Study on SO42−/Cl− Erosion Resistance and Mechanism of Recycled Concrete Containing Municipal Solid Waste Incineration (MSWI) Powder
Abstract
:1. Introduction
2. Materials and Test Methods
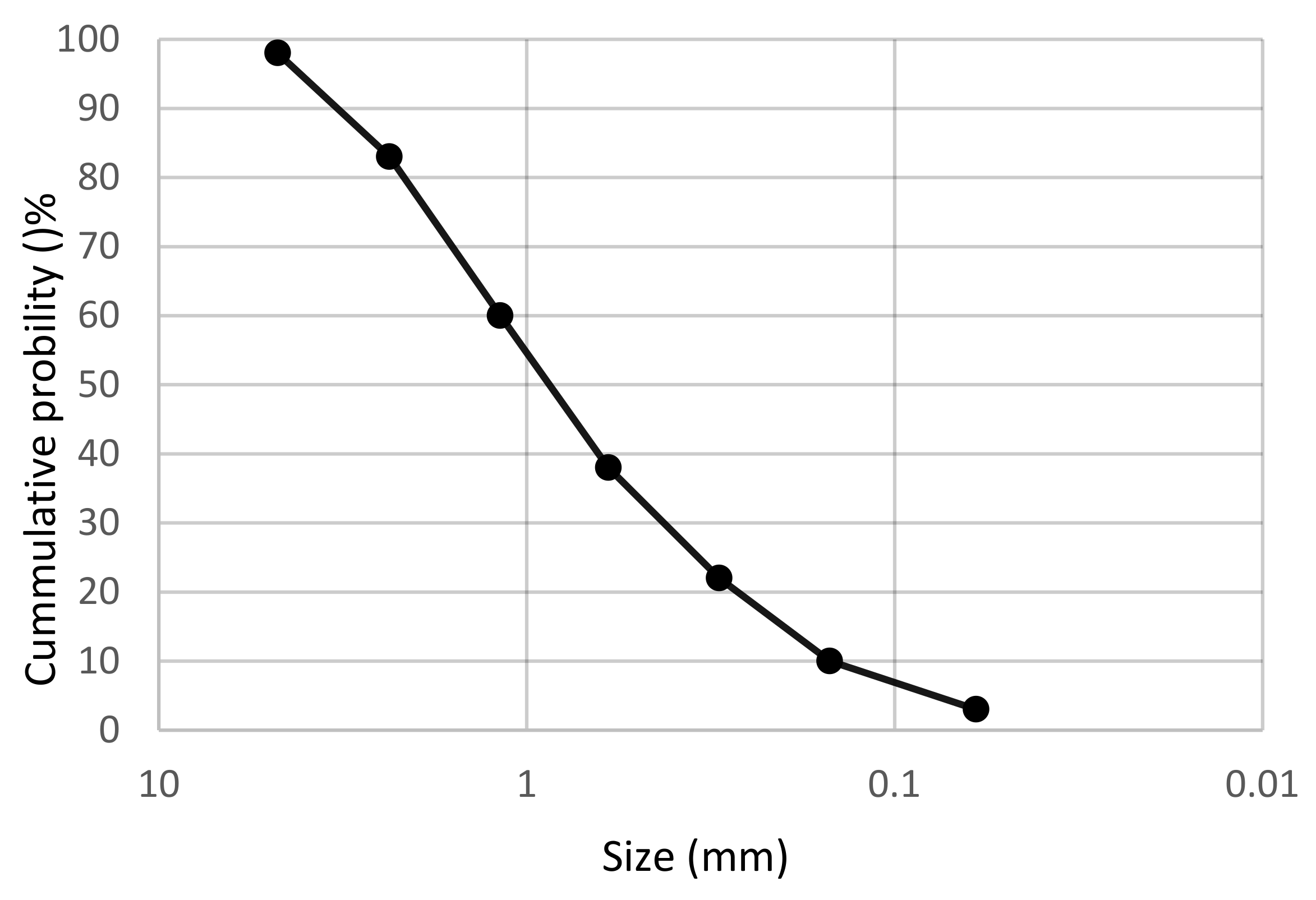
2.1. Material
2.2. Test Method
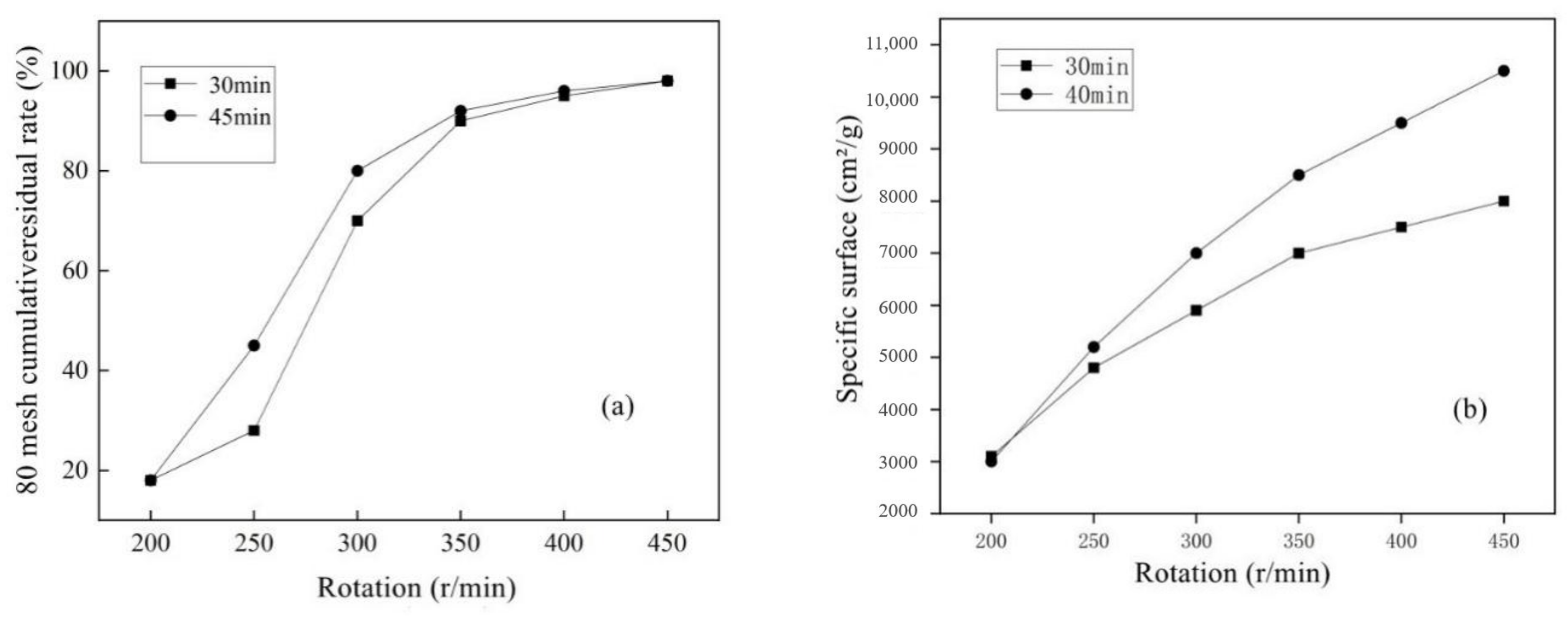
2.2.1. Determination of Pretreatment Process
2.2.2. Strength Test of MSWI Slag Regenerated Micro-Powder
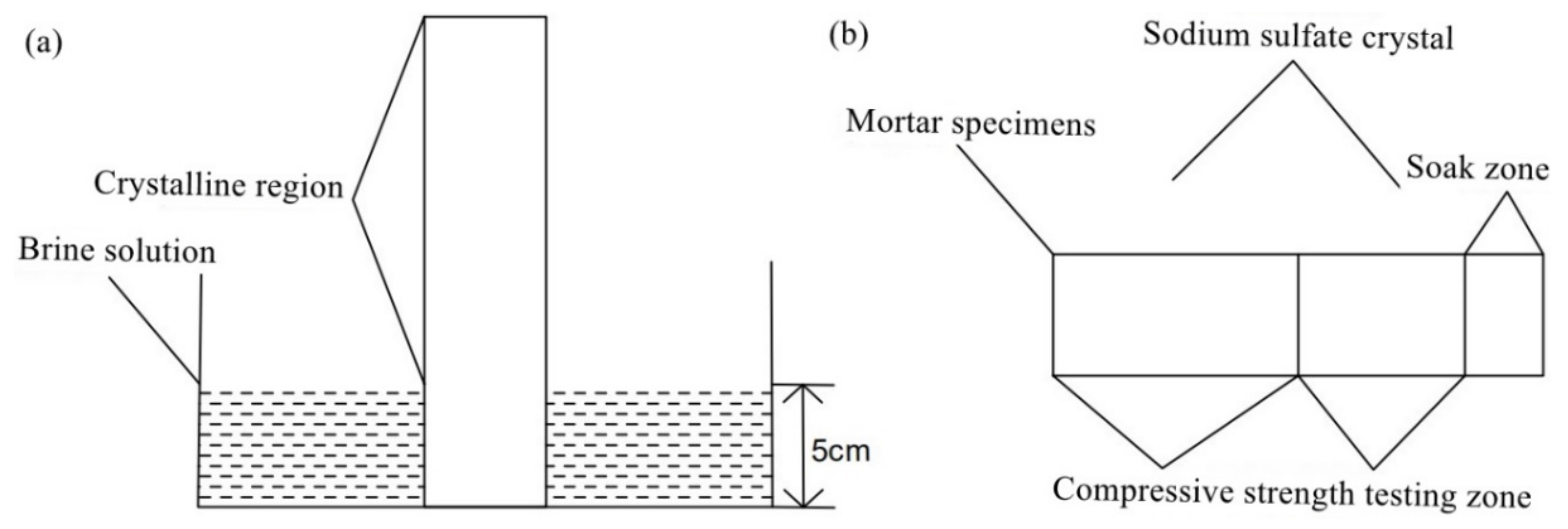
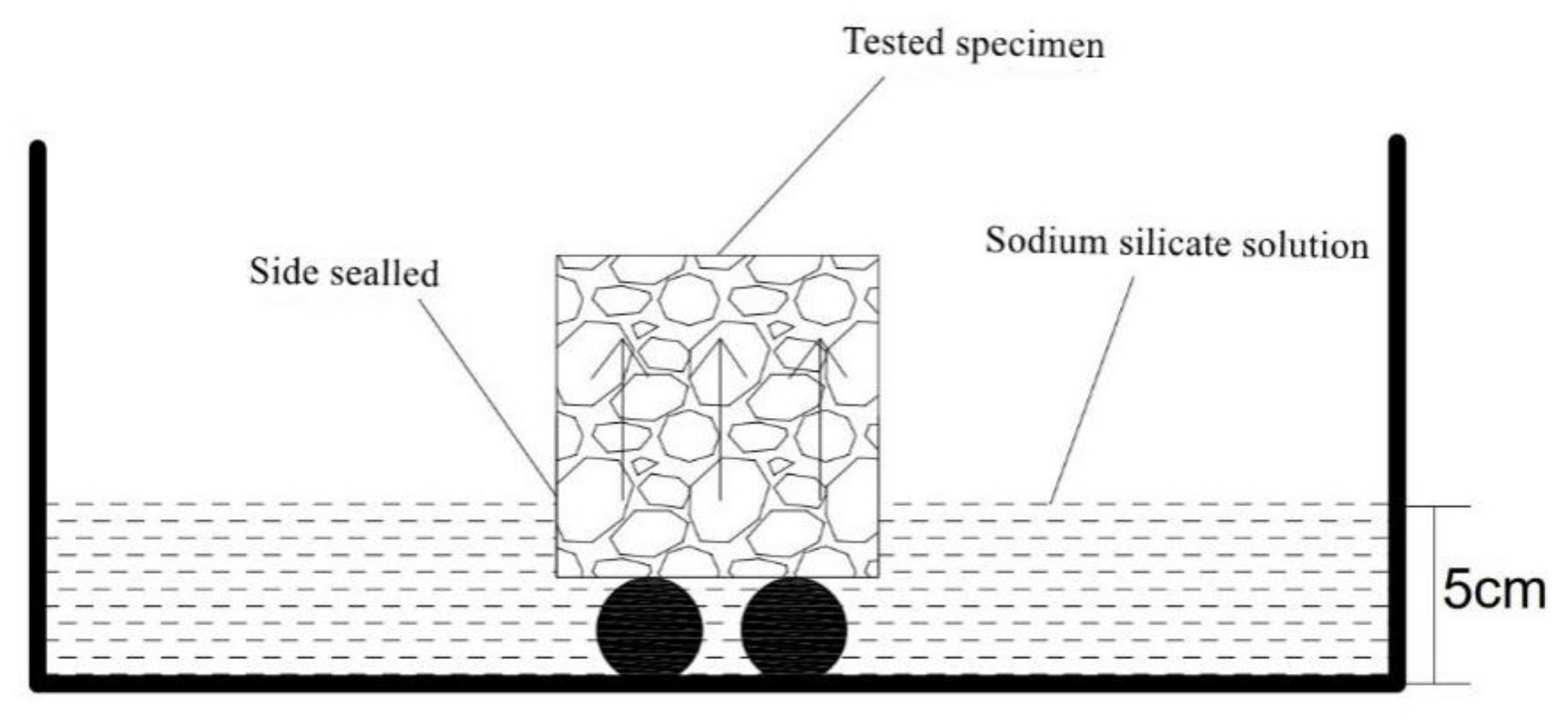
2.2.3. Erosion Test of Mortar Containing MSWI
