Controlling Shock-Induced Energy Release Characteristics of PTFE/Al by Adding Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
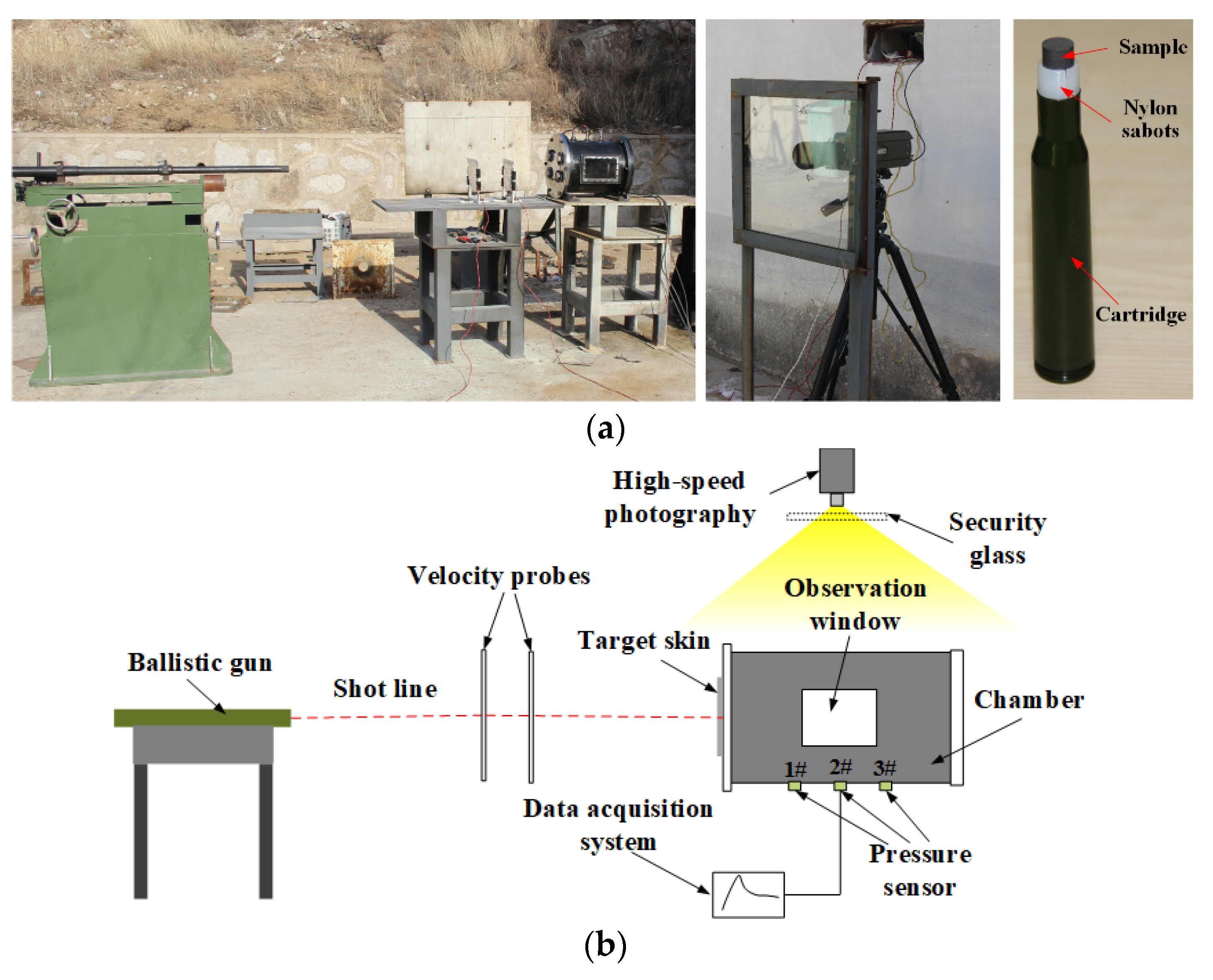
2.2. Experimental Setup
3. Results and Discussion
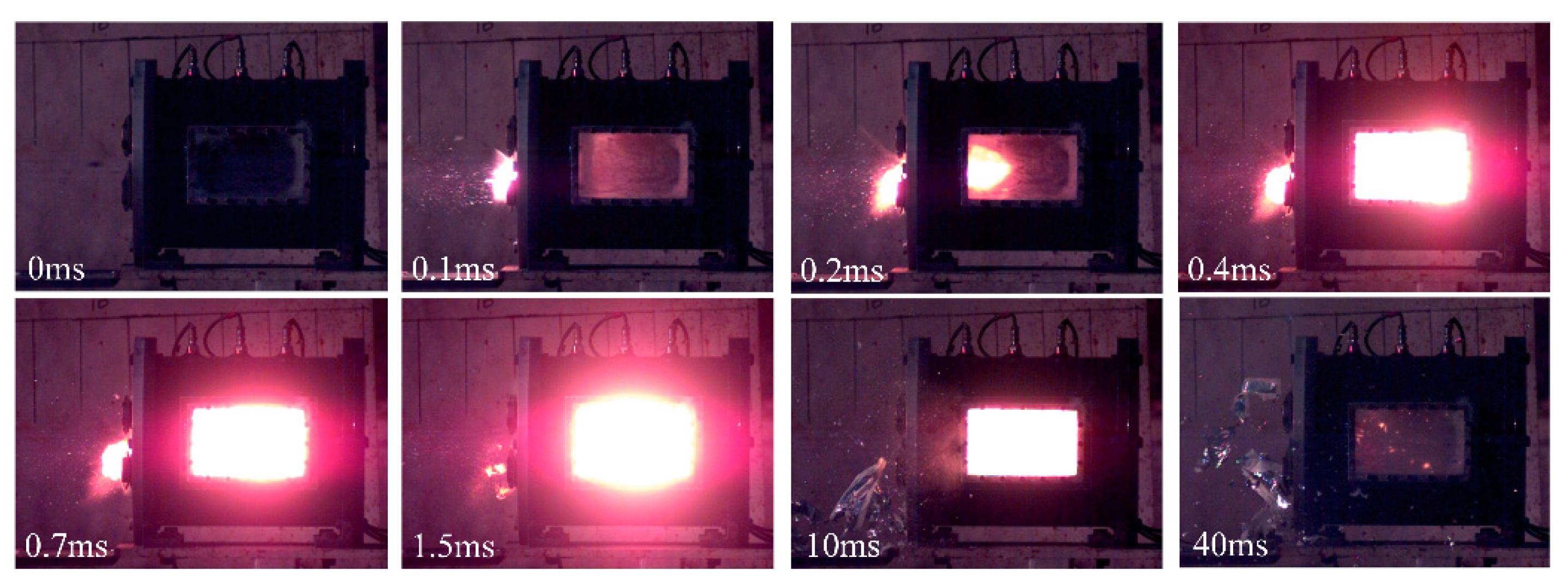
3.1. Typical Shock-Induced Energy Release Characteristics
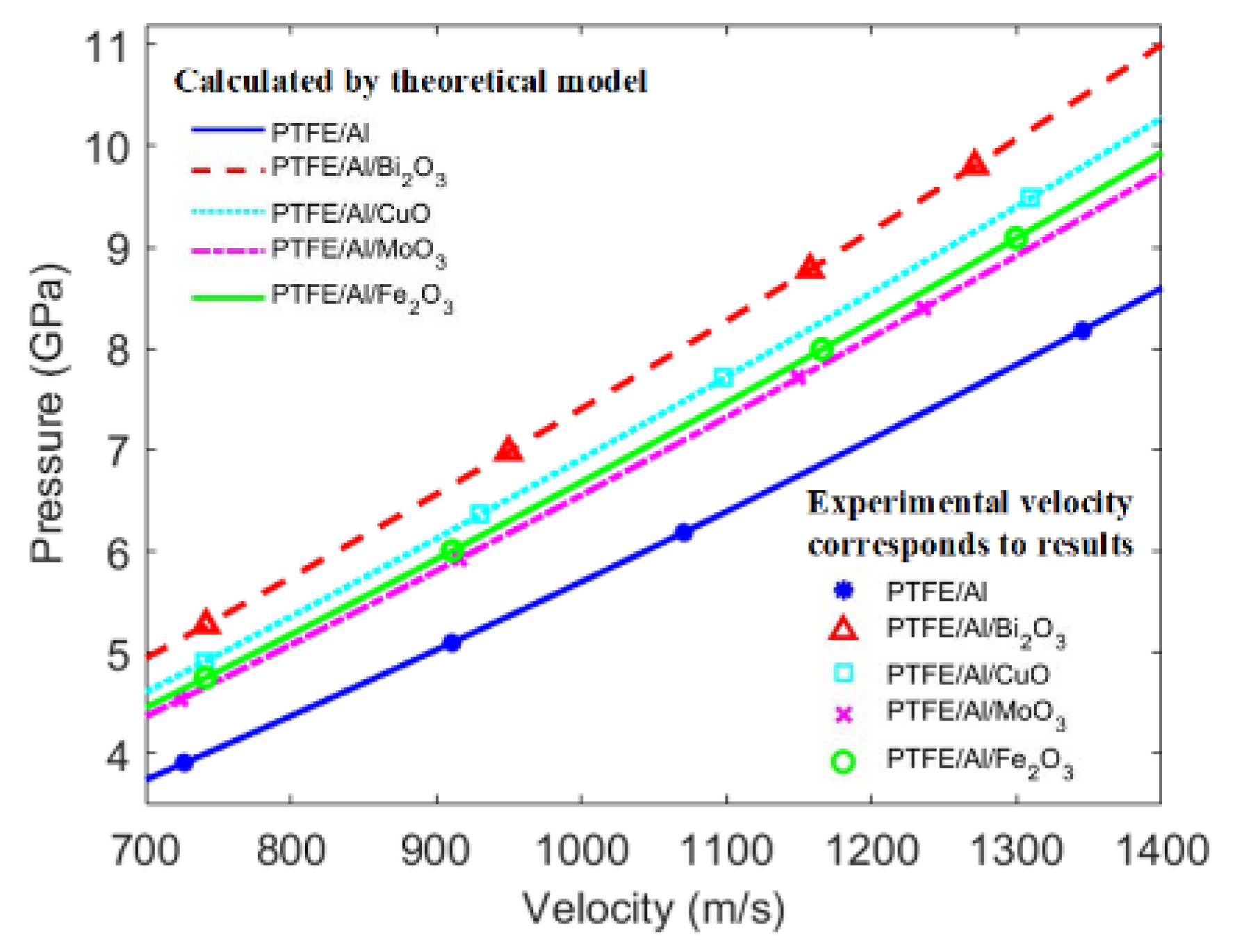
3.2. Analytical Model of Shock-Induced Energy Release Characteristics
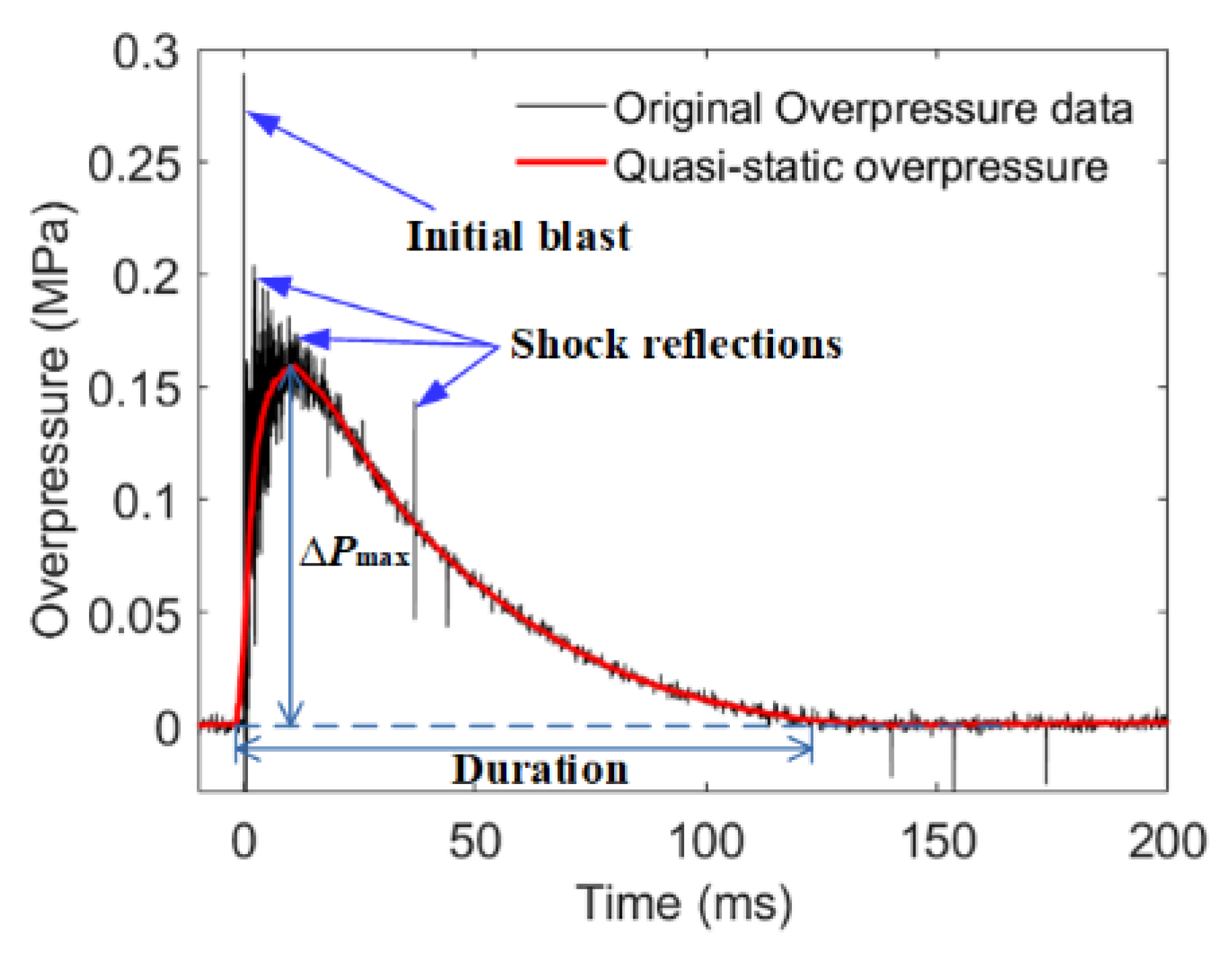
3.3. Overpressure Characteristics
3.4. Energy Release Efficiency of PTFE/Al/Oxide
3.5. Controlling Mechanism of Oxides on Energy Release Characteristics
4. Conclusions
- (a)
- The experimental results indicate that the oxides controlled the shock-induced energy release characteristics, and this controlling effect was affected by the impact velocity. With a lower impact velocity (usually lower than 750 m/s), the energy release characteristics of the PTFE/Al was significantly enhanced by MoO3, by 1.99 times. The oxides also presented a significant influence on the overpressure duration of the PTFE/Al-based energetic materials.
- (b)
- The analytical model for PTFE/Al/oxide shock-induced energy release indicated that the oxides dominated the energy release characteristics by affecting the apparent activation energy and impact shock pressure of the energetic materials. Oxides with a high-sensitivity corresponding thermite, or with a high density could enhance the energy release performance of PTFE/Al/oxide.
- (c)
- The mechanism of oxides controlling the shock-induced energetic behaviors of PTFE/Al energetic materials was revealed. It indicated that oxides improved the continuous reaction ability of energetic materials after shock wave unloading. The controlling effects of different oxides was determined by the chemical and physical properties of the corresponding thermites.
