Time and Crack Width Dependent Model of Chloride Transportation in Engineered Cementitious Composites (ECC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Properties of ECC
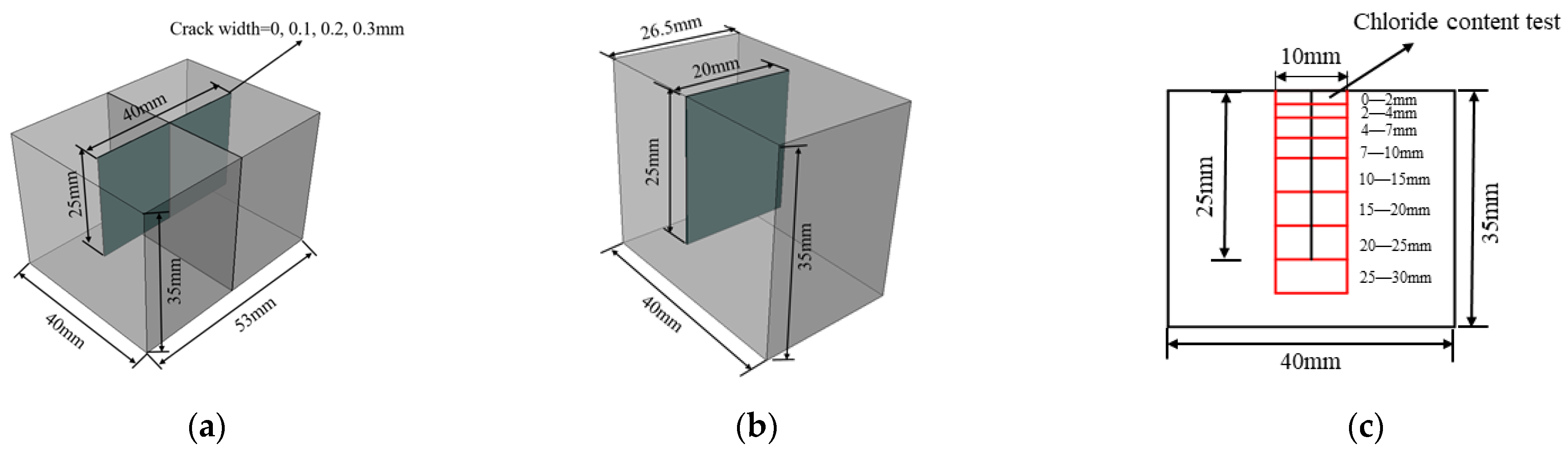
2.2. Specimens and Exposure Conditions
2.3. Free Chloride Content Measurement
2.4. Analysis of Variance (ANOVA)
3. Experimental Results
3.1. Chloride Transport Model Development
3.1.1. Fundamental of Chloride Transport Models
3.1.2. Model the Surface Chloride Concentration
3.1.3. Model the Apparent Chloride Diffusion Coefficient
3.1.4. Model Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrade, C.; Alonso, C.; Molina, F.J. Cover cracking as a function of bar corrosion: Part I—experimental test. Mater. Struct. 1993, 26, 453–464. [Google Scholar] [CrossRef]
- Zeng, Y.; Gu, X.; Liu, X.; Zhang, W. Degradation of Mechanical Behavior of Corroded Prestressing Wires Subjected to High-Cycle Fatigue Loading. J. Bridge Eng. 2017, 22, 04017004. [Google Scholar]
- Gu, X.; Guo, H.; Zhou, B.; Zhang, W.; Jiang, C. Corrosion non-uniformity of steel bars and reliability of corroded RC beams. Eng. Struct. 2018, 167, 188–202. [Google Scholar] [CrossRef]
- Shi, X.; Ning, X.; Fortune, K.; Jing, G. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Alexander, M.; Thomas, M. Service life prediction and performance testing—Current developments and practical applications. Cem. Concr. Res. 2015, 78, 155–164. [Google Scholar] [CrossRef]
- Basheer, L.; Kropp, J.; Cleland, D.J. Assessment of the durability of concrete from its permeation properties: A review. Constr. Build. Mater. 2001, 15, 93–103. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Gu, C.; Su, H.; Li, V.C. Influence of micro-cracking on the permeability of engineered cementitious composites. Cem. Concr. Compos. 2016, 72, 104–113. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Y.; Yu, J.; Xiao, J.; Xu, S. Feasibility study of strain hardening magnesium oxychloride cement-based composites. Constr. Build. Mater. 2018, 165, 750–760. [Google Scholar] [CrossRef]
- Zhang, J.; Leung, C.K.; Gao, Y. Simulation of crack propagation of fiber reinforced cementitious composite under direct tension. Eng. Fract. Mech. 2011, 78, 2439–2454. [Google Scholar] [CrossRef]
- Li, V.C.; Leung, C. Steady-State and Multiple Cracking of Short Random Fiber Composites. J. Eng. Mech. 1992, 118, 2246–2264. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Gu, C.; Su, H.; Li, V. Self-healing of microcracks in Engineered Cementitious Composites under sulfate and chloride environment. Constr. Build. Mater. 2017, 153, 948–956. [Google Scholar] [CrossRef]
- Lu, C.; Li, V.C.; Leung, C.K.Y. Flaw characterization and correlation with cracking strength in Engineered Cementitious Composites (ECC). Cem. Concr. Res. 2018, 107, 64–74. [Google Scholar] [CrossRef]
- Sahmaran, M.; University, G. Influence of Microcracking on the Transport Properties of Engineered Cementitious Composites. In Proceedings of the International Balkans Conference on Challenges of Civil Engineering, Tirana, Albania, 23–25 May 2011. [Google Scholar]
