LTA and FAU-X Iron-Enriched Zeolites: Use for Phosphate Removal from Aqueous Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Zeolite Samples
2.2. Obtaining of Iron Form of Zeolites LTA and FAU-X
2.3. Physicochemical Characterization
2.4. Influence of the pH on Phosphate Adsorption
2.5. Equilibrium Phosphate Adsorption
2.6. Kinetic of Phosphate Adsorption
2.7. Phosphate Fractioning
2.8. Zeolites Regeneration
3. Results and Discussions
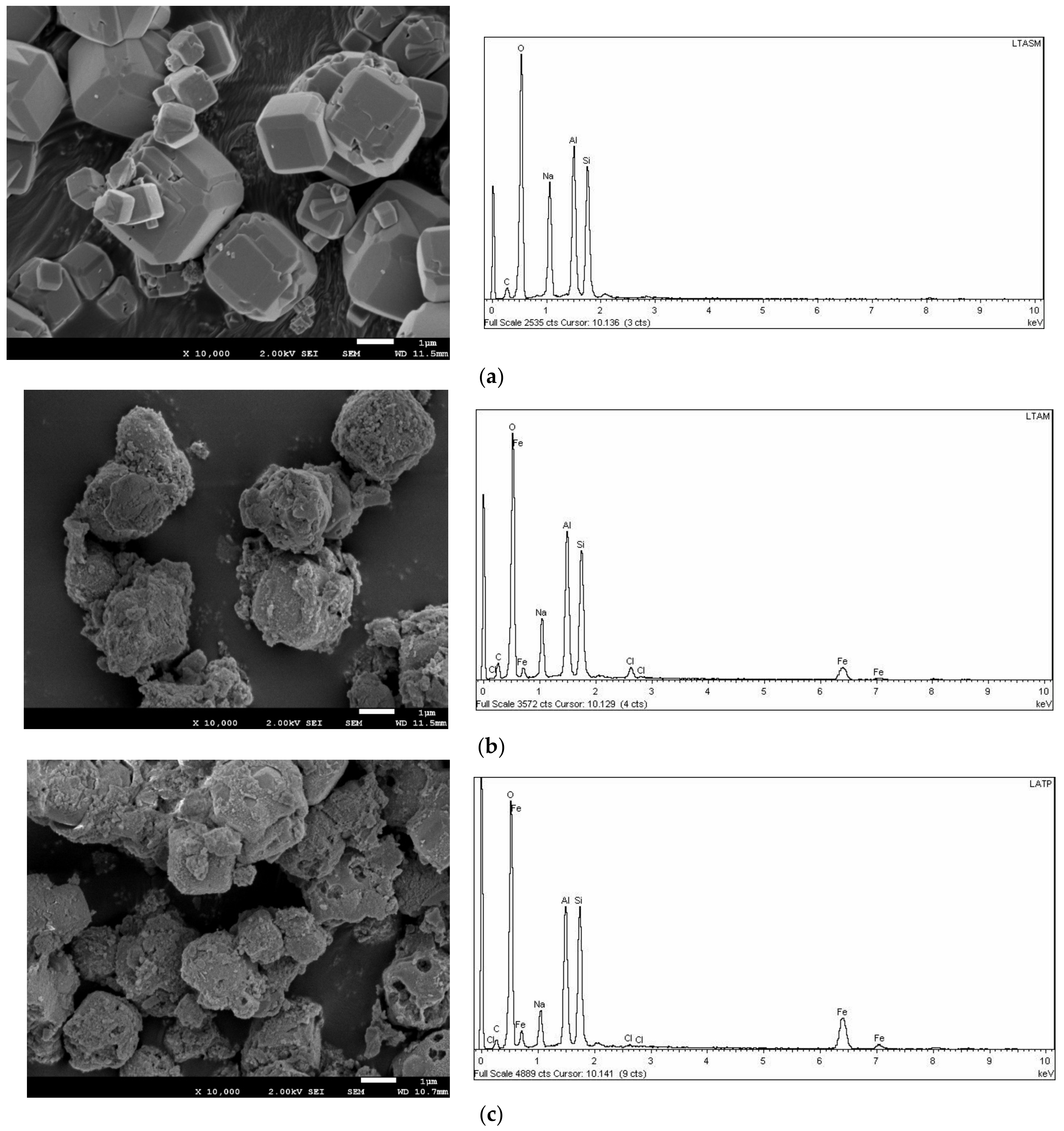
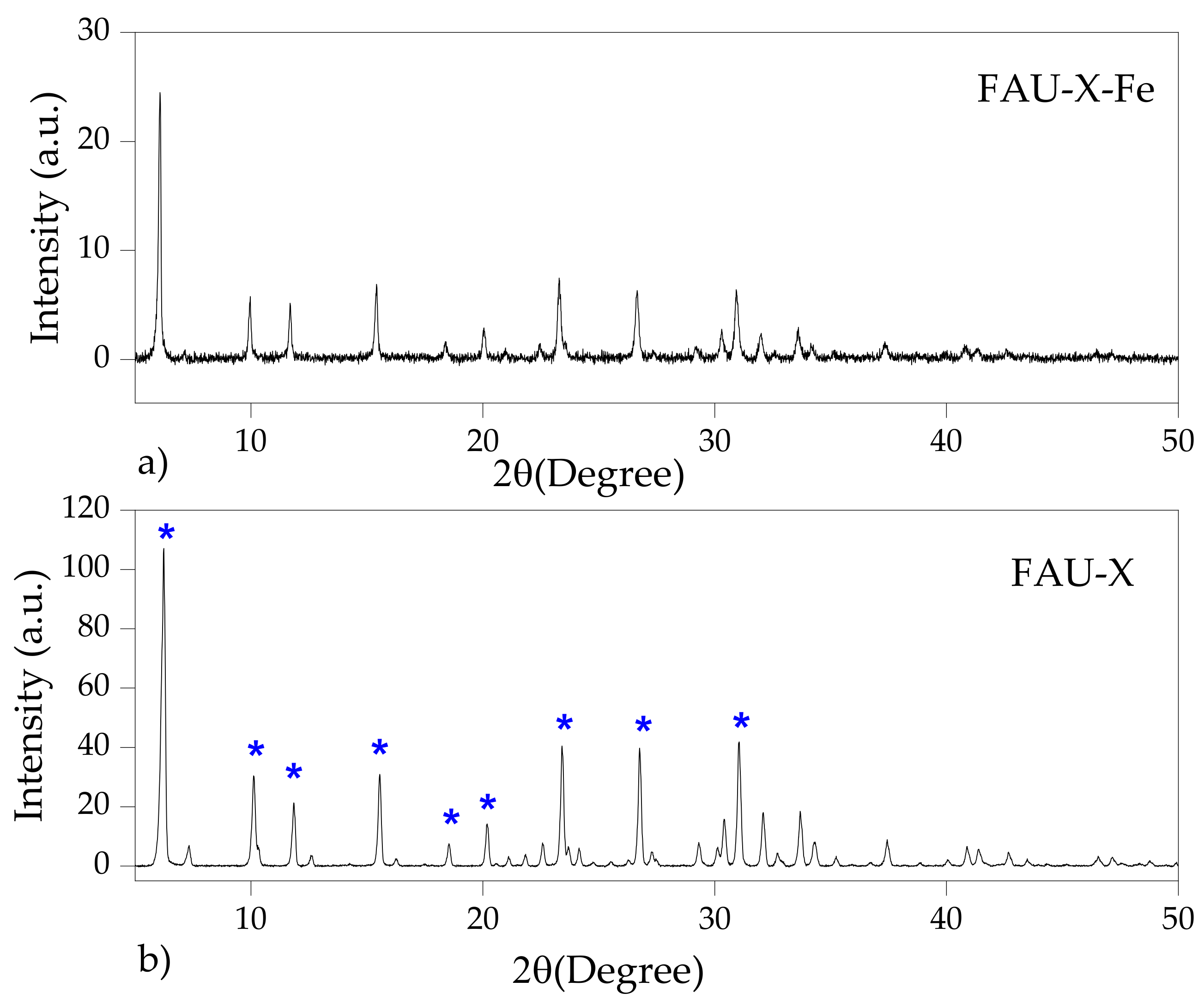
3.1. Zeolites Characterization
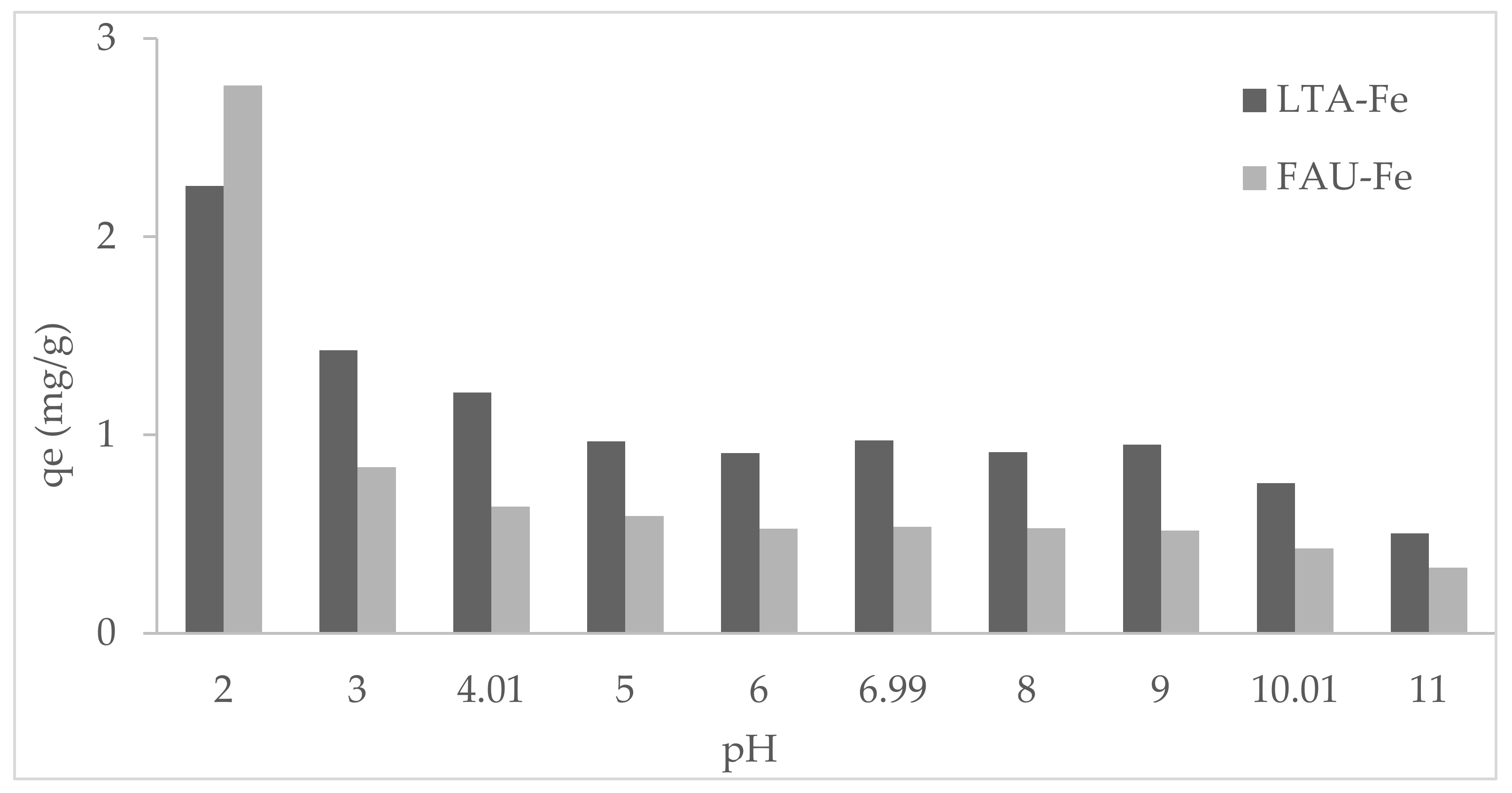
3.2. Effect of pH on Phosphate Adsorption
3.3. Phosphate Adsorption Isotherms: Thermodynamical Characterization
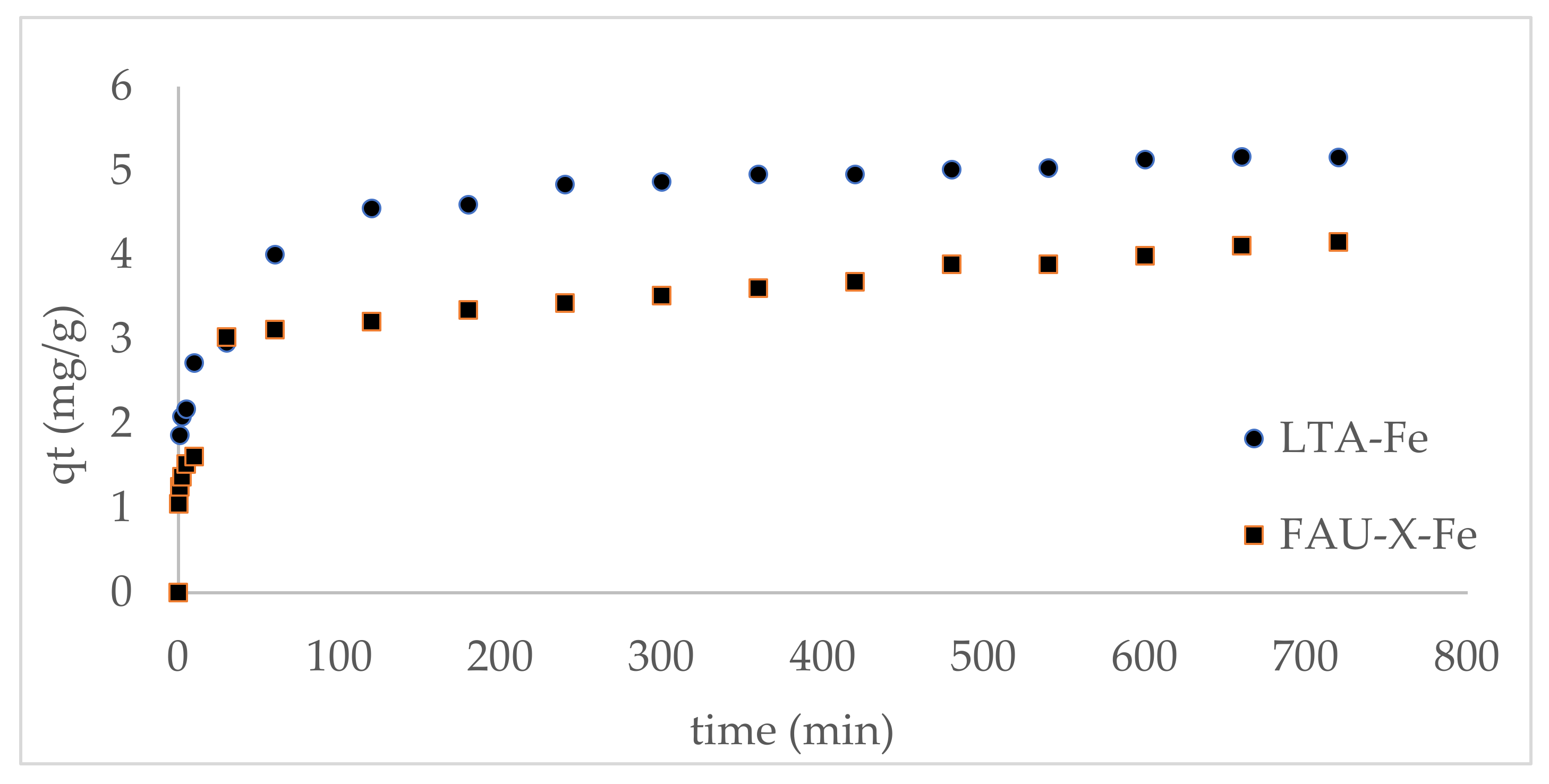
3.4. Kinetic of Phosphate Adsorption Processes
3.5. Phosphate Fractioning
3.6. Phosphate Desorption Processes
3.7. Advantages and Disadvantages of Phosphate Adsorption Using Hydrothermally Synthetized LTA-Fe and FAU-X-Fe Zeolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Modification of a Natural Zeolite with Fe(III) for Simultaneous Phosphate and Ammonium Removal from Aqueous Solutions. J. Chem. Technol. Biotechnol. 2016, 91, 1737–1746. [Google Scholar] [CrossRef]
- Khan, F.; Ansari, A.A. Eutrophication: An Ecological Vision. Bot. Rev. 2005, 71, 449–482. [Google Scholar] [CrossRef]
- Nanda, M.; Kansal, A.; Cordell, D. Managing Agricultural Vulnerability to Phosphorus Scarcity through Bottom-up Assessment of Regional-Scale Opportunities. Agric. Syst. 2020, 184, 102910. [Google Scholar] [CrossRef]
