Biphenylene: A Two−Dimensional Graphene−Based Coating with Superior Anti−Corrosion Performance
Abstract
:1. Introduction
2. Computational Methodologies
3. Results and Discussion
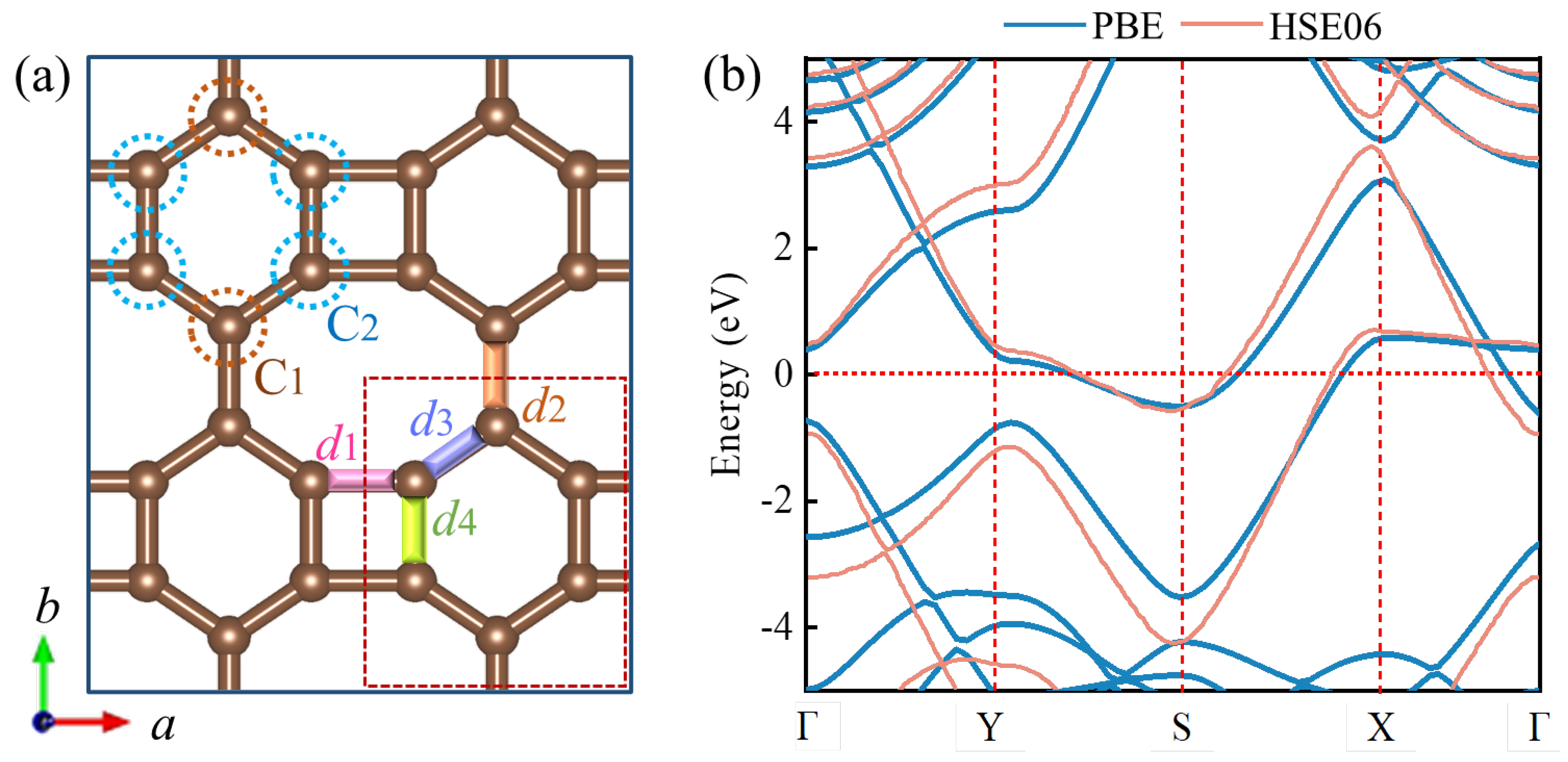
3.1. Geometric Structure of Biphenylene
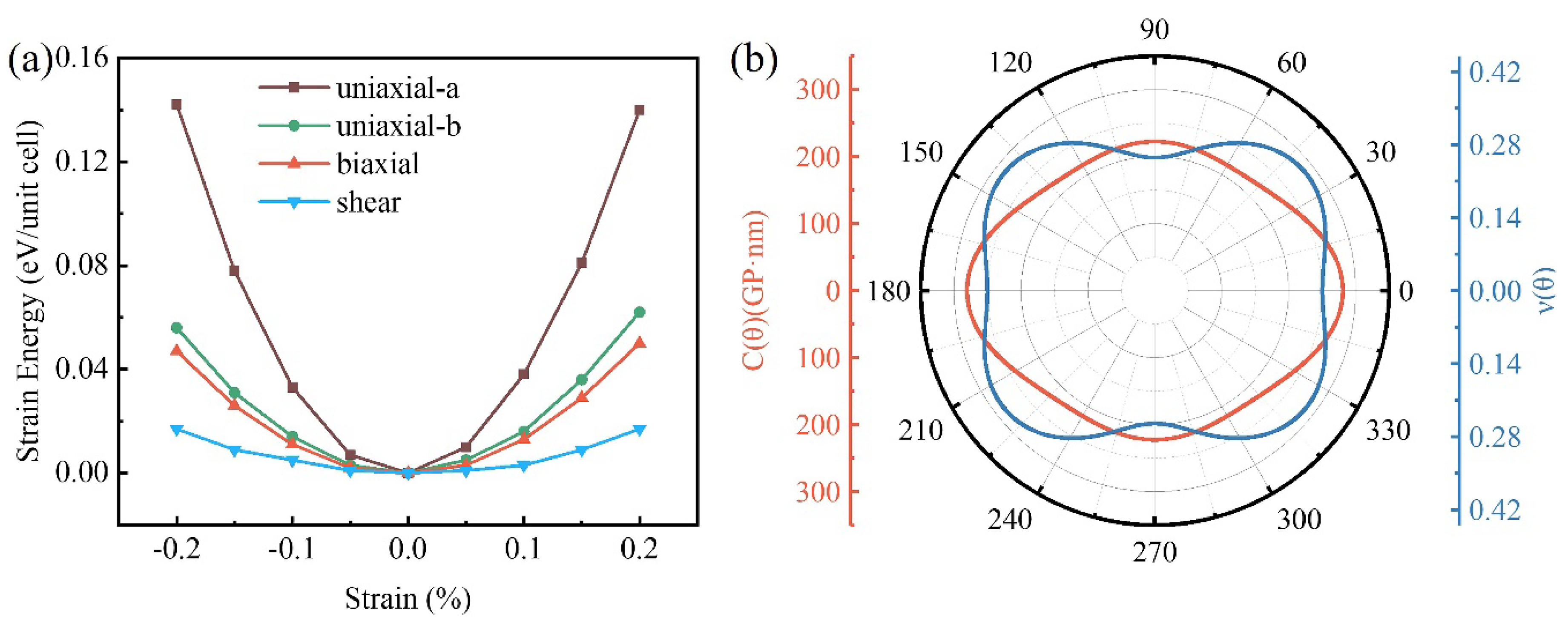
3.2. Mechanical Properties of Biphenylene
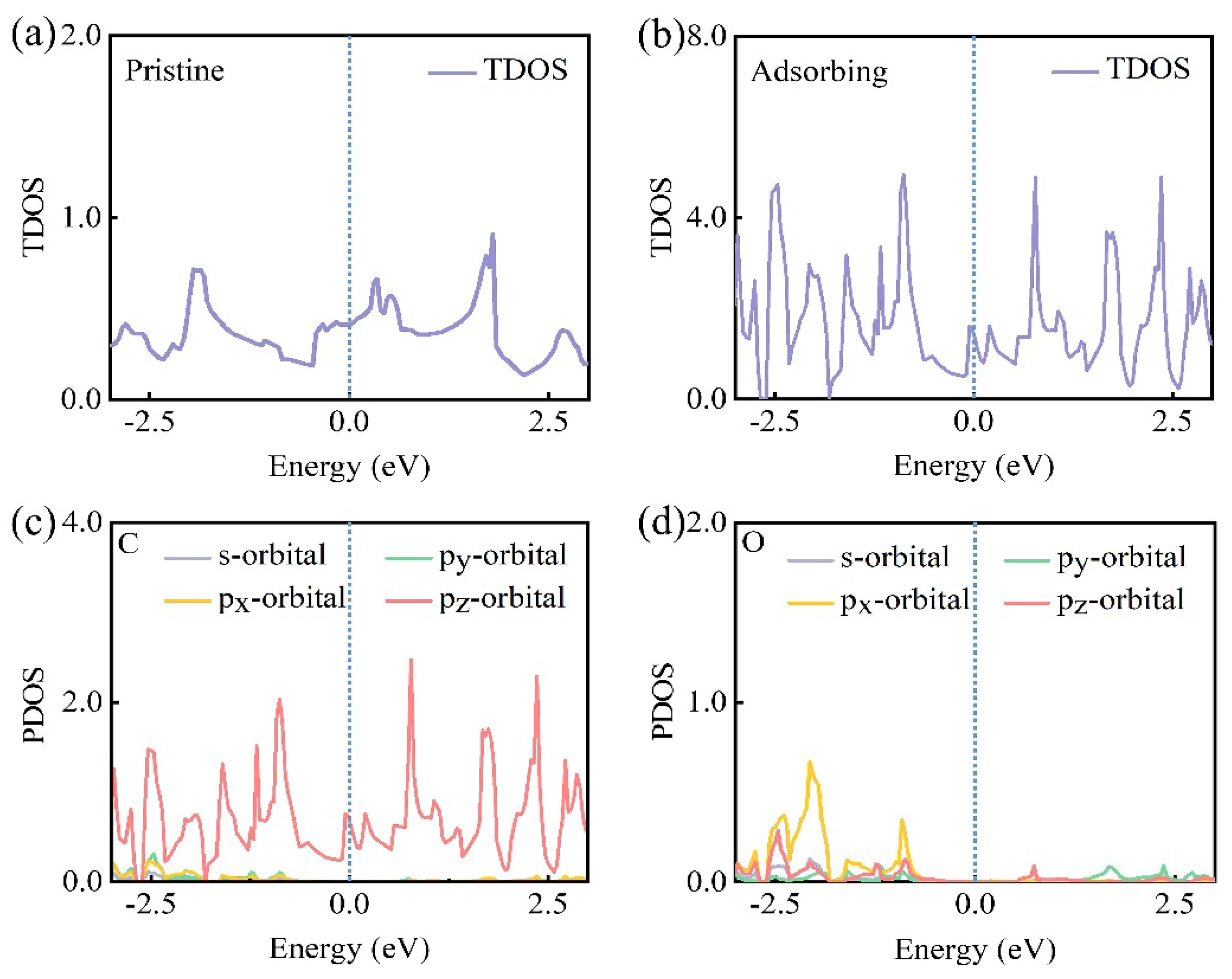
3.3. Single O Adsorption on Biphenylene
3.4. Electronic Properties of Biphenylene
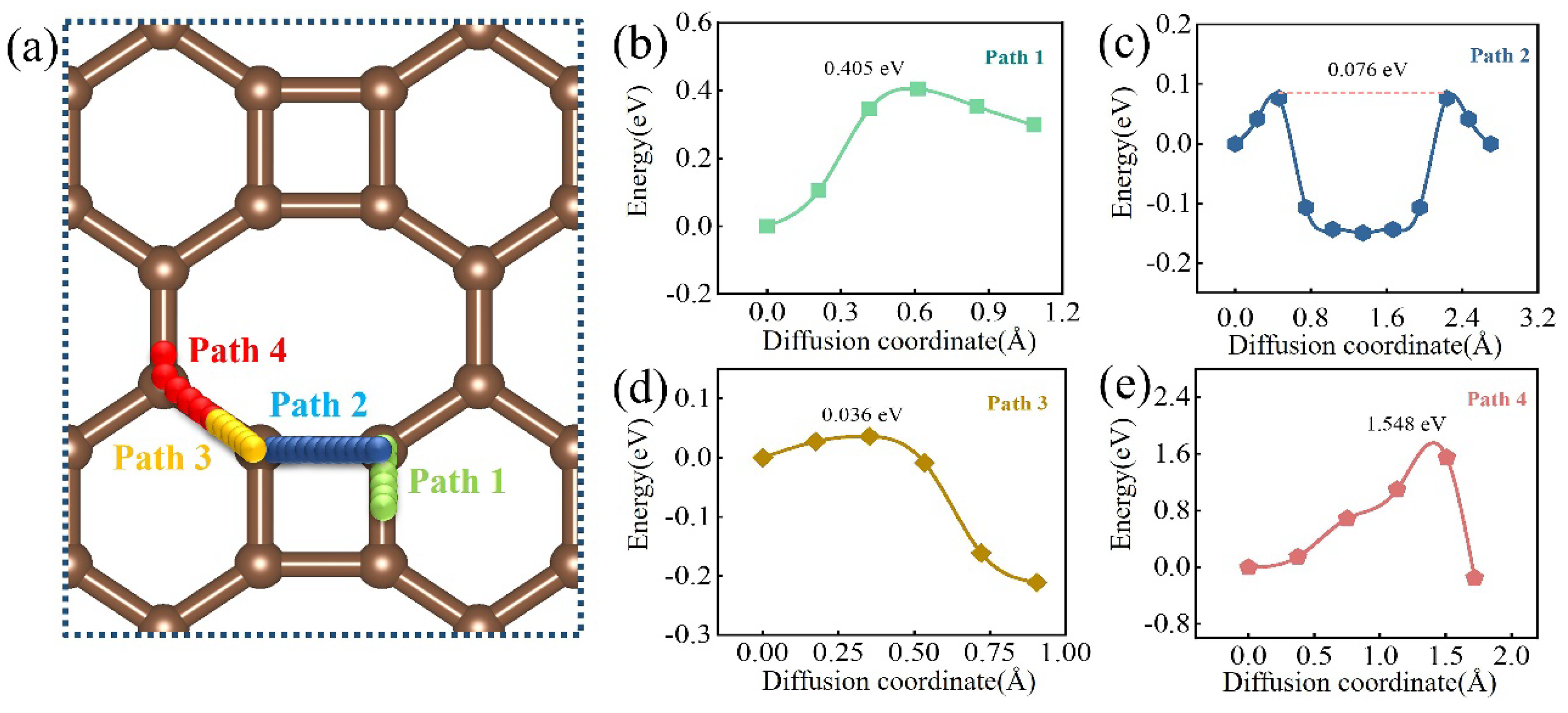
3.5. O Diffusion Behavior on Biphenylene
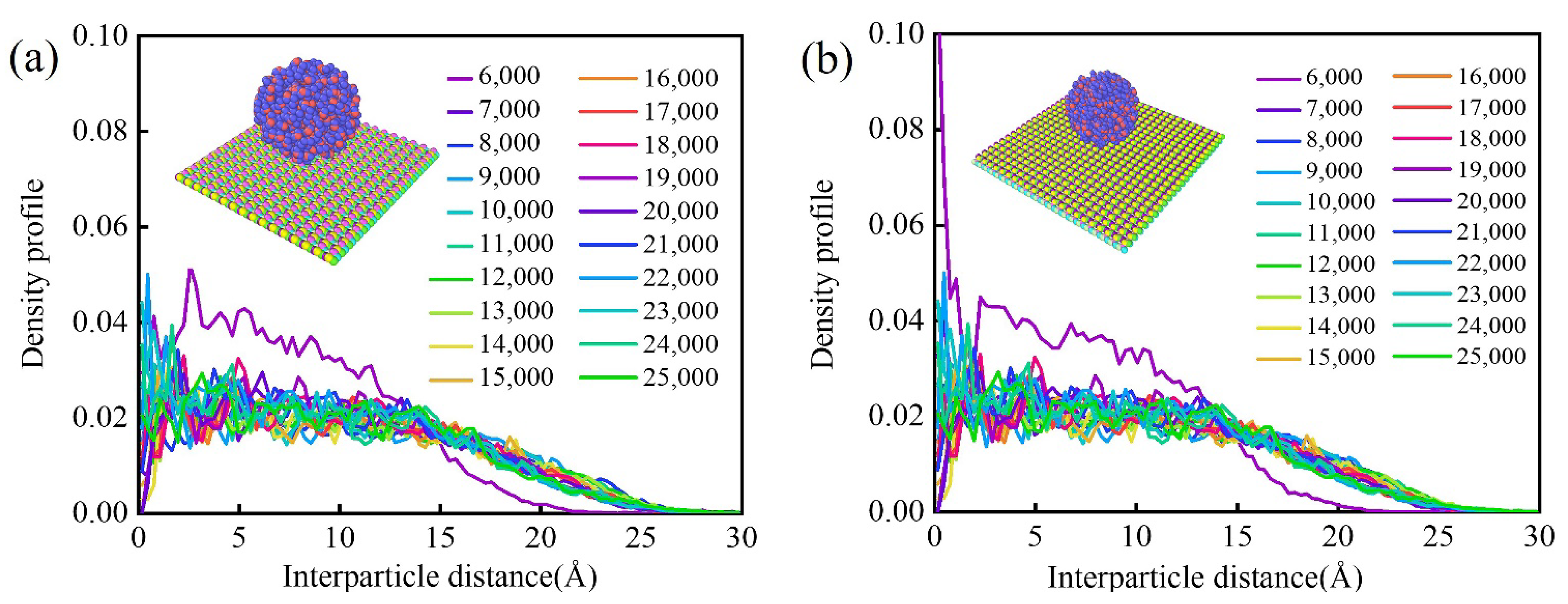
3.6. Wettability of Water Molecules on Monolayer Biphenylene
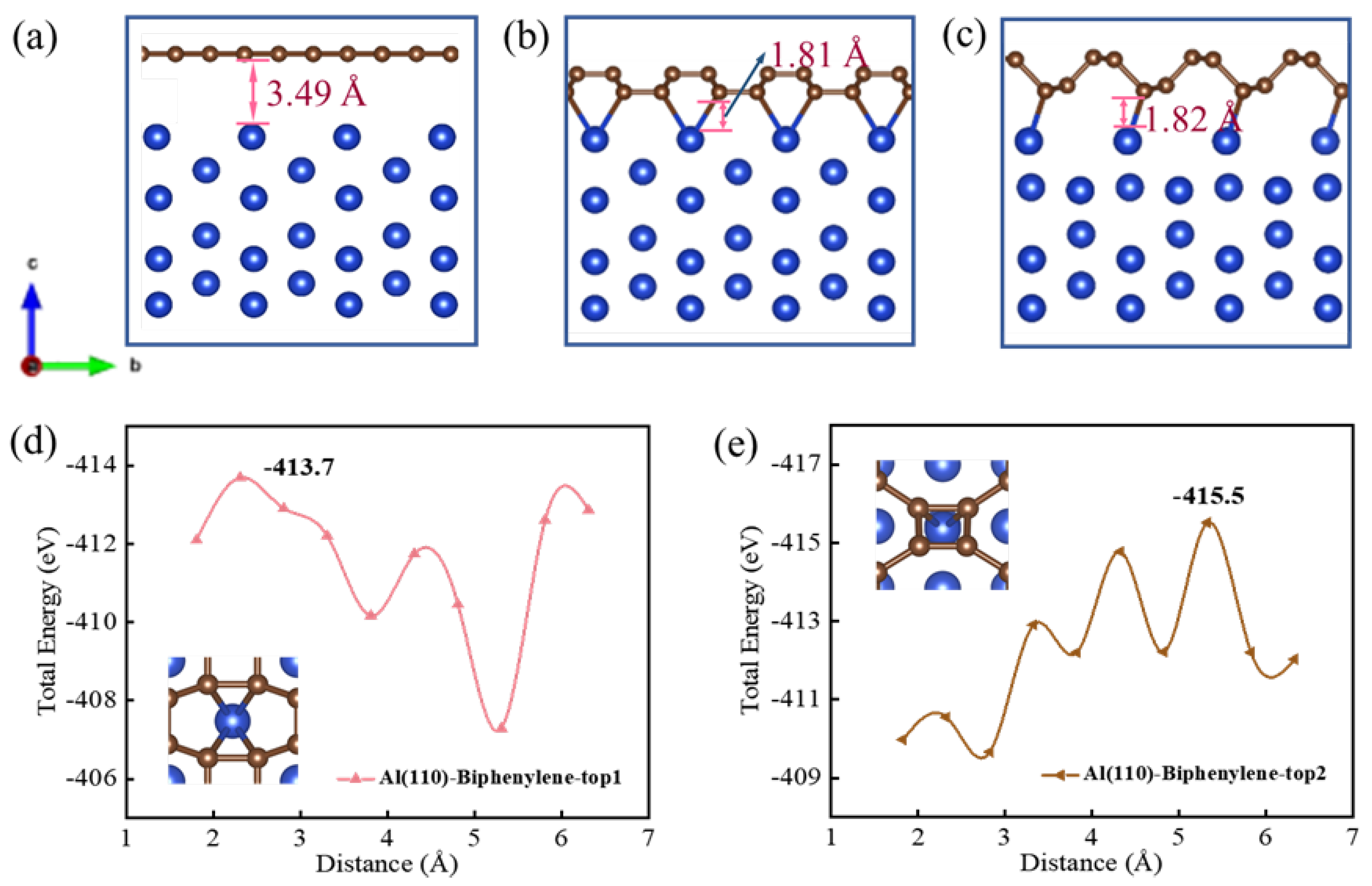
3.7. The Binding Properties of Biphenylene with Aluminum Substrate
4. Conclusions
- (1)
- The particular structure of periodic tetragonal, hexagonal, and octagonal carbon rings endows its stable mechanical properties, ample adsorption sites, strong O atom adsorption, and excellent electronic properties.
