Polymorphic Biological and Inorganic Functional Nanomaterials
Abstract
:1. Introduction
2. Functional Protein Nanomaterials: Amyloids
2.1. Amyloid Fibrils: From Inducing Disease to Becoming a Biomaterial with Diverse Applications
- (1)
- The assembly properties of amyloid and its polymer subunits do not change under the influence of major chemical modifications;
- (2)
- comparable isomorphism can be obtained from various monomeric units;
- (3)
- condensed state is formed via noncovalent interaction (…); and
- (4)
- Under specific conditions gel or liquid crystals can form.
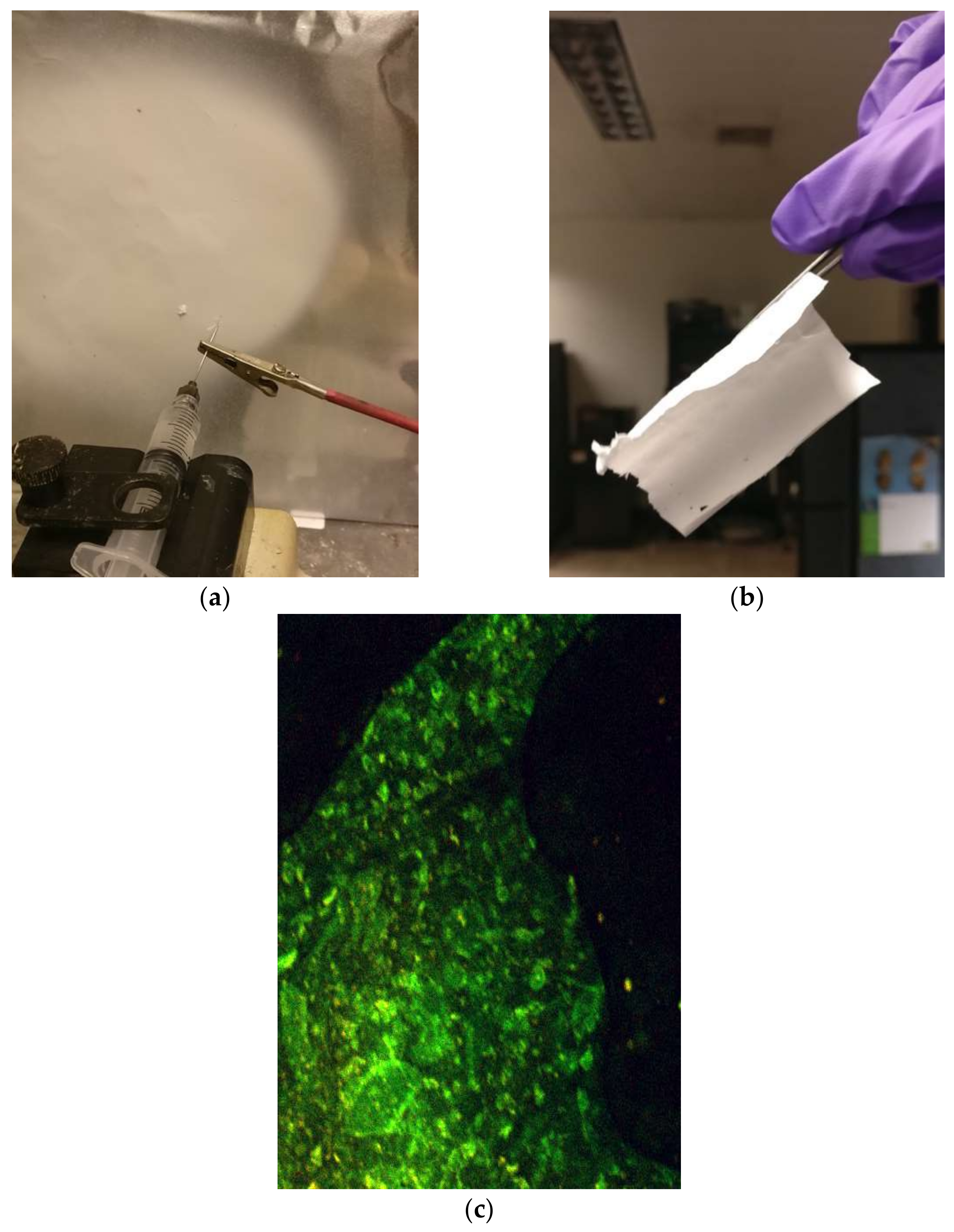
2.2. Recent Breakthrough: Novel Process for Amyloid from Wheat Flour
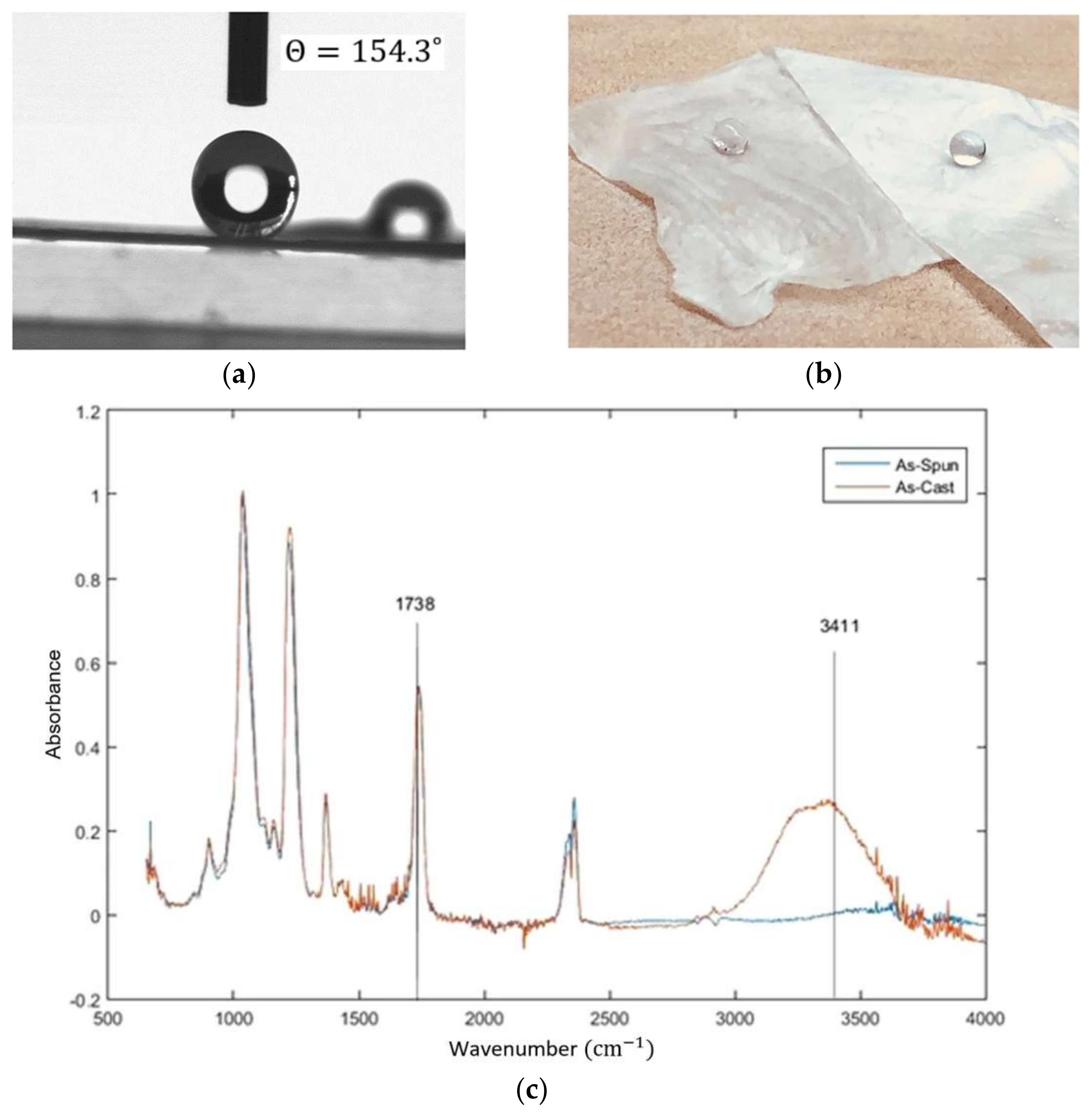
2.3. Amyloid Composites with Super-Hydrophobic CA Electrospun Mats
- Materials: cellulose acetate (MW 30,000), amyloid fibrils (prepared from bovine insulin), acetic acid, acetone;
- A total of 3 mL of 15 wt.% CA solution was mixed with 1 mL of amyloid fibril-containing solution;
- Electrospinning: 1 mL/hr flow rate, 7 cm working distance, 20 kV voltage.