- (d)
- This study fills the gap in the theoretical study of PTFE/Al shock-induced energy release behaviors and has great guiding significance for the design and application of energetic materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granier, J.J.; Pantoya, M.L. Laser ignition of nanocomposite thermites. Combust. Flame 2004, 138, 373–383. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Yu, Q.; Liu, Z.; Yu, W. Impact-induced initiation and energy release behavior of reactive materials. J. Appl. Phys. 2011, 110, 074904. [Google Scholar] [CrossRef]
- Martin, L.; Santanu, C. Theoretical Study of Elementary Steps in the Reactions between Aluminum and Teflon Fragments under Combustive Environments. J. Phys. Chem. A 2009, 113, 5933–5941. [Google Scholar] [CrossRef]
- Ames, R.G. Energy Release Characteristics of Impact-Initiated Energetic Materials. Mater. Res. Soc. Symp. Proc. 2006, 896, 321–333. [Google Scholar] [CrossRef]
- Ames, R.G. A Standardized Evaluation Technique for Reactive Warhead Fragments. Int. Symp. Ballist 2007, 23, 49–58. [Google Scholar]
- Ames, R.G. Vented chamber calorimetry for impact-initiated energetic materials. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, Nevada, 10–13 January 2005; p. 279. [Google Scholar] [CrossRef]
- Chen, C.; Tang, E.; Zhu, W.; Han, Y.; Gao, Q. Modified model of Al/PTFE projectile impact reaction energy release considering energy loss. Exp. Therm. Fluid Sci. 2020, 116, 110132. [Google Scholar] [CrossRef]
- Yu, Z.S.; Fang, X.; Li, Y.; Wu, J.X.; Wu, S.Z.; Zhang, J.; Ren, J.K.; Zhong, M.S.; Chen, L.P.; Yao, M. Investigation on the Reaction Energy, Dynamic Mechanical Behaviors, and Impact-Induced Reaction Characteristics of PTFE/Al with Different TiH2 Percentages. Materials 2018, 11, 2008. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.X.; Li, Y.C.; Huang, J.Y.; Wu, J.X.; Wu, S.Z.; Liu, Q.; Wang, R.Q.; Feng, B. Effect of addition of HTa to Al/PTFE under quasi-static compression on the properties of the developed energetic composite material. RSC Adv. 2021, 11, 8540–8545. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.C.; Huang, J.Y.; Wu, J.X.; Liu, Q.; Wu, S.Z.; Gao, Z.R.; Zhang, S.; Yang, L. The effect of al particle size on thermal decomposition, mechanical strength and sensitivity of Al/ZrH2/PTFE composite. Def. Technol. 2021, 17, 829–835. [Google Scholar] [CrossRef]