- Li, V.C.; Wang, S.; Wu, C. Tensile strain-hardening behavior of polyvinyl alcohol engineered cementitious composite (PVA-ECC). ACI Mater. J. 2001, 98, 483–492. [Google Scholar]
- Li, V.C.; Wu, C.; Wang, S.X.; Ogawa, A.; Saito, T. Interface tailoring for strain-hardening polyvinyl alcohol-engineered cementitious composite (PVA-ECC). ACI Mater. J. 2002, 99, 463–472. [Google Scholar]
- Hou, L.-j.; Xu, S.; Zhang, X.-f.; Chen, D. Shear behaviors of reinforced ultrahigh toughness cementitious composite slender beams with stirrups. J. Mater. Civ. Eng. 2014, 26, 466–475. [Google Scholar] [CrossRef]
- Fan, S.; Li, M. X-ray computed microtomography of three-dimensional microcracks and self-healing in engineered cementitious composites. Smart Mater. Struct. 2014, 24, 015021. [Google Scholar] [CrossRef]
- Lee, B.J.; Hyun, J.H.; Kim, Y.Y.; Shin, K.J. Chloride Permeability of Damaged High-Performance Fiber-Reinforced Cement Composite by Repeated Compressive Loads. Materials 2014, 7, 5802–5815. [Google Scholar] [CrossRef]
- Yu, B.; Ma, Q.; Huang, H.C.; Chen, Z. Probabilistic prediction model for chloride diffusion coefficient of concrete in terms of material parameters. Constr. Build. Mater. 2019, 215, 941–957. [Google Scholar] [CrossRef]
- Chidiac, S.E.; Shafikhani, M. Electrical resistivity model for quantifying concrete chloride diffusion coefficient. Cem. Concr. Compos. 2020, 113, 103707. [Google Scholar] [CrossRef]
- Al-Sodani, K.; Al-Zahrani, M.M.; Maslehuddin, M.; Al-Amoudi, O.; Al-Dulaijan, S.U. Chloride diffusion models for Type I and fly ash cement concrete exposed to field and laboratory conditions. Mar. Struct. 2021, 76, 102900. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, X.; Wu, L. Prediction model of chloride diffusion in concrete considering the coupling effects of coarse aggregate and steel reinforcement exposed to marine tidal environment. Constr. Build. Mater. 2019, 216, 40–57. [Google Scholar] [CrossRef]
- Chalee, W.; Jaturapitakkul, C.; Chindaprasirt, P. Predicting the chloride penetration of fly ash concrete in seawater. Mar. Struct. 2009, 22, 341–353. [Google Scholar] [CrossRef]
- Mangat, P.S.; Limbachiya, M.C. Effect of initial curing on chloride diffusion in concrete repair materials. Cem. Concr. Res. 1999, 29, 1475–1485. [Google Scholar] [CrossRef]
- Violetta, B. Life-365 Service Life Prediction Model. Concr. Int. 2002, 24, 53–57. [Google Scholar]
- Zhang, J.; Pei, T.; Chang, J.; Xie, S.; Zhao, Y. Experimental study on chloride penetration in cracked engineered cementitious composite under soak-dry cycles. Constr. Build. Mater. 2021, 307, 124980. [Google Scholar] [CrossRef]
- Ma, H.; Yi, C.; Wu, C. Review and outlook on durability of engineered cementitious composite (ECC). Constr. Build. Mater. 2021, 287, 122719. [Google Scholar] [CrossRef]
- Şahmaran, M.; Li, V.C. Durability properties of micro-cracked ECC containing high volumes fly ash. Cem. Concr. Res. 2009, 39, 1033–1043. [Google Scholar] [CrossRef]
- Samaha, H.R.; Hover, K.C. Influence of microcracking on the mass transport properties of concrete. ACI Mater. J. 1992, 89, 416–424. [Google Scholar]
