Versatile Zirconium Oxide (ZrO2) Sol-Gel Development for the Micro-Structuring of Various Substrates (Nature and Shape) by Optical and Nano-Imprint Lithography
Abstract
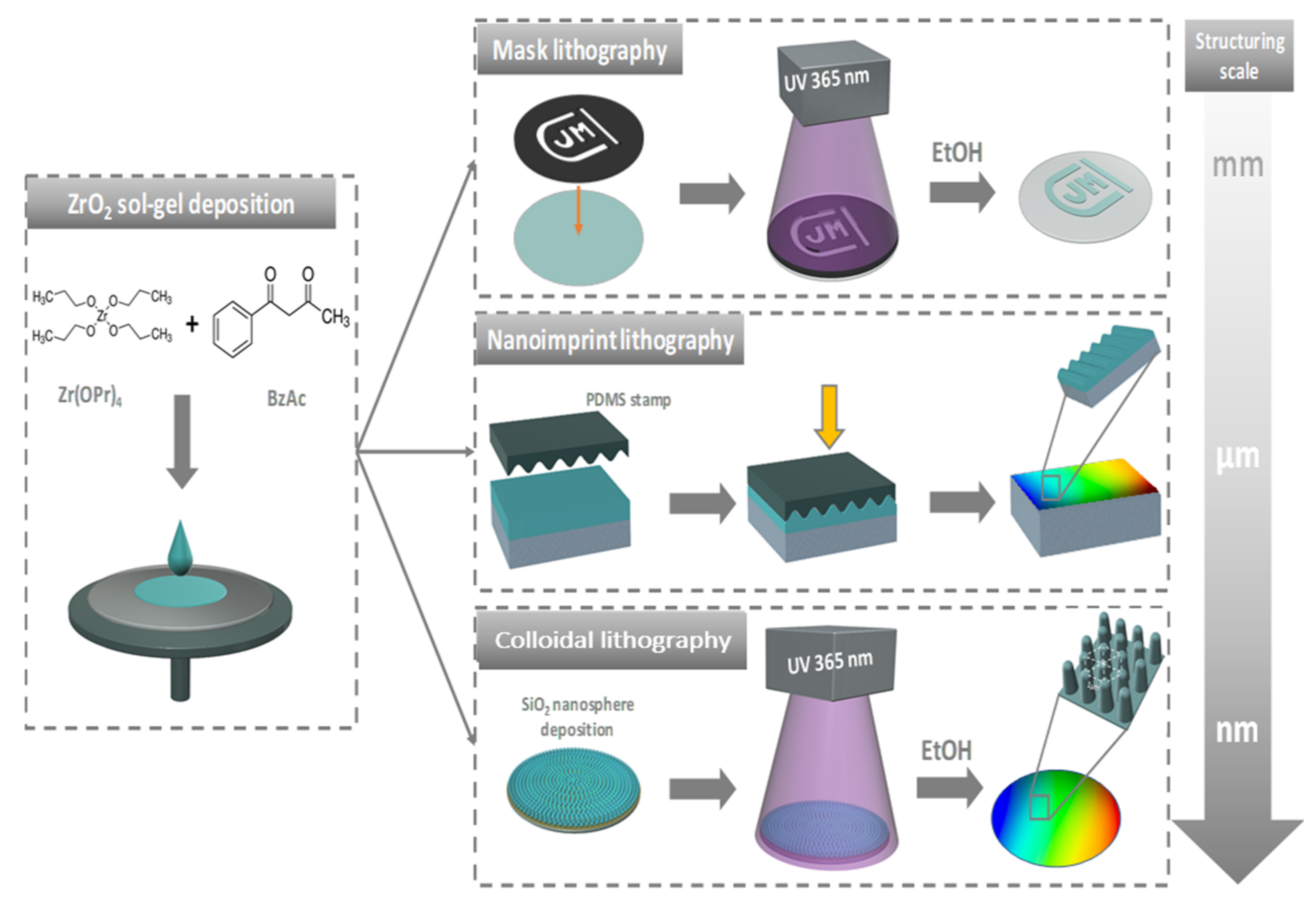
:1. Introduction
2. Materials and Methods
2.1. Sol-Gel
2.2. Optical Lithography
2.2.1. Macroscopic Mask Lithography
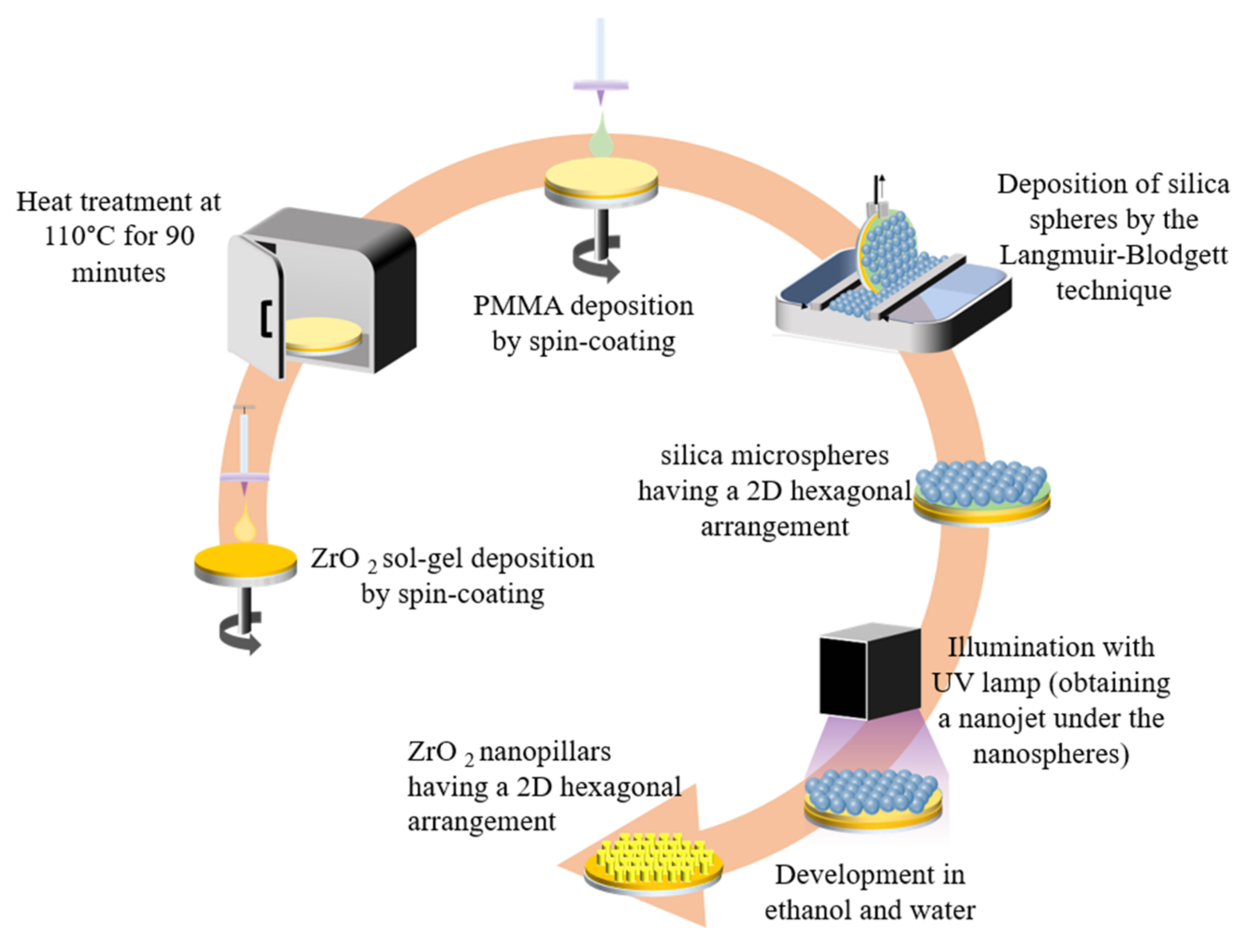
2.2.2. Colloidal Lithography
2.3. Nano-Imprint Lithography
2.4. Characterizations
3. Results and Discussion
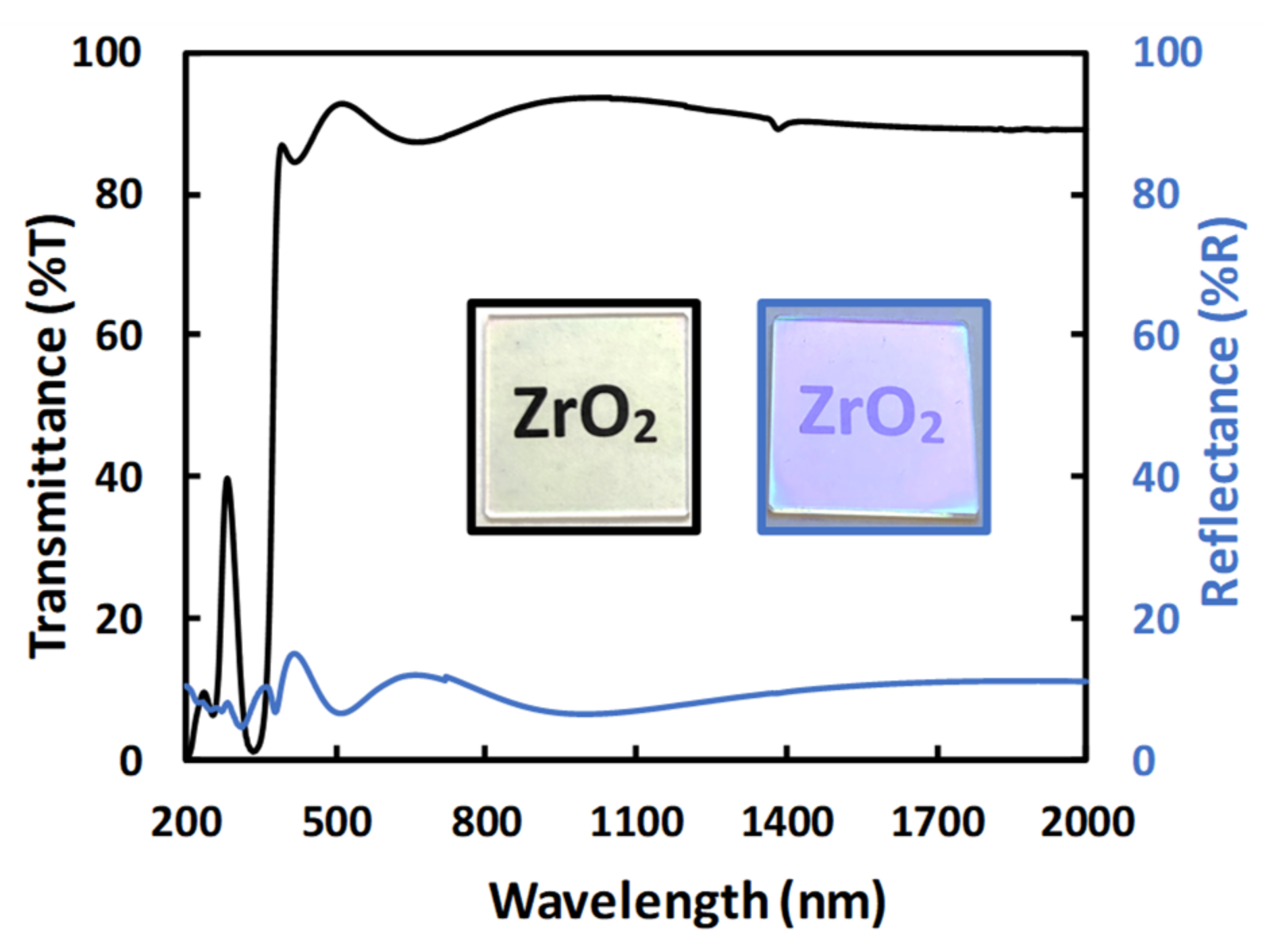
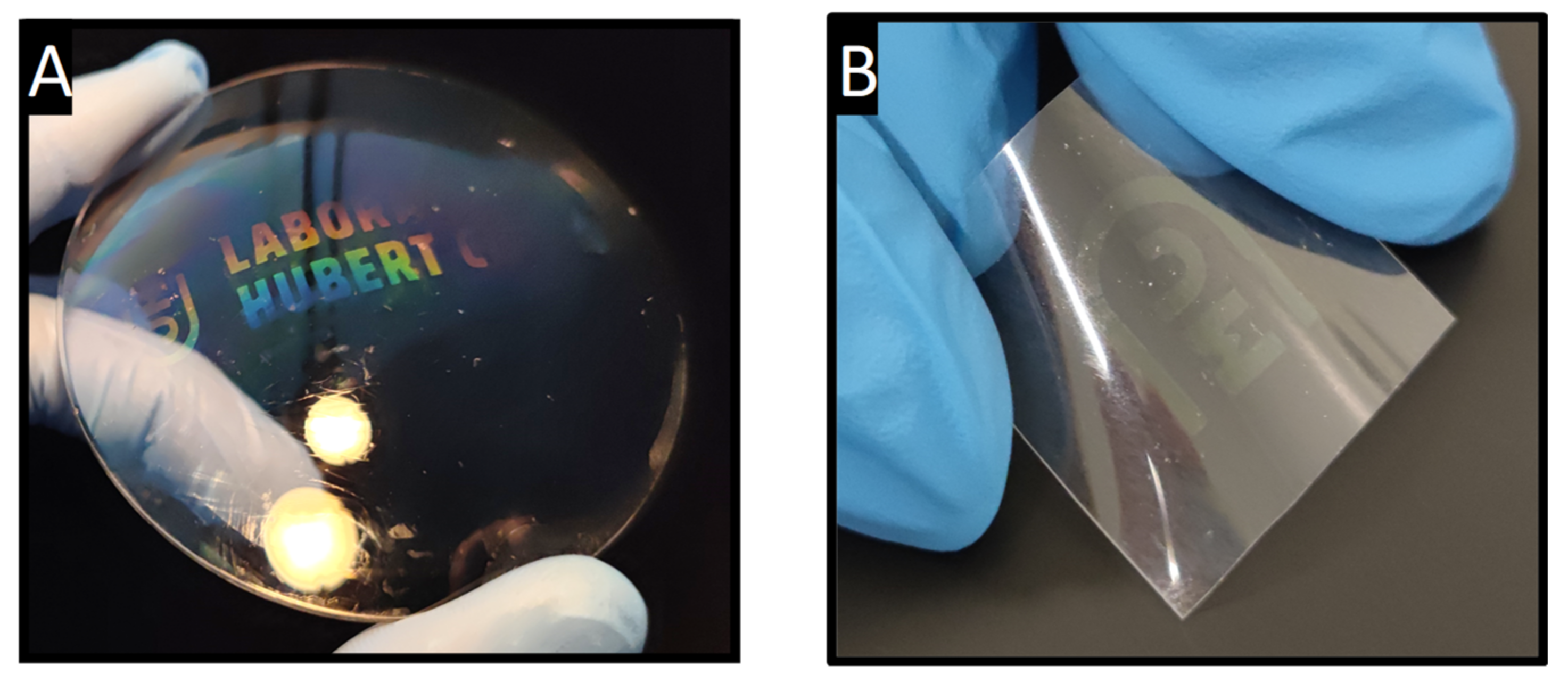
3.1. ZrO2 Layers
3.2. Micro-Nanostructuration of ZrO2 Xerogel Films
3.2.1. Optical Lithography
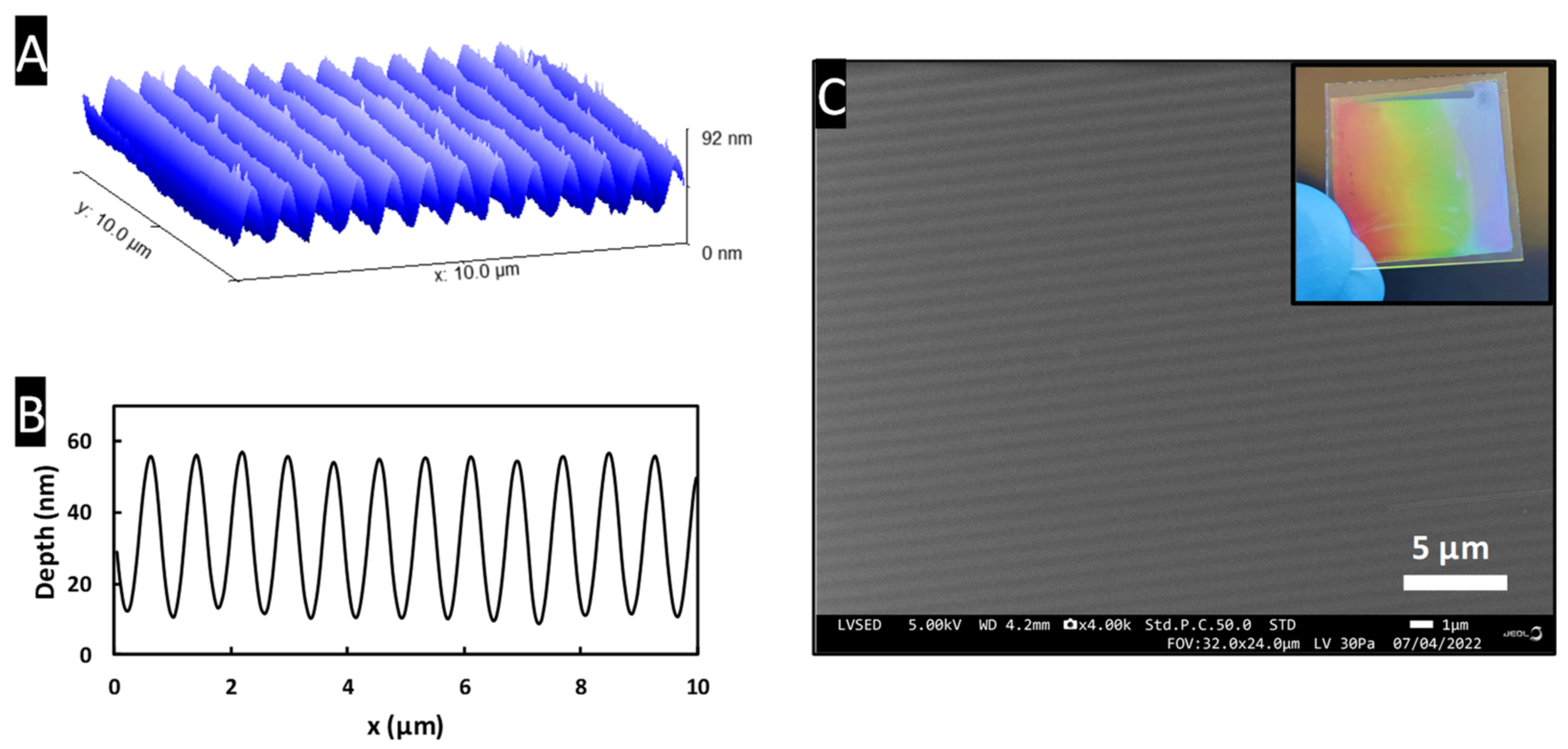
3.2.2. Nano-Imprint Lithography
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, P.J.; Bendavid, A. Properties of zirconium oxide films prepared by filtered cathodic vacuum arc deposition and pulsed DC substrate bias. Thin Solid Film. 2010, 518, 5078–5082. [Google Scholar] [CrossRef]
- Gan, Z.; Yu, G.; Zhao, Z.; Tan, C.M.; Tay, B.K. Mechanical properties of zirconia thin films deposited by filtered cathodic vacuum arc. J. Am. Ceram. Soc. 2005, 88, 2227–2229. [Google Scholar] [CrossRef]
- Sui, J.H.; Cai, W. Formation of ZrO2 coating on the NiTi alloys for improving their surface properties. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2006, 251, 402–406. [Google Scholar] [CrossRef]
