Heat Treatment of Aluminum Alloys with the Natural Combination of Dopants
Abstract
:1. Introduction
2. Materials and Methods
3. Results
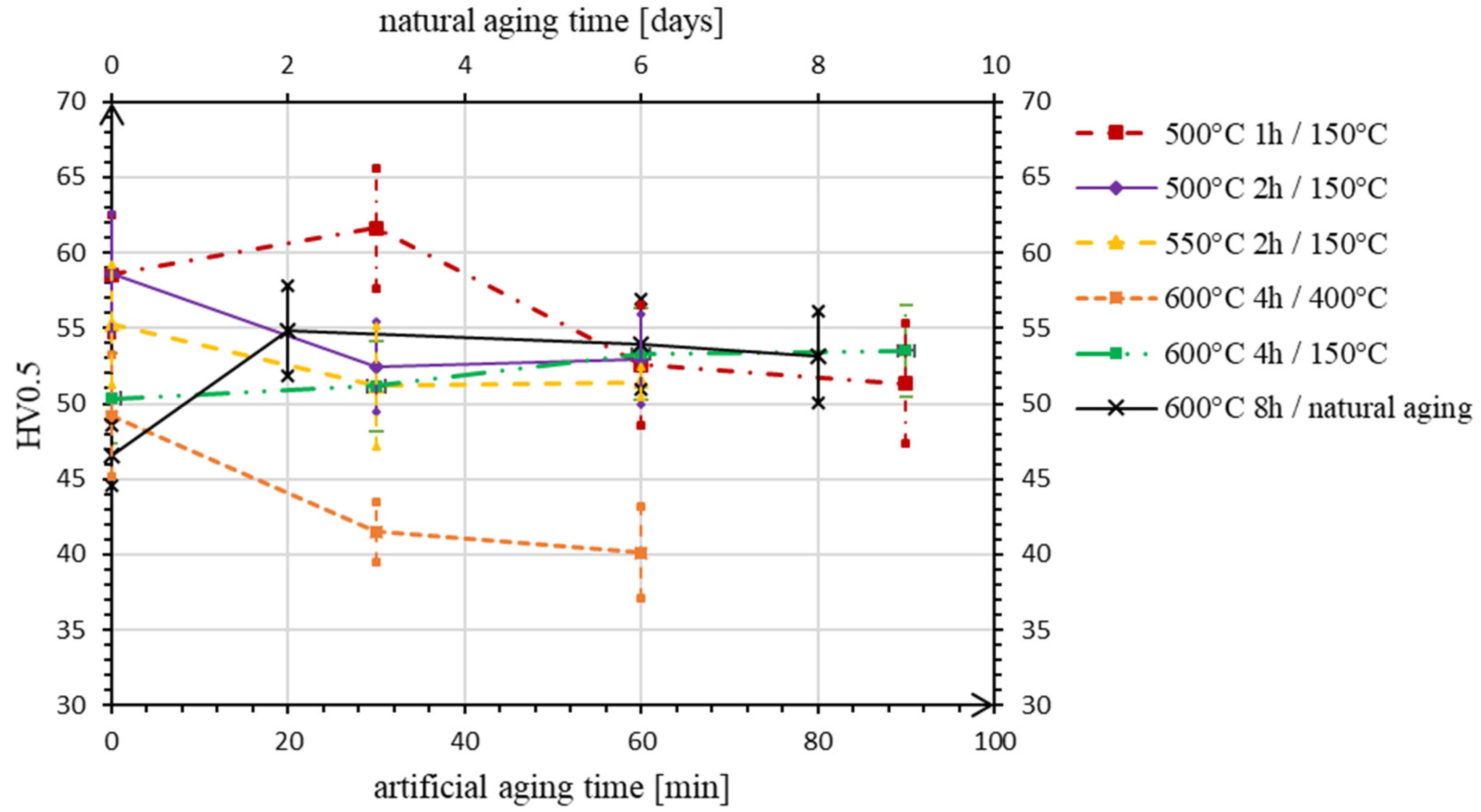
3.1. Mechanical Properties
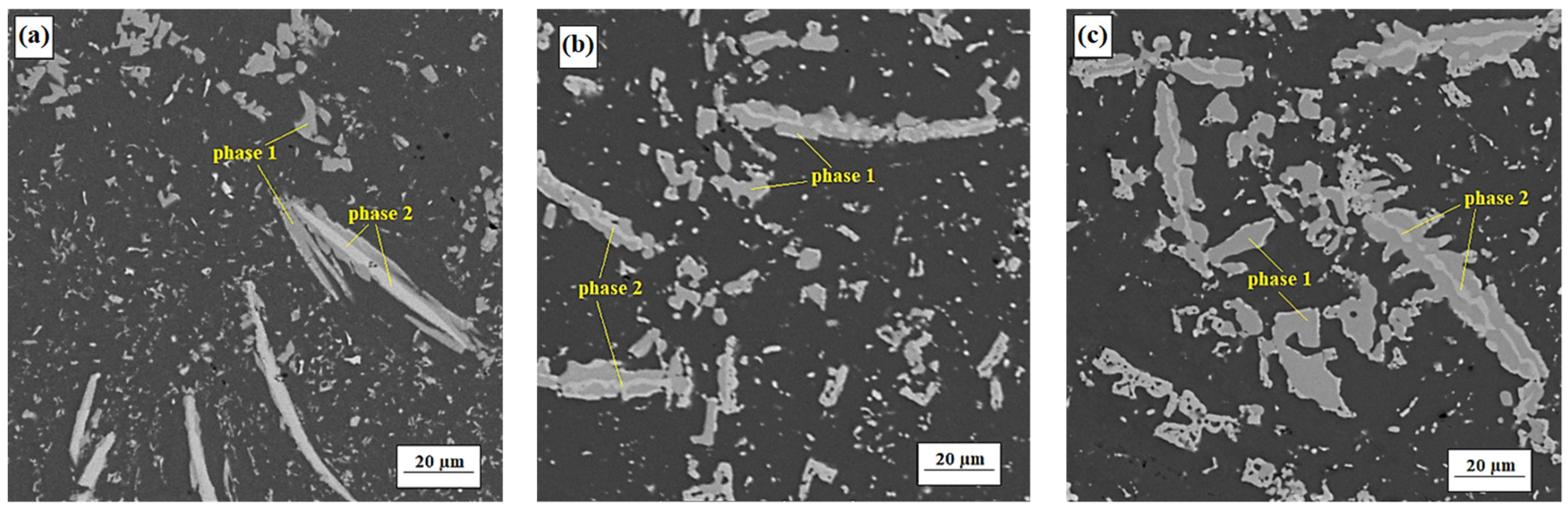
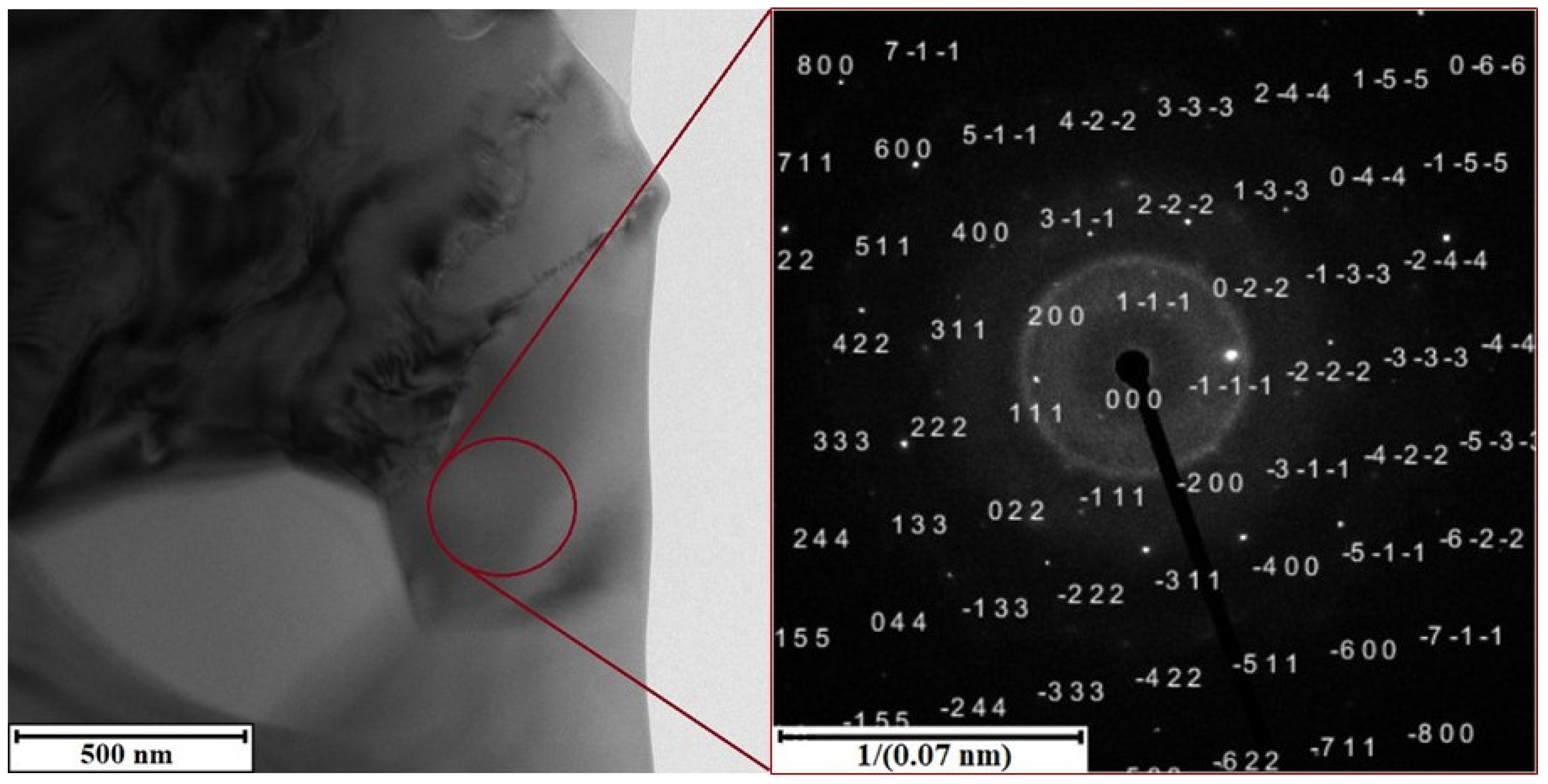
3.2. Microstructure and Chemical Composition
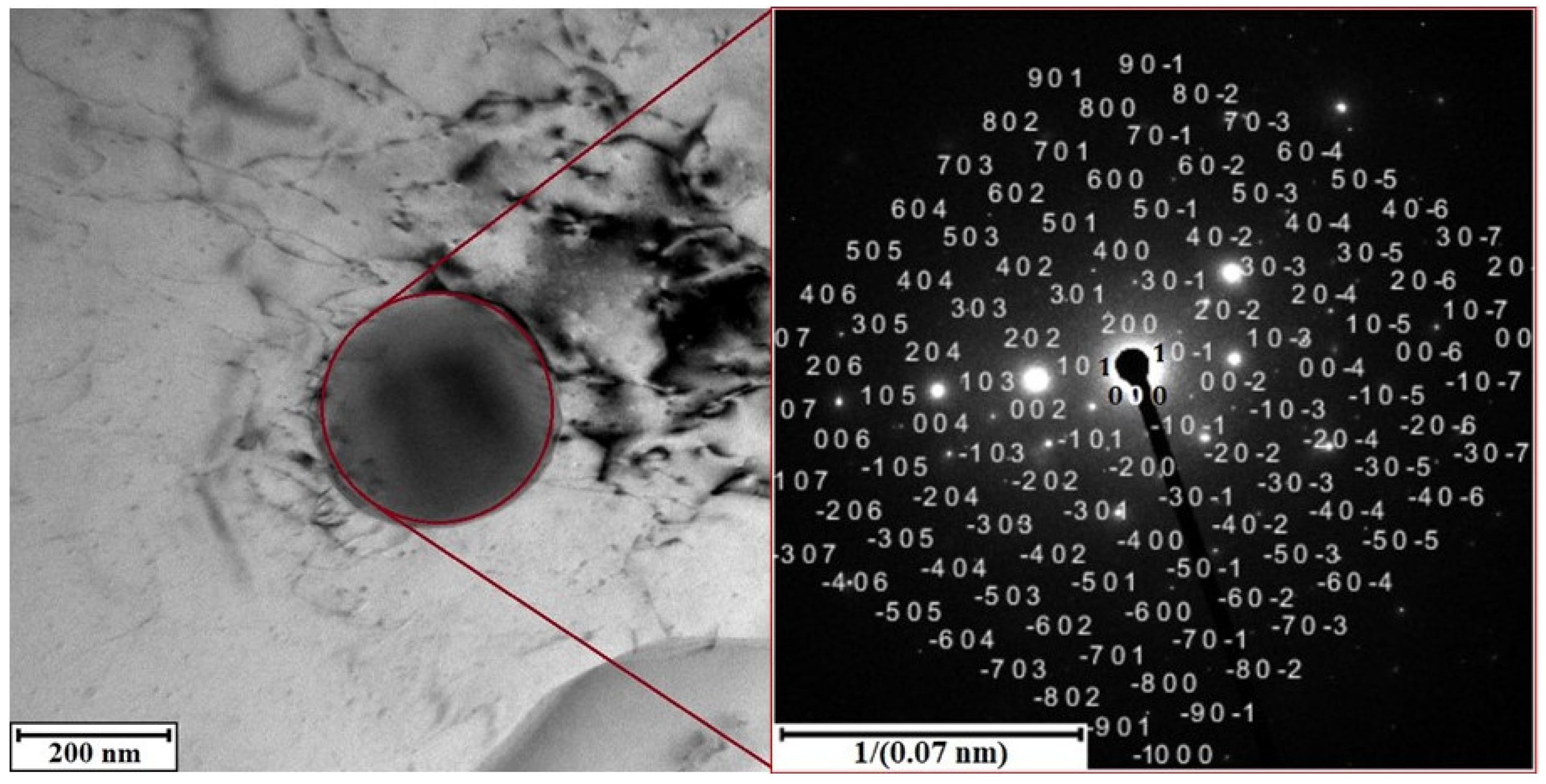
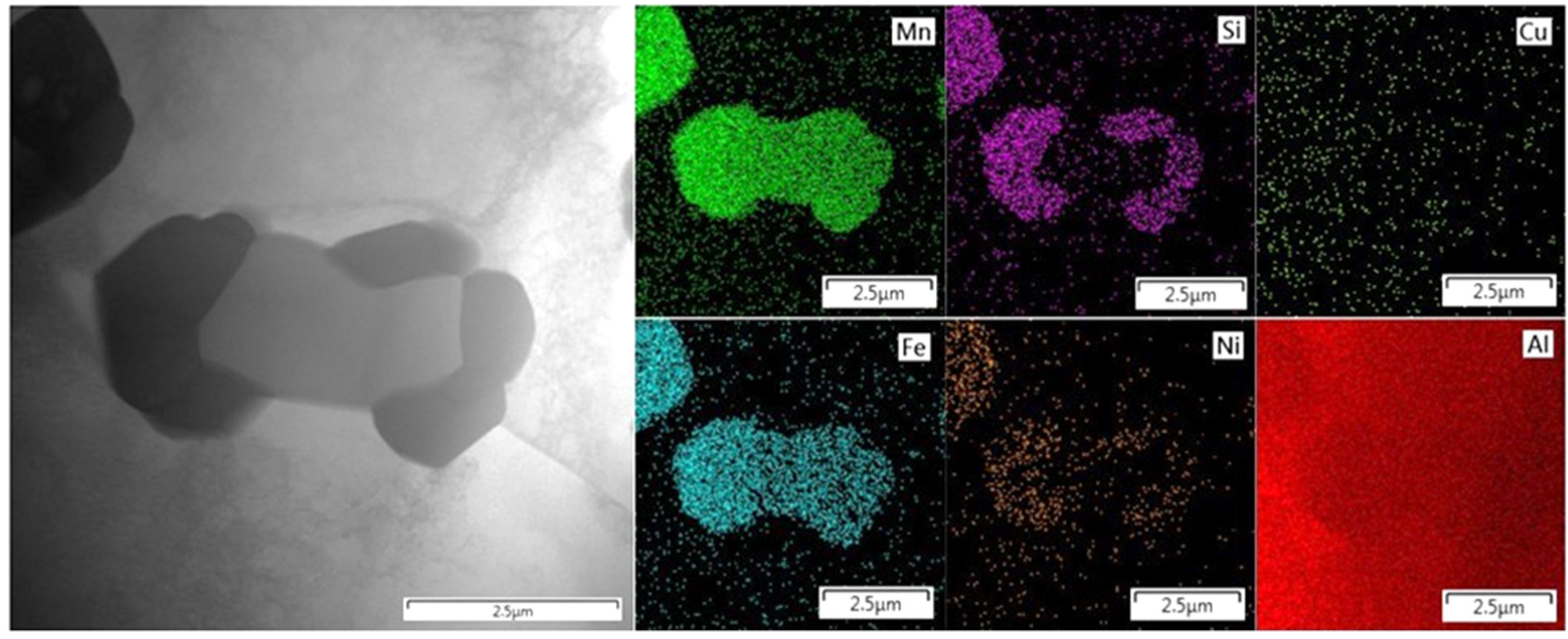
3.2.1. Alloy S1
3.2.2. Alloy S2
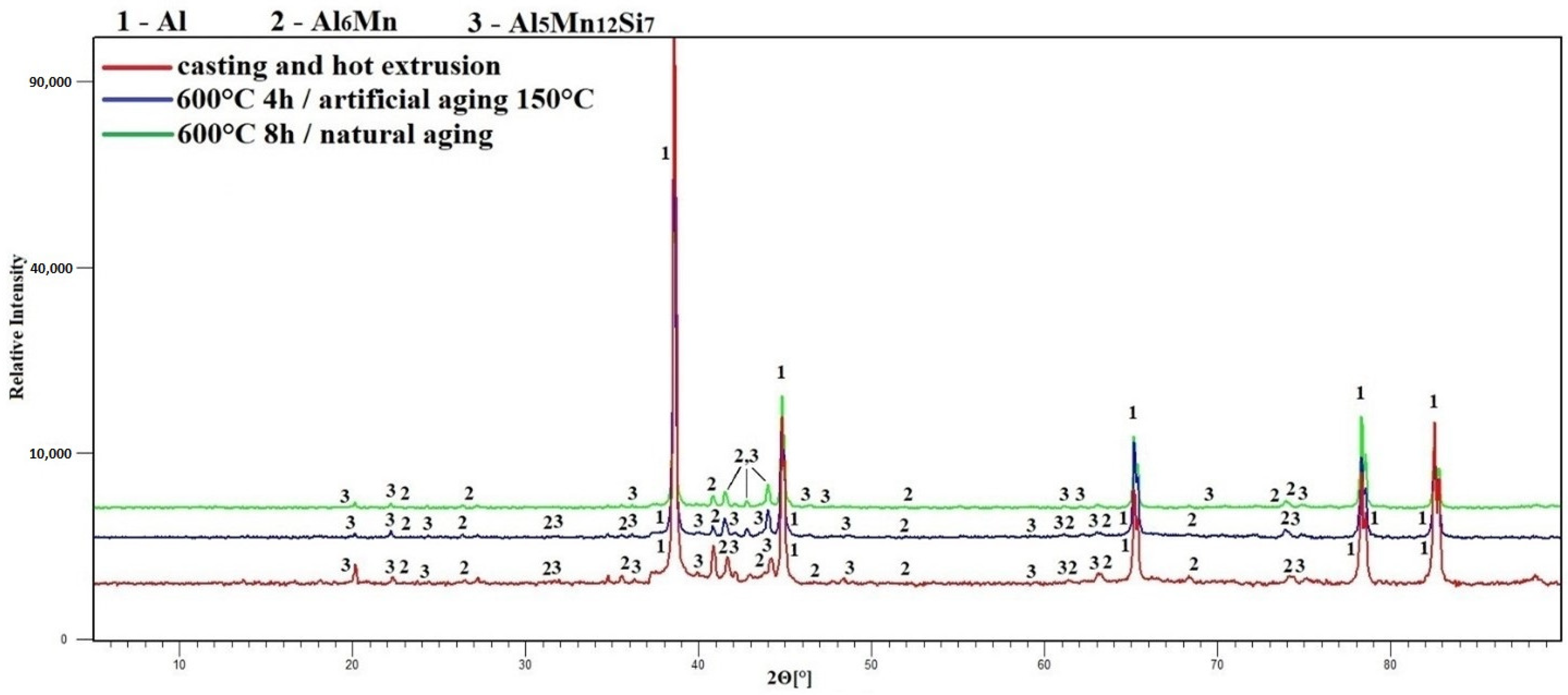
3.3. Phase Composition
4. Discussion
5. Conclusions
- -
- Two new wrought aluminum alloys were prepared, in which the product of an aluminothermic reduction of ferromanganese deep-sea nodules was used as the alloying mixture. The alloy samples were subjected to the set of heat treatments;
- -
- After comparing the mechanical properties, phase, and chemical composition, slight changes in the microstructure are found, but it does not lead to a significant increase in hardness;
- -
- None of the tested heat treatment conditions lead to a significant hardening, even though the precipitation is observed. It shows that the amount of copper, nickel, and other elements, which could ensure precipitation strengthening, is not high enough to form the suitable intermetallic phases in sufficient amounts;