- (2)
- Biphenylene coating with a dense oxygen−rich layer and appropriate wettability could isolate the matrix from corrosive media.
- (3)
- The charge transfers of O atoms while adsorbing at biphenylene are +0.477 and +0.420 e, indicating the preferable adsorption properties in oxygen−rich layer construction.
- (4)
- The rigid binding of the biphenylene coating to the aluminum substrate with the energy of 413.7 and 415.5 eV enhances the durability of the anti-corrosion material and its resistance to external interference.
- (5)
- This paper systematically expounds the superior anti−corrosion properties of biphenylene and the potential to replace graphene as a better anti-corrosion coating. The research was based on theoretical calculation; our practical conclusions remain be verified in subsequent work.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Marcus, P.; Oudar, J. Corrosion Mechanisms in Theory and Practice, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Hazlewood, P.E.; Singh, P.M.; Hsieh, J.S. Corrosion behavior of carbon steels in sulfide-containing caustic solutions. Ind. Eng. Chem. Res. 2006, 45, 7789–7794. [Google Scholar] [CrossRef]
- Newman, R.C.; Corcoran, S.G.; Erlebacher, J.; Aziz, M.J.; Sieradzki, K. Alloy corrosion. MRS Bull. 1999, 24, 24–28. [Google Scholar] [CrossRef]
- Chandrappa, K.G.; Venkatesha, T.V. Generation of nanostructured CuO by electrochemical method and its Zn–Ni–CuO composite thin films for corrosion protection. Mater. Corros. 2013, 64, 831–839. [Google Scholar] [CrossRef]
- Szabo, S.; Bakos, I. Cathodic protection with sacrificial anodes. Corros. Rev. 2006, 24, 231–280. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Hintze, P.E.; Calle, L.M. Electrochemical properties and corrosion protection of organosilane self-assembled monolayers on aluminum 2024-T3. Electrochim. Acta 2006, 51, 1761–1766. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Stoloff, N.S. Mater. Iron aluminides: Present status and future prospects. Sci. Eng. A 1998, 258, 1–14. [Google Scholar] [CrossRef]
