Nanotube Functionalization: Investigation, Methods and Demonstrated Applications
Abstract
:1. Introduction
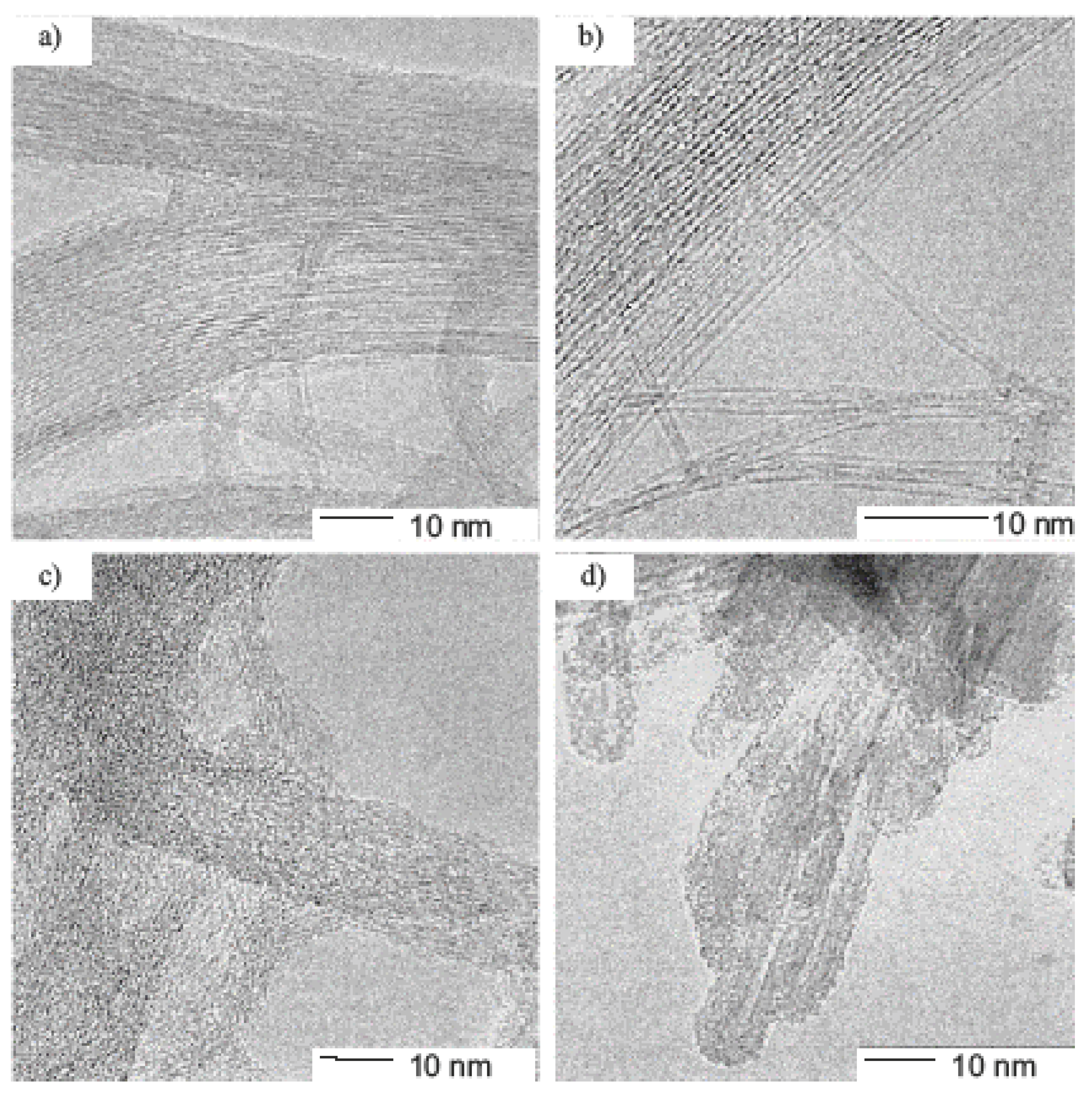
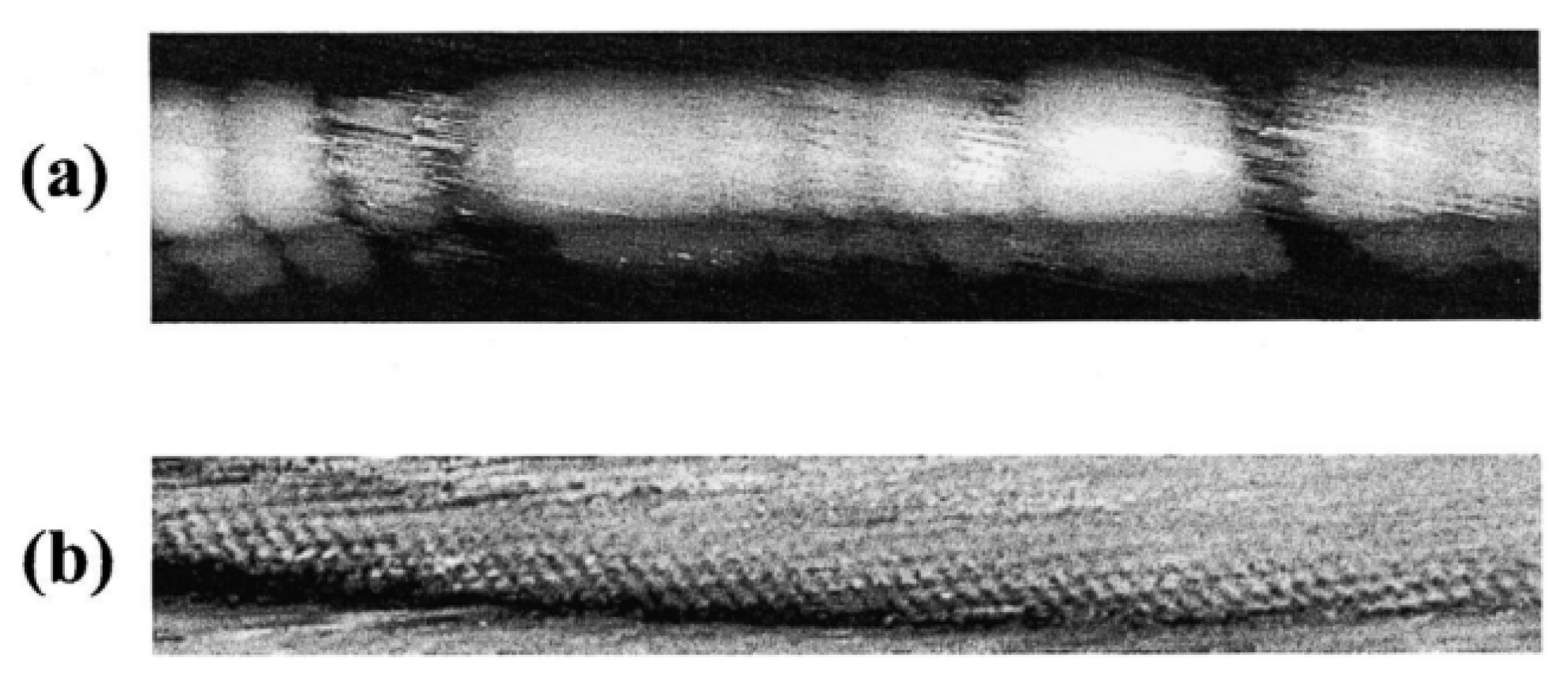
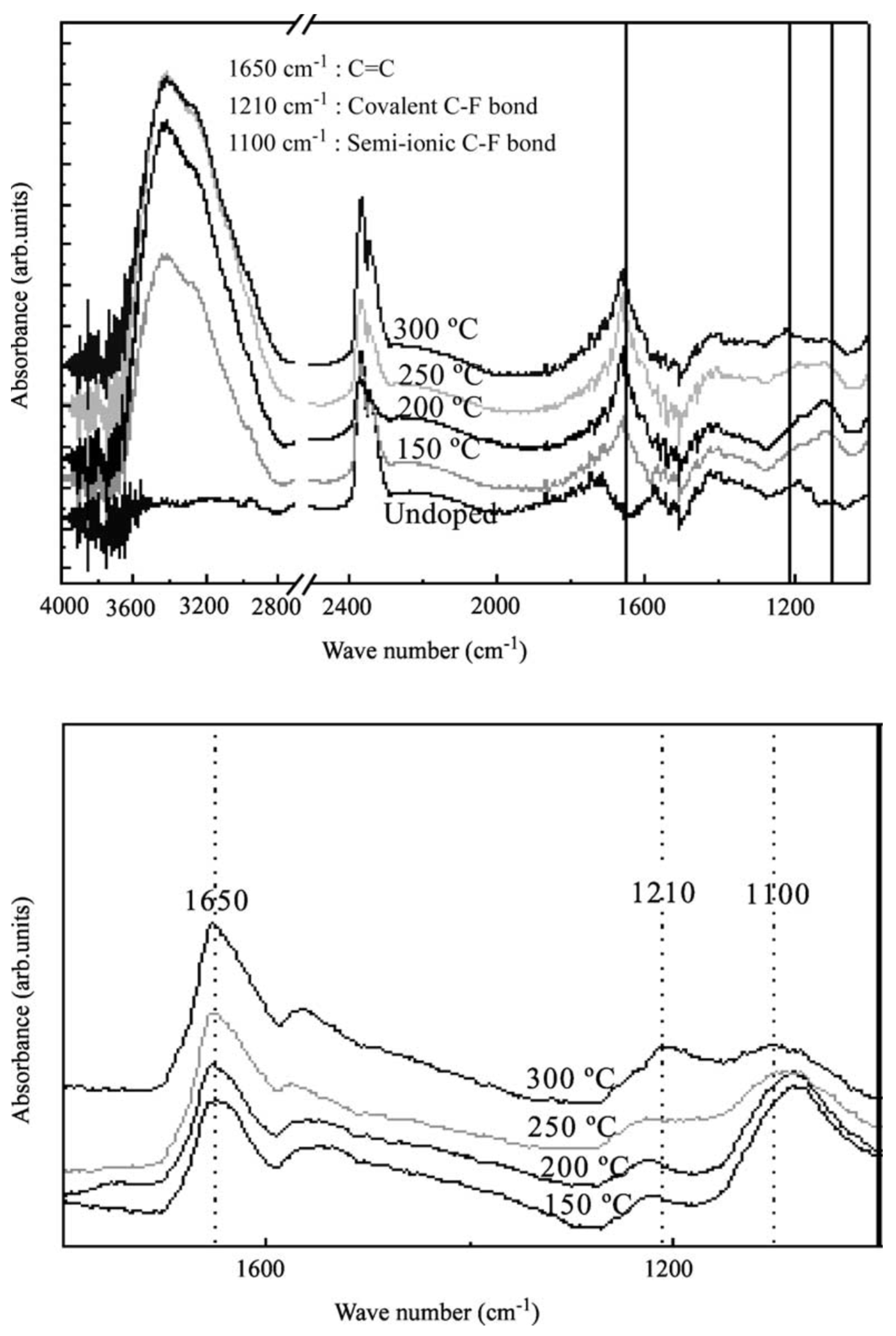
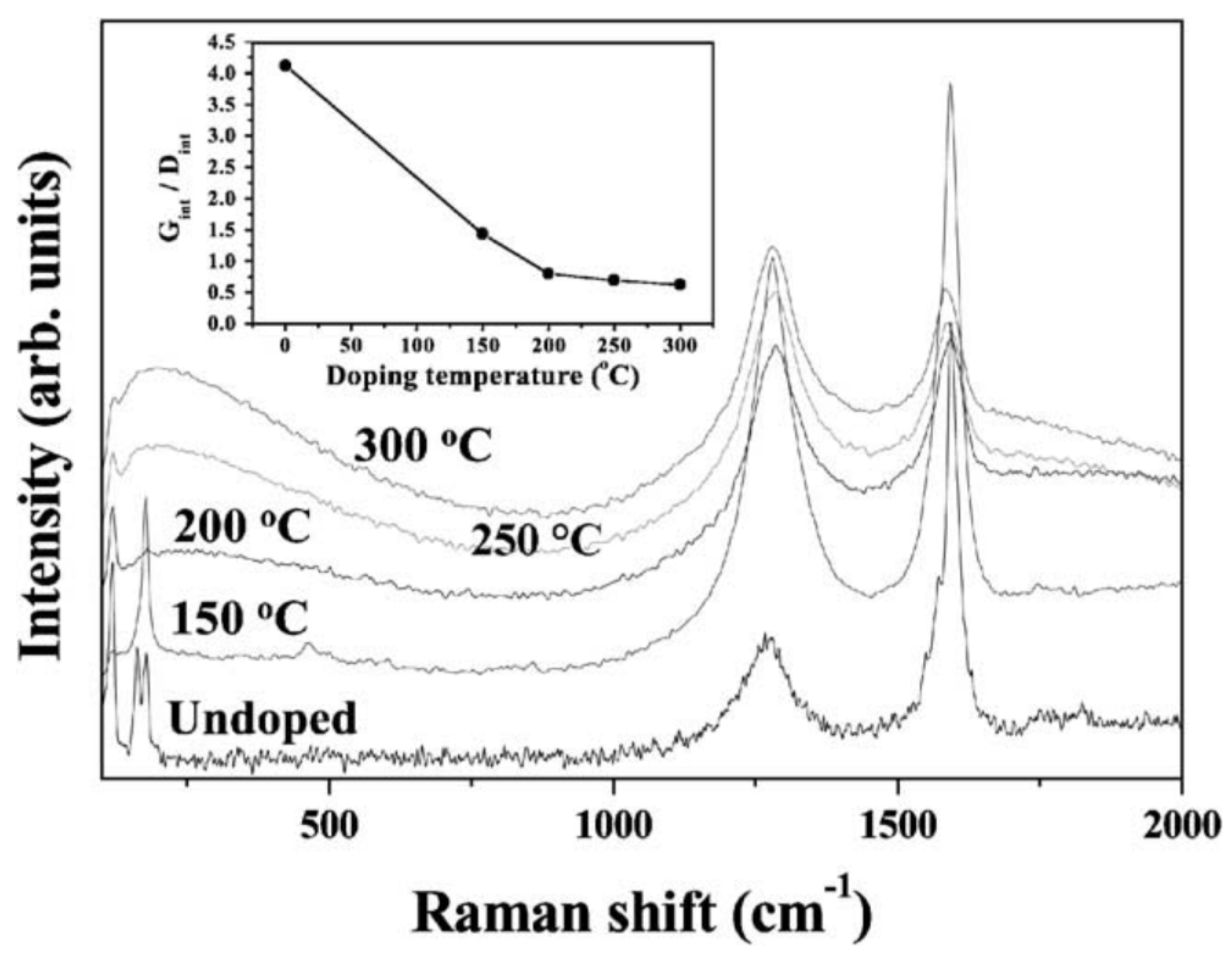
2. Microscopy and Spectroscopy of Functionalized Carbon Nanotubes
- (a)
- exterior covalently attached functional groups,
- (b)
- exterior physisorbed moieties,
- (c)
- heteroatoms directly incorporated into the sp2 mesh,
- (d)
- endohedral filling of SWCNTs.
3. Modification of Carbon Nanotubes
3.1. Covalent Modification
3.2. Non-Covalent Modification
3.3. Substitution of Atoms in CNTs
4. Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNT | Carbon nanotube |
| CTAB | Cetyltrimethyl ammonium bromide |
| CVD | Chemical vapor deposition |
| DGC | Density gradient centrifugation |
| DGU | Density gradient ultracentrifugation |
| DNA | Deoxyribonucleic acid |
| DOC | Sodium deoxycholate |
| EELS | Electron energy loss spectroscopy |
| F-SWCNT | Fluorinated single-walled carbon nanotube |
| FTIR | Fourier-transformed infrared spectra |
| GCE | Glassy carbon electrode |
| MWCNT | Multi-walled carbon nanotube |
| NP | Nanoparticles |
| PNA | Peptide nucleic acid |
| SC | Sodium cholate |
| SDBS | Sodium dodecyl benzene sulfonate |
| SDS | Sodium dodecyl sulphate |
| siRNA | Small interfering ribonucleic acid |
| STEM | Scanning transmission electron microscopy |
| STM | Scanning tunnelling microscopy |
| SWCNT | Single-walled carbon nanotube |
| TEM | Transmission electron microscopy |
| TX | Triton X |
| UV | Ultraviolet |
| XPS | X-ray photoelectron spectra |
References
- Kharlamova, M.V. Electronic Properties of Pristine and Modified Single-Walled Carbon Nanotubes. Phys.-Uspekhi 2013, 56, 1047–1073. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Advances in Tailoring the Electronic Properties of Single-Walled Carbon Nanotubes. Prog. Mater. Sci. 2016, 77, 125–211. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Liu, M.; Staniforth, M.; Zheng, Y.; Xiang, R.; Chiashi, S.; Anisimov, A.; Kauppinen, E.I.; Maruyama, S.; Lloyd-Hughes, J. Intertube Excitonic Coupling in Nanotube Van Der Waals Heterostructures. Adv. Funct. Mater. 2022, 32, 2104969. [Google Scholar] [CrossRef]
- Kashtiban, R.J.; Burdanova, M.G.; Vasylenko, A.; Wynn, J.; Medeiros, P.V.C.; Ramasse, Q.; Morris, A.J.; Quigley, D.; Lloyd-Hughes, J.; Sloan, J. Linear and Helical Cesium Iodide Atomic Chains in Ultranarrow Single-Walled Carbon Nanotubes: Impact on Optical Properties. ACS Nano 2021, 15, 13389–13398. [Google Scholar] [CrossRef] [PubMed]
- Burdanova, M.G.; Tsapenko, A.P.; Kharlamova, M.V.; Kauppinen, E.I.; Gorshunov, B.P.; Kono, J.; Lloyd-Hughes, J. A Review of the Terahertz Conductivity and Photoconductivity of Carbon Nanotubes and Heteronanotubes. Adv. Opt. Mater. 2021, 9, 2101042. [Google Scholar] [CrossRef]
- Jouni, M.; Fedorko, P.; Celle, C.; Djurado, D.; Chenevier, P.; Faure-Vincent, J. Conductivity vs Functionalization in Single-Walled Carbon Nanotube Films. SN Appl. Sci. 2022, 4, 132. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Applications of Filled Single-Walled Carbon Nanotubes: Progress, Challenges, and Perspectives. Nanomaterials 2021, 11, 2863. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Katyba, G.M.; Kashtiban, R.; Komandin, G.A.; Butler-Caddle, E.; Staniforth, M.; Mkrtchyan, A.A.; Krasnikov, D.V.; Gladush, Y.G.; Sloan, J.; et al. Ultrafast, High Modulation Depth Terahertz Modulators Based on Carbon Nanotube Thin Films. Carbon 2021, 173, 245–252. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Tsapenko, A.P.; Satco, D.A.; Kashtiban, R.; Mosley, C.D.W.; Monti, M.; Staniforth, M.; Sloan, J.; Gladush, Y.G.; Nasibulin, A.G.; et al. Giant Negative Terahertz Photoconductivity in Controllably Doped Carbon Nanotube Networks. ACS Photonics 2019, 6, 1058–1066. [Google Scholar] [CrossRef]
