbable collagen membrane;
- Despite the volume maintenance effect of ABBMs, approximately 27.8% of grafted volume resorption was noted at T2, and there was no significant difference between the Bio-Oss and A-Oss groups, even in the quality of the regenerated bone;
- The grafted volume loss (approximately 43.2%) continued up to T3 and did not differ between the Bio-Oss and A-Oss groups.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDP s) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.; Caplanis, N.; Lozada, J.L. Simultaneous vertical guided bone regeneration and guided tissue regeneration in the posterior maxilla using recombinant human platelet-derived growth factor: A case report. J. Oral Implantol. 2009, 35, 251–256. [Google Scholar] [CrossRef]
- Adeyemo, W.; Reuther, T.; Bloch, W.; Korkmaz, Y.; Fischer, J.H.; Zöller, J.E.; Kuebler, A.C. Healing of onlay mandibular bone grafts covered with collagen membrane or bovine bone substitutes: A microscopical and immunohistochemical study in the sheep. Int. J. Oral Maxillofac. Surg. 2008, 37, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S.; Kaleem, A. Use of sintered xenograft over allograft for ridge augmentation: Technique note. J. Oral Maxillofac. Surg. 2014, 72, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Chappuis, V.; Kuchler, U.; Bornstein, M.; Wittneben, J.; Buser, R.; Cavusoglu, Y.; Belser, U.C. Long-term stability of early implant placement with contour augmentation. J. Dent. Res. 2013, 92, 176S–182S. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Bosshardt, D.D.; Gruber, R.; Buser, D. Long-term stability of contour augmentation in the esthetic zone: Histologic and histomorphometric evaluation of 12 human biopsies 14 to 80 months after augmentation. J. Periodontol. 2014, 85, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Hwang, K.G.; Jun, S.H.; Tallarico, M.; Kwon, A.M.; Park, C.J. Radiologic comparative analysis between saline and platelet-rich fibrin filling after hydraulic transcrestal sinus lifting without adjunctive bone graft: A randomized controlled trial. Clin. Oral Implant. Res. 2020, 31, 1087–1093. [Google Scholar] [CrossRef]
- Nevins, M.; Nevins, M.L.; Kim, S.-W.; Schupbach, P.; Kim, D.M. The use of mucograft collagen matrix to augment the zone of keratinized tissue around teeth: A pilot study. Int. J. Periodontics Restor. Dent. 2011, 31, 367–373. [Google Scholar]
- Lee, J.S.; Shin, H.K.; Yun, J.H.; Cho, K.S. Randomized clinical trial of maxillary sinus grafting using deproteinized porcine and bovine bone mineral. Clin. Implant. Dent. Relat. Res. 2017, 19, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Susin, C.; Lee, J.; Fiorini, T.; Koo, K.T.; Schüpbach, P.; Angst, P.D.; Finger Stadler, A.; Wikesjö, U.M. Screening of candidate biomaterials for alveolar augmentation using a critical-size rat calvaria defect model. J. Clin. Periodontol. 2018, 45, 884–893. [Google Scholar] [CrossRef]
- Amara, H.B.; Lee, J.-W.; Kim, J.-J.; Kang, Y.-M.; Kang, E.-J.; Koo, K.-T. Influence of rhBMP-2 on Guided Bone Regeneration for Placement and Functional Loading of Dental Implants: A Radiographic and Histologic Study in Dogs. Int. J. Oral Maxillofac. Implant. 2017, 32, e265–e276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, J.S.; Kim, H.S.; Um, S.H.; Rhee, S.H. Preparation of a novel anorganic bovine bone xenograft with enhanced bioactivity and osteoconductivity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Kang, T.; Fien, M.J. Titanium mesh as an alternative to a membrane for ridge augmentation. J. Oral Maxillofac. Surg. 2012, 70, 803–810. [Google Scholar] [CrossRef]