3. Functional Inorganic Nanomaterials
3.1. Nanowires of α-MoO3
3.2. The 4D Functionality of Bio-Nano-Materials-Volatolomics: Headspace Chemo-Sensing
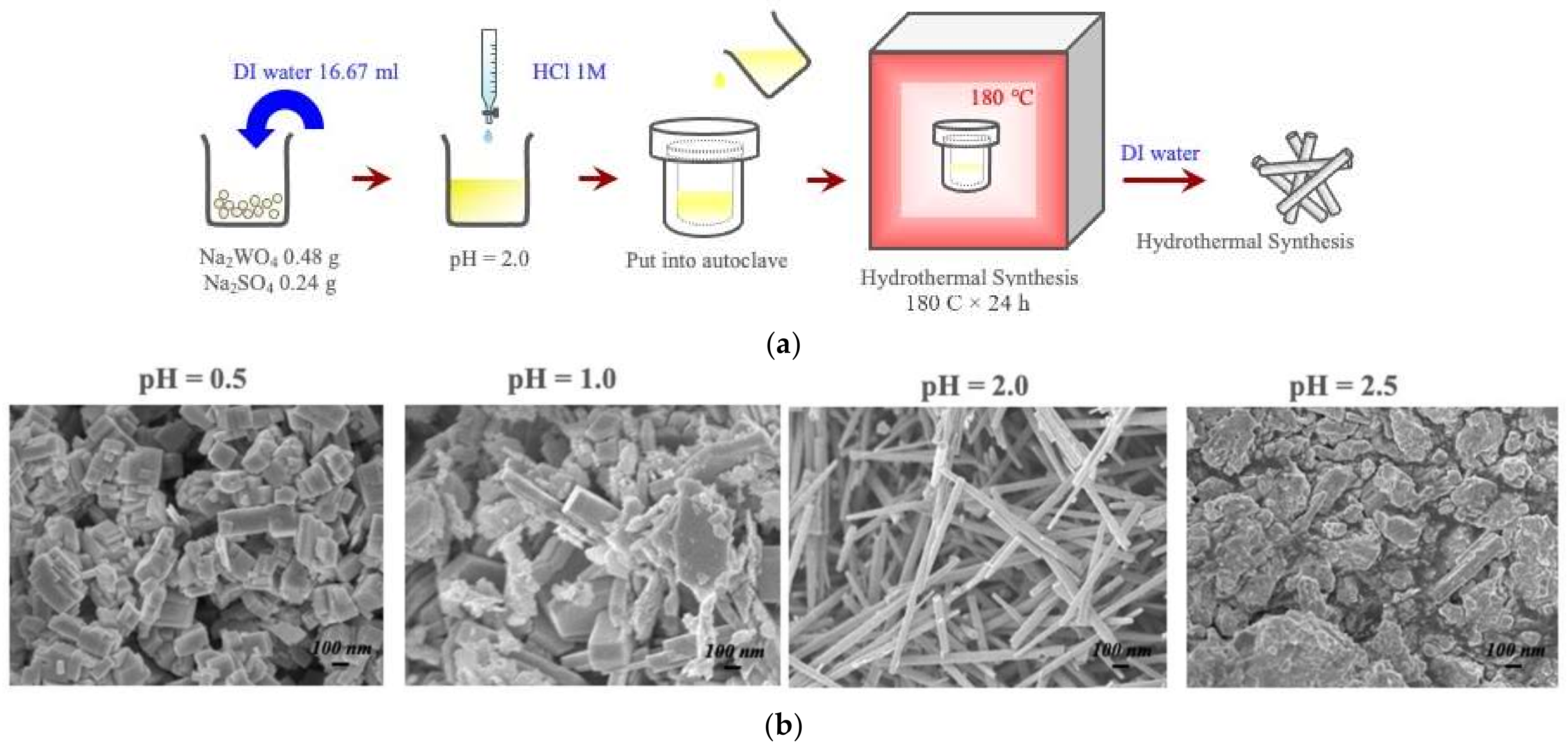
3.3. Hydrothermal Synthesis of Metastable Oxide Phases-Nanowires of Hexagonal-WO3
3.4. Electron Lithography for Nanowire Growth—The Case of γ-WO3
4. Smart and Hybrid Scaffolds
4.1. The 3D Scaffolds of Self-Supported Photocatalysts for Water Clean Up and CO2 Capture
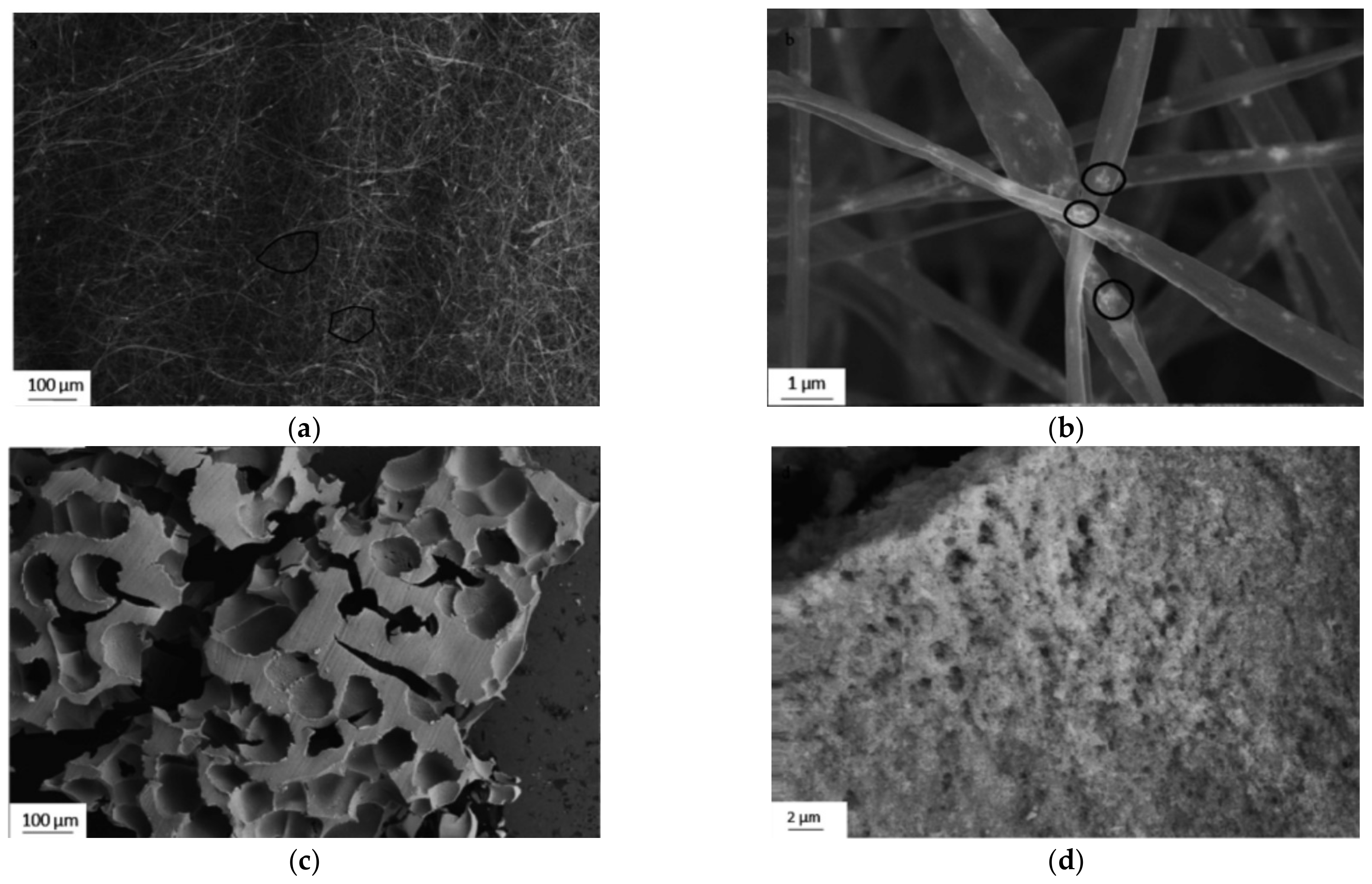
4.2. The 3D Fabrication of Nanogrids
4.3. Nanogrids on Amyloid
4.4. TiO2 Nanogrids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ke, P.C.; Zhou, R.; Serpell, L.C.; Riek, R.; Knowles, T.P.J.; Mezzenga, R. Half a century of amyloids:past, present, and future. Chem. Soc. Rev. 2020, 49, 5473–5509. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Mezzenga, R. Amyloid Fibrils as Building Blocks for Natural and Artificial Functional Materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, L.D.; Blakeman, D.; Lutter, L.; Serpell, C.; Tuite, M.; Serpell, L.; Xue, W. Quantification of amyloid fibril polymorphism by nano-morphometry reveals the individuality of filament assembly. Comm. Chem. 2020, 3, 125. [Google Scholar] [CrossRef]
- Hessick, E.; Pawar, M.; Souchereau, R.; Schmitz, E.; Gouma, P.I. Novel, Inexpensive, and Scalable Amyloid Fibril Formation Method. Materials 2022, 16, 1766. [Google Scholar] [CrossRef]
- Schultz, M. Rudolf Virchow. Emerg. Infect. Dis. 2008, 14, 1480. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B. The Pleated Sheet, A New Layer Configuration of Polypeptide Chains. Proc. Natl. Acad. Sci. USA 1951, 37, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Gras, S.L. Amyloid Fibrils: From Disease to Design. New Biomaterial Applications for Self-Assembling Cross-β Fibrils. Aust. J. Chem. 2007, 60, 333–342. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Wang, X.; An, B.; Cui, M.; Pu, J.; Wei, S.; Xue, S.; Ye, H.; Zhao, Y.; et al. Patterned Amyloid Materials Integrating Robustness and Genetically Programmable Functionality. Nano Lett. 2019, 19, 8399–8408. [Google Scholar] [CrossRef]