- Millán-Ramos, B.; Morquecho-Marín, D.; Silva-Bermudez, P.; Ramírez-Ortega, D.; Depablos-Rivera, O.; García-López, J.; Fernández-Lizárraga, M.; Victoria-Hernández, J.; Letzig, D.; Almaguer-Flores, A.; et al. Biocompatibility and electrochemical evaluation of ZrO2 thin films deposited by reactive magnetron sputtering on MgZnCa alloy. J. Magnes. Alloy. 2021, 9, 2019–2038. [Google Scholar] [CrossRef]
- Bodurov, I.; Vlaeva, I.; Viraneva, A.; Yovcheva, T.; Sainov, S. Modified design of a laser refractometer. Nanosci. Nanotechnol. 2016, 16, 31–33. [Google Scholar]
- Khojier, K.; Savaloni, H.; Jafari, F. Structural, electrical, and decorative properties of sputtered zirconium thin films during post-annealing process. J. Theor. Appl. Phys. 2013, 7, 55. [Google Scholar] [CrossRef]
- Venkataraj, S.; Geurts, J.; Weis, H.; Kappertz, O.; Njoroge, W.K.; Jayavel, R.; Wuttig, M. Structural and optical properties of thin lead oxide films produced by reactive direct current magnetron sputtering. J. Vac. Sci. Technol. A 2001, 19, 2870–2878. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Nataraj, S.K.; Sannaikar, M.S.; Inamdar, S.R. Photoluminescence properties of zirconium oxide (ZrO2) nanoparticles. AIP Conf. Proc. 2020, 2274, 020002. [Google Scholar] [CrossRef]
- King, A.; Singh, R.; Nayak, B.B. Phase and photoluminescence analysis of dual-color emissive Eu3+-doped ZrO2 nanoparticles for advanced security features in anti-counterfeiting. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127715. [Google Scholar] [CrossRef]