- Gladush, Y.; Mkrtchyan, A.A.; Kopylova, D.S.; Ivanenko, A.; Nyushkov, B.; Kobtsev, S.; Kokhanovskiy, A.; Khegai, A.; Melkumov, M.; Burdanova, M.; et al. Ionic Liquid Gated Carbon Nanotube Saturable Absorber for Switchable Pulse Generation. Nano Lett. 2019, 19, 5836–5843. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the Science and Technology of Carbon Nanotubes and Their Composites: A Review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Burghard, M.; Kern, K. Reversible Sidewall Osmylation of Individual Carbon Nanotubes. Nano Lett. 2003, 3, 613–615. [Google Scholar] [CrossRef]
- Paloniemi, H.; Ääritalo, T.; Laiho, T.; Liuke, H.; Kocharova, N.; Haapakka, K.; Terzi, F.; Seeber, R.; Lukkari, J. Water-Soluble Full-Length Single-Wall Carbon Nanotube Polyelectrolytes: Preparation and Characterization. J. Phys. Chem. B 2005, 109, 8634–8642. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, N.; Tomonari, Y.; Murakami, H. Water-Soluble Single-Walled Carbon Nanotubes via Noncovalent Sidewall-Functionalization with a Pyrene-Carrying Ammonium Ion. Chem. Lett. 2002, 31, 638–639. [Google Scholar] [CrossRef]
- Guldi, D.M.; Rahman, G.M.A.; Jux, N.; Balbinot, D.; Hartnagel, U.; Tagmatarchis, N.; Prato, M. Functional Single-Wall Carbon Nanotube Nanohybrids-Associating SWNTs with Water-Soluble Enzyme Model Systems. J. Am. Chem. Soc. 2005, 127, 9830–9838. [Google Scholar] [CrossRef]
- Kitaygorodskiy, A.; Wang, W.; Xie, S.Y.; Lin, Y.; Shiral Fernando, K.A.; Wang, X.; Qu, L.; Chen, B.; Sun, Y.P. NMR Detection of Single-Walled Carbon Nanotubes in Solution. J. Am. Chem. Soc. 2005, 127, 7517–7520. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Biomolecule-Functionalized Carbon Nanotubes: Applications in Nanobioelectronics. Chemphyschem 2004, 5, 1084–1104. [Google Scholar] [CrossRef]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually Suspended Single-Walled Carbon Nanotubes in Various Surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Matarredona, O.; Rhoads, H.; Li, Z.; Harwell, J.H.; Balzano, L.; Resasco, D.E. Dispersion of Single-Walled Carbon Nanotubes in Aqueous Solutions of the Anionic Surfactant NaDDBS. J. Phys. Chem. B 2003, 107, 13357–13367. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High Weight Fraction Surfactant Solubilization of Single-Wall Carbon Nanotubes in Water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- Monthioux, M.; Flahaut, E.; Cleuziou, J.P. Hybrid Carbon Nanotubes: Strategy, Progress, and Perspectives. J. Mater. Res. 2006, 21, 2774–2793. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically Functionalized Carbon Nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Bahr, J.L.; Tour, J.M. Covalent Chemistry of Single-Wall Carbon Nanotubes. J. Mater. Chem. 2002, 12, 1952–1958. [Google Scholar] [CrossRef]
- Banerjee, S.; Kahn, M.G.C.; Wong, S.S. Rational Chemical Strategies for Carbon Nanotube Functionalization. Chem.–A Eur. J. 2003, 9, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Hemraj-Benny, T.; Wong, S.S. Covalent Surface Chemistry of Single-Walled Carbon Nanotubes. Adv. Mater. 2005, 17, 17–29. [Google Scholar] [CrossRef]
- Controlled Synthesis and Modification of Carbon Nanotubes and C60: Carbon Nanostructures for Advanced Polymeric Composite Materials-Dai-2001-Advanced Materials-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/1521-4095%28200107%2913%3A12/13%3C899%3A%3AAID-ADMA899%3E3.0.CO%3B2-G (accessed on 24 July 2022).