- Wu, J.X.; Liu, Q.; Feng, B.; Yin, Q.; Li, Y.C.; Wu, S.Z.; Yu, Z.S.; Huang, J.Y.; Ren, X.X. Improving the energy release characteristics of PTFE/Al by doping magnesium hydride. Def. Technol. 2022, 18, 219–228. [Google Scholar] [CrossRef]
- Wu, J.X.; Wang, H.X.; Fang, X.; Li, Y.C.; Mao, Y.M.; Yang, L.; Yin, Q.; Wu, S.Z.; Yao, M.; Song, J.X. Investigation on the Thermal Behavior, Mechanical Properties and Reaction Characteristics of Al-PTFE Composites Enhanced by Ni Particle. Materials 2018, 11, 1741. [Google Scholar] [CrossRef] [Green Version]
- Mozaffari, A.; Manesh, H.D.; Janghorban, K. Evaluation of mechanical properties and structure of multilayered Al/Ni composites produced by accumulative roll bonding (ARB) process. J. Alloys Compd. 2010, 489, 103–109. [Google Scholar] [CrossRef]
- Patselov, A.; Greenberg, B.; Gladkovskii, S.; Lavrikov, R.; Borodin, E. Layered metal-intermetallic composites in Ti-Al system: Strength under static and dynamic load. AASRI Procedia 2012, 3, 107–112. [Google Scholar] [CrossRef]
- Ding, L.L.; Zhou, J.Y.; Tang, W.H.; Ran, X.W.; Hu, Y.X. Impact Energy Release Characteristics of PTFE/Al/CuO Reactive Materials Measured by a New Energy Release Testing Device. Polymers 2019, 11, 149. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Geng, B.Q.; Sun, T.; Yu, Q.B.; Wang, H.F. Impact-Induced Reaction Characteristic and the Enhanced Sensitivity of PTFE/Al/Bi2O3 Composites. Polymers 2019, 11, 2049. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Liu, J.X.; Zhang, S.; Xue, X.Y.; He, C.; Wu, Z.Y.; Yang, M.; Li, S.K. Influence of multi-oxidants on reaction characteristics of PTFE-Al-XmOY reactive material. Mater. Design. 2020, 186, 108325. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.Y.; Fang, X.; Li, Y.C.; Yu, Z.S.; Gao, Z.R.; Wu, S.Z.; Yang, L.; Wu, J.X.; Kui, J.Y. Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite. Materials 2018, 11, 2451. [Google Scholar] [CrossRef] [Green Version]
- Glavier, L.; Taton, G.; Ducere, J.M.; Baijot, V.; Pinon, S.; Calais, T.; Esteve, A.; Rouhani, M.D.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Feng, S.S.; Wang, C.L.; Huang, G.Y. Experimental Study on the Reaction Zone Distribution of Impact-Induced Reactive Materials. Propellants Explos. Pyrotech. 2017, 42, 896–905. [Google Scholar] [CrossRef]