- -
- The alloying elements are bound in stable manganese-rich intermetallics, or in the aluminum-based solid–solution matrix;
- -
- A qualitative description of the heat treatment conditions, which can ensure the correct processing, was given, and the physical parameters for quantitative determination of these conditions, such as the phase transformation on heating, were identified.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Reykhard, L.Y.; Shulga, N.A. Fe-Mn nodule morphotypes from the NE Clarion-Clipperton Fracture Zone, Pacific Ocean: Comparison of mineralogy, geochemistry and genesis. Ore Geol. Rev. 2019, 110, 102933. [Google Scholar] [CrossRef]
- Haynes, B.W.; Law, S.L.; Barron, D.C.; Kramer, G.W.; Maeda, Y.; Magyar, M.J. Pacific Manganese Nodules: Characterization and Processing; United States Government Printing Office: Washington, DC, USA, 1985; p. 48. [Google Scholar]
- Blöthe, M.; Wegorzewski, A.; Müller, C.; Simon, F.; Kuhn, T.; Schippers, A. Manganese-Cycling Microbial Communities Inside Deep-Sea Manganese Nodules. Environ. Sci. Technol. 2015, 49, 7692–7700. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.R.; Conrad, T.A.; Dunham, R.E. Seamount Characteristics and Mine-Site Model Applied to Exploration- and Mining-Lease-Block Selection for Cobalt-Rich Ferromanganese Crusts. Mar. Georesources Geotechnol. 2009, 27, 160–176. [Google Scholar] [CrossRef]
- Senanayake, G. Acid leaching of metals from deep-sea manganese nodules—A critical review of fundamentals and applications. Miner. Eng. 2011, 24, 1379–1396. [Google Scholar] [CrossRef]
- Sommerfeld, M.; Friedmann, D.; Kuhn, T.; Friedrich, B. “Zero-Waste”: A Sustainable Approach on Pyrometallurgical Processing of Manganese Nodule Slags. Minerals 2018, 8, 544. [Google Scholar] [CrossRef]
- Vu, H.; Jandová, J.; Lisá, K.; Vranka, F. Leaching of manganese deep ocean nodules in FeSO4–H2SO4–H2O solutions. Hydrometallurgy 2005, 77, 147–153. [Google Scholar] [CrossRef]
- Jandová, J.; Lisá, K.; Vu, H.; Vranka, F. Separation of copper and cobalt–nickel sulphide concentrates during processing of manganese deep ocean nodules. Hydrometallurgy 2005, 77, 75–79. [Google Scholar] [CrossRef]