- Shajahan, S.; Basu, A. Corrosion, oxidation and wear study of electro-co-deposited ZrO2-TiO2 reinforced Ni-W coatings. Surf. Coat. Technol. 2020, 393, 125729. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.; Nie, X. Deposition and properties of zirconia coatings on a zirconium alloy produced by pulsed DC plasma electrolytic oxidation. Surf. Coat. Technol. 2013, 221, 150–157. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, T.; Zhang, D.; Fan, S.; Shao, J.; Fan, Z. Laser conditioning of ZrO2:Y2O3/SiO2 mirror coatings prepared by E-beam evaporation. Appl. Surf. Sci. 2005, 239, 171–175. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Shen, J.; Wu, G.; Wang, J.; Chen, L. ZrO2 Thin films and ZrO2/SiO2 optical reflection filters deposited by sol–gel method. Mater. Lett. 2000, 45, 311–314. [Google Scholar] [CrossRef]
- Pirvaram, A.; Talebzadeh, N.; Rostami, M.; Leung, S.N.; O’Brien, P.G. Evaluation of a ZrO2/ZrO2-aerogel one-dimensional photonic crystal as an optical filter for thermophotovoltaic applications. Therm. Sci. Eng. Prog. 2021, 25, 100968. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Moradi, M.; Saeidi, F.S. The nearly perfect optical filter composed of [SiO2/ZrO2] Stacks using one-dimensional photonic crystals. J. Nanostruct. 2021, 11, 618–627. [Google Scholar] [CrossRef]
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An Overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef]