- Davis, J.J.; Coleman, K.S.; Azamian, B.R.; Bagshaw, C.B.; Green, M.L.H. Chemical and Biochemical Sensing with Modified Single Walled Carbon Nanotubes. Chem.–A Eur. J. 2003, 9, 3732–3739. [Google Scholar] [CrossRef]
- De La Torre, G.; Blau, W.; Torres, T. A Survey on the Functionalization of Single-Walled Nanotubes. The Chemical of Phthalocyanine Moieties. Nanotechnology 2003, 14, 765. [Google Scholar] [CrossRef]
- Dyke, C.A.; Tour, J.M. Solvent-Free Functionalization of Carbon Nanotubes. J. Am. Chem. Soc. 2003, 125, 1156–1157. [Google Scholar] [CrossRef]
- Dyke, C.A.; Tour, J.M. Overcoming the Insolubility of Carbon Nanotubes Through High Degrees of Sidewall Functionalization. Chem.–A Eur. J. 2004, 10, 812–817. [Google Scholar] [CrossRef]
- Fischer, J.E. Chemical Doping of Single-Wall Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Écija, T.; Vargas-Quesada, B.; Chinchilla-Rodríguez, Z. Identification and Visualization of the Intellectual Structure and the Main Research Lines in Nanoscience and Nanotechnology at the Worldwide Level. J. Nanoparticle Res. 2017, 19, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilding, J.; Grulke, E.A.; Zhang, Z.G.; Lockwood, F. Dispersion of Carbon Nanotubes in Liquids. J. Dispers. Sci. Technol. 2007, 24, 1–41. [Google Scholar] [CrossRef]
- Atzrodt, J.; Derdau, V.; Kerr, W.; Reid, M. Applications of Hydrogen Isotopes in the Life Sciences. Angew. Chem. Int. Ed. 2017, 23, 627–628. [Google Scholar] [CrossRef]
- Hirsch, A.; Vostrowsky, O. Functionalization of Carbon Nanotubes. Top. Curr. Chem. 2005, 245, 193–237. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z. Curved Pi-Conjugation, Aromaticity, and the Related Chemistry of Small Fullerenes (<C60) and Single-Walled Carbon Nanotubes. Chem. Rev. 2005, 105, 3643–3696. [Google Scholar] [CrossRef]
- Niyogi, S.; Hamon, M.A.; Hu, H.; Zhao, B.; Bhowmik, P.; Sen, R.; Itkis, M.E.; Haddon, R.C. Chemistry of Single-Walled Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1105–1113. [Google Scholar] [CrossRef] [Green Version]
- Lukzen, N.N.; Ivanov, K.L.; Morozov, V.A.; Sagdeev, R.Z.; Kattnig, D.; Grampp, G. Chemical Polarization of Electrons of Spin-Correlated Radical Ion Pairs in Nanotubes. Dokl. Phys. Chem. 2006, 409, 233–236. [Google Scholar] [CrossRef]
- Sinnott, S.B. Chemical Functionalization of Carbon Nanotubes. J. Nanosci. Nanotechnol. 2002, 2, 113–123. [Google Scholar] [CrossRef]
- Sun, Y.P.; Fu, K.; Lin, Y.; Huang, W. Functionalized Carbon Nanotubes: Properties and Applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Georgakilas, V.; Prato, M. Soluble Carbon Nanotubes. Chem.–A Eur. J. 2003, 9, 4000–4008. [Google Scholar] [CrossRef] [PubMed]
- Terrones, M. Science and Technology of the Twenty-First Century: Synthesis, Properties, and Applications of Carbon Nanotubes. Annu. Rev. Mater. Res. 2003, 33, 419–501. [Google Scholar] [CrossRef]
- Mickelson, E.T.; Huffman, C.B.; Rinzler, A.G.; Smalley, R.E.; Hauge, R.H.; Margrave, J.L. Fluorination of Single-Wall Carbon Nanotubes. Chem. Phys. Lett. 1998, 296, 188–194. [Google Scholar] [CrossRef]
- Plank, N. Functionalisation of Carbon Nanotubes for Molecular Electronics. Microelectron. Eng. 2004, 73–74, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.F.; Chiang, I.W.; Mickelson, E.T.; Hauge, R.H.; Margrave, J.L.; Wang, X.; Scuseria, G.E.; Radloff, C.; Halas, N.J. Insight into the Mechanism of Sidewall Functionalization of Single-Walled Nanotubes: An STM Study. Chem. Phys. Lett. 1999, 313, 445–450. [Google Scholar] [CrossRef]
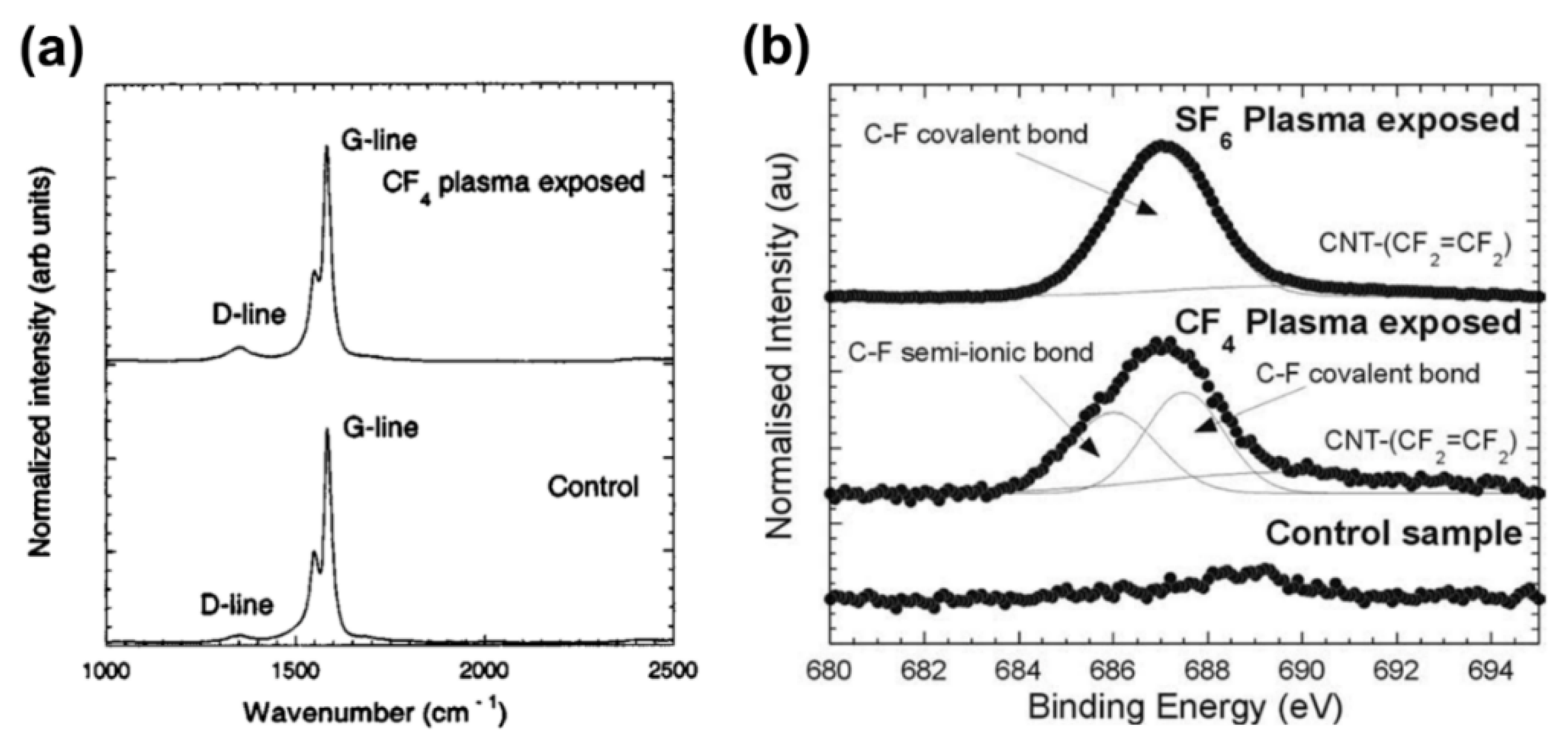
- An, K.H.; Heo, J.G.; Jeon, K.G.; Bae, D.J.; Jo, C.; Yang, C.W.; Park, C.Y.; Lee, Y.H.; Lee, Y.S.; Chung, Y.S. X-ray Photoemission Spectroscopy Study of Fluorinated Single-Walled Carbon Nanotubes. Appl. Phys. Lett. 2002, 80, 4235. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Cho, T.H.; Lee, B.K.; Rho, J.S.; An, K.H.; Lee, Y.H. Surface Properties of Fluorinated Single-Walled Carbon Nanotubes. J. Fluor. Chem. 2003, 120, 99–104. [Google Scholar] [CrossRef]
- Plank, N.O.V.; Jiang, L.; Cheung, R. Fluorination of Carbon Nanotubes in CF4 Plasma. Appl. Phys. Lett. 2003, 83, 2426. [Google Scholar] [CrossRef]
- Esumi, K.; Ishigami, M.; Nakajima, A.; Sawada, K.; Honda, H. Chemical Treatment of Carbon Nanotubes. Carbon 1996, 34, 279–281. [Google Scholar] [CrossRef]
- Yu, R.; Chen, L.; Liu, Q.; Lin, J.; Tan, K.L.; Ng, S.C.; Chan, H.S.O.; Xu, G.Q.; Hor, T.S.A. Platinum Deposition on Carbon Nanotubes via Chemical Modification. Chem. Mater. 1998, 10, 718–722. [Google Scholar] [CrossRef]
- Wang, Z.; Shirley, M.D.; Meikle, S.T.; Whitby, R.L.D.; Mikhalovsky, S.V. The Surface Acidity of Acid Oxidised Multi-Walled Carbon Nanotubes and the Influence of in-Situ Generated Fulvic Acids on Their Stability in Aqueous Dispersions. Carbon 2009, 47, 73–79. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H. Chemical Modification of Multiwalled Carbon Nanotubes for Sorption of Zn2+ from Aqueous Solution. Chem. Eng. J. 2008, 139, 462–468. [Google Scholar] [CrossRef]
- Hernadi, K.; Siska, A.; Thiên-Nga, L.; Forró, L.; Kiricsi, I. Reactivity of Different Kinds of Carbon during Oxidative Purification of Catalytically Prepared Carbon Nanotubes. Solid State Ion. 2001, 141, 203–209. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, J.; Varadan, V.K. Functionalization of Carbon Nanotubes by Potassium Permanganate Assisted with Phase Transfer Catalyst. Smart Mater. Struct. 2002, 11, 962–965. [Google Scholar] [CrossRef]
- Lu, C.; Liu, C.; Rao, G.P. Comparisons of Sorbent Cost for the Removal of Ni2+ from Aqueous Solution by Carbon Nanotubes and Granular Activated Carbon. J. Hazard. Mater. 2008, 151, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Zhang, Q.; Zhao, M.Q.; Wei, F. The Release of Free Standing Vertically-Aligned Carbon Nanotube Arrays from a Substrate Using CO2 Oxidation. Carbon 2010, 5, 1441–1450. [Google Scholar] [CrossRef]
- Lu, M.; Lu, M.; He, Q.; Li, Y.; Xu, C.; Ji, K.; Dai, Z. Influence of Carbon Dioxide Plasma Treatment on the Dry Adhesion of Vertical Aligned Carbon Nanotube Arrays. Nanotechnology 2020, 31, 345701. [Google Scholar] [CrossRef]
- Ran, M.; Sun, W.; Liu, Y.; Chu, W.; Jiang, C. Functionalization of Multi-Walled Carbon Nanotubes Using Water-Assisted Chemical Vapor Deposition. J. Solid State Chem. 2013, 197, 517–522. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Ebbesen, T.W.; Ichihashi, T.; Iijima, S.; Tanigaki, K.; Hiura, H. Opening Carbon Nanotubes with Oxygen and Implications for Filling. Nature 1993, 362, 522–525. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, C.; Dou, S.; Liu, D.; Wang, S. Oxidized Carbon Nanotubes as an Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. RSC Adv. 2015, 5, 41901–41904. [Google Scholar] [CrossRef]
- Ye, B.; Kim, S.I.; Lee, M.; Ezazi, M.; Kim, H.D.; Kwon, G.; Lee, D.H. Synthesis of Oxygen Functionalized Carbon Nanotubes and Their Application for Selective Catalytic Reduction of NOx with NH3. RSC Adv. 2020, 10, 16700–16708. [Google Scholar] [CrossRef] [PubMed]
- Morales-Lara, F.; Pérez-Mendoza, M.J.; Altmajer-Vaz, D.; García-Román, M.; Melguizo, M.; López-Garzón, F.J.; Domingo-García, M. Functionalization of Multiwall Carbon Nanotubes by Ozone at Basic PH. Comparison with Oxygen Plasma and Ozone in Gas Phase. J. Phys. Chem. C 2013, 117, 11647–11655. [Google Scholar] [CrossRef]