- European Commission Study on the Review of the List of Critical Raw Materials. Available online: https://op.europa.eu/en/publication-detail/-/publication/08fdab5f-9766-11e7-b92d-01aa75ed71a1 (accessed on 1 June 2020).
- Awual, M.R. Efficient Phosphate Removal from Water for Controlling Eutrophication Using Novel Composite Adsorbent. J. Clean. Prod. 2019, 228, 1311–1319. [Google Scholar] [CrossRef]
- Guaya, D.; Jiménez, R.; Sarango, J.; Valderrama, C.; Cortina, J.L. Iron-Doped Natural Clays: Low-Cost Inorganic Adsorbents for Phosphate Recovering from Simulated Urban Treated Wastewater. J. Water Process Eng. 2021, 43, 102274. [Google Scholar] [CrossRef]
- Hermassi, M.; Valderrama, C.; Moreno, N.; Font, O.; Querol, X.; Batis, N.H.; Cortina, J.L. Fly Ash as Reactive Sorbent for Phosphate Removal from Treated Waste Water as a Potential Slow Release Fertilizer. J. Environ. Chem. Eng. 2017, 5, 160–169. [Google Scholar] [CrossRef]
- Awual, M.R.; Jyo, A. Assessing of Phosphorus Removal by Polymeric Anion Exchangers. Desalination 2011, 281, 111–117. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous Phosphate and Ammonium Removal from Aqueous Solution by a Hydrated Aluminum Oxide Modified Natural Zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Guaya, D.; Mendoza, A.; Valderrama, C.; Farran, A.; Sauras-Yera, T.; Cortina, J.L. Use of Nutrient-Enriched Zeolite (NEZ) from Urban Wastewaters in Amended Soils: Evaluation of Plant Availability of Mineral Elements. Sci. Total Environ. 2020, 727, 138646. [Google Scholar] [CrossRef]