- Louis, P.J.; Gutta, R.; Said-Al-Naief, N.; Bartolucci, A.A. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J. Oral Maxillofac. Surg. 2008, 66, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef]
- Jung, G.-U.; Jeon, J.-Y.; Hwang, K.-G.; Park, C.-J. Preliminary evaluation of a three-dimensional, customized, and preformed titanium mesh in peri-implant alveolar bone regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 181. [Google Scholar] [CrossRef]
- Lee, S.-H.; Moon, J.-H.; Jeong, C.-M.; Bae, E.-B.; Park, C.-E.; Jeon, G.-R.; Lee, J.-J.; Jeon, Y.-C.; Huh, J.-B. The mechanical properties and biometrical effect of 3D preformed titanium membrane for guided bone regeneration on alveolar bone defect. BioMed Res. Int. 2017, 2017, 7102123. [Google Scholar] [CrossRef]
- Tallarico, M.; Ceruso, F.M.; Muzzi, L.; Meloni, S.M.; Kim, Y.-J.; Gargari, M.; Martinolli, M. Effect of simultaneous immediate implant placement and guided bone reconstruction with ultra-fine titanium mesh membranes on radiographic and clinical parameters after 18 months of loading. Materials 2019, 12, 1710. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265. [Google Scholar]
- Liyanage, L.; Lee, N.J.; Cook, T.; Herrmann, H.C.; Jagasia, D.; Litt, H.; Han, Y. The impact of gender on cardiovascular system calcification in very elderly patients with severe aortic stenosis. Int. J. Cardiovasc. Imaging 2016, 32, 173–179. [Google Scholar] [CrossRef]
- Lodewick, T.M.; Arnoldussen, C.W.; Lahaye, M.J.; van Mierlo, K.M.; Neumann, U.P.; Beets-Tan, R.G.; Dejong, C.H.; van Dam, R.M. Fast and accurate liver volumetry prior to hepatectomy. HPB 2016, 18, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.-M.; Lee, J.-K.; Choi, S.-H.; Kim, Y.-K.; Kim, B.-J.; Lee, J.-H. Maxillary sinus augmentation with calcium phosphate double-coated anorganic bovine bone: Comparative multicenter randomized clinical trial with histological and radiographic evaluation. Implant. Dent. 2019, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ohe, J.Y.; Kim, G.T.; Lee, J.W.; Al Nawas, B.; Jung, J.; Kwon, Y.D. Volume stability of hydroxyapatite and β-tricalcium phosphate biphasic bone graft material in maxillary sinus floor elevation: A radiographic study using 3D cone beam computed tomography. Clin. Oral Implant. Res. 2016, 27, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Wui, H.; Jung, R.E.; Hämmerle, C.H.; Benic, G.I. Influence of blinded wound closure on the volume stability of different GBR materials: An in vitro cone-beam computed tomographic examination. Clin. Oral Implant. Res. 2016, 27, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Benic, G.I.; Valmaseda-Castellón, E.; Hämmerle, C.H.; Jung, R.E. Influence of wound closure on the volume stability of particulate and non-particulate GBR materials: An in vitro cone-beam computed tomographic examination. Part II. Clin. Oral Implant. Res. 2017, 28, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Maghaireh, H.; Grusovin, M.G.; Ziounas, I.; Worthington, H.V. Soft tissue management for dental implants: What are the most effective techniques? A Cochrane systematic review. Eur. J. Oral. Implant. 2012, 5, 221–238. [Google Scholar]