- Peng, K.; Liu, L.Q.; Li, H.; Meyer, H.; Zhang, Z. Room Temperature Functionalization of Carbon Nanotubes Using an Ozone/Water Vapor Mixture. Carbon 2011, 1, 70–76. [Google Scholar] [CrossRef]
- Simmons, J.M.; Nichols, B.M.; Baker, S.E.; Marcus, M.S.; Castellini, O.M.; Lee, C.S.; Hamers, R.J.; Eriksson, M.A. Effect of Ozone Oxidation on Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.M.; Manickam, S. Improved Functionalization and Recovery of Carboxylated Carbon Nanotubes Using the Acoustic Cavitation Approach. Chem. Phys. Lett. 2013, 557, 97–101. [Google Scholar] [CrossRef]
- Martín, O.; Gutierrez, H.R.; Maroto-Valiente, A.; Terrones, M.; Blanco, T.; Baselga, J. An Efficient Method for the Carboxylation of Few-Wall Carbon Nanotubes with Little Damage to Their Sidewalls. Mater. Chem. Phys. 2013, 140, 499–507. [Google Scholar] [CrossRef]
- Grujicic, M.; Cao, G.; Rao, A.M.; Tritt, T.M.; Nayak, S. UV-Light Enhanced Oxidation of Carbon Nanotubes. Appl. Surf. Sci. 2003, 214, 289–303. [Google Scholar] [CrossRef]
- Dutta, D.; Dubey, R.; Yadav, J.; Shami, T.C.; Rao, K.U.B.; Dutta, D.; Dubey, R.; Yadav, J.; Shami, T.C.; Rao, K.U.B. Preparation of Spongy Microspheres Consisting of Functionalized Multiwalled Carbon Nanotubes. New Carbon Mater. 2011, 26, 98–102. [Google Scholar] [CrossRef]
- Zhou, W.; Ooi, Y.H.; Russo, R.; Papanek, P.; Luzzi, D.E.; Fischer, J.E.; Bronikowski, M.J.; Willis, P.A.; Smalley, R.E. Structural Characterization and Diameter-Dependent Oxidative Stability of Single Wall Carbon Nanotubes Synthesized by the Catalytic Decomposition of CO. Chem. Phys. Lett. 2001, 350, 6–14. [Google Scholar] [CrossRef]
- Singh, D.K.; Iyer, P.K.; Giri, P.K. Diameter Dependence of Oxidative Stability in Multiwalled Carbon Nanotubes: Role of Defects and Effect of Vacuum Annealing. J. Appl. Phys. 2010, 108, 084313. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Wu, S.; Ni, K.; Tan, Z.; Xu, J.; Tao, Z.; Zhu, Y. Diameter-Sensitive Breakdown of Single-Walled Carbon Nanotubes upon KOH Activation. ChemPhysChem 2017, 18, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Avilés, F.; Sierra-Chi, C.A.; Nistal, A.; May-Pat, A.; Rubio, F.; Rubio, J. Influence of Silane Concentration on the Silanization of Multiwall Carbon Nanotubes. Carbon 2013, 57, 520–529. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.K.; Tang, B.Z. Functionalization of Carbon Nanotubes Using a Silane Coupling Agent. Carbon 2006, 44, 3232–3238. [Google Scholar] [CrossRef] [Green Version]
- Yaghoubi, A.; Alavi Nikje, M.M. Silanization of Multi-Walled Carbon Nanotubes and the Study of Its Effects on the Properties of Polyurethane Rigid Foam Nanocomposites. Compos. Part A Appl. Sci. Manuf. 2018, 109, 338–344. [Google Scholar] [CrossRef]
- Hamon, M.A.; Hui, H.; Bhowmik, P.; Itkis, H.M.E.; Haddon, R.C. Ester-Functionalized Soluble Single-Walled Carbon Nanotubes. Appl. Phys. A 2002, 74, 333–338. [Google Scholar] [CrossRef]
- Malikov, E.Y.; Muradov, M.B.; Akperov, O.H.; Eyvazova, G.M.; Puskás, R.; Madarász, D.; Nagy, L.; Kukovecz, Á.; Kónya, Z. Synthesis and Characterization of Polyvinyl Alcohol Based Multiwalled Carbon Nanotube Nanocomposites. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 61, 129–134. [Google Scholar] [CrossRef]
- Basiuk, E.V.; Basiuk, V.A.; Bañuelos, J.G.; Saniger-Blesa, J.M.; Pokrovskiy, V.A.; Gromovoy, T.Y.; Mischanchuk, A.V.; Mischanchuk, B.G. Interaction of Oxidized Single-Walled Carbon Nanotubes with Vaporous Aliphatic Amines. J. Phys. Chem. B 2002, 106, 1588–1597. [Google Scholar] [CrossRef]
- Lavorgna, M.; Romeo, V.; Martone, A.; Zarrelli, M.; Giordano, M.; Buonocore, G.G.; Qu, M.Z.; Fei, G.X.; Xia, H.S. Silanization and Silica Enrichment of Multiwalled Carbon Nanotubes: Synergistic Effects on the Thermal-Mechanical Properties of Epoxy Nanocomposites. Eur. Polym. J. 2013, 49, 428–438. [Google Scholar] [CrossRef]
- Gammoudi, H.; Belkhiria, F.; Helali, S.; ben Assaker, I.; Gammoudi, I.; Morote, F.; Souissi, A.; Karyaoui, M.; Amlouk, M.; Cohen-Bouhacina, T.; et al. Chemically Grafted of Single-Walled Carbon Nanotubes onto a Functionalized Silicon Surface. J. Alloy Compd. 2017, 694, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Chua, T.P.; Mariatti, M.; Azizan, A.; Rashid, A.A. Effects of Surface-Functionalized Multi-Walled Carbon Nanotubes on the Properties of Poly(Dimethyl Siloxane) Nanocomposites. Compos. Sci. Technol. 2010, 70, 671–677. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, C.; Li, H.; Du, Z. Fabrication of Silica Nanoparticles on the Surface of Functionalized Multi-Walled Carbon Nanotubes. Carbon 2011, 1, 126–132. [Google Scholar] [CrossRef]
- Valentini, L.; Macan, J.; Armentano, I.; Mengoni, F.; Kenny, J.M. Modification of Fluorinated Single-Walled Carbon Nanotubes with Aminosilane Molecules. Carbon 2006, 44, 2196–2201. [Google Scholar] [CrossRef]
- Lin, T.W.; Salzmann, C.G.; Shao, L.D.; Yu, C.H.; Green, M.L.H.; Tsang, S.C. Polyethylene Glycol Grafting and Attachment of Encapsulated Magnetic Iron Oxide Silica Nanoparticles onto Chlorosilanized Single-Wall Carbon Nanotubes. Carbon 2009, 6, 1415–1420. [Google Scholar] [CrossRef]
- Cui, J.B.; Daghlian, C.P.; Gibson, U.J. Solubility and Electrical Transport Properties of Thiolated Single-Walled Carbon Nanotubes. J. Appl. Phys. 2005, 98, 044320. [Google Scholar] [CrossRef]
- Lee, Y.S. Syntheses and Properties of Fluorinated Carbon Materials. J. Fluor. Chem. 2007, 128, 392–403. [Google Scholar] [CrossRef]
- Adamska, M.; Narkiewicz, U. Fluorination of Carbon Nanotubes—A Review. J. Fluor. Chem. 2017, 200, 179–189. [Google Scholar] [CrossRef]
- Pulikkathara, M.X.; Khabashesku, V.N. Covalent Sidewall Functionalization of Single-Walled Carbon Nanotubes by Amino Acids. Russ. Chem. Bull. 2009, 57, 1054–1062. [Google Scholar] [CrossRef]
- Kónya, Z.; Vesselenyi, I.; Niesz, K.; Kukovecz, A.; Demortier, A.; Fonseca, A.; Delhalle, J.; Mekhalif, Z.; Nagy, J.B.; Koós, A.A.; et al. Large Scale Production of Short Functionalized Carbon Nanotubes. Chem. Phys. Lett. 2002, 360, 429–435. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, S.J.; Lee, W.J.; Lee, J.G.; Haddon, R.C.; Reucroft, P.J. X-ray Photoelectron Spectroscopic Studies of Surface Modified Single-Walled Carbon Nanotube Material. Appl. Surf. Sci. 2001, 181, 121–127. [Google Scholar] [CrossRef]
- Duesberg, G.S.; Graupner, R.; Downes, P.; Minett, A.; Ley, L.; Roth, S.; Nicoloso, N. Hydrothermal Functionalisation of Single-Walled Carbon Nanotubes. Synth. Met. 2004, 142, 263–266. [Google Scholar] [CrossRef]
- Barthos, R.; Méhn, D.; Demortier, A.; Pierard, N.; Morciaux, Y.; Demortier, G.; Fonseca, A.; Nagy, J.B. Functionalization of Single-Walled Carbon Nanotubes by Using Alkyl-Halides. Carbon 2005, 43, 321–325. [Google Scholar] [CrossRef]
- Mazov, I.; Krasnikov, D.; Stadnichenko, A.; Kuznetsov, V.; Romanenko, A.; Anikeeva, O.; Tkachev, E. Direct Vapor-Phase Bromination of Multiwall Carbon Nanotubes. J. Nanotechnol. 2012, 2012, 954084. [Google Scholar] [CrossRef] [Green Version]
- Bulusheva, L.G.; Okotrub, A.V.; Flahaut, E.; Asanov, I.P.; Gevko, P.N.; Koroteev, V.O.; Fedoseeva, Y.V.; Yaya, A.; Ewels, C.P. Bromination of Double-Walled Carbon Nanotubes. Chem. Mater. 2012, 24, 2708–2715. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, Y.; Jiao, Q.; Cao, Y. Hydrogenation of Multi-walled Carbon Nanotubes in Ethylenediamine. Fuller. Nanotub. Carbon Nanostruct. 2010, 18, 14–23. [Google Scholar] [CrossRef]