- Wang, M.; Gao, J. Atomic layer deposition of ZnO thin film on ZrO2 dental implant surface for enhanced antibacterial and bioactive performance. Mater. Lett. 2021, 285, 128854. [Google Scholar] [CrossRef]
- Kusano, E. Homologous substrate-temperature dependence of structure and properties of TiO2, ZrO2, and HfO2 thin films deposited by reactive sputtering. J. Vac. Sci. Technol. A 2019, 37, 051508. [Google Scholar] [CrossRef]
- Patel, U.S.; Patel, K.H.; Chauhan, K.V.; Chawla, A.K.; Rawal, S.K. Investigation of various properties for zirconium oxide films synthesized by sputtering. Procedia Technol. 2016, 23, 336–343. [Google Scholar] [CrossRef]
- Houska, J.; Rezek, J.; Cerstvy, R. Dependence of the ZrO2 growth on the crystal orientation: Growth Simulations and magnetron sputtering. Appl. Surf. Sci. 2022, 572, 151422. [Google Scholar] [CrossRef]
- Beer, S.M.J.; Samelor, D.; Abdel Aal, A.; Etzkorn, J.; Rogalla, D.; Turgambaeva, A.E.; Esvan, J.; Kostka, A.; Vahlas, C.; Devi, A. Direct liquid injection chemical vapor deposition of ZrO2 films from a heteroleptic Zr precursor: Interplay between film characteristics and corrosion protection of stainless steel. J. Mater. Res. Technol. 2021, 13, 1599–1614. [Google Scholar] [CrossRef]
- Espinoza-Pérez, L.J.; López-Honorato, E.; González, L.A. Development of ZrO2 and YSZ coatings deposited by PE-CVD below 800 °C for the protection of Ni alloys. Ceram. Int. 2020, 46, 15621–15630. [Google Scholar] [CrossRef]