- Nishida, E.; Miyaji, H.; Takita, H.; Kanayama, I.; Tsuji, M.; Akasaka, T.; Sugaya, T.; Sakagami, R.; Kawanami, M. Graphene oxide coating facilitates the bioactivity of scaffold material for tissue engineering. Jpn. J. Appl. Phys. 2014, 53, 06JD04. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Jena, B.K.; Bhattacharjee, S.; Besra, L. Development of oxidation and corrosion resistance hydrophobic graphene oxide-polymer composite coating on copper. Surf. Coat. Technol. 2013, 232, 475–481. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Yuan, Z.; Rong, J.; Feng, J.; Muzammil, I.; Yu, X.; Zhang, Y.; Zhan, Z. DHQ-graphene: A novel two-dimensional defective graphene for corrosion-resistant coating. J. Mater. Chem. A 2019, 7, 8967–8974. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Hofmann, M.; Chang, K.W.; Jhu, J.G.; Li, Y.Y.; Chen, K.Y.; Yang, C.C.; Chang, W.S.; Chen, L.C. Complete Corrosion Inhibition through Graphene Defect Passivation. ACS Nano 2013, 8, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a long-term metal oxidation barrier: Worse than nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, Z.; Shenoy, G.J.; Li, L.; Liu, H. Enhanced room-temperature corrosion of copper in the presence of graphene. ACS Nano 2013, 7, 6939–6947. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Z.; Rong, J.; Zhang, Y.; Zhong, Y.; Feng, J.; Yu, X.; Zhan, Z. Planar net-τ: A new high-performance metallic carbon anode material for lithium-ion batteries. Carbon 2018, 142, 438–444. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.F.; Zhang, X.; Zhu, Q.; Dong, H.; Zhao, M.; Oganov, A.R. Phagraphene: A low-energy graphene allotrope composed of 5-6-7 carbon rings with distorted Dirac cones. Nano Lett. 2015, 15, 6182–6186. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.H.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Balaban, A.T.; Rentia, C.C.; Ciupitu, E. Chemical graphs. 