- Krueger, A. New Carbon Materials: Biological Applications of Functionalized Nanodiamond Materials. Chem.—A Eur. J. 2008, 14, 1382–1390. [Google Scholar] [CrossRef]
- Marshall, M.W.; Popa-Nita, S.; Shapter, J.G. Measurement of Functionalised Carbon Nanotube Carboxylic Acid Groups Using a Simple Chemical Process. Carbon 2006, 44, 1137–1141. [Google Scholar] [CrossRef]
- Nikitin, A.; Li, X.; Zhang, Z.; Ogasawara, H.; Dai, H.; Nilsson, A. Hydrogen Storage in Carbon Nanotubes through the Formation of Stable C−H Bonds. Nano Lett. 2007, 8, 162–167. [Google Scholar] [CrossRef]
- Froudakis, G.E. Hydrogen Storage in Nanotubes & Nanostructures. Mater. Today 2011, 14, 324–328. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of Hydrogen in Single-Walled Carbon Nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Pantarotto, D.; Singh, R.; McCarthy, D.; Erhardt, M.; Briand, J.-P.; Prato, M.; Kostarelos, K.; Bianco, A.; Pantarotto, D.-C.D.; Prato, M.; et al. Functionalized Carbon Nanotubes for Plasmid DNA Gene Delivery. Angew. Chem. Int. Ed. 2004, 43, 5242–5246. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Kim, K.; Zhang, J.; Escalada, A.; Tunuguntla, R.; Comolli, L.R.; Allen, F.I.; Shnyrova, A.V.; Cho, K.R.; Munoz, D.; et al. Stochastic Transport through Carbon Nanotubes in Lipid Bilayers and Live Cell Membranes. Nature 2014, 514, 612–615. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Yu, W. Functionalization Methods of Carbon Nanotubes and Its Applications. Carbon Nanotub. Appl. Electron Devices 2011, 41, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Kis, A.; Zettl, A.; Bertozzi, C.R. A Cell Nanoinjector Based on Carbon Nanotubes. Proc. Natl. Acad. Sci. USA 2007, 104, 8218–8222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintmire, J.W.; Dunlap, B.I.; White, C.T.; Nielsen, P.E.; Engholm, M.; Berg, R.H.; Buchardt, O.S.; Verschueren, A.R.M.; Dekker, C.; Derycke, V.; et al. Carbon Nanotubes with DNA Recognition. Nature 2002, 420, 761. [Google Scholar] [CrossRef]
- Awasthi, K.; Singh, D.P.; Singh, S. Attachment of Biomolecules (Protein and DNA) to Amino-Functionalized Carbon Nanotubes. New Carbon Mater. 2009, 24, 301–306. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Villa, C.H.; Dao, T.; Ahearn, I.; Fehrenbacher, N.; Casey, E.; Rey, D.A.; Korontsvit, T.; Zakhaleva, V.; Batt, C.A.; Philips, M.R.; et al. Single-Walled Carbon Nanotubes Deliver Peptide Antigen into Dendritic Cells and Enhance IgG Responses to Tumor-Associated Antigens. ACS Nano 2011, 5, 5300. [Google Scholar] [CrossRef]
- Lu, Q.; Moore, J.M.; Huang, G.; Mount, A.S.; Rao, A.M.; Larcom, L.L.; Ke, P.C. RNA Polymer Translocation with Single-Walled Carbon Nanotubes. Nano Lett. 2004, 4, 2473–2477. [Google Scholar] [CrossRef]
- Duan, H.G.; Jha, A.; Li, X.; Tiwari, V.; Ye, H.; Nayak, P.K.; Zhu, X.L.; Li, Z.; Martinez, T.J.; Thorwart, M.; et al. Intermolecular Vibrations Mediate Ultrafast Singlet Fission. Sci. Adv. 2020, 6, 52–70. [Google Scholar] [CrossRef]
- Piao, Y.; Meany, B.; Powell, L.R.; Valley, N.; Kwon, H.; Schatz, G.C.; Wang, Y. Brightening of Carbon Nanotube Photoluminescence through the Incorporation of Sp3 Defects. Nat. Chem. 2013, 5, 840–845. [Google Scholar] [CrossRef] [PubMed]
- White, B.; Banerjee, S.; O’Brien, S.; Turro, N.J.; Herman, I.P. Zeta-Potential Measurements of Surfactant-Wrapped Individual Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2007, 111, 13684–13690. [Google Scholar] [CrossRef]
- McDonald, T.J.; Engtrakul, C.; Jones, M.; Rumbles, G.; Heben, M.J. Kinetics of PL Quenching during Single-Walled Carbon Nanotube Rebundling and Diameter-Dependent Surfactant Interactions. J. Phys. Chem. B 2006, 110, 25339–25346. [Google Scholar] [CrossRef] [PubMed]
- Britz, D.A.; Khlobystov, A.N. Noncovalent Interactions of Molecules with Single Walled Carbon Nanotubes. Chem. Soc. Rev. 2006, 35, 637–659. [Google Scholar] [CrossRef]
- Priya, B.R.; Byrne, H.J. Investigation of Sodium Dodecyl Benzene Sulfonate Assisted Dispersion and Debundling of Single-Wall Carbon Nanotubes. J. Phys. Chem. C 2007, 112, 332–337. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.Q.; Qian, W.Z.; Zhang, Y.Y.; Wei, F. The Road for Nanomaterials Industry: A Review of Carbon Nanotube Production, Post-Treatment, and Bulk Applications for Composites and Energy Storage. Small 2013, 9, 1237–1265. [Google Scholar] [CrossRef]
- Wenseleers, W.; Vlasov, I.L.; Goovaerts, E.; Obraztsova, E.D.; Lobach, A.S.; Bouwen, A. Efficient Isolation and Solubilization of Pristine Single-Walled Nanotubes in Bile Salt Micelles. Adv. Funct. Mater. 2004, 14, 1105–1112. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.H.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, P.; Barnes, B.; Fortner, J.; Wang, Y. Cleanly Removable Surfactant for Carbon Nanotubes. Chem. Mater. 2021, 33, 4551–4557. [Google Scholar] [CrossRef]
- Rastogi, R.; Kaushal, R.; Tripathi, S.K.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative Study of Carbon Nanotube Dispersion Using Surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Lee, S.J.; Bae, T.S.; Watari, F.; Uo, M.; Lee, M.H. Aqueous Dispersion of Surfactant-Modified Multiwalled Carbon Nanotubes and Their Application as an Antibacterial Agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Yun, D.J.; Kim, J.M.; Ra, H.; Byun, S.; Kim, H.; Park, G.S.; Park, S.; Rhee, S.W. The Physical/Chemical Properties and Electrode Performance Variations of SWNT Films in Consequence of Solution Based Surfactant Elimination Processes. Org. Electron. 2013, 14, 2962–2972. [Google Scholar] [CrossRef]
- Rossi, J.E.; Soule, K.J.; Cleveland, E.; Schmucker, S.W.; Cress, C.D.; Cox, N.D.; Merrill, A.; Landi, B.J. Removal of Sodium Dodecyl Sulfate Surfactant from Aqueous Dispersions of Single-Wall Carbon Nanotubes. J. Colloid Interface Sci. 2017, 495, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urper, O.; Çakmak, İ.; Karatepe, N. Fabrication of Carbon Nanotube Transparent Conductive Films by Vacuum Filtration Method. Mater. Lett. 2018, 223, 210–214. [Google Scholar] [CrossRef]
- Billing, B.K.; Mayank; Agnihotri, P.K.; Singh, N. Development of Pyrene-Stacked Carbon Nanotube-Based Hybrid: Measurement of NO3- Ions Using Fluorescence Spectroscopy. Analyst 2018, 143, 3343–3352. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Galli, F.; Janssen, K.G.H.; Jiang, L.; van der Linden, H.J.; de Geus, D.C.; Voskamp, P.; Kuil, M.E.; Olsthoorn, R.C.L.; Oosterkamp, T.H.; et al. Stable Single-Walled Carbon Nanotube-Streptavidin Complex for Biorecognition. J. Phys. Chem. C 2010, 114, 4345–4352. [Google Scholar] [CrossRef]
- Meredith, M.T.; Minson, M.; Hickey, D.; Artyushkova, K.; Glatzhofer, D.T.; Minteer, S.D. Anthracene-Modified Multi-Walled Carbon Nanotubes as Direct Electron Transfer Scaffolds for Enzymatic Oxygen Reduction. ACS Catal. 2011, 1, 1683–1690. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; Ma, Y.; Li, Y.; Du, F.; Chen, Y. Noncovalent Nanohybrid of Ferrocene with Single-Walled Carbon Nanotubes and Its Enhanced Electrochemical Property. Chem. Phys. Lett. 2006, 420, 416–420. [Google Scholar] [CrossRef]