- Ortega, A.; Maqueda, L.P.; Criado, J.M. The problem of discerning Avrami-Erofeev kinetic models from the new controlled rate thermal analysis with constant acceleration of the transformation. Thermochim. Acta 1995, 254, 147–152. [Google Scholar] [CrossRef]
- Meyers, M.A. Dynamic Behavior of Materials; University of California: San Diego, CA, USA, 1994. [Google Scholar]
- Zhang, X.F.; Shi, A.S.; Qiao, L.; Zhang, J.; Zhang, Y.G.; Guan, Z.W. Experimental study on impact-initiated characters of multifunctional energetic structural materials. J. Appl. Phys. 2013, 113, 083508. [Google Scholar] [CrossRef]
- Umbrajkar, S.M.; Schoenitz, M.; Dreizin, E.L. Exothermic Reactions in Al-CuO Nanocomposites. Thermochim. Acta 2006, 451, 34–43. [Google Scholar] [CrossRef]
- Rehwoldt, M.C.; Yang, Y.; Wang, H.Y.; Holdren, S.; Zachariah, M.R. Ignition of Nanoscale Titanium/Potassium Perchlorate Pyrotechnic Powder: Reaction Mechanism Study. J. Phys. Chem. C 2018, 20, 10792–10800. [Google Scholar] [CrossRef]
- Fredenburg, D.A.; Thadhani, N.N. High-pressure equation of state properties of bismuth oxide. J. Appl. Phys. 2011, 110, 063510. [Google Scholar] [CrossRef]
- Williams, R.A.; Patel, J.V.; Ermoline, A.; Schoenitz, M.; Dreizin, E.L. Correlation of optical emission and pressure generated upon ignition of fully-dense nanocomposite thermite powders. Combust. Flame 2013, 160, 734–741. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, X.F.; Tan, M.T.; Liu, C.; Wu, X. The Energy Release Characteristics of Shock-Induced Chemical Reaction of Al/Ni Composites. J. Phys. Chem. C 2016, 120, 24551–24559. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, D.; He, S.; Guo, H.; Zheng, Y.; Zhang, Y.; Wang, H. Shock-Induced Energy Release Performances of PTFE/Al/Oxide. Materials 2022, 15, 3042. [Google Scholar] [CrossRef]

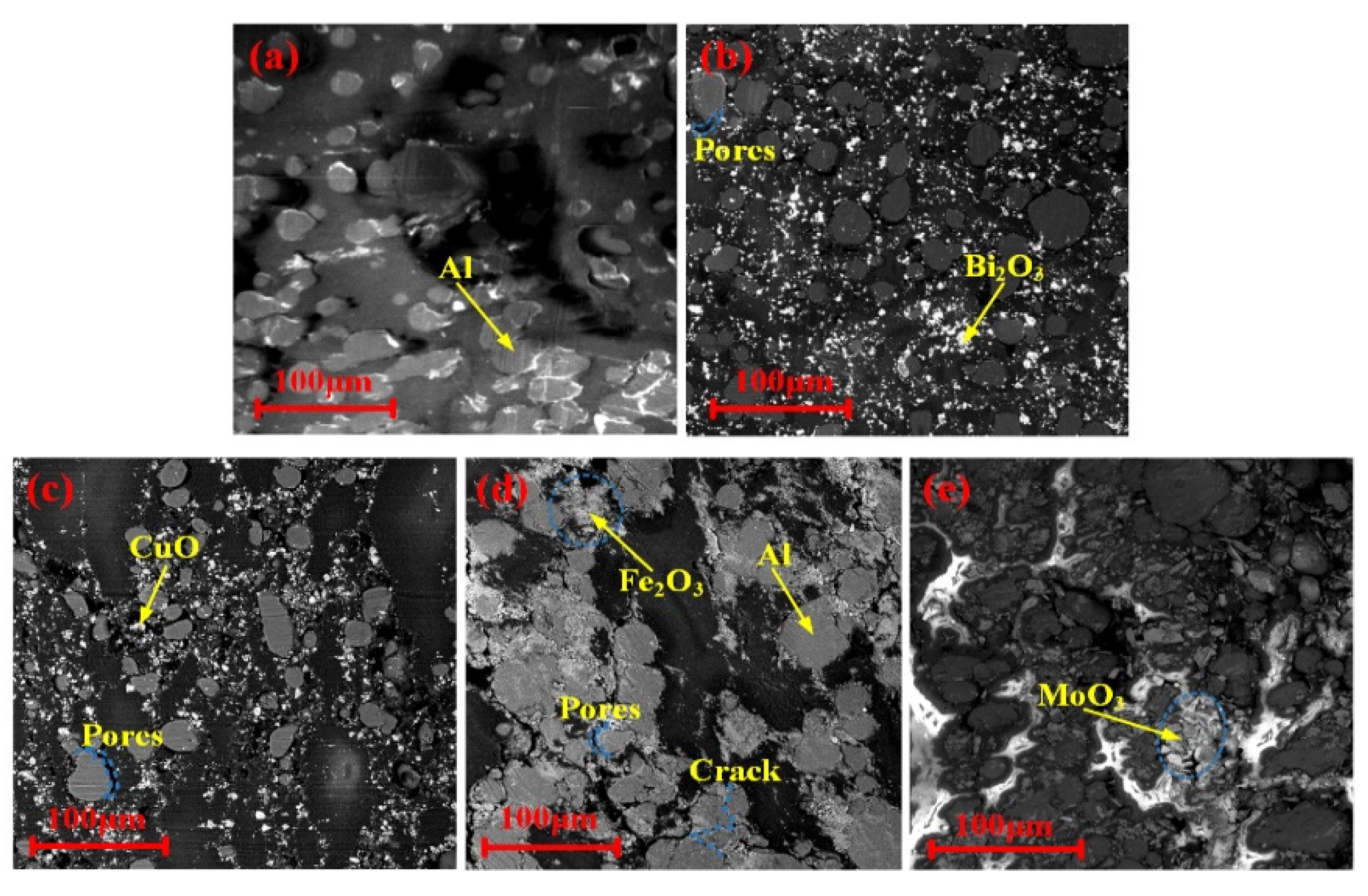


| Mixture | Chemical Reaction Equation | Stoichiometric Ratio | Theoretical ΔH (J/g) |
|---|---|---|---|
| Al/PTFE | 4Al + 3C2F4→4AlF3 + 6C | 26.5/73.5 | 8530 |
| Al/Bi2O3 | 2Al + Bi2O3→Al2O3 + 3Bi | 10.4/89.6 | 2115 |
| Al/CuO | 2Al + 3CuO→Al2O3 + 3Cu | 18.4/81.6 | 4072 |
| Al/MoO3 | 2Al + MoO3→Al2O3 + Mo | 27.3/72.7 | 4698 |
| Al/Fe2O3 | 2Al + Fe2O3→Al2O3 + 2Fe | 25.3/74.7 | 3156 |
| Type | Oxide | PTFE/Al/Oxide a | ρTMD/ρab (g/cm3) | Relative Density | Et c (kJ/g) |