- Cherny, I.; Gazit, E. Amyloids: Not only pathological agents but also ordered nanomaterials. Angew. Chem. Int. Ed. 2008, 47, 4062–4069. [Google Scholar] [CrossRef]
- Conway, K.A.; Harper, J.D.; Lansbury, P.T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998, 4, 1318. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein misfolding, evolution and disease. Trends Biochem. Sci. 1999, 24, 329–332. [Google Scholar] [CrossRef]
- Rambaran, R.N.; Serpell, L.C. Amyloid fibrils. Prion 2008, 2, 112–117. [Google Scholar] [CrossRef]
- Glenner, G.G.; Ein, D.; Eanes, E.D.; Bladen, H.A.; Terry, W.; Page, D.L. Creation of “amyloid” fibrils from Bence Jones proteins in vitro. Science 1971, 174, 712–714. [Google Scholar] [CrossRef]
- Smith, J.F.; Knowles, T.P.; Dobson, C.M.; MacPhee, C.E.; Welland, M.E. Characterization of the nanoscale properties of individual amyloid fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 15806–15811. [Google Scholar] [CrossRef] [Green Version]
- Knowles, T.P.; Fitzpatrick, A.W.; Meehan, S.; Mott, H.R.; Vendruscolo, M.; Dobson, C.M.; Welland, M.E. Role of intermolecular forces in defining material properties of protein nanofibrils. Science 2007, 318, 1900–1903. [Google Scholar] [CrossRef]
- Knowles, T.P.; Buehler, M.J. Nanomechanics of functional and pathological amyloid materials. Nat. Nanotechnol. 2011, 6, 469. [Google Scholar] [CrossRef]
- Adamcik, J.; Lara, C.; Usov, I.; Jeong, J.S.; Ruggeri, F.S.; Dietler, G.; Mezzenga, R. Measurement of intrinsic properties of amyloid fibrils by the peak force QNM method. Nanoscale 2012, 4, 4426–4429. [Google Scholar] [CrossRef] [Green Version]
- Gilead, S.; Gazit, E. Self-organization of Short Peptide Fragments: From Amyloid Fibrils to Nanoscale Supramolecular Assemblies. Supramol. Chem. 2005, 17, 87–92. [Google Scholar] [CrossRef]
- Gazit, E. Mechanistic studies of the process of amyloid fibrils formation by the use of peptide fragments and analogues: Implications for the design of fibrillization inhibitors. Curr. Med. Chem. 2002, 9, 1725–1735. [Google Scholar] [CrossRef]