- Huang, X.J.; Im, H.S.; Lee, D.H.; Kim, H.S.; Choi, Y.K. Ferrocene Functionalized Single-Walled Carbon Nanotube Bundles. Hybrid Interdigitated Construction Film for l-Glutamate Detection. J. Phys. Chem. C 2006, 111, 1200–1206. [Google Scholar] [CrossRef]
- Khripin, C.Y.; Fagan, J.A.; Zheng, M. Spontaneous Partition of Carbon Nanotubes in Polymer-Modified Aqueous Phases. J. Am. Chem. Soc. 2013, 135, 6822–6825. [Google Scholar] [CrossRef]
- Subbaiyan, N.K.; Cambré, S.; Parra-Vasquez, A.N.G.; Hároz, E.H.; Doorn, S.K.; Duque, J.G. Role of Surfactants and Salt in Aqueous Two-Phase Separation of Carbon Nanotubes toward Simple Chirality Isolation. ACS Nano 2014, 8, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Defillet, J.; Avramenko, M.; Martinati, M.; López Carrillo, M.Á.; van der Elst, D.; Wenseleers, W.; Cambré, S. The Role of the Bile Salt Surfactant Sodium Deoxycholate in Aqueous Two-Phase Separation of Single-Wall Carbon Nanotubes Revealed by Systematic Parameter Variations. Carbon 2022, 195, 349–363. [Google Scholar] [CrossRef]
- Namasivayam, M.; Andersson, M.R.; Shapter, J.G.; Koloor, R.; Ayatollahi, R.; Petrů, M.; Petrů, P. A Comparative Study on the Role of Polyvinylpyrrolidone Molecular Weight on the Functionalization of Various Carbon Nanotubes and Their Composites. Polymers 2021, 13, 2447. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Riviere, N.A.; Inman, A.O.; Wang, Y.Y.; Nemanich, R.J. Surfactant Effects on Carbon Nanotube Interactions with Human Keratinocytes. Nanomedicine 2005, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, T.; Ntim, S.A.; Ji, Z.; George, S.; Meng, H.; Zhang, H.; Castranova, V.; Mitra, S.; Nel, A.E. Quantitative Techniques for Assessing and Controlling the Dispersion and Biological Effects of Multiwalled Carbon Nanotubes in Mammalian Tissue Culture Cells. ACS Nano 2010, 4, 7241–7252. [Google Scholar] [CrossRef] [Green Version]
- Vaisman, L.; Marom, G.; Wagner, H.D. Dispersions of Surface-Modified Carbon Nanotubes in Water-Soluble and Water-Insoluble Polymers. Adv. Funct. Mater. 2006, 16, 357–363. [Google Scholar] [CrossRef]
- Manasrah, A.D.; Laoui, T.; Zaidi, S.J.; Atieh, M.A. Effect of PEG Functionalized Carbon Nanotubes on the Enhancement of Thermal and Physical Properties of Nanofluids. Exp. Therm. Fluid Sci. 2017, 84, 231–241. [Google Scholar] [CrossRef]
- Sharmeen, S.; Rahman, A.F.M.M.; Lubna, M.M.; Salem, K.S.; Islam, R.; Khan, M.A. Polyethylene Glycol Functionalized Carbon Nanotubes/Gelatin-Chitosan Nanocomposite: An Approach for Significant Drug Release. Bioact. Mater. 2018, 3, 236–244. [Google Scholar] [CrossRef]
- Taghavi, S.; Nia, A.H.; Abnous, K.; Ramezani, M. Polyethylenimine-Functionalized Carbon Nanotubes Tagged with AS1411 Aptamer for Combination Gene and Drug Delivery into Human Gastric Cancer Cells. Int. J. Pharm. 2017, 516, 301–312. [Google Scholar] [CrossRef]
- Cavuslar, O.; Unal, H. Self-Assembly of DNA Wrapped Carbon Nanotubes and Asymmetrical Cyanine Dyes into Fluorescent Nanohybrids. RSC Adv. 2015, 5, 22380–22389. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.H.; Kim, N.Y.; Ryou, M.H.; Bae, H.; Kim, J.H.; Lee, Y.G.; Lee, S.Y. Nanofibrous Conductive Binders Based on DNA-Wrapped Carbon Nanotubes for Lithium Battery Electrodes. iScience 2020, 23, 101739. [Google Scholar] [CrossRef] [PubMed]
- McMorrow, J.; Freeley, M.; Palma, M. DNA-Wrapped Single-Walled Carbon Nanotube Assemblies. Ind. Eng. Chem. Res. 2017, 56, 5302–5308. [Google Scholar] [CrossRef]
- Streit, J.K.; Fagan, J.A.; Zheng, M. A Low Energy Route to DNA-Wrapped Carbon Nanotubes via Replacement of Bile Salt Surfactants. Anal. Chem. 2017, 89, 10496–10503. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Pantarotto, D.; McCarthy, D.; Chaloin, O.; Hoebeke, J.; Partidos, C.D.; Briand, J.P.; Prato, M.; Bianco, A.; Kostarelos, K. Binding and Condensation of Plasmid DNA onto Functionalized Carbon Nanotubes: Toward the Construction of Nanotube-Based Gene Delivery Vectors. J. Am. Chem. Soc. 2005, 127, 4388–4396. [Google Scholar] [CrossRef] [PubMed]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroǧlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Carbon Nanotubes: Biomedical Applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar] [CrossRef] [Green Version]
- Shkodra, B.; Petrelli, M.; Costa Angeli, M.A.; Garoli, D.; Nakatsuka, N.; Lugli, P.; Petti, L. Electrolyte-Gated Carbon Nanotube Field-Effect Transistor-Based Biosensors: Principles and Applications. Appl. Phys. Rev. 2021, 8, 041325. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Kharlamova, M.V.; Kramberger, C.; Nikitin, M.P. Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials 2021, 11, 3020. [Google Scholar] [CrossRef]
- Wu, B.; Ou, Z.; Xing, D. Functional Single-Walled Carbon Nanotubes/Chitosan Conjugate for Tumor Cells Targeting. In Eighth International Conference on Photonics and Imaging in Biology and Medicine (PIBM 2009); SPIE: Bellingham, WA, USA, 2009; Volume 7519, pp. 142–149. [Google Scholar]
- Varkouhi, A.K.; Foillard, S.; Lammers, T.; Schiffelers, R.M.; Doris, E.; Hennink, W.E.; Storm, G. SiRNA Delivery with Functionalized Carbon Nanotubes. Int. J. Pharm. 2011, 416, 419–425. [Google Scholar] [CrossRef]
- Alidori, S.; Asqiriba, K.; Londero, P.; Bergkvist, M.; Leona, M.; Scheinberg, D.A.; McDevitt, M.R. Deploying RNA and DNA with Functionalized Carbon Nanotubes. J. Phys. Chem. C 2013, 117, 5982–5992. [Google Scholar] [CrossRef] [Green Version]
- De Juan, A.; Pouillon, Y.; Ruiz-González, L.; Torres-Pardo, A.; Casado, S.; Martín, N.; Rubio, Á.; Pérez, E.M. Mechanically Interlocked Single-Wall Carbon Nanotubes. Angew. Chem. Int. Ed. 2014, 53, 5394–5400. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Zhou, Y.; Zhang, X.; Cai, B.; Qiu, J. Flowing Nitrogen Assisted-Arc Discharge Synthesis of Nitrogen-Doped Single-Walled Carbon Nanohorns. Appl. Surf. Sci. 2013, 277, 88–93. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, M.R.; Jeong, S.H.; Kim, S.U.; Lee, O.J.; Lee, K.H.; Suh, J.H.; Park, C.K. High-Yield Synthesis of Multi-Walled Carbon Nanotubes by Arc Discharge in Liquid Nitrogen. Appl. Phys. A 2003, 76, 285–286. [Google Scholar] [CrossRef]
- Mo, Z.; Liao, S.; Zheng, Y.; Fu, Z. Preparation of Nitrogen-Doped Carbon Nanotube Arrays and Their Catalysis towards Cathodic Oxygen Reduction in Acidic and Alkaline Media. Carbon 2012, 50, 2620–2627. [Google Scholar] [CrossRef]
- Sharifi, T.; Nitze, F.; Barzegar, H.R.; Tai, C.W.; Mazurkiewicz, M.; Malolepszy, A.; Stobinski, L.; Wågberg, T. Nitrogen Doped Multi Walled Carbon Nanotubes Produced by CVD-Correlating XPS and Raman Spectroscopy for the Study of Nitrogen Inclusion. Carbon 2012, 50, 3535–3541. [Google Scholar] [CrossRef]
- Tao, X.Y.; Zhang, X.B.; Sun, F.Y.; Cheng, J.P.; Liu, F.; Luo, Z.Q. Large-Scale CVD Synthesis of Nitrogen-Doped Multi-Walled Carbon Nanotubes with Controllable Nitrogen Content on a CoxMg1−xMoO4 Catalyst. Diam. Relat. Mater. 2007, 3, 425–430. [Google Scholar] [CrossRef]
- She, X.; Yang, D.; Jing, D.; Yuan, F.; Yang, W.; Guo, L.; Che, Y. Nitrogen-Doped One-Dimensional (1D) Macroporous Carbonaceous Nanotube Arrays and Their Application in Electrocatalytic Oxygen Reduction Reactions. Nanoscale 2014, 6, 11057–11061. [Google Scholar] [CrossRef]