- Hermassi, M.; Valderrama, C.; Moreno, N.; Font, O.; Querol, X.; Batis, N.; Cortina, J.L. Powdered Ca-Activated Zeolite for Phosphate Removal from Treated Waste-Water. J. Chem. Technol. Biotechnol. 2016, 91, 1962–1971. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Li, X.; Zhang, Y.; Han, L.; Chen, Y. Phase and Morphology Control of LTA/FAU Zeolites by Adding Trace Amounts of Inorganic Ions. Ceram. Int. 2013, 39, 5997–6003. [Google Scholar] [CrossRef]
- Khaleque, A.; Alam, M.M.; Hoque, M.; Mondal, S.; Haider, J.B.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Zhou, J.L.; Ahmed, M.B.; et al. Zeolite Synthesis from Low-Cost Materials and Environmental Applications: A Review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Kannangara, I.; Jayawardhana, Y.; Munasinghe, E.; Rajapakse, A.; Bandara, A.; Weerasooriya, R.; Jayarathna, L. Synthesis and Characterization of Nano Zeolite-A with Aid of Sodium Dodecyl Sulfate (SDS) as Particle Size-Controlling Agent. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124427. [Google Scholar] [CrossRef]
- Mohd Nazir, L.S.; Yeong, Y.F.; Chew, T.L. Controlled Growth of Faujasite Zeolite with NaX Topology by Manipulating Solution Aging and Na2O/Al2O3 Ratios. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124803. [Google Scholar] [CrossRef]
- Suvaci, E.; Özel, E. Hydrothermal Synthesis. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; ISBN 978-0-12-822233-1. [Google Scholar]