- Shin, H.; Jeong, D.-K.; Lee, J.; Sung, M.M.; Kim, J. Formation of TiO2 and ZrO2 nanotubes using atomic layer deposition with ultraprecise control of the wall thickness. Adv. Mater. 2004, 16, 1197–1200. [Google Scholar] [CrossRef]
- Cassir, M.; Goubin, F.; Bernay, C.; Vernoux, P.; Lincot, D. Synthesis of ZrO2 thin films by atomic layer deposition: Growth kinetics, structural and electrical properties. Appl. Surf. Sci. 2002, 193, 120–128. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Sun, Y.; Yuan, J.C.-C.; Mathew, M.T.; Sukotjo, C.; Takoudis, C.G. In vitro corrosion behavior of coated Ti6Al4V with TiO2, ZrO2, and TiO2/ZrO2 mixed nanofilms using atomic layer deposition for dental implants. Surf. Coat. Technol. 2022, 444, 128686. [Google Scholar] [CrossRef]
- Tarafdar, A.; Panda, A.B.; Pramanik, P. Synthesis of ZrO2–SiO2 Mesocomposite with High ZrO2 content via a novel sol–gel method. Microporous Mesoporous Mater. 2005, 84, 223–228. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, C.; Lin, J. Phase transformation and photoluminescence properties of nanocrystalline ZrO2 powders prepared via the pechini-type sol−gel process. J. Phys. Chem. C 2007, 111, 3300–3307. [Google Scholar] [CrossRef]
- Della Giustina, G.; Garoli, D.; Romanato, F.; Brusatin, G. Zirconia based functional sol–gel resist for UV and high resolution lithography. Microelectron. Eng. 2013, 110, 436–440. [Google Scholar] [CrossRef]