- Novák, P.; Vu, N.H.; Šulcová, L.; Kopeček, J.; Laufek, F.; Tsepeleva, A.; Dvořák, P.; Michalcová, A. Structure and Properties of Alloys Obtained by Aluminothermic Reduction of Deep-Sea Nodules. Materials 2021, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A. Materials for Architects and Builders, 6th ed.; Routledge: London, UK, 2019. [Google Scholar]
- Totten, G.E.; MacKenzie, D.S. (Eds.) Handbook of Aluminum: Alloy Production and Materials Manufacturing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Lumley, R. (Ed.) Fundamentals of Aluminium Metallurgy: Recent Advances, 1st ed.; Woodhead Publishing: Cambridge, MA, USA, 2018. [Google Scholar]
- Čapek, J.; Kubásek, J.; Pinc, J.; Drahokoupil, J.; Čavojský, M.; Vojtěch, D. Extrusion of the biodegradable ZnMg0.8Ca0.2 alloy—The influence of extrusion parameters on microstructure and mechanical characteristics. J. Mech. Behav. Biomed. Mater. 2020, 108, 103796. [Google Scholar] [CrossRef] [PubMed]
- Mehl, R.F. Atlas of Microstructures of Industrial Alloys, Metals Handbook, 8th ed.; Lyman, T., Ed.; American Society for Metals: Metals Park, OH, USA, 1972; pp. 241–273. [Google Scholar]