- Wetzel, R.; Shivaprasad, S.; Williams, A.D. Plasticity of amyloid fibrils. Biochemistry 2007, 46, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. TIFS Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Osborne, T.B. The Proteins of the Wheat Kernel; Carnegie Institution: Washington, DC, USA, 1907. [Google Scholar]
- Nilsson, M.R. Techniques to study amyloid fibril formation in vitro. Methods 2004, 34, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta BBA Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Mikaeili, F.; Gouma, P.I. Super Water-Repellent Cellulose Acetate Mats. Sci. Rep. 2018, 8, 12472. [Google Scholar] [CrossRef]
- Thacker, D.; Sanagavarapu, K.; Frohm, B.; Meisl, G.; Knowles, P.J.; Linse, S. The role of fibril structure and surface hydrophobicity in secondary nucleation of amyloid fibrils. Proc. Natl. Acad. Sci. USA 2020, 117, 25272–25283. [Google Scholar] [CrossRef]
- Sawicka, K.M.; Prasad, A.K.; Gouma, P.I. Metal Oxide Nanowires for Use in Chemical Sensing Applications. Sens. Lett. 2005, 3, 1–5. [Google Scholar] [CrossRef]
- Gouma, P.; Kalyanasundaram, K.; Bishop, A. Electrospun Single Crystal MoO3 Nanowires for Bio-Chem sensing probes. J. Mater. Res. Nanowires Nanotub. Spec. Issue 2006, 21, 2904–2910. [Google Scholar]
- Gouma, P.I.; Mills, M.J. Anatase to Rutile Transformation in Titania Powders. J. Am. Ceram. Soc. 2001, 84, 619–622. [Google Scholar] [CrossRef]
- Gouma, P.-I.; Haynes, A.S.; Kalyanasundaram, K. Electrospun Single Crystal MoO3 Nanowires for Bio-Chem Sensing Probes. U.S. Patent 7981215, 19 July 2011. [Google Scholar]
- Canepa, P.; Gautam, G.S.; Hannah, D.C.; Malik, R.; Liu, M.; Gallagher, K.G.; Persson, K.A.; Ceder, G. Odyssey of Multivalent Cathode Materials: Open Questions and Future Challenges. Chem. Rev. 2017, 117, 4287–4341. [Google Scholar] [CrossRef]
- Yu, B.; Gouma, P. Isoprene Sensor MS Report; The Ohio State University: Columbus, OH, USA, 2018. [Google Scholar]
- Sood, S.; Kisslinger, K.; Gouma, P. Nanowire Growth by an Electron-Beam-Induced Massive Phase Transformation. J. Am. Ceram. Soc. 2014, 97, 3733–3736. [Google Scholar] [CrossRef]