- Guaya, D.; Valderrama, C.; Farran, A.; Sauras, T.; Cortina, J.L. Valorisation of N and P from Waste Water by Using Natural Reactive Hybrid Sorbents: Nutrients (N,P,K) Release Evaluation in Amended Soils by Dynamic Experiments. Sci. Total Environ. 2018, 612, 728–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, A.; López, C.M.; García, L.V.; Casanova, J.; Goldwasser, M.R. Improvements in the Synthesis of Zeolites with Low Si/Al Ratio from Venezuelan Sodium Silicate for an Environmentally Friendly Process. Ing. E Investig. 2016, 36, 62–69. [Google Scholar] [CrossRef]
- Guaya, D.; Hermassi, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Recovery of Ammonium and Phosphate from Treated Urban Wastewater by Using Potassium Clinoptilolite Impregnated Hydrated Metal Oxides as N-P-K Fertilizer. J. Environ. Chem. Eng. 2016, 4, 3519–3526. [Google Scholar] [CrossRef]
- McCrady, M.H. American Journal of Public Health. In Standard Methods for the Examination of Water and Wastewater, 12th ed.; American Public Health Association: New York, NY, USA, 2011. [Google Scholar]
- Hieltjes, A.H.M.; Lijklema, L. Fractionation of Inorganic Phosphates in Calcareous Sediments. J. Environ. Qual. 1980, 9, 405–407. [Google Scholar] [CrossRef]
- Monteiro, W.F.; Diz, F.M.; Andrieu, L.; Morrone, F.B.; Ligabue, R.A.; Bernardo-Gusmão, K.; de Souza, M.O.; Schwanke, A.J. Waste to Health: Ag-LTA Zeolites Obtained by Green Synthesis from Diatom and Rice-Based Residues with Antitumoral Activity. Microporous Mesoporous Mater. 2020, 307, 110508. [Google Scholar] [CrossRef]