- Strobel, K.; Sweet, E.; Easton, M.; Nie, J.; Couper, M. Dispersoid Phases in 6xxx Series Aluminium Alloys. In Proceedings of the 7th Pacific Rim International Conference on Advanced Materials and Processing, Cairns, Australia, 2–6 August 2010; pp. 926–929. [Google Scholar]
- Donnadieu, P.; Lapasset, G.; Sanders, T.H. Manganese-induced ordering in the α-(Al-Mn-Fe-Si) approximant phase. Philos. Mag. Lett. 1994, 70, 319–326. [Google Scholar] [CrossRef]
- Yoo, J.E.; Shan, A.; Moon, I.G.; Maeng, S.J. A study on composition and crystal structure of dispersoids in AlMgSi alloys. J. Mater. Sci. 1999, 34, 2679–2683. [Google Scholar] [CrossRef]
- Li, Y.; Muggerud, A.M.F.; Olsen, A.; Furu, T. Precipitation of Partially coherent α-Al(Mn,Fe)Si dispersoids and their strengthening effect in AA 3003 alloy. Acta Mater. 2012, 60, 1004–1014. [Google Scholar] [CrossRef]
- Li, Y.J.; Arnberg, L. Quantitative study on the precipitation behavior of dispersoids in DC-cast AA3003 alloy during heating and homogenization. Acta Mater. 2003, 51, 3415–3428. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, W.; Hao, X.; Qiu, F.; Zhao, Q. Improving Elevated-Temperature Strength of an Al–Mn–Si Alloy by Strain-Induced Precipitation. Metals 2018, 8, 446. [Google Scholar] [CrossRef]