- Wang, K.; Jansen, D.C.; Shah, S.P.; Karr, A.F. Permeability study of cracked concrete. Cem. Concr. Res. 1997, 27, 381–393. [Google Scholar] [CrossRef]
- Takewaka, K.; Yamaguchi, T.; Maeda, S. Simulation Model for Deterioration of Concrete Structures due to Chloride Attack. J. Adv. Concr. Technol. 2003, 1, 139–146. [Google Scholar] [CrossRef]
- Rs, A.; Xh, A.; Yl, B.; Zz, C.; Pz, A.; Fei, W.C. Chloride diffusion behavior of engineered cementitious composite under dry-wet cycles. Constr. Build. Mater. 2020, 260, 119943. [Google Scholar]
- den Heede, P.V.; Maes, M.; Belie, N.D. Influence of active crack width control on the chloride penetration resistance and global warming potential of slabs made with fly ash + silica fume concrete. Constr. Build. Mater. 2014, 67, 74–80. [Google Scholar] [CrossRef]
- Poulsen, E.; Mejlbro, L. Diffusion of Chloride in Concrete: Theory and Application; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Tang, L.; Gulikers, J. On the mathematics of time-dependent apparent chloride diffusion coefficient in concrete. Cem. Concr. Res. 2007, 37, 589–595. [Google Scholar]
- Zhang, J.; Kong, W.D. Chloride diffusivity analysis of existing concrete based on Fick’s second law. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2010, 25, 142–146. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chiu, H.J.; Chan, Y.W.; Chang, Y.C.; Yang, C.C. The correlation between air-borne salt and chlorides cumulated on concrete surface in the marine atmosphere zone in North Taiwan. J. Mar. Sci. Technol. 2013, 21, 4. [Google Scholar]
- Zhang, J.; Zhao, J.; Zhang, Y.; Gao, Y.; Zheng, Y. Instantaneous chloride diffusion coefficient and its time dependency of concrete exposed to a marine tidal environment. Constr. Build. Mater. 2018, 167, 225–234. [Google Scholar] [CrossRef]
- Shakouri, M.; Trejo, D. A time-variant model of surface chloride build-up for improved service life predictions. Cem. Concr. Compos. 2017, 84, 99–110. [Google Scholar] [CrossRef]
- Pack, S.W.; Jung, M.S.; Song, H.W.; Kim, S.H.; Ann, K.Y. Prediction of time dependent chloride transport in concrete structures exposed to a marine environment. Cem. Concr. Res. 2010, 40, 302–312. [Google Scholar] [CrossRef]
- Liu, Q.F.; Hu, Z.; Lu, X.Y.; Yang, J.; Sun, W. Prediction of Chloride Distribution for Offshore Concrete Based on Statistical Analysis. Materials 2020, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yu, M.; Hu, Y.; Yang, L.; Ma, H. Influence of data acquisition and processing on surface chloride concentration of marine concrete. Constr. Build. Mater. 2020, 273, 121705. [Google Scholar] [CrossRef]
- Muthulingam, S.; Rao, B.N. Consistent models for estimating chloride ingress parameters in fly ash concrete. J. Build. Eng. 2015, 3, 24–38. [Google Scholar] [CrossRef]
- Lfy, A.; Lu, W.B.; Bo, Y.C. Time-varying behavior and its coupling effects with environmental conditions and cementitious material types on surface chloride concentration of marine concrete. Constr. Build. Mater. 2021, 303, 124578. [Google Scholar]