- Sharma, P.; Yeo, J.; Han, M.H.; Cho, C.H. NaA Zeolite Cubic Crystal Formation and Deformation: Cubes with Crystalline Core, Simultaneous Growth of Surface and Core Crystals, and Layer-by-Layer Destruction. RSC Adv. 2012, 2, 7809–7823. [Google Scholar] [CrossRef]
- Pillai, R.S.; Peter, S.A.; Jasra, R.V. CO2 and N2 Adsorption in Alkali Metal Ion Exchanged X-Faujasite: Grand Canonical Monte Carlo Simulation and Equilibrium Adsorption Studies. Microporous Mesoporous Mater. 2012, 162, 143–151. [Google Scholar] [CrossRef]
- Oliveira, R.C.P.; Vasić, M.; Santos, D.M.F.; Babić, B.; Hercigonja, R.; Sequeira, C.A.C.; Šljukić, B. Performance Assessment of a Direct Borohydride-Peroxide Fuel Cell with Pd-Impregnated Faujasite X Zeolite as Anode Electrocatalyst. Electrochim. Acta 2018, 269, 517–525. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L.; Li, D.; Xin, Q.; Jiao, R.; Hou, X.; Zhang, Y.; Li, H. Enhanced Phenol Degradation at near Neutral PH Achieved by Core-Shell Hierarchical 4A Zeolite/Fe@Cu Catalyst. J. Environ. Chem. Eng. 2020, 8, 103933. [Google Scholar] [CrossRef]
- Noroozi, R.; Al-Musawi, T.J.; Kazemian, H.; Kalhori, E.M.; Zarrabi, M. Removal of Cyanide Using Surface-Modified Linde Type-A Zeolite Nanoparticles as an Efficient and Eco-Friendly Material. J. Water Process Eng. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- International Zeolite Association Database of Zeolites Structure. Available online: https://asia.iza-structure.org/IZA-SC/ftc_table.php (accessed on 29 April 2022).