|---|---|---|---|---|---|
| A | / | 73.5/26.5 | 2.33/2.28 | 97.6% | 8.528 |
| B | Bi2O3 | 57.1/22.9/20.0 | 2.74/2.73 | 99.6% | 7.098 |
| C | CuO | 55.5/24.5/20.0 | 2.69/2.64 | 98.1% | 7.418 |
| M | MoO3 | 53.3/26.7/20.0 | 2.63/2.53 | 96.2% | 7.467 |
| F | Fe2O3 | 53.8/26.2/20.0 | 2.65/2.48 | 93.6% | 7.586 |
| Oxide Type | Bi2O3 | CuO | Fe2O3 | MoO3 |
|---|---|---|---|---|
| Cv (J/(mol K)) | 236 | 530 | 662 | 521 |
| Ea 1 (kJ/mol) | 201.5 | 349.5 | 425.4 | 252.3 |
| Sample | Impact Velocity (m/s) | ΔPmax (MPa) | Duration (ms) | Impulse (s kPa) |
|---|---|---|---|---|
| A | 726.80 | 0.0598 | 126.76 | 3.6795 |
| B | 741.48 | 0.0602 | 118.88 | 3.2596 |
| C | 739.97 | 0.0987 | 182.44 | 7.1655 |
| M | 723.98 | 0.1190 | 138.96 | 6.5227 |
| F | 741.07 | 0.0434 | 166.72 | 3.1393 |
| A | 910.54 | 0.1466 | 139.74 | 8.2577 |
| B | 949.80 | 0.1619 | 117.04 | 6.7877 |
| C | 930.28 | 0.1456 | 126.40 | 7.6622 |
| M | 915.29 | 0.1397 | 106.38 | 6.4186 |
| F | 911.87 | 0.0953 | 198.96 | 8.5054 |
| A | 1070.43 | 0.1745 | 127.42 | 9.5816 |
| B | 1157.47 | 0.1767 | 125.70 | 8.9589 |
| C | 1098.18 | 0.1544 | 123.02 | 7.7877 |
| M | 1149.03 | 0.2023 | 152.02 | 10.883 |
| F | 1165.57 | 0.1503 | 129.08 | 8.1513 |
| A | 1345.80 | 0.1917 | 131.30 | 9.1400 |
| B | 1270.97 | 0.2029 | 114.77 | 9.3705 |
| C | 1309.50 | 0.1978 | 115.84 | 8.7886 |
| M | 1235.94 | 0.2078 | 127.32 | 9.7313 |
| F | 1299.46 | 0.2150 | 124.12 | 9.8345 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Cai, Y.; Shi, D.; Chen, P.; Liu, R.; Wang, H. Controlling Shock-Induced Energy Release Characteristics of PTFE/Al by Adding Oxides. Materials 2022, 15, 5502. https://doi.org/10.3390/ma15165502
Yuan Y, Cai Y, Shi D, Chen P, Liu R, Wang H. Controlling Shock-Induced Energy Release Characteristics of PTFE/Al by Adding Oxides. Materials. 2022; 15(16):5502. https://doi.org/10.3390/ma15165502
Chicago/Turabian StyleYuan, Ying, Yiqiang Cai, Dongfang Shi, Pengwan Chen, Rui Liu, and Haifu Wang. 2022. "Controlling Shock-Induced Energy Release Characteristics of PTFE/Al by Adding Oxides" Materials 15, no. 16: 5502. https://doi.org/10.3390/ma15165502
APA StyleYuan, Y., Cai, Y., Shi, D., Chen, P., Liu, R., & Wang, H. (2022). Controlling Shock-Induced Energy Release Characteristics of PTFE/Al by Adding Oxides. Materials, 15(16), 5502. https://doi.org/10.3390/ma15165502