- Chen, L.; Xia, K.; Huang, L.; Li, L.; Pei, L.; Fei, S. Facile Synthesis and Hydrogen Storage Application of Nitrogen-Doped Carbon Nanotubes with Bamboo-like Structure. Int. J. Hydrog. Energy 2013, 38, 3297–3303. [Google Scholar] [CrossRef]
- Shi, W.; Venkatachalam, K.; Gavalas, V.; Qian, D.; Andrews, R.; Bachas, L.G.; Chopra, N. The Role of Plasma Treatment on Electrochemical Capacitance of Undoped and Nitrogen Doped Carbon Nanotubes. Nanomater. Energy 2015, 2, 71–81. [Google Scholar] [CrossRef]
- Du, Z.; Wang, S.; Kong, C.; Deng, Q.; Wang, G.; Liang, C.; Tang, H. Microwave Plasma Synthesized Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction. J. Solid State Electrochem. 2015, 19, 1541–1549. [Google Scholar] [CrossRef]
- Chan, L.H.; Hong, K.H.; Xiao, D.Q.; Lin, T.C.; Lai, S.H.; Hsieh, W.J.; Shih, H.C. Resolution of the Binding Configuration in Nitrogen-Doped Carbon Nanotubes. Phys. Rev. B-Condens. Matter Mater. Phys. 2004, 70, 125408. [Google Scholar] [CrossRef]
- Nagaiah, T.C.; Kundu, S.; Bron, M.; Muhler, M.; Schuhmann, W. Nitrogen-Doped Carbon Nanotubes as a Cathode Catalyst for the Oxygen Reduction Reaction in Alkaline Medium. Electrochem. Commun. 2010, 3, 338–341. [Google Scholar] [CrossRef]
- Vikkisk, M.; Kruusenberg, I.; Ratso, S.; Joost, U.; Shulg, E.; Kink, I.; Rauwel, P.; Tammeveski, K. Enhanced Electrocatalytic Activity of Nitrogen-Doped Multi-Walled Carbon Nanotubes towards the Oxygen Reduction Reaction in Alkaline Media. RSC Adv. 2015, 5, 59495–59505. [Google Scholar] [CrossRef]
- Keskar, G.; Rao, R.; Luo, J.; Hudson, J.; Chen, J.; Rao, A.M. Growth, Nitrogen Doping and Characterization of Isolated Single-Wall Carbon Nanotubes Using Liquid Precursors. CPL 2005, 412, 269–273. [Google Scholar] [CrossRef]
- Mickelson, E.T.; Chiang, I.W.; Zimmerman, J.L.; Boul, P.J.; Lozano, J.; Liu, J.; Smalley, R.E.; Hauge, R.H.; Margrave, J.L. Solvation of Fluorinated Single-Wall Carbon Nanotubes in Alcohol Solvents. J. Phys. Chem. B 1999, 103, 4318–4322. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Dubois, M.; Guérin, K.; Bonnet, P.; Kharbache, H.; Masin, F.; Kharitonov, A.P.; Hamwi, A. Effect of Curvature on C–F Bonding in Fluorinated Carbons: From Fullerene and Derivatives to Graphite. Phys. Chem. Chem. Phys. 2010, 12, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Pehrsson, P.E.; Zhao, W.; Baldwin, J.W.; Song, C.; Liu, J.; Kooi, S.; Zheng, B. Thermal Fluorination and Annealing of Single-Wall Carbon Nanotubes. J. Phys. Chem. B 2003, 107, 5690–5695. [Google Scholar] [CrossRef]
- An, K.H.; Park, K.A.; Heo, J.G.; Lee, J.Y.; Jeon, K.K.; Lim, S.C.; Yang, C.W.; Lee, Y.S.; Lee, Y.H. Structural Transformation of Fluorinated Carbon Nanotubes Induced by in Situ Electron-Beam Irradiation. J. Am. Chem. Soc. 2003, 125, 3057–3061. [Google Scholar] [CrossRef]
- Chamssedine, F.; Guérin, K.; Dubois, M.; Disa, E.; Petit, E.; El Fawal, Z.; Hamwi, A. Fluorination of Single Walled Carbon Nanotubes at Low Temperature: Towards the Reversible Fluorine Storage into Carbon Nanotubes. J. Fluor. Chem. 2011, 132, 1072–1078. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Añez, L.; Primera, J.; Silva, P.; Etienne-Calas, S.; Anglaret, E. Photoluminescent Single Wall Carbon Nanotube–Silica Composite Gels. Carbon 2008, 46, 1253–1255. [Google Scholar] [CrossRef]
- Rafailov, P.M.; Thomsen, C.; Monev, M.; Dettlaff-Weglikowska, U.; Roth, S. Electrochemical Functionalization of SWNT Bundles in Acid and Salt Media as Observed by Raman and X-ray Photoelectron Spectroscopy. Physica Status Solidi (b) 2008, 245, 1967–1970. [Google Scholar] [CrossRef]
- Qian, Z.; Ma, J.; Zhou, J.; Lin, P.; Chen, C.; Chen, J.; Feng, H. Facile Synthesis of Halogenated Multi-Walled Carbon Nanotubes and Their Unusual Photoluminescence. J. Mater. Chem. 2012, 22, 22113–22119. [Google Scholar] [CrossRef]
- Abdelkader, V.K.; Scelfo, S.; García-Gallarín, C.; Godino-Salido, M.L.; Domingo-García, M.; López-Garzón, F.J.; Pérez-Mendoza, M. Carbon Tetrachloride Cold Plasma for Extensive Chlorination of Carbon Nanotubes. J. Phys. Chem. C 2013, 117, 16677–16685. [Google Scholar] [CrossRef]
- Vejpravova, J.; Pacakova, B.; Kalbac, M. Magnetic Impurities in Single-Walled Carbon Nanotubes and Graphene: A Review. Analyst 2016, 141, 2639–2656. [Google Scholar] [CrossRef]
- Lee, R.S.; Kim, H.J.; Fischer, J.E.; Thess, A.; Smalley, R.E. Conductivity Enhancement in Single-Walled Carbon Nanotube Bundles Doped with K and Br. Nature 1997, 388, 255–257. [Google Scholar] [CrossRef]
- Jin, Z.; Xu, G.Q.; Goh, S.H. A Preferentially Ordered Accumulation of Bromine on Multi-Wall Carbon Nanotubes. Carbon 2000, 38, 1135–1139. [Google Scholar] [CrossRef]
- Unger, E.; Graham, A.; Kreupl, F.; Liebau, M.; Hoenlein, W. Electrochemical Functionalization of Multi-Walled Carbon Nanotubes for Solvation and Purification. Curr. Appl. Phys. 2002, 2, 107–111. [Google Scholar] [CrossRef]
- Colomer, J.F.; Marega, R.; Traboulsi, H.; Meneghetti, M.; van Tendeloo, G.; Bonifazi, D. Microwave-Assisted Bromination of Double-Walled Carbon Nanotubes. Chem. Mater. 2009, 21, 4747–4749. [Google Scholar] [CrossRef]
- Abdelkader, V.K.; Domingo-García, M.; Melguizo, M.; López-Garzón, R.; Javier López-Garzón, F.; Pérez-Mendoza, M. Covalent Bromination of Multi-Walled Carbon Nanotubes by Iodine Bromide and Cold Plasma Treatments. Carbon 2015, 93, 276–285. [Google Scholar] [CrossRef]
- Moradi, L.; Etesami, I. New Route for Bromination of Multiwalled Carbon Nanotubes under Mild and Efficient Conditions. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 213–218. [Google Scholar] [CrossRef]
- Milowska, K.Z.; Krzywiecki, M.; Payne, M.C.; Janas, D. Effective Doping of Single-Walled Carbon Nanotube Films with Bromine under Ultrasound. Mater. Des. 2022, 213, 110310. [Google Scholar] [CrossRef]
- Coleman, K.S.; Chakraborty, A.K.; Bailey, S.R.; Sloan, J.; Alexander, M. Iodination of Single-Walled Carbon Nanotubes. Chem. Mater. 2007, 19, 1076–1081. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, J.; Vajtai, R.; Ajayan, P.M.; Barrera, E.V. Iodine Doped Carbon Nanotube Cables Exceeding Specific Electrical Conductivity of Metals. Sci. Rep. 2011, 1, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Lin, P.; Ma, J.; Shan, X.; Feng, H.; Chen, C.; Chen, J.; Qian, Z. Facile Synthesis of Halogenated Carbon Quantum Dots as an Important Intermediate for Surface Modification. RSC Adv. 2013, 3, 9625–9628. [Google Scholar] [CrossRef]
- Inani, H.; Mustonen, K.; Markevich, A.; Ding, E.X.; Tripathi, M.; Hussain, A.; Mangler, C.; Kauppinen, E.I.; Susi, T.; Kotakoski, J. Silicon Substitution in Nanotubes and Graphene via Intermittent Vacancies. J. Phys. Chem. C 2019, 123, 13136–13140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.S.; Green, A.A.; Hulvat, J.F.; Stupp, S.I.; Hersam, M.C. Sorting Carbon Nanotubes by Electronic Structure Using Density Differentiation. Nat. Nanotechnol. 2006, 1, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Antaris, A.L.; Seo, J.W.T.; Green, A.A.; Hersam, M.C. Sorting Single-Walled Carbon Nanotubes by Electronic Type Using Nonionic, Biocompatible Block Copolymers. ACS Nano 2010, 4, 4725–4732. [Google Scholar] [CrossRef]
- Fagan, J.A.; Khripin, C.Y.; Silvera Batista, C.A.; Simpson, J.R.; Hároz, E.H.; Hight Walker, A.R.; Zheng, M. Isolation of Specific Small-Diameter Single-Wall Carbon Nanotube Species via Aqueous Two-Phase Extraction. Adv. Mater. 2014, 26, 2800–2804. [Google Scholar] [CrossRef]