- Ma, B.; Fernandez-Martinez, A.; Mancini, A.; Lothenbach, B. Spectroscopic Investigations on Structural Incorporation Pathways of FeIII into Zeolite Frameworks in Cement-Relevant Environments. Cem. Concr. Res. 2021, 140, 106304. [Google Scholar] [CrossRef]
- Chubar, N.; Gerda, V.; Szlachta, M.; Yablokova, G. Effect of Fe Oxidation State (+2 versus +3) in Precursor on the Structure of Fe Oxides/Carbonates-Based Composites Examined by XPS, FTIR and EXAFS. Solid State Sci. 2021, 121, 106752. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Asiri, A.M.; Rahman, M.M. Cleaning the Arsenic(V) Contaminated Water for Safe-Guarding the Public Health Using Novel Composite Material. Compos. Part B Eng. 2019, 171, 294–301. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T.; Suzuki, S.; Shiwaku, H. Ultimate Selenium(IV) Monitoring and Removal from Water Using a New Class of Organic Ligand Based Composite Adsorbent. J. Hazard. Mater. 2015, 291, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X. The Unit Problem in the Thermodynamic Calculation of Adsorption Using the Langmuir Equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Yang, Y.; Zhang, Y.; Lei, X.; Yuan, D. Application of FeMgMn Layered Double Hydroxides for Phosphate Anions Adsorptive Removal from Water. Appl. Clay Sci. 2021, 200, 105903. [Google Scholar] [CrossRef]
- Deng, L.; Shi, Z. Synthesis and Characterization of a Novel Mg–Al Hydrotalcite-Loaded Kaolin Clay and Its Adsorption Properties for Phosphate in Aqueous Solution. J. Alloy. Compd. 2015, 637, 188–196. [Google Scholar] [CrossRef]
- Valderrama, C.; Barios, J.I.; Caetano, M.; Farran, A.; Cortina, J.L. Kinetic Evaluation of Phenol/Aniline Mixtures Adsorption from Aqueous Solutions onto Activated Carbon and Hypercrosslinked Polymeric Resin (MN200). React. Funct. Polym. 2010, 70, 142–150. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Simultaneous Nutrients (N,P) Removal by Using a Hybrid Inorganic Sorbent Impregnated with Hydrated Manganese Oxide. J. Environ. Chem. Eng. 2017, 5, 1516–1525. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Zhu, J.; Wang, D.; Meng, H.; Wang, H.; Li, J. Simultaneous Adsorption of Phosphate and Zinc by Lanthanum Modified Zeolite. Environ. Technol. Innov. 2021, 24, 101906. [Google Scholar] [CrossRef]
- Zamparas, M.; Gianni, A.; Stathi, P.; Deligiannakis, Y.; Zacharias, I. Removal of Phosphate from Natural Waters Using Innovative Modified Bentonites. Appl. Clay Sci. 2012, 62–63, 101–106. [Google Scholar] [CrossRef]
- Moharami, S.; Jalali, M. Removal of Phosphorus from Aqueous Solution by Iranian Natural Adsorbents. Chem. Eng. J. 2013, 223, 328–339. [Google Scholar] [CrossRef]
- Yaghoobi-Rahni, S.; Rezaei, B.; Mirghaffari, N. Bentonite Surface Modification and Characterization for High Selective Phosphate Adsorption from Aqueous Media and Its Application for Wastewater Treatments. J. Water Reuse Desalinat. 2017, 7, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.G.; Xu, Y.Y.; Yu, H.Q.; Xin, X.D.; Wei, Q.; Du, B. Adsorption of Phosphate from Aqueous Solution by Hydroxy-Aluminum, Hydroxy-Iron and Hydroxy-Iron-Aluminum Pillared Bentonites. J. Hazard. Mater. 2010, 179, 244–250. [Google Scholar] [CrossRef]
- Mdlalose, L.; Balogun, M.; Setshedi, K.; Chimuka, L.; Chetty, A. Adsorption of Phosphates Using Transition Metals-Modified Bentonite Clay. Sep. Sci. Technol. 2019, 54, 2397–2408. [Google Scholar] [CrossRef]