- Jodhani, G.; Topcu, S.; Bishop-Haynes, A.; Lee, J.; Gouma, P.I. Self-supported Nano-WO3 Foams formed by Self-Assembly of Non-Woven Mats. J. Adv. Nanomater. 2016, 1, 57. [Google Scholar] [CrossRef]
- Challagulla, K.S.; Venkatesh, T.A. Electromechanical Response of Piezoelectric Foams. Acta Mater. 2012, 60, 2111–2127. [Google Scholar] [CrossRef]
- Nair, M.; Luo, Z.; Heller, A. Rates of photocatalytic oxidation of crude oil on salt water on buoyant, cenosphere-attached titanium dioxide. Ind. Eng. Chem. Res. 1993, 32, 2318–2323. [Google Scholar] [CrossRef]
- Gouma, P.; Lee, J. Fiber Mats Coated with Nanogrid Visible Spectrum Photocatalysts. U.S. Patent 20150129472A1; NanogridsTM IP’:Patent application PCT/US2012/044327 based on R8345/2011: Hydrocarbon Recovery and Oil Spill Remediation Using Nanostructured Fiber Mats; including provisional patent application 61/501,436; R8396: Nanogrids for Water Remediation including provisional patent application. 61/544,122, 2011,
- Gouma, P.I.; Lee, J. Photocatalytic nanomats clean up produced water from fracking. Transl. Mater. Res. 2014, 1, 025002. [Google Scholar] [CrossRef]
- Yourey, J.E.; Bartlett, B.M. Electrochemical deposition and photoelectrochemistry of CuWO4, a promising photoanode for water oxidation. J. Mater. Chem. 2011, 21, 7651–7660. [Google Scholar] [CrossRef]
- Lee, J.; Gouma, P.I. Tailored 3D CuO Nanogrid Formation. J. Nanomater. 2011, 2011, 863631. [Google Scholar] [CrossRef] [Green Version]
- Sood, S.; Divya, S.; Gouma, P. High Throughput Electrospinning of 3D Nano Fibrous Mats. J. Nanoeng. Nanomanuf. 2014, 4, 39–44. [Google Scholar] [CrossRef]
- Li, D.; Furukawa, H.; Deng, H.; Liu, C.; Yaghi, O.M.; Eisenberg, D.S. Designed amyloid fibers as materials for selective carbon dioxide capture. Proc. Natl. Acad. Sci. USA 2014, 111, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topcu, S.; Jodhani, G.; Gouma, P.I. Optimized nanostructured TiO2 photocatalysts. Front. Mater. 2016, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Sibu, C.P.; Kumar, R.S.; Mukundan, P.; Warrier, K.G.K. Structural modifications and associated properties of lanthamum oxide doped sol-gel nanosized titanium oxide. Chem. Mater. 2002, 14, 2876–2881. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef]