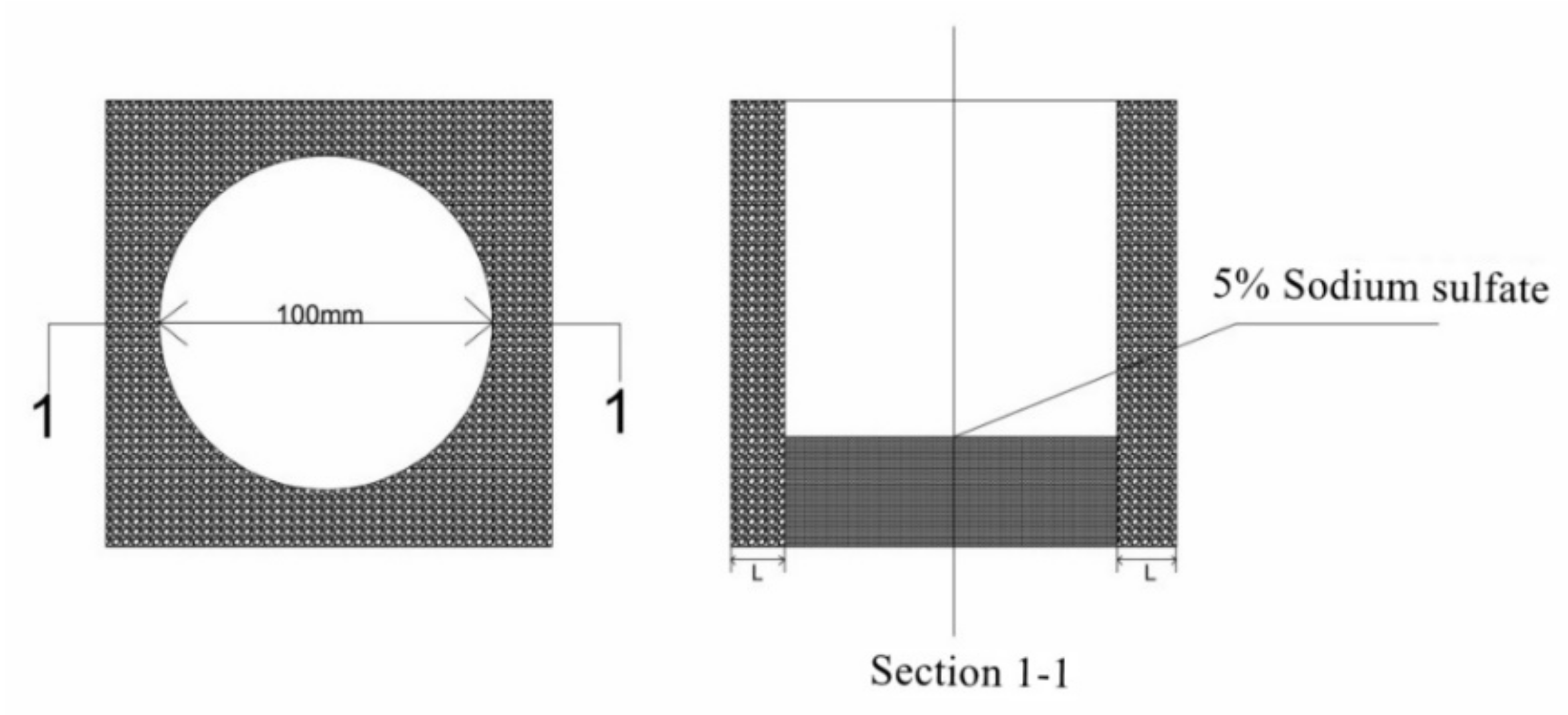
2.2.4. Strength of Recycled Concrete Containing MSWI
3. Results and Discussion
3.1. Determination of Pretreatment Process
3.2. Strength Test Results of Mortar Containing MSWI
3.3. Test Results of Cl− and SO42− Erosion Resistance of Mortar Containing MSWI
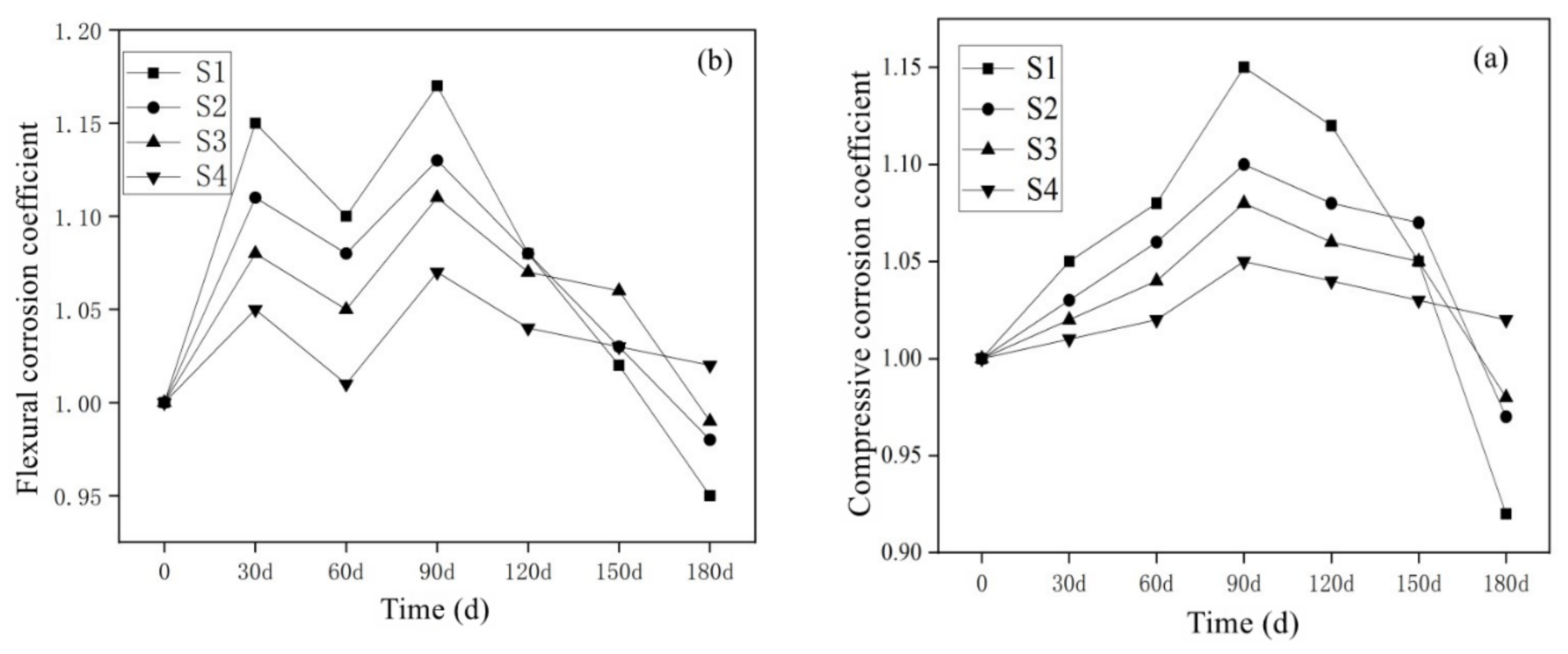
3.3.1. Results of SO42− Corrosion Resistance of Mortar Containing MSWI
3.3.2. Results of Cl− Corrosion Resistance of Mortar Containing MSWI
3.4. MSWI Recycled Concrete
4. Conclusions
- With the increase in MSWI powder content, the compressive strength and flexural strength of mortar specimens at various ages showed a decreasing trend, and the reduction in flexural strength was larger than that of compressive strength. The micro-powder had little influence on the strength of recycled mortar in the early stage, but it had significant influences on the strength in the later stage. The strength activity index of regenerated powder was about 55% when the content of regenerated powder was 30%.