- Gao, W.; Kono, J. Science and Applications of Wafer-Scale Crystalline Carbon Nanotube Films Prepared through Controlled Vacuum Filtration. R. Soc. Open Sci. 2019, 6, 181605. [Google Scholar] [CrossRef] [Green Version]
- Thostenson, E.T.; Chou, T.W. Processing-Structure-Multi-Functional Property Relationship in Carbon Nanotube/Epoxy Composites. Carbon 2006, 44, 3022–3029. [Google Scholar] [CrossRef]
- Ci, L.; Bai, J.B. The Reinforcement Role of Carbon Nanotubes in Epoxy Composites with Different Matrix Stiffness. Compos. Sci. Technol. 2006, 66, 3–4, 599–603. [Google Scholar] [CrossRef]
- Roy, S.; Petrova, R.S.; Mitra, S. Effect of Carbon Nanotube (CNT) Functionalization in Epoxy-CNT Composites. Nanotechnol. Rev. 2018, 7, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S. Surface Functionalization of Carbon Nanotubes: Fabrication and Applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
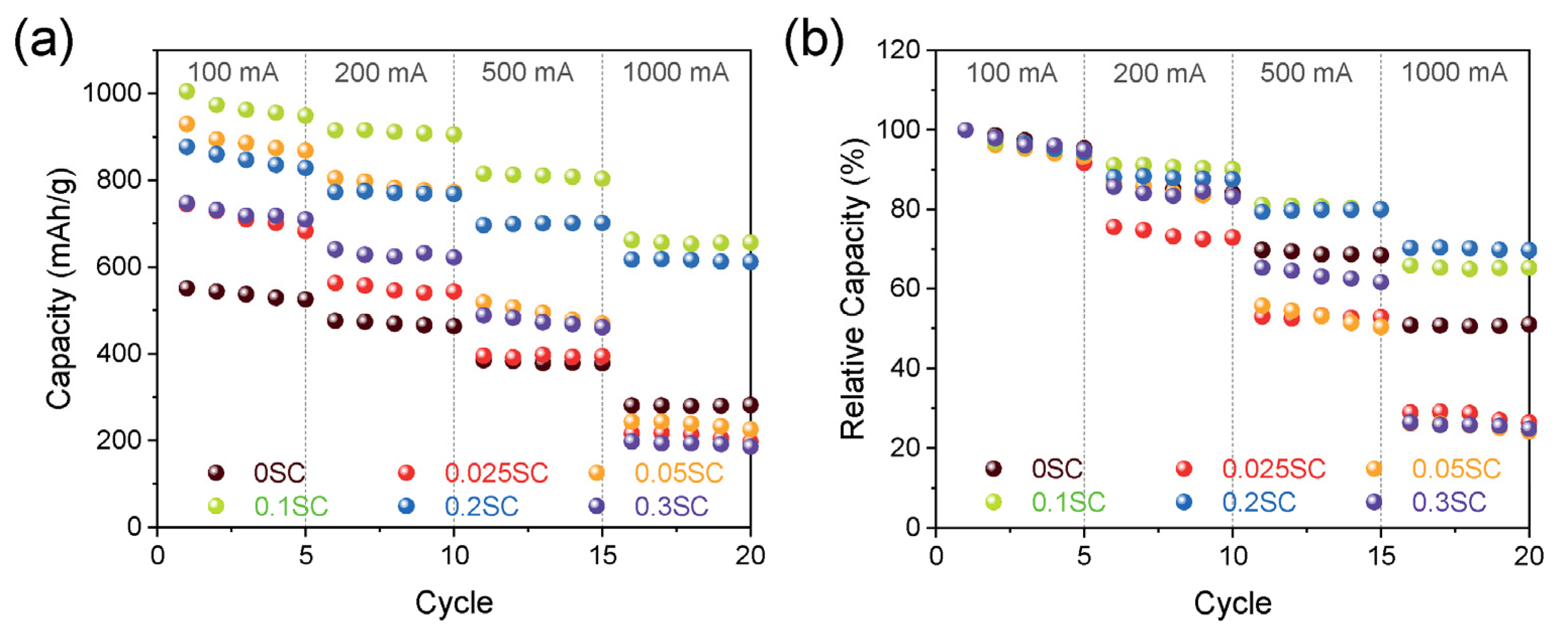
- Ku, N.; Cheon, J.; Lee, K.; Jung, Y.; Yoon, S.Y.; Kim, T. Hydrophilic and Conductive Carbon Nanotube Fibers for High-Performance Lithium-Ion Batteries. Materials 2021, 14, 7822. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Moreno, N.; Gervasio, D.; Godínez García, A.; Pérez Robles, J.F. Polybenzimidazole-Multiwall Carbon Nanotubes Composite Membranes for Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2015, 300, 229–237. [Google Scholar] [CrossRef]
- Kumar, T.H.V.; Sundramoorthy, A.K. Non-Enzymatic Electrochemical Detection of Urea on Silver Nanoparticles Anchored Nitrogen-Doped Single-Walled Carbon Nanotube Modified Electrode. J. Electrochem. Soc. 2018, 165, B3006–B3016. [Google Scholar] [CrossRef] [Green Version]
- Ben Messaoud, N.; Ghica, M.E.; Dridi, C.; ben Ali, M.; Brett, C.M.A. Electrochemical Sensor Based on Multiwalled Carbon Nanotube and Gold Nanoparticle Modified Electrode for the Sensitive Detection of Bisphenol A. Sens. Actuators B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Kangkamano, T.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Chitosan Cryogel with Embedded Gold Nanoparticles Decorated Multiwalled Carbon Nanotubes Modified Electrode for Highly Sensitive Flow Based Non-Enzymatic Glucose Sensor. Sens. Actuators B Chem. 2017, 246, 854–863. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Jayanth Babu, K.; Balaguru Rayappan, J.B.; Krishnan, U.M. Fabrication of Mediator-Free Hybrid Nano-Interfaced Electrochemical Biosensor for Monitoring Cancer Cell Proliferation. Biosens. Bioelectron. 2017, 87, 832–841. [Google Scholar] [CrossRef]
- Chen, C.; Ran, R.; Yang, Z.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.H. An Efficient Flexible Electrochemical Glucose Sensor Based on Carbon Nanotubes/Carbonized Silk Fabrics Decorated with Pt Microspheres. Sens. Actuators B Chem. 2018, 256, 63–70. [Google Scholar] [CrossRef]
- Magar, H.S.; Ghica, M.E.; Abbas, M.N.; Brett, C.M.A. A Novel Sensitive Amperometric Choline Biosensor Based on Multiwalled Carbon Nanotubes and Gold Nanoparticles. Talanta 2017, 167, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, J.; Lai, L.; Yu, F.; Ying, X.; Ye, B.C.; Li, Y. A Free-Standing Electrochemical Sensor Based on Graphene Foam-Carbon Nanotube Composite Coupled with Gold Nanoparticles and Its Sensing Application for Electrochemical Determination of Dopamine and Uric Acid. J. Electroanal. Chem. 2017, 801, 129–134. [Google Scholar] [CrossRef]
- He, X.; Fujimura, N.; Lloyd, J.M.; Erickson, K.J.; Talin, A.A.; Zhang, Q.; Gao, W.; Jiang, Q.; Kawano, Y.; Hauge, R.H.; et al. Carbon Nanotube Terahertz Detector. Nano Lett. 2014, 14, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, G.; Liu, H.; Fu, H.; Fan, J.; Wang, K.; Chen, Y.; Li, B.; Zhang, C.; Zhi, X.; et al. Identification of Volatile Biomarkers of Gastric Cancer Cells and Ultrasensitive Electrochemical Detection Based on Sensing Interface of Au-Ag Alloy Coated MWCNTs. Theranostics 2014, 4, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janudin, N.; Abdullah, N.; Wan Yunus, W.M.Z.; Yasin, F.M.; Yaacob, M.H.; Mohamad Saidi, N.; Kasim, N.A.M. Effect of Functionalized Carbon Nanotubes in the Detection of Benzene at Room Temperature. J. Nanotechnol. 2018, 2018, 2107898. [Google Scholar] [CrossRef] [Green Version]
- Su, H.C.; Tran, T.T.; Bosze, W.; Myung, N.V. Chemiresistive Sensor Arrays for Detection of Air Pollutants Based on Carbon Nanotubes Functionalized with Porphyrin and Phthalocyanine Derivatives. Sens. Actuators Rep. 2020, 2, 100011. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug Delivery with Carbon Nanotubes for in Vivo Cancer Treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Cai, W.; He, L.; Nakayama, N.; Chen, K.; Sun, X.; Chen, X.; Dai, H. In Vivo Biodistribution and Highly Efficient Tumour Targeting of Carbon Nanotubes in Mice. Nat. Nanotechnol. 2006, 2, 47–52. [Google Scholar] [CrossRef]
- Bhirde, A.A.; Patel, S.; Sousa, A.A.; Patel, V.; Molinolo, A.A.; Ji, Y.; Leapman, R.D.; Gutkind, J.S.; Rusling, J.F. Distribution and Clearance of PEG-Single-Walled Carbon Nanotube Cancer Drug Delivery Vehicles in Mice. Nanomedicine 2010, 5, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bai, Y.; Yan, B. Functionalized Carbon Nanotubes for Potential Medicinal Applications. Drug Discov. Today 2010, 15, 428. [Google Scholar] [CrossRef] [Green Version]
- Martincic, M.; Tobias, G. Filled Carbon Nanotubes in Biomedical Imaging and Drug Delivery. Expert Opin. Drug Deliv. 2015, 12, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Kapralov, A.A.; Feng, W.H.; Amoscato, A.A.; Yanamala, N.; Balasubramanian, K.; Winnica, D.E.; Kisin, E.R.; Kotchey, G.P.; Gou, P.; Sparvero, L.J.; et al. Adsorption of Surfactant Lipids by Single-Walled Carbon Nanotubes in Mouse Lung upon Pharyngeal Aspiration. ACS Nano 2012, 6, 4147–4156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Li, Y.; Yao, Y.C.; Aydin, F.; Zhan, C.; Chen, Y.; Elimelech, M.; Pham, T.A.; Noy, A. Strong Differential Monovalent Anion Selectivity in Narrow Diameter Carbon Nanotube Porins. ACS Nano 2020, 14, 6269–6275. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Aydin, F.; Quan, J.; Chen, X.; Yao, Y.C.; Zhan, C.; Chen, Y.; Pham, T.A.; Noy, A. Water-Ion Permselectivity of Narrow-Diameter Carbon Nanotubes. Sci. Adv. 2020, 6, 9966–9982. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced Water Permeability and Tunable Ion Selectivity in Subnanometer Carbon Nanotube Porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.M.; Wong, C.P.P.; Tan, T.L.; Lai, C.W. Functionalized Carbon Nanotubes for Adsorptive Removal of Water Pollutants. Mater. Sci. Eng. B 2018, 236, 61–69. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharlamova, M.V.; Paukov, M.; Burdanova, M.G. Nanotube Functionalization: Investigation, Methods and Demonstrated Applications. Materials 2022, 15, 5386. https://doi.org/10.3390/ma15155386
Kharlamova MV, Paukov M, Burdanova MG. Nanotube Functionalization: Investigation, Methods and Demonstrated Applications. Materials. 2022; 15(15):5386. https://doi.org/10.3390/ma15155386
Chicago/Turabian StyleKharlamova, Marianna V., Maksim Paukov, and Maria G. Burdanova. 2022. "Nanotube Functionalization: Investigation, Methods and Demonstrated Applications" Materials 15, no. 15: 5386. https://doi.org/10.3390/ma15155386