- Topcu, S. TiO2 and Ce-TiO2 Photocatalysts for Water Remediation and Energy Applications. Ph.D Thesis, The Graduate School, Stony Brook University, Stony Brook, NY, USA, 2016. [Google Scholar]
- Gouma, P.; Mills, M. Electron Microscopy of TiO2-Based Thick Films for Gas Sensors; CRC Press: Boca Raton, FL, USA, 1997; pp. 491–494. [Google Scholar]
- Chen, W.T.; Chan, A.; Jovic, V.; Sun-Waterhouse, D.; Murai, K.-I.; Idriss, H.; Waterhouse, G.I.N. Effect of the TiO crystallite size, TiO2 polymorph and test conditions on the photo-oxidation rate of aqueous methylene blue. Top. Catal. 2015, 58, 85–102. [Google Scholar] [CrossRef]
- Xu, S.; Ng, J.; Jianhong Du, A.; Liu, J.; Delai Sun, D. Highly efficient TiO2 nanotube photocatalyst for simultaneous hydrogen production and copper removal from water. Int. J. Hydrog. Energy 2011, 36, 6538–6545. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Watthanaarun, J.; Pavarajarn, V.; Supaphol, P. Titanium (IV) oxide nanofibers by combined sol-gel and electrospinning techniques: Preliminary report on effects of preparation conditions and secondary metal dopant. Sci. Technol. Adv. Mater. 2005, 6, 240–245. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. 2012, 13, 169–189. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilmore, T.; Gouma, P.-I. Polymorphic Biological and Inorganic Functional Nanomaterials. Materials 2022, 15, 5355. https://doi.org/10.3390/ma15155355
Gilmore T, Gouma P-I. Polymorphic Biological and Inorganic Functional Nanomaterials. Materials. 2022; 15(15):5355. https://doi.org/10.3390/ma15155355
Chicago/Turabian StyleGilmore, Tessa, and Pelagia-Irene Gouma. 2022. "Polymorphic Biological and Inorganic Functional Nanomaterials" Materials 15, no. 15: 5355. https://doi.org/10.3390/ma15155355
APA StyleGilmore, T., & Gouma, P.-I. (2022). Polymorphic Biological and Inorganic Functional Nanomaterials. Materials, 15(15), 5355. https://doi.org/10.3390/ma15155355