- Jemt, T. Implant failures and age at the time of surgery: A retrospective study on implant treatment in 2915 partially edentulous jaws. Clin. Implant. Dent. Relat. Res. 2019, 21, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implant. 2009, 24, 218–236. [Google Scholar]
- Scheyer, E.T.; Heard, R.; Janakievski, J.; Mandelaris, G.; Nevins, M.L.; Pickering, S.R.; Richardson, C.R.; Pope, B.; Toback, G.; Velásquez, D.; et al. A randomized, controlled, multicentre clinical trial of post-extraction alveolar ridge preservation. J. Clin. Periodontol. 2016, 43, 1188–1199. [Google Scholar] [CrossRef]
- Wang, H.-L.; Misch, C.; Neiva, R.F. “Sandwich” bone augmentation technique: Rationale and report of pilot cases. Int. J. Periodontics Restor. Dent. 2004, 24, 232–245. [Google Scholar]
- Sarnachiaro, G.O.; Chu, S.J.; Sarnachiaro, E.; Gotta, S.L.; Tarnow, D.P. Immediate implant placement into extraction sockets with labial plate dehiscence defects: A clinical case series. Clin. Implant. Dent. Relat. Res. 2016, 18, 821–829. [Google Scholar] [CrossRef]
- Meloni, S.M.; Jovanovic, S.A.; Urban, I.; Baldoni, E.; Pisano, M.; Tallarico, M. Horizontal ridge augmentation using GBR with a native collagen membrane and 1:1 ratio of particulate xenograft and autologous bone: A 3-year after final loading prospective clinical study. Clin. Implant. Dent. Relat. Res. 2019, 21, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. Histological and histomorphometric analysis of bone tissue after guided bone regeneration with non-resorbable membranes vs resorbable membranes and titanium mesh. Clin. Implant. Dent. Relat. Res. 2019, 21, 693–701. [Google Scholar] [CrossRef]
- Proussaefs, P.; Lozada, J. Use of titanium mesh for staged localized alveolar ridge augmentation: Clinical and histologic-histomorphometric evaluation. J. Oral Implantol. 2006, 32, 237–247. [Google Scholar] [CrossRef]
- Sagheb, K.; Schiegnitz, E.; Moergel, M.; Walter, C.; Al-Nawas, B.; Wagner, W. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int. J. Implant. Dent. 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]




| ABBMs | Patient | Site (FDI) | Implant Body (mm) | Defect Size (mm) | Grafted Volume (cc) | Probing Depth (mm) | Titanium Mesh | Collagen Membrane | Sequelae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (M:F) | Age (Year) | Width | Length | Width | Length | T0 | T1 | T2 | T3 | C:H | Type 1:2 | OG:OM | Class I:II | ||||
| Bio-Oss group | 1 | F | 63 | 36 | 5 | 7 | 6 | 3 | 1.14 | 0.91 | 0.80 | 3.5 | H | 2 | M | ||
| 2 | Dropped | ||||||||||||||||
| 3 | M | 56 | 16 | 4.5 | 8.5 | 5 | 4.5 | 1.53 | 1.11 | 0.89 | 2 | H | 1 | G | |||
| 4 | M | 60 | 23 | 4 | 8.5 | 6.5 | 4.5 | 1.91 | 1.40 | 1.10 | 3 | C | 2 | G | I | ||
| 5 | F | 54 | 42 | 4 | 8.5 | 4 | 4 | 0.00 | 1.16 | 0.83 | 0.60 | 2.5 | H | 1 | M | ||
| 6 | F | 55 | 11 | 4 | 10 | 4 | 4 | 2.41 | 1.32 | 0.85 | 3 | H | 1 | M | |||
| 7 | M | 51 | 16 | 4.5 | 8.5 | 4.5 | 3 | 1.33 | 0.91 | 0.67 | 2 | H | 1 | G | |||
| 8 | F | 28 | 12 | 4 | 8.5 | 5.5. | 3 | 2.37 | 1.94 | 1.75 | 2 | C | 2 | M | |||