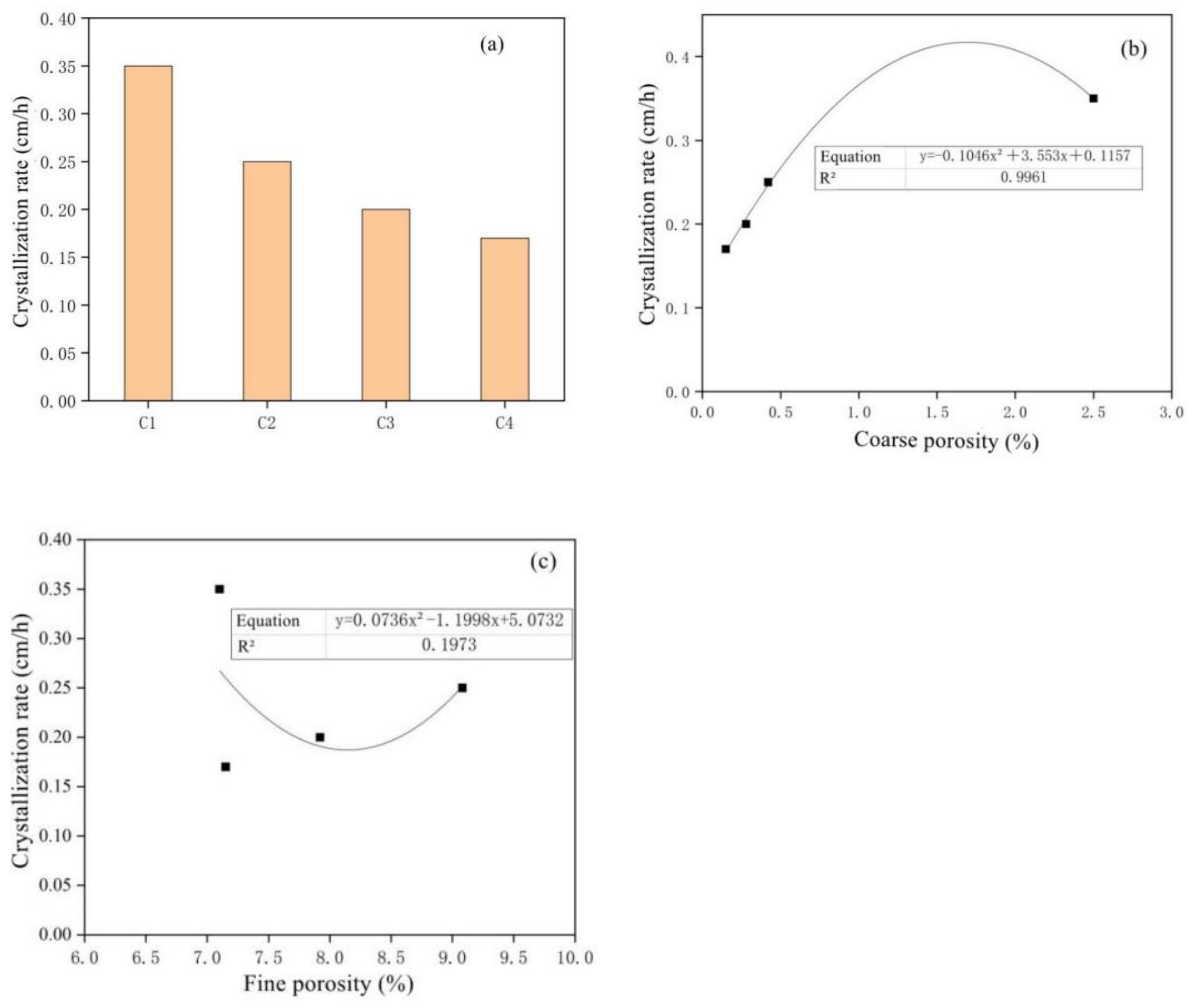
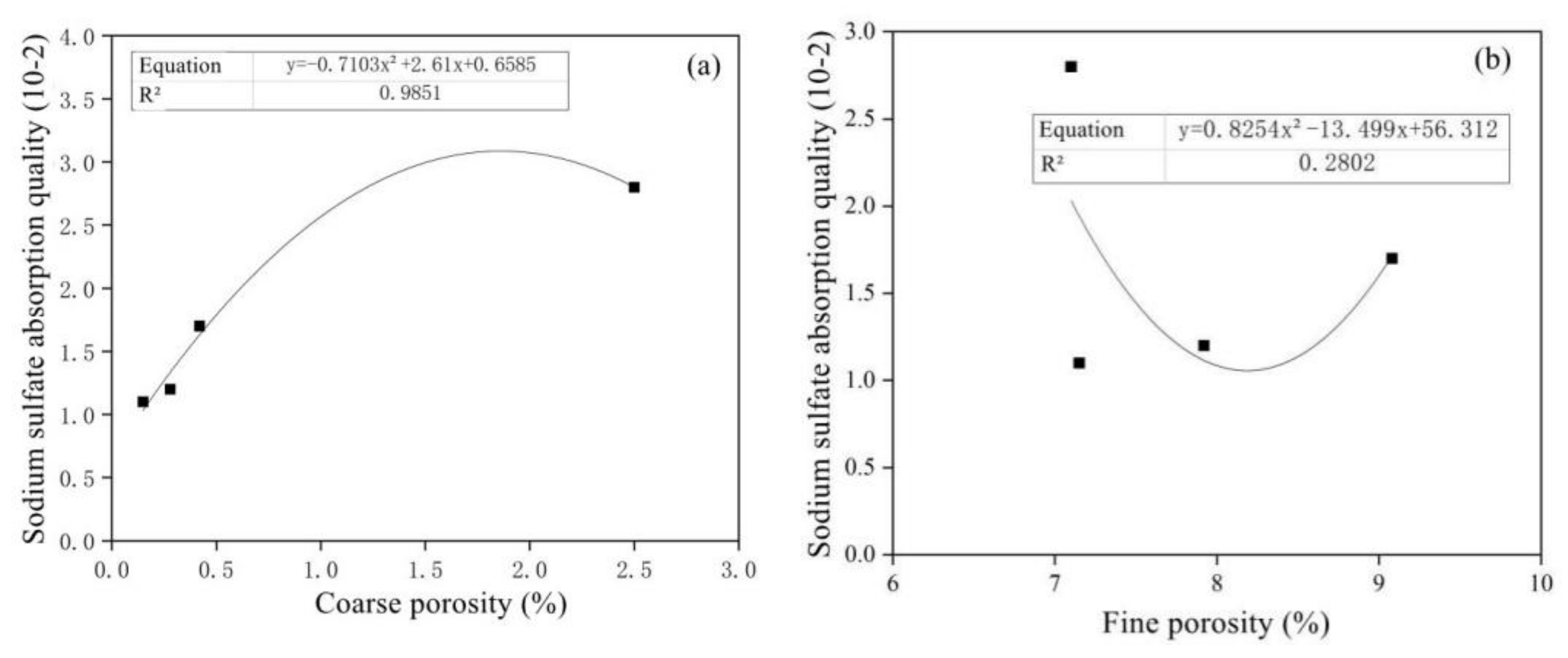
- With the passage of the sample’s half-soaking time in sodium sulfate solutions, the mass loss rate of mortar decreased gradually with the increase in MSWI content, while the corrosion resistance coefficient increased first and then decreased. Under the condition of constant w/c ratio, the salt crystal erosion resistance of mortar can be improved effectively with the addition of MSWI powder.