- Lobanov, M.; Zorina, M. Metody Opredeleniya Koeffitsiyentov Diffuzii; Izdatel’stvo Ural’skogo Universiteta: Yekaterinburg, Russia, 2017. [Google Scholar]
- Kakurin, Y.; Kakurina, N.; Zakharov, A. Metodika opredeleniya koeffitsiyenta zernogranichnoy diffuzii primesi v metallakh na osnove chislennogo resheniya po modeli Fishera. Inž. vestn. Dona 2013, 26, 95. [Google Scholar]
- Dolgopolov, N.A. Zernogranichnaya Diffuziya Medi v Alyuminii i v Splavakh Alyuminiy–Med’ i Alyuminiy–Tseriy. Ph.D. Dissertation, NUST MISiS, Moscow, Russia, 2014. [Google Scholar]
- Czerwinski, F. Thermal Stability of Aluminum Alloys. Materials 2020, 13, 3441. [Google Scholar] [CrossRef]
- de Campos, M.F. Diffusion Coefficients of Interest for the Simulation of Heat Treatment in Rare-Earth Transition Metal Magnets. Mater. Sci. Forum 2012, 727–728, 163–168. [Google Scholar] [CrossRef]




| Element | Mn | Fe | Si | Al | Mg | Ca | Na | Cu | Ni | Ti | Zn | Co |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | 30.57 | 4.41 | 3.53 | 2.16 | 1.87 | 1.84 | 1.64 | 1.18 | 1.14 | 0.35 | 0.14 | 0.13 |
| Element | Mn | Fe | Al | Si | Cu | Ni | Mo | Co | P |
|---|---|---|---|---|---|---|---|---|---|