- Kintaka, K.; Nishii, J.; Tohge, N. Diffraction gratings of photosensitive ZrO2 gel films fabricated with the two-ultraviolet-beam interference method. Appl. Opt. 2000, 39, 489–493. [Google Scholar] [CrossRef]
- Yamada, I.; Ikeda, Y. Sol-gel zirconia diffraction grating using a soft imprinting process. Appl. Opt. 2017, 56, 5054–5059. [Google Scholar] [CrossRef]
- Ridaoui, H.; Wieder, F.; Ponche, A.; Soppera, O. Direct ArF laser photopatterning of metal oxide nanostructures prepared by the sol-gel route. Nanotechnology 2010, 21, 065303. [Google Scholar] [CrossRef]
- Park, H.-H.; Zhang, X.; Lee, S.-W.; Kim, K.; Choi, D.-G.; Choi, J.-H.; Lee, J.; Lee, E.-S.; Park, H.-H.; Hill, R.H.; et al. Facile nanopatterning of zirconium dioxide films via direct ultraviolet-assisted nanoimprint lithography. J. Mater. Chem. 2010, 21, 657–662. [Google Scholar] [CrossRef]
- Dinachali, S.S.; Saifullah, M.S.M.; Ganesan, R.; Thian, E.S.; He, C. A universal scheme for patterning of oxides via thermal nanoimprint lithography. Adv. Funct. Mater. 2013, 23, 2201–2211. [Google Scholar] [CrossRef]
- Hochedel, M.; Bichotte, M.; Arnould, F.; Celle, F.; Veillas, C.; Pouit, T.; Dubost, L.; Kämpfe, T.; Dellea, O.; Crespo-Monteiro, N.; et al. Microstructuring technology for large and cylindrical receivers for Concentrated Solar Plants (CSP). Microelectron. Eng. 2021, 248, 111616. [Google Scholar] [CrossRef]