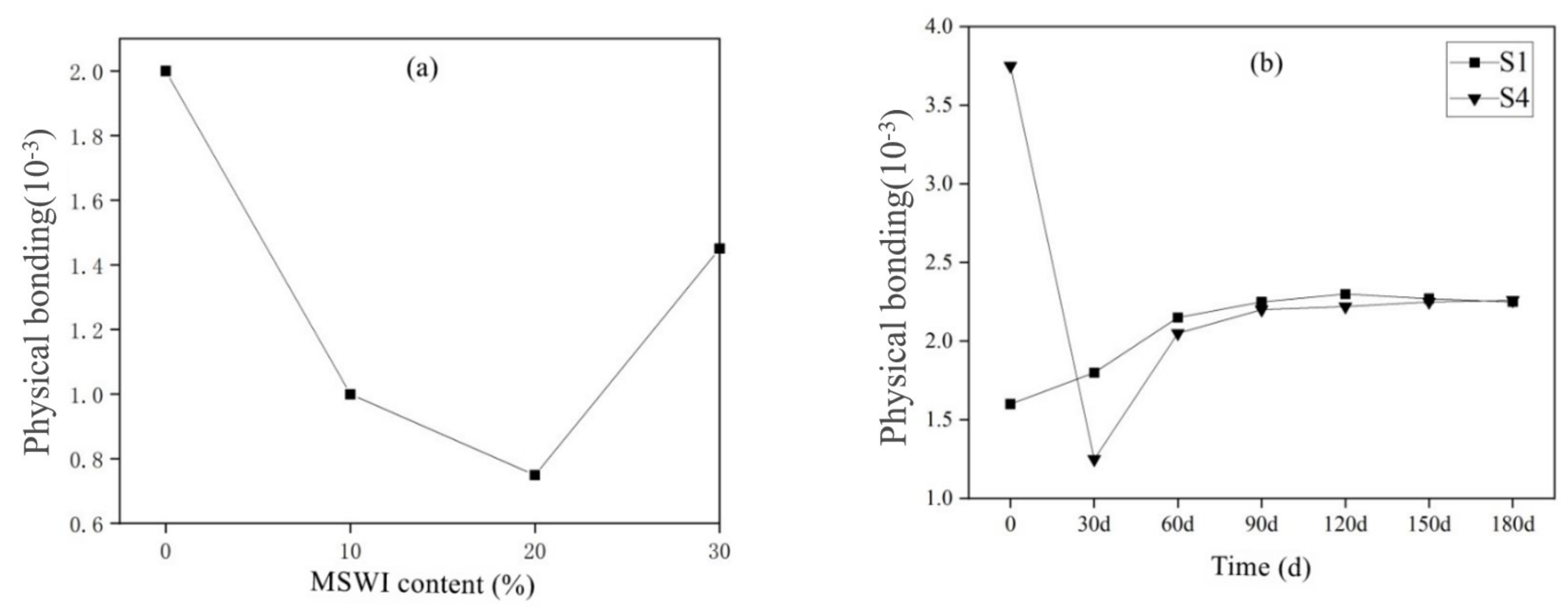
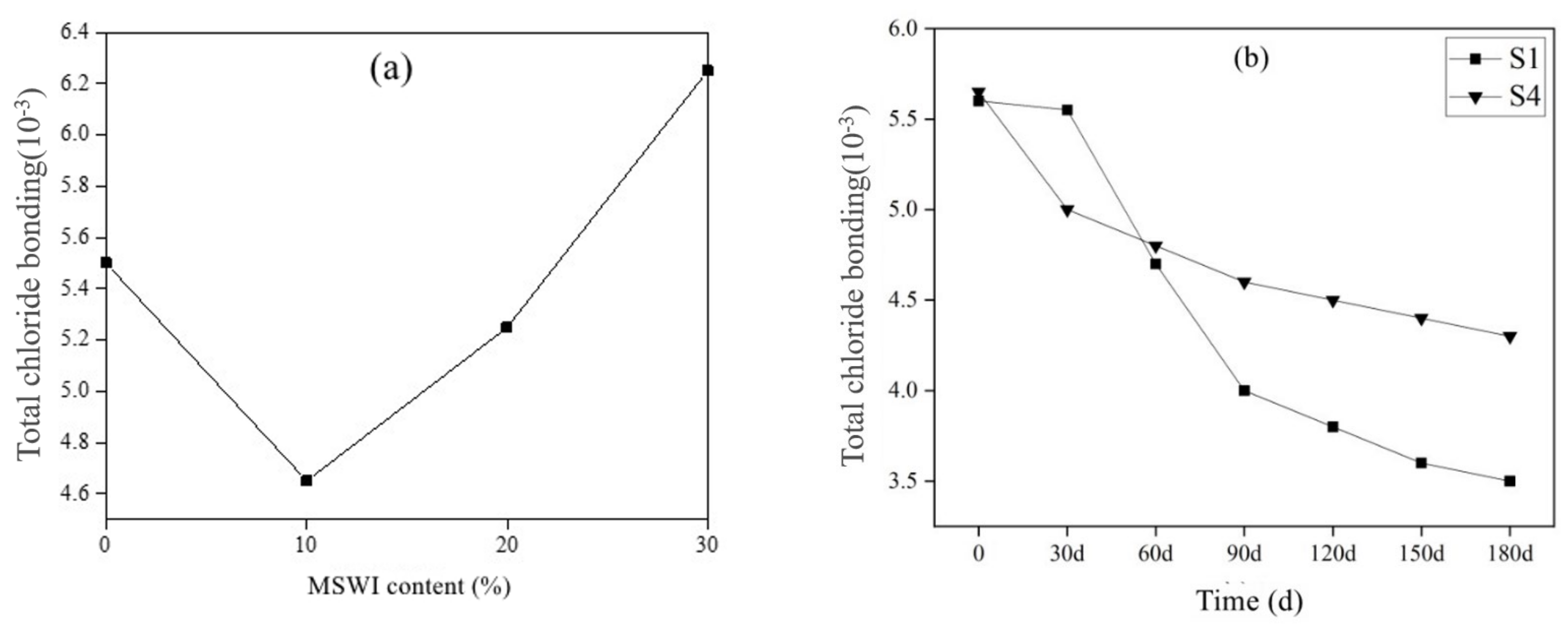
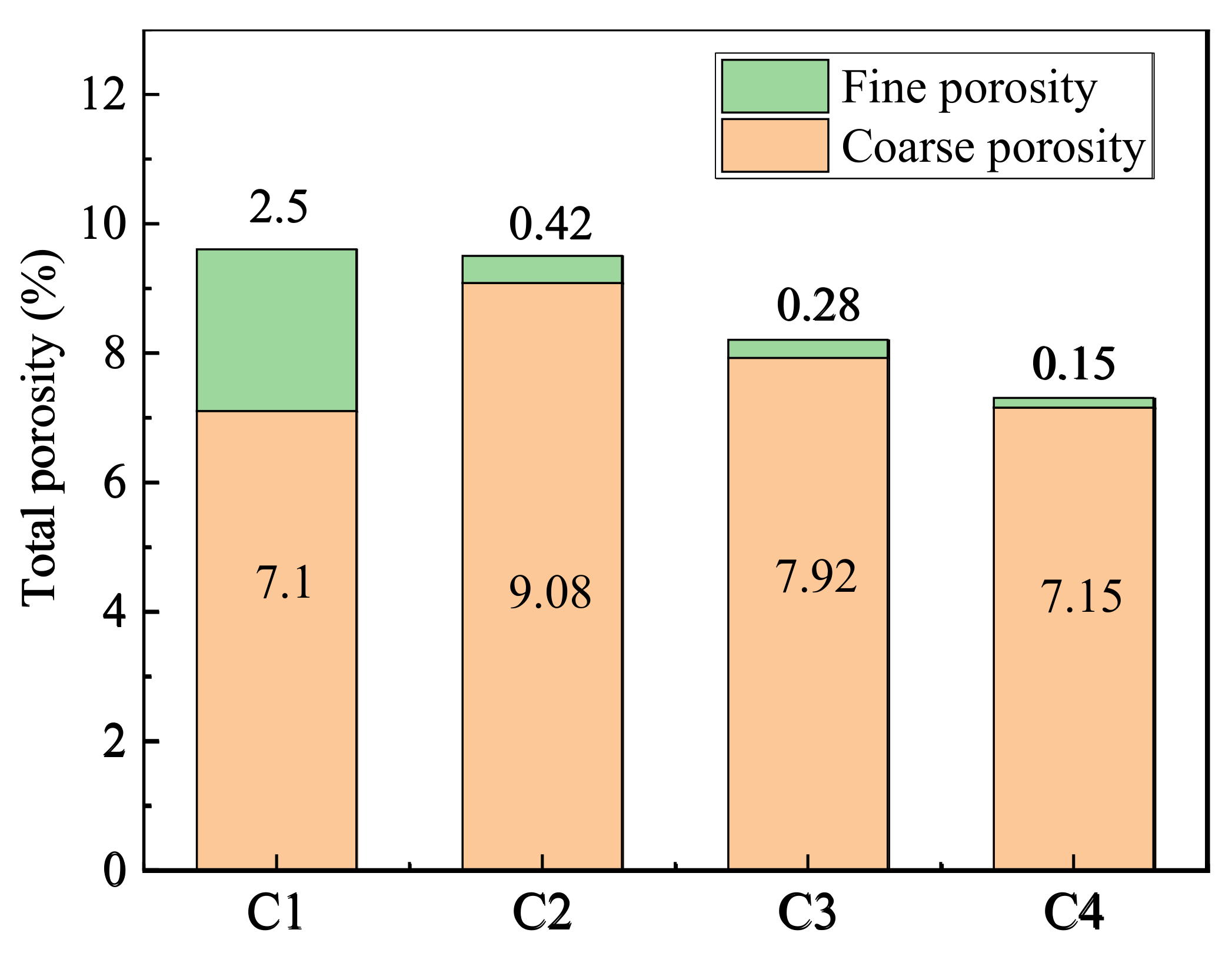
- The cement-based materials containing MSWI had a high ability to bind chloride ions. With the aging of curing, the performance of cement-based materials to bind chloride ions decreased. The total porosity, coarse capillary porosity and fine capillary porosity of recycled concrete decreased with increasing MSWI powder content.
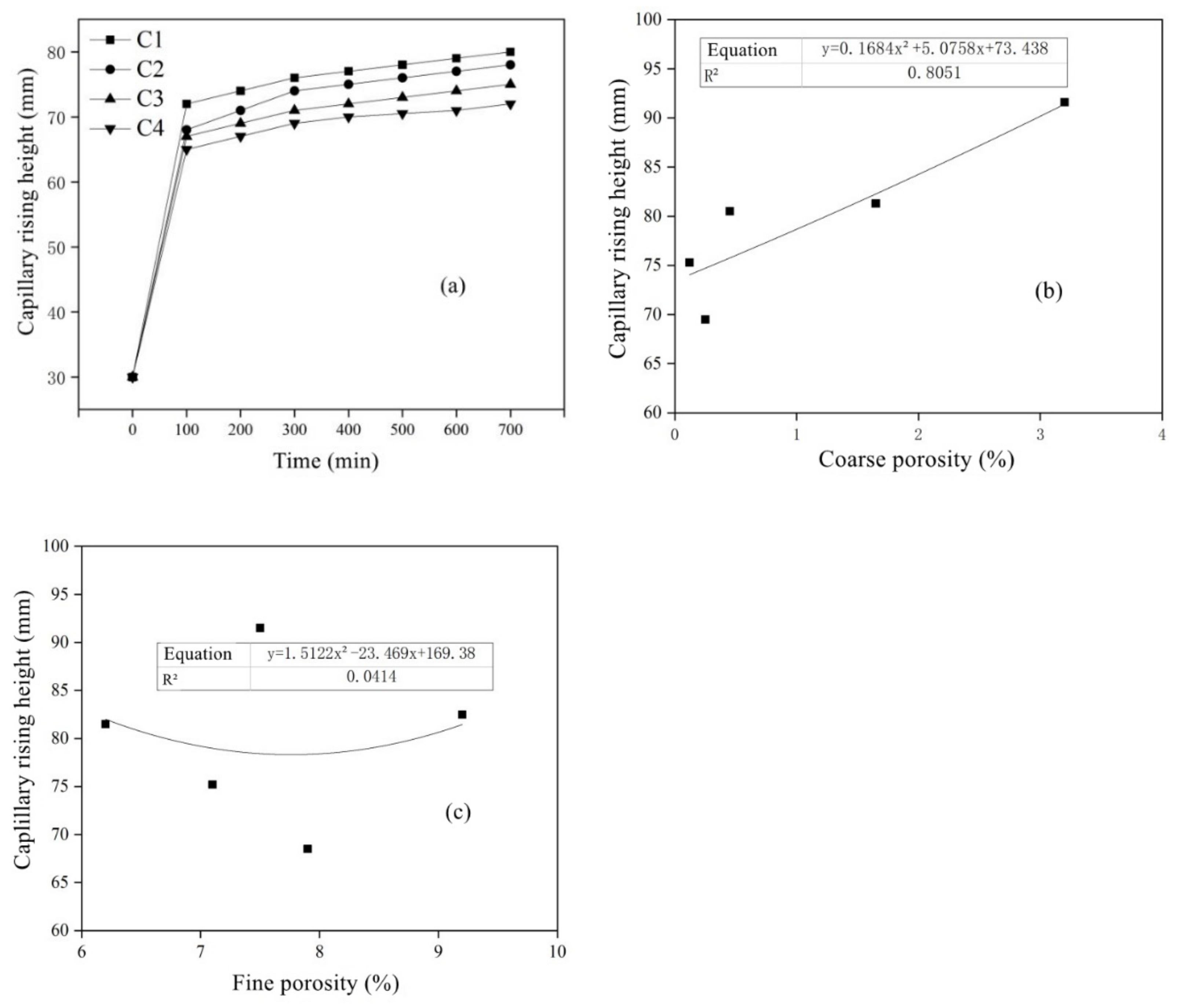
- The amount of MSWI powder can affect the capillary height, absorption and crystallization rate of sulfate solution in concrete. The capillary rise height, osmotic crystallization rate and 5% sodium sulfate solution were linearly correlated with capillary porosity, but they were linearly independent of capillary porosity; thus, capillary porosity played a key role in capillary transport, migration and the crystallization of sulfate solution in recycled concrete.