| wt. % | 57.71 | 15.36 | 9.31 | 8.00 | 3.93 | 4.08 | 0.21 | 0.54 | 0.49 |
| Element | Al | Mn | Fe | Si | Cu | Ni | Zn | P | Co | Mo |
|---|---|---|---|---|---|---|---|---|---|---|
| Alloy S1 | 98.70 | 0.31 | 0.07 | 0.71 | 0.04 | 0.03 | 0.01 | 0.01 | - | - |
| Alloy S2 | 92.25 | 4.23 | 1.24 | 1.35 | 0.28 | 0.37 | 0.01 | 0.03 | 0.05 | 0.02 |
| Experiment No | Solution Annealing | Temperature of Artificial Aging [°C] | |
|---|---|---|---|
| Temperature [°C] | Time [h] | ||
| 1 | 500 | 1 | 150 |
| 2 | 500 | 2 | 150 |
| 550 | 2 | ||
| 3 | 600 | 4 | 400 |
| 4 | 600 | 4 | 150 |
| 5 | 600 | 8 | Natural aging |
| After Casting and Hot Extrusion | After Artificial Aging No. 4 | After Natural Aging | ||||
|---|---|---|---|---|---|---|
| Element | Matrix | Phase 1 Al6(Fe, Mn) | Matrix | Phase 1 Al6(Fe, Mn) | Matrix | Phase 1 Al6(Fe, Mn) |
| Al | 100 | 97.9 | 99.9 | 99.4 | 99.9 | 97 |
| Mn | 0.3 | 0.1 | 0.2 | 0.12 | 0.2 | |
| Fe | 1.8 | 0.4 | 0.03 | 1.8 | ||
| Ni | 0.8 | |||||
| After Hot Extrusion | After Artificial Aging No. 4 | |||||
|---|---|---|---|---|---|---|
| Element | Matrix | Phase 1 Al6(Fe, Mn) | Phase 2 Al5Mn12Si7 | Matrix | Phase 1 Al6(Fe, Mn) | Phase 2 Al5Mn12Si7 |