- Shavdina, O.; Berthod, L.; Kampfe, T.; Reynaud, S.; Veillas, C.; Verrier, I.; Langlet, M.; Vocanson, F.; Fugier, P.; Jourlin, Y. Large area fabrication of periodic TiO2 nanopillars using microsphere photolithography on a photopatternable sol–gel film. Langmuir 2015, 31, 7877–7884. [Google Scholar] [CrossRef]
- Ai, B.; Yu, Y.; Möhwald, H.; Zhang, G.; Yang, B. Plasmonic films based on colloidal lithography. Adv. Colloid Interface Sci. 2014, 206, 5–16. [Google Scholar] [CrossRef]
- Valour, A.; Usuga Higuita, M.A.; Crespo-Monteiro, N.; Reynaud, S.; Hochedel, M.; Jamon, D.; Donnet, C.; Jourlin, Y. Micro–nanostructured TiN thin film: Synthesis from a photo-patternable TiO2 sol–gel coating and rapid thermal nitridation. J. Phys. Chem. C 2020, 123, 25480–25488. [Google Scholar] [CrossRef]
- Briche, S.; Tebby, Z.; Riassetto, D.; Messaoud, M.; Gamet, E.; Pernot, E.; Roussel, H.; Dellea, O.; Jourlin, Y.; Langlet, M. New insights in photo-patterned sol-gel-derived TiO2 films. J. Mater. Sci 2011, 46, 1474–1486. [Google Scholar] [CrossRef]
- Bardosova, M.; Pemble, M.E.; Povey, I.M.; Tredgold, R.H. The Langmuir-Blodgett approach to making colloidal photonic crystals from silica spheres. Adv. Mater. 2010, 22, 3104–3124. [Google Scholar] [CrossRef]
- Oda, S.; Uchiyama, H.; Kozuka, H. Thermoplasticity of Sol–Gel-derived titanoxanes chemically modified with benzoylacetone. J. Sol. Gel Sci. Technol. 2014, 70, 441–450. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, G.; Zhang, W.; Feng, Z.; Lin, L.; Zheng, Z. Low-cost micro-lens arrays fabricated by photosensitive sol–gel and multi-beam laser interference. Photonics Nanostruct. Fundam. Appl. 2012, 10, 667–673. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-Monteiro, N.; Valour, A.; Vallejo-Otero, V.; Traynar, M.; Reynaud, S.; Gamet, E.; Jourlin, Y. Versatile Zirconium Oxide (ZrO2) Sol-Gel Development for the Micro-Structuring of Various Substrates (Nature and Shape) by Optical and Nano-Imprint Lithography. Materials 2022, 15, 5596. https://doi.org/10.3390/ma15165596
Crespo-Monteiro N, Valour A, Vallejo-Otero V, Traynar M, Reynaud S, Gamet E, Jourlin Y. Versatile Zirconium Oxide (ZrO2) Sol-Gel Development for the Micro-Structuring of Various Substrates (Nature and Shape) by Optical and Nano-Imprint Lithography. Materials. 2022; 15(16):5596. https://doi.org/10.3390/ma15165596
Chicago/Turabian StyleCrespo-Monteiro, Nicolas, Arnaud Valour, Victor Vallejo-Otero, Marie Traynar, Stéphanie Reynaud, Emilie Gamet, and Yves Jourlin. 2022. "Versatile Zirconium Oxide (ZrO2) Sol-Gel Development for the Micro-Structuring of Various Substrates (Nature and Shape) by Optical and Nano-Imprint Lithography" Materials 15, no. 16: 5596. https://doi.org/10.3390/ma15165596
APA StyleCrespo-Monteiro, N., Valour, A., Vallejo-Otero, V., Traynar, M., Reynaud, S., Gamet, E., & Jourlin, Y. (2022). Versatile Zirconium Oxide (ZrO2) Sol-Gel Development for the Micro-Structuring of Various Substrates (Nature and Shape) by Optical and Nano-Imprint Lithography. Materials, 15(16), 5596. https://doi.org/10.3390/ma15165596