- The MSWI powder had a certain strength activity, and it was feasible to replace part of the cement as a mineral admixture. It was not only beneficial to reduce the environmental problems caused by MSWI bottom slag, in line with the concept of green and sustainable development, but it also can make concrete more dense and improve the SO42−/Cl− erosion resistance of concrete.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, H.; Jin, Z.; Wang, P.; Wang, J.; Liu, J. Comprehensive resistance of fair-faced concrete suffering from sulfate attack under marine environments. Constr. Build. Mater. 2021, 277, 122312. [Google Scholar]
- Zhang, W.; Xiao, B.; Hu, D.; Zhou, H.; Zheng, J.; Zhang, F. Deterioration of the performance of cement mortar lining due to sulfate attack. Environ. Geotech. 2020, 40, 1–10. [Google Scholar] [CrossRef]
- Dehwah, H.A.F. Effect of sulfate concentration and associated cation type on concrete deterioration and morphological changes in cement hydrates. Constr. Build. Mater. 2007, 21, 29–39. [Google Scholar] [CrossRef]
- Shahmansouri, A.A.; Akbarzadeh Bengar, H.; AzariJafari, H. Life cycle assessment of eco-friendly concrete mixtures incorporating natural zeolite in sulfate-aggressive environment. Constr. Build. Mater. 2021, 268, 121136. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Y.; Diao, J.; Zhang, G.; Chen, C.; Wu, D. MSWI Bottom Ash Application to Resist Sulfate Attack on Concrete. Appl. Sci. 2019, 9, 5091. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Navarro, C.; Doehne, E.; Sebastian, E. How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cem. Concr. Res. 2000, 30, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Tsui, N.; Flatt, R.J.; Scherer, G.W. Crystallization damage by sodium sulfate. J. Cult. Herit. 2003, 4, 109–115. [Google Scholar] [CrossRef]
- Thaulow, N.; Sahu, S. Mechanism of concrete deterioration due to salt crystallization. Mater. Charact. 2004, 53, 123–129. [Google Scholar] [CrossRef]
- Valero, M.; Sultimova, N.B.; Houston, J.E.; Levin, P.P. Naproxen sodium salt photochemistry in aqueous sodium dodecyl sulfate (SDS) ellipsoidal micelles. J. Mol. Liq. 2021, 324, 114724. [Google Scholar] [CrossRef]
- Alavia, W.; Lovera, J.A.; Graber, T.A.; Azúa, D.; Soto, I. Modeling of the density, viscosity and electrical conductivity of aqueous solutions saturated in boric acid in presence of lithium sulfate or sodium sulfate at 293.15 to 313.15 K. Fluid Phase Equilib. 2021, 532, 112864. [Google Scholar] [CrossRef]
- Yang, S.; Han, M.; Chen, X.; Song, J.; Yang, J. Influence of sulfate crystallization on bond-slip behavior between deformed rebar and concrete subjected to combined actions of dry-wet cycle and freeze-thaw cycle. Constr. Build. Mater. 2022, 345, 128368. [Google Scholar] [CrossRef]
- Khodaparast, S.; Marcos, J.; Sharratt, W.N.; Tyagi, G.; Cabral, J.T. Surface-Induced Crystallization of Sodium Dodecyl Sulfate (SDS) Micellar Solutions in Confinement. Langmuir 2020, 37, 230–239. [Google Scholar] [CrossRef]
- Benavente, D.; del Cura, M.G.; Garcıa-Guinea, J.; Sánchez-Moral, S.; Ordóñez, S. Role of pore structure in salt crystallisation in unsaturated porous stone. J. Cryst. Growth 2004, 260, 532–544. [Google Scholar] [CrossRef]
- Benavente, D.; Martínez-Martínez, J.; Cueto, N.; García-del-Cura, M.A. Salt weathering in dual-porosity building dolostones. Eng. Geol. 2007, 94, 215–226. [Google Scholar] [CrossRef]
- Cardell, C.; Benavente, D.; Rodríguez-Gordillo, J. Weathering of limestone building material by mixed sulfate solutions. Characterization of stone microstructure, reaction products and decay forms. Mater. Charact. 2008, 59, 1371–1385. [Google Scholar] [CrossRef]
- Pradhan, B. Corrosion behavior of steel reinforcement in concrete exposed to composite chloride-sulfate environment. Constr. Build. Mater. 2014, 72, 398–410. [Google Scholar] [CrossRef]
- Grengg, C.; Mittermayr, F.; Baldermann, A.; Böttcher, M.E.; Leis, A.; Koraimann, G.; Grunert, P.; Dietzel, M. Microbiologically induced concrete corrosion: A case study from a combined sewer network. Cem. Concr. Res. 2015, 77, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, F.; Pradhan, B. Influence of sulfate ion and associated cation type on steel reinforcement corrosion in concrete powder aqueous solution in the presence of chloride ions. Cem. Concr. Res. 2017, 91, 73–86. [Google Scholar] [CrossRef]
- Huber, B.; Hilbig, H.; Drewes, J.E.; Müller, E. Evaluation of concrete corrosion after short- and long-term exposure to chemically and microbially generated sulfuric acid. Cem. Concr. Res. 2017, 94, 36–48. [Google Scholar] [CrossRef]
- Grengg, C.; Mittermayr, F.; Ukrainczyk, N.; Koraimann, G.; Kienesberger, S.; Dietzel, M. Advances in concrete materials for sewer systems affected by microbial induced concrete corrosion: A review. Water Res. 2018, 134, 341–352. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Wang, Z. Influence of cement components on the ability of concrete to bind chloride ions. J. Chin. Ceram. Soc. 2000, 28, 570–574. (In Chinese) [Google Scholar]
- Page, C.L.; Short, N.R.; El Tarras, A. Diffusion of chloride ions in hardened cement pastes. Cem. Concr. Res. 1981, 11, 395–406. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Kayyali, O.A.; Haque, M.N. The C1−/OH− ratio in chloride-contaminated concrete—A most important criterion. Mag. Concr. Res. 1995, 47, 235–242. [Google Scholar] [CrossRef]
- Liu, F.; You, Z.; Yang, X.; Wang, H. Macro-micro degradation process of fly ash concrete under alternation of freeze-thaw cycles subjected to sulfate and carbonation. Constr. Build. Mater. 2018, 181, 369–380. [Google Scholar] [CrossRef]
- Reddy, B.; Glass, G.K.; Lim, P.J.; Buenfeld, N.R. On the corrosion risk presented by chloride bound in concrete. Cem. Concr. Compos. 2002, 24, 1–5. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. The influence of chloride binding on the chloride induced corrosion risk in reinforced concrete. Corros. Sci. 2000, 42, 329–344. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. The presentation of the chloride threshold level for corrosion of steel in concrete. Corros. Sci. 1997, 39, 1001–1013. [Google Scholar] [CrossRef]
- Hossack, A.M.; Thomas, M.D. Varying fly ash and slag contents in Portland limestone cement mortars exposed to external sulfates. Constr. Build. Mater. 2015, 78, 333–341. [Google Scholar] [CrossRef]
- Mou, W. Popularize ferric aluminate cement to solve the world problem of cement concrete corrosion in Marine engineering. China Build. Mater. 2020, 10, 16–17. [Google Scholar]
- Karakoc, M.B.; Türkmen, İ.; Maraş, M.M.; Kantarci, F.; Demirboğa, R. Sulfate resistance of ferrochrome slag based geopolymer concrete. Ceram. Int. 2016, 42, 1254–1260. [Google Scholar] [CrossRef]
- Yue, X. Experimental study on strength enhancement and sulfate resistance of soil-cement by waste silica powder. Highw. Autom. Appl. 2020, 4, 66–69. (In Chinese) [Google Scholar]
- Dong, Y.; Zhang, G.; Li, W.; Wang, L.; Liu, C.; Zou, Y. Experimental study on strength characteristics of regenerated micro powder from municipal solid waste incineration residue. J. Civ. Environ. Eng. 2019, 41, 174–180. [Google Scholar]
- GB/T17671-1999; Test Method for Strength of Cement Mortar. National Standard of the People’s Republic of China: Beijing, China, 1999.