- Zelmanov, G.; Semiat, R. The Influence of Competitive Inorganic Ions on Phosphate Removal from Water by Adsorption on Iron (Fe+3) Oxide/Hydroxide Nanoparticles-Based Agglomerates. J. Water Process Eng. 2015, 5, 143–152. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, R.; Ma, L.; Fu, H.; Lin, X.; Parker, S.C.; Molinari, M. Adsorption of Phosphate and Cadmium on Iron (Oxyhydr)Oxides: A Comparative Study on Ferrihydrite, Goethite, and Hematite. Geoderma 2021, 383, 114799. [Google Scholar] [CrossRef]




| Zeolite | SiO2/Al2O3 | Na2O/SiO2 | H2O/Na2O | Na2O3Si∙9H2O a (g) | NaAlO2 b (g) | H2O c (g) |
|---|---|---|---|---|---|---|
| LTA | 2 | 3 | 40 | 50 | 179 | 310 |
| FAU-X | 4 | 2 | 46 | 56 | 99 | 222 |
| Zeolite | O (%) | Na (%) | Al (%) | Si (%) | Cl (%) | Fe (%) |
|---|---|---|---|---|---|---|
| LTA | 58.9 | 14.1 | 13.3 | 13.7 | <lq * | <lq * |
| LTA-Fe | 60.9 | 7.7 | 12.3 | 12.4 | 1.3 | 5.3 |
| FAU-X | 58.7 | 14.8 | 12.3 | 14.2 | <lq * | <lq * |
| FAU-X-Fe | 60.7 | 7.3 | 11.6 | 13.9 | 0.3 | 6.2 |
| Zeolite | qe * (mg.g−1) |
|---|---|
| LTA | 0.3 |
| LTA-Fe | 0.9 |
| FAU-X | 0.2 |
| FAU-X-Fe | 0.8 |
| Zeolite | Langmuir * | Freundlich * | ||||
|---|---|---|---|---|---|---|
(mg.g−1) | (L.mg−1) | R2 | (mg.g−1) | 1/n | R2 | |
| LTA-Fe | 18.5 | 0.007 | 0.99 | 0.37 | 0.59 | 0.97 |
| FAU-X-Fe | 17.5 | 0.006 | 0.99 | 0.41 | 0.53 | 0.95 |
| Zeolite | Temperature (K) | ln | R2 | ΔG0 * (kJ mol−1) | ΔS0 * (kJ mol−1 K−1) | ΔH0 * (kJ mol−1) |
|---|---|---|---|---|---|---|
| LTA-Fe | 315.15 | 6.44 | 0.95 | −4.88 | 0.18 | 39.80 |
| 319.15 | 6.70 | −5.05 | ||||
| 323.15 | 6.81 | −5.16 | ||||
| FAU-X-Fe | 315.15 | 8.81 | 0.97 | −5.70 | 0.15 | 24.01 |
| 319.15 | 8.89 | −5.80 | ||||
| 323.15 | 9.04 | −5.91 |
| Kinetic Model | Kinetic Parameter * | LTA-Fe | FAU-X-Fe |
|---|---|---|---|
| Pseudo-first-order | qe (mg g−1) | 3.51 | 2.89 |
| k1 (h−1) | 0.12 | 0.13 | |
| R2 | 0.85 | 0.92 | |
| Pseudo-second-order | qe (mg g−1) | 5.76 | 4.61 |
| k2 (g mg−1 h−1) | 0.030 | 0.047 | |
| R2 | 0.99 | 0.99 | |
| Intraparticle diffusion | kt1 (mg g−1 h−1/2) | 2.64 | 3.39 |
| R2 | 0.84 | 0.90 | |
| kt2 (mg g−1 h−1/2) | 5 × 10−1 | 2 × 10−1 | |
| R2 | 0.90 | 0.88 | |
| kt3 (mg g−1 h−1/2) | 6 × 10−1 | 5 × 10−1 | |
| R2 | 0.93 | 0.99 | |
| HPDF film diffusion | Df (m2 s−1) | 9.27 × 10−11 | 2.45 × 10−15 |
| R2 | 0.96 | 0.95 | |
| HPDM particle diffusion | Dp (m2 s−1) | 2.42 × 10−15 | 2.11 × 10−15 |
| R2 | 0.95 | 0.97 |
| Zeolite | qe * (mg·g−1) | LB-P * | (Fe-Al)-P * | (Na)-P * | R-P * | ||||
|---|---|---|---|---|---|---|---|---|---|
| (mg·g−1) | % | (mg·g−1) | % | (mg·g−1) | % | (mg·g−1) | % | ||
| LTA-Fe | 15.6 ± 0.4 | 4.5 ± 0.2 | 28 | 10.0 ± 0.1 | 64 | 0.8 ± 0.1 | 5 | 0.4 ± 0.0 | 3 |
| FAU-X-Fe | 12.1 ± 0.6 | 4.0 ± 0.1 | 33 | 7.1 ± 0.2 | 59 | 0.7 ± 0.1 | 6 | 0.3 ± 0.0 | 2 |
| Adsorbent | Description | Isotherm Models | Kinetic Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Langmuir | Freundlich | Pseudo-First-Order | Pseudo-Second-Order | ||||||||
| 1/n | R2 | R2 | Ref. | ||||||||
| (mg g−1) | (L mg−1) | (m g−1) | (min−1) | g mg−1 min−1 | |||||||
| Synthetic zeolites | Hydrothermally synthetized | LTA-Fe | 18.5 | 0.007 | 0.6 | 0.4 | 0.12 | 0.85 | 0.03 | 0.99 | This study |