| 9 | F | 63 | 46 | 5 | 7 | 7 | 4 | 1.72 | 0.91 | 0.70 | 4 | C | 2 | M | II | ||
| A-Oss group | 1 | M | 30 | 46 | 5 | 10 | 5 | 3 | 1.53 | 1.29 | 1.17 | 3 | H | 1 | G | ||
| 2 | F | 54 | 16 | 4.5 | 8.5 | 6.5 | 5 | 2.04 | 1.51 | 1.20 | 3.5 | H | 2 | G | |||
| 3 | M | 50 | 42 | 4 | 8.5 | 6 | 3.5 | 1.98 | 1.67 | 1.25 | 2 | C | 2 | M | |||
| 4 | Dropped | ||||||||||||||||
| 5 | M | 53 | 35 | 4 | 10 | 6 | 6 | 0.00 | 1.73 | 1.39 | 1.18 | 2 | H | 2 | M | ||
| 6 | F | 64 | 15 | 4 | 8.5 | 6 | 7 | 2.12 | 1.60 | 1.37 | 2 | H | 2 | G | |||
| 7 | F | 65 | 25 | 4 | 10 | 6.5 | 2.5 | 1.73 | 1.29 | 1.18 | 1.5 | C | 2 | M | |||
| 8 | F | 72 | 21 | 4 | 8.5 | 4 | 5.5 | 2.03 | 1.42 | 0.87 | 3 | H | 1 | M | |||
| 9 | M | 50 | 33 | 4 | 8.5 | 6 | 4 | 2.33 | 1.51 | 0.97 | 4 | C | 2 | M | I | ||
| Variables | Bio-Oss | A-Oss | p-Value | |
|---|---|---|---|---|
| Sex | (Male) | 3 (37.5) | 4 (50.0) | 0.6143 |
| (Female) | 5 (62.5) | 4 (50.0) | ||
| Age | (Years) | 53.8 ± 10.8 | 54.8 ± 12.2 | 0.9258 |
| Surgical site | (Maxilla) | 5 (62.5) | 4 (50.0) | 0.6143 |
| (Mandible) | 3 (37.5) | 4 (50.0) | ||
| (Anterior) | 4 (50.0) | 3 (37.5) | 0.6143 | |
| (Posterior) | 4 (50.0) | 5 (62.5) | ||
| Defect | (Width) | 5.3 ± 1.1 | 5.8 ± 0.8 | 0.1542 |
| (Height) | 3.8 ± 0.6 | 4.6 ± 1.5 | 0.0892 | |
| Implant (mm) | (Width) | 4.4 ± 0.4 | 4.2 ± 0.4 | 0.0850 |
| (Length) | 8.3 ± 0.9 | 9.1 ± 0.7 | 0.0050 * | |
| Probing depth (mm) | (mm) | 2.8 ± 0.7 | 2.6 ± 0.8 | 0.5001 |
| Titanium mesh | (Cover cap) | 3 (37.5) | 4 (50.0) | 0.6143 |
| (Healing cap) | 5 (62.5) | 4 (50.0) | ||
| Type I (Buccal only) | 4 (50.0) | 2 (25.0) | 0.2369 | |
| Type II (Bucco-proximal) | 4 (50.0) | 6 (75.0) | ||
| Sequelae | (Yes) | 2 (25.0) | 1 (12.5) | 0.4103 |
| Estimates | T Value | p-Value | |
|---|---|---|---|
| T2—T3 | −0.2244 | −5.02 | 0.0007 * |
| T2—T1 | −2.3194 | −15.62 | <0.0001 * |
| T3—T1 | −2.0950 | −16.87 | <0.0001 * |
| Effects | Estimates | T Value | p-Value | |
|---|---|---|---|---|
| Group | −0.2112 | −1.12 | 0.2906 | |
| Time | (T2) | −2.4125 | −11.49 | <0.0001 *** |
| (T3) | −2.2475 | −12.80 | <0.0001 *** | |
| Group × Time † | (T2) | 0.1863 | 0.63 | 0.5460 |
| (T3) | 0.3050 | 1.23 | 0.2505 | |
| Age | −0.0045 | −4.17 | 0.0024 ** | |
| Sequelae | (Yes) | −0.1184 | −2.79 | 0.0210 * |
| Defect | (Width) | 0.0796 | 4.58 | 0.0013 ** |
| Surgical site | (Posterior) | −0.0624 | −2.17 | 0.0583 |
| (Maxilla) | 0.0684 | 2.77 | 0.0218 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Jun, S.H.; Tallarico, M.; Park, J.-B.; Park, D.-H.; Hwang, K.-G.; Park, C.-J. A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials 2022, 15, 5294. https://doi.org/10.3390/ma15155294
Lim J, Jun SH, Tallarico M, Park J-B, Park D-H, Hwang K-G, Park C-J. A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials. 2022; 15(15):5294. https://doi.org/10.3390/ma15155294
Chicago/Turabian StyleLim, JaeHyung, Sang Ho Jun, Marco Tallarico, Jun-Beom Park, Dae-Ho Park, Kyung-Gyun Hwang, and Chang-Joo Park. 2022. "A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes" Materials 15, no. 15: 5294. https://doi.org/10.3390/ma15155294
APA StyleLim, J., Jun, S. H., Tallarico, M., Park, J.-B., Park, D.-H., Hwang, K.-G., & Park, C.-J. (2022). A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials, 15(15), 5294. https://doi.org/10.3390/ma15155294