- Luping, T.; Nilsson, L.-O. Chloride binding capacity and binding isotherms of OPC pastes and mortars. Cem. Concr. Res. 1993, 23, 247–253. [Google Scholar] [CrossRef]
- JGJ 55-2000; Code for Design of Ordinary Concrete. National Standards Committee of China: Beijing, China, 2000.
- Luo, R.; Cai, Y.; Wang, C. Properties of ground slag slurry combined with chloride ion extravasation. J. Build. Mater. 2001, 4, 148–153. (In Chinese) [Google Scholar]
- Florea, M.V.A.; Brouwers, H.J.H. Chloride binding related to hydration products: Part I: Ordinary Portland Cement. Cem. Concr. Res. 2012, 42, 282–290. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Electrokinetic potential of hydrated cement in relation to adsorption of chlorides. Cem. Concr. Res. 2009, 39, 340–344. [Google Scholar] [CrossRef]
- Zibara, H.; Hooton, R.D.; Thomas, M.D.A.; Stanish, K. Influence of the C/S and C/A ratios of hydration products on the chloride ion binding capacity of lime-SF and lime-MK mixtures. Cem. Concr. Res. 2008, 38, 422–426. [Google Scholar] [CrossRef]
- Garbev, K.; Stemmermann, P.; Black, L.; Breen, C.; Yarwood, J.; Gasharova, B.J. Structural features of C–S–H (I) and its carbonation in air—A Raman spectroscopic study. Part I: Fresh phases. J. Am. Ceram. Soc. 2007, 90, 900–907. [Google Scholar] [CrossRef]
- Potgieter-Vermaak, S.S.; Potgieter, J.H.; Van Grieken, R. The application of Raman spectrometry to investigate and characterize cement, Part I: A review. Cem. Concr. Res. 2006, 36, 656–662. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]





| Composition | SiO2 | CaO | Al2O3 | Na2O | Fe2O3 | SO3 | K2O | MgO | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 48.41 | 14.78 | 11.99 | 3.25 | 5.40 | 1.86 | 1.42 | 1.78 | 0.76 |
| MSWI (%) | Cement (kg/m3) | MSWI (kg/m3) | Sand (kg/m3) | Water (kg/m3) |
|---|---|---|---|---|
| 0 | 450 | 0 | 1350 | 225 |
| 10 | 405 | 45 | 1350 | 225 |
| 12.5 | 393.75 | 56.25 | 1350 | 225 |
| 15 | 382.5 | 67.5 | 1350 | 225 |
| 17.5 | 371.25 | 78.75 | 1350 | 225 |
| 20 | 360 | 90 | 1350 | 225 |
| 22.5 | 348.75 | 101.25 | 1350 | 225 |
| 25 | 337.5 | 112.5 | 1350 | 225 |
| 27.5 | 326.75 | 123.75 | 1350 | 225 |
| 30 | 315 | 135 | 1350 | 225 |
| Number | MSWI (%) | Cement (kg/m3) | MSWI (kg/m3) | Sand (kg/m3) | Water (kg/m3) |
|---|---|---|---|---|---|
| S1 | 0 | 450 | 0 | 1350 | 225 |
| S2 | 10 | 405 | 45 | 1350 | 225 |
| S3 | 20 | 360 | 90 | 1350 | 225 |
| S4 | 30 | 315 | 135 | 1350 | 225 |
| Number | MSWI (%) | Cement (kg/m3) | MSWI (kg/m3) | Water (kg/m3) | Sand (kg/m3) | Gravel (kg/m3) | Super-Plasticizer (kg/m3) |
|---|---|---|---|---|---|---|---|
| C1 | 0 | 330 | 0 | 130 | 614 | 1248 | 2.65 |
| C2 | 10 | 297 | 33 | 130 | 614 | 1248 | 2.65 |
| C3 | 20 | 264 | 66 | 130 | 614 | 1248 | 2.65 |
| C4 | 30 | 231 | 99 | 130 | 614 | 1248 | 2.65 |
| MSWI Content (%) | Compressive Strength (MPa) | Strength Activity Index (%) | |||
|---|---|---|---|---|---|
| 3 d | 7 d | 14 d | 28 d | ||
| 0 | 29.37 | 43.65 | 53.35 | 57.61 | 100 |
| 10.0 | 23.31 | 34.64 | 42.17 | 45.52 | 79.03 |
| 12.5 | 22.76 | 34.35 | 41.92 | 43.89 | 76.22 |
| 15.0 | 19.36 | 31.59 | 40.60 | 42.19 | 73.25 |
| 17.5 | 19.14 | 28.02 | 40.57 | 41.33 | 73.51 |
| 20.0 | 18.57 | 27.41 | 34.62 | 36.35 | 62.99 |
| 22.5 | 18.03 | 27.05 | 34.26 | 35.67 | 61.92 |
| 25.0 | 17.45 | 26.13 | 34.20 | 35.28 | 61.28 |
| 27.0 | 15.15 | 23.90 | 30.96 | 32.90 | 57.12 |
| 30 | 15.04 | 22.83 | 30.20 | 32.15 | 55.80 |
| MSWI Content (%) | Flexural Strength (MPa) | Strength Activity Index (%) |
|---|---|---|
| 10 | 5.21 | 58 |
| 12.5 | 5.13 | 57 |
| 15 | 5.00 | 55.5 |
| 17.5 | 5.02 | 55.8 |
| 20 | 4.98 | 55.3 |
| 22.5 | 4.91 | 54.6 |
| 25 | 4.82 | 53.4 |
| 27.5 | 4.62 | 51.6 |
| 30 | 4.49 | 49.8 |
| Number | W/B Ratio | MSWI Content (%) | Compressive Strength (MPa) |
|---|---|---|---|
| C1 | 0.4 | 0 | 39.5 |
| C2 | 0.4 | 10 | 33.7 |
| C3 | 0.4 | 20 | 27.4 |
| C4 | 0.4 | 30 | 23.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Ma, Y.; Peng, N.; Qiu, J. Study on SO42−/Cl− Erosion Resistance and Mechanism of Recycled Concrete Containing Municipal Solid Waste Incineration (MSWI) Powder. Materials 2022, 15, 5352. https://doi.org/10.3390/ma15155352
Dong Y, Ma Y, Peng N, Qiu J. Study on SO42−/Cl− Erosion Resistance and Mechanism of Recycled Concrete Containing Municipal Solid Waste Incineration (MSWI) Powder. Materials. 2022; 15(15):5352. https://doi.org/10.3390/ma15155352
Chicago/Turabian StyleDong, Yun, Yuanshan Ma, Ningbo Peng, and Jianchun Qiu. 2022. "Study on SO42−/Cl− Erosion Resistance and Mechanism of Recycled Concrete Containing Municipal Solid Waste Incineration (MSWI) Powder" Materials 15, no. 15: 5352. https://doi.org/10.3390/ma15155352