| FAU-X-Fe | 17.5 | 0.006 | 0.5 | 0.4 | 0.13 | 0.92 | 0.04 | 0.99 | |||
| Natural zeolites | Natural clinoptilolite | ZN | 0.6 | 0.01 | 0.47 | 0.02 | - | - | - | - | [9] |
| Z-Al | 7.0 | 0.02 | 0.32 | 0.85 | 0.2 | 0.93 | 0.6 | 0.9 | |||
| Z-Fe | 3.4 | 0.02 | 0.25 | 0.59 | 0.1 | 0.92 | 0.2 | 0.99 | [1] | ||
| Z-Mn | 5.6 | 0.01 | 0.34 | 0.95 | - | - | - | - | [38] | ||
| Synthetic zeolite | From fly ash with lanthanum | LMZ | 2.31 | 3.09 | 0.59 | 1.54 | - | - | - | - | [39] |
| Natural clays | Natural form | C1 | 21.4 | 0.0018 | 0.7 | 0.1 | 0.33 | 0.94 | 0.14 | 0.99 | [6] |
| C2 | 20.9 | 0.0098 | 0.8 | 0.1 | 0.15 | 0.79 | 0.22 | 0.97 | |||
| Modified form | C1-Fe | 38.0 | 0.0018 | 0.6 | 0.3 | 0.09 | 0.73 | 0.01 | 1.00 | ||
| C2-Fe | 37.6 | 0.0012 | 0.6 | 0.3 | 0.15 | 0.77 | 0.06 | 0.99 | |||
| Modified bentonite | Zn-containing bentonite clay | 4.12 | 1.1 | 0.96 | 2.2 | - | - | - | >0.99 | [40] | |
| Pillared bentonite by Fe | 11.15 | 0.6 | 0.81 | 4.4 | - | - | - | >0.99 | |||
| Natural clays | Bentonite from Iran | 0.369 | 0.01 | 0.58 | 12.85 | - | - | - | - | [41] | |
| Zeolite from Iran | 0.627 | 0.007 | 0.64 | 12.63 | - | - | - | - | |||
| Kaolinite from Iran | 0.624 | 0.005 | 0.62 | 11.94 | - | - | - | - | |||
| Modified bentonite | Pillared bentonite by Fe/Al | 8.33 | 0.03 | 0.26 | 0.18 | - | - | - | - | [42] | |
| Na-Bentonites | Pillared bentonite with Al | 12.7 | 1.61 | 0.22 | 7.56 | - | - | - | 1 | [43] | |
| Pillared bentonite with Fe | 11.2 | 1.83 | 0.16 | 7.43 | - | - | - | 0.99 | |||
| Pillared bentonite with Fe-Al | 10.5 | 1.25 | 0.21 | 5.54 | - | - | - | 1 | |||
| Metals-modified bentonite clay | Bentonite (Bent) modified with Fe, Co and Ni | Fe-Bent | 20.88 | 0.111 | 0.11 | 9.86 | 0.0090 | 0.956 | 0.0040 | 0.996 | [44] |
| Co-Bent | 46.95 | 0.648 | 0.12 | 23.03 | 0.0020 | 0.928 | 0.0034 | 0.981 | |||
| Ni-Bent | 29.07 | 0.496 | 0.11 | 13.44 | 0.0070 | 0.963 | 0.0091 | 0.965 | |||
| Bent | 6.57 | 0.281 | 0.15 | 2.44 | 0.0023 | 0.927 | 0.024 | 0.998 | |||
| Iron oxide/hydroxide nanoparticles-based agglomerates | Iron nanoparticles | AggFe | 122.0 | - | - | - | - | - | - | - | [45] |
| Iron (oxyhydr)oxides | Ferrihydrite | Fh | 57 | - | - | - | - | - | - | - | [46] |
| Goethite | Gt | 9.5 | - | - | - | - | - | - | - | ||
| Hematite | Hm | 4.75 | - | - | - | - | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guaya, D.; Cobos, H.; Camacho, J.; López, C.M.; Valderrama, C.; Cortina, J.L. LTA and FAU-X Iron-Enriched Zeolites: Use for Phosphate Removal from Aqueous Medium. Materials 2022, 15, 5418. https://doi.org/10.3390/ma15155418
Guaya D, Cobos H, Camacho J, López CM, Valderrama C, Cortina JL. LTA and FAU-X Iron-Enriched Zeolites: Use for Phosphate Removal from Aqueous Medium. Materials. 2022; 15(15):5418. https://doi.org/10.3390/ma15155418
Chicago/Turabian StyleGuaya, Diana, Hernán Cobos, Jhulissa Camacho, Carmen Milena López, César Valderrama, and José Luis Cortina. 2022. "LTA and FAU-X Iron-Enriched Zeolites: Use for Phosphate Removal from Aqueous Medium" Materials 15, no. 15: 5418. https://doi.org/10.3390/ma15155418
APA StyleGuaya, D., Cobos, H., Camacho, J., López, C. M., Valderrama, C., & Cortina, J. L. (2022). LTA and FAU-X Iron-Enriched Zeolites: Use for Phosphate Removal from Aqueous Medium. Materials, 15(15), 5418. https://doi.org/10.3390/ma15155418