| Al | 99.3 | 86.3 | 76.3 | 99.3 | 85.3 | 78.4 |
| Mn | 0.7 | 10.2 | 17.8 | 0.4 | 12.3 | 13.3 |
| Fe | 3.5 | 2.7 | 0.2 | 2.4 | 3 | |
| Si | 3.2 | 4.8 | ||||
| After Natural Aging | ||||||
| Element | Matrix | Phase 1 Al6(Fe, Mn) | Phase 2 Al5Mn12Si7 | |||
| Al | 99 | 85 | 78.2 | |||
| Mn | 0.5 | 12.1 | 13.6 | |||
| Fe | 0.1 | 2.3 | 2.3 | |||
| Si | 0.3 | 0.4 | 5.2 | |||
| Cu | 0.2 | 0.1 | 0.1 | |||
| Ni | 0.1 | 0.5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsepeleva, A.; Novák, P.; Kolesnichenko, E.; Michalcová, A.; Kačenka, Z.; Kubásek, J. Heat Treatment of Aluminum Alloys with the Natural Combination of Dopants. Materials 2022, 15, 5541. https://doi.org/10.3390/ma15165541
Tsepeleva A, Novák P, Kolesnichenko E, Michalcová A, Kačenka Z, Kubásek J. Heat Treatment of Aluminum Alloys with the Natural Combination of Dopants. Materials. 2022; 15(16):5541. https://doi.org/10.3390/ma15165541
Chicago/Turabian StyleTsepeleva, Alisa, Pavel Novák, Evdokim Kolesnichenko, Alena Michalcová, Zdeněk Kačenka, and Jiří Kubásek. 2022. "Heat Treatment of Aluminum Alloys with the Natural Combination of Dopants" Materials 15, no. 16: 5541. https://doi.org/10.3390/ma15165541