- Liu, Z.; Wang, Y.; Wang, J.; Liu, C.; Jiang, J.; Li, H. Experiment and simulation of chloride ion transport and binding in concrete under the coupling of diffusion and convection. J. Build. Eng. 2022, 45, 103610. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Yu, R.; Huang, Y.; Cheng, S. Understanding the chloride binding and diffusion behaviors of marine concrete based on Portland limestone cement-alumina enriched pozzolans. Constr. Build. Mater. 2019, 198, 207–217. [Google Scholar] [CrossRef]
- Li, C.Z.; Song, X.B.; Jiang, L. A time-dependent chloride diffusion model for predicting initial corrosion time of reinforced concrete with slag addition. Cem. Concr. Res. 2021, 145, 106455. [Google Scholar] [CrossRef]
- Yang, L.F.; Cai, R.; Yu, B. Investigation of computational model for surface chloride concentration of concrete in marine atmosphere zone. Ocean Eng. 2017, 138, 105–111. [Google Scholar] [CrossRef]
- Su, L.; Niu, D.; Huang, D.; Luo, Y.; Qiao, H.; Zhang, Y. Chloride diffusion behavior and microstructure of basalt-polypropylene hybrid fiber reinforced concrete in salt spray environment. Constr. Build. Mater. 2022, 324, 126716. [Google Scholar] [CrossRef]
- Petcherdchoo, A. Time dependent models of apparent diffusion coefficient and surface chloride for chloride transport in fly ash concrete. Constr. Build. Mater. 2013, 38, 497–507. [Google Scholar] [CrossRef]










| Materials | Cement | Fly Ash | Sand | HPMC | Water | Water Reducer | PVA Fibers | ECC |
|---|---|---|---|---|---|---|---|---|
| Quantity | 568 | 682 | 455 | 0.5 | 325 | 10 | 26 | 2066.57 |
| Tensile Strength (GPa) | Tensile Modulus (GPa) | Diameter (µm) | Length (mm) | Elongation (%) | Density (g/cm3) |
|---|---|---|---|---|---|
| 1.56 | 41 | 40 | 12 | 6.5 | 1.3 |
| Test Number | Crack Width (mm) | Exposure Period (Days) |
|---|---|---|
| CW-0 | 0 | 30, 60, 90, 120 |
| CW-1 | 0.1 | |
| CW-2 | 0.2 | |
| CW-3 | 0.3 |
| F | p | |
|---|---|---|
| Exposure time | 73.61717 | 1.18382 × 10−6 |
| Crack width | 32.35408 | 3.76888 × 10−6 |
| Proposed model | 52.98563 | 1.62782 × 10−6 |
| F | p | |
|---|---|---|
| Exposure time | 1487.09451 | 1.90548 × 10−12 |
| Crack width | 44.77743 | 9.81442 × 10−6 |
| Proposed model | 765.93597 | 1.14968 × 10−11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, L.; Qiao, L.; Sun, R.; Lu, W.; Guan, Y.; Gao, N.; Hu, X.; Li, Z.; Wang, L.; Tian, Y.; et al. Time and Crack Width Dependent Model of Chloride Transportation in Engineered Cementitious Composites (ECC). Materials 2022, 15, 5611. https://doi.org/10.3390/ma15165611
Bu L, Qiao L, Sun R, Lu W, Guan Y, Gao N, Hu X, Li Z, Wang L, Tian Y, et al. Time and Crack Width Dependent Model of Chloride Transportation in Engineered Cementitious Composites (ECC). Materials. 2022; 15(16):5611. https://doi.org/10.3390/ma15165611
Chicago/Turabian StyleBu, Linglai, Lei Qiao, Renjuan Sun, Wei Lu, Yanhua Guan, Nan Gao, Xinlei Hu, Zhenhuan Li, Lin Wang, Yuhe Tian, and et al. 2022. "Time and Crack Width Dependent Model of Chloride Transportation in Engineered Cementitious Composites (ECC)" Materials 15, no. 16: 5611. https://doi.org/10.3390/ma15165611