6. estimation of relative stability of several planar and tridimensional lattices for elementary carbon. Rev. Roum. Chim. 1968, 13, 231–247. [Google Scholar]
- Fan, Q.; Yan, L.; Tripp, M.W.; Krejci, O.; Dimosthenous, S.; Kachel, S.R.; Chen, M.; Foster, A.S.; Koert, U.; Liljeroth, P.; et al. Biphenylene network: A nonbenzenoid carbon allotrope. Science 2021, 372, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yang, R.; Xie, K.; Yu, Z.; Zheng, Y.; Zhang, R.; Chen, L.; Wu, B.R.; Su, W.S.; Wang, S. Electronic and optical properties of hydrogen-terminated biphenylene nanoribbons: A first-principles study. Phys. Chem. Chem. Phys. 2021, 24, 357–365. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogneous electron gas. Phys. Rev. Sect. B 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yue, W. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B Condens. Matter Mater. Phys. 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B Condens. Matter Mater. Phys. 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jonsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ren, C.; Xu, Y.; Yu, J.; Wang, S.; Sun, M. A first principles investigation on the structural, mechanical, electronic, and catalytic properties of biphenylene. Sci. Rep. 2021, 11, 19008. [Google Scholar] [CrossRef] [PubMed]
- Veeravenkata, H.P.; Jain, A. Density functional theory driven phononic thermal conductivity prediction of biphenylene: A comparison with graphene. Carbon 2021, 183, 893–898. [Google Scholar] [CrossRef]
- Liu, T.; Jing, Y.; Li, Y. Two-dimensional biphenylene: A graphene allotrope with superior activity toward electrochemical oxygen reduction reaction. J. Phys. Chem. Lett. 2021, 12, 12230–12234. [Google Scholar] [CrossRef]
- Mak, T.C.W.; Trotter, J. 1. The crystal and molecular structure of biphenylene. J. Chem. Soc. Resumed 1962, 1, 1–8. [Google Scholar] [CrossRef]
- Liao, Y.; Shi, X.Z.; Ouyang, T.; Zhang, C.; Tang, C.; He, C.; Zhong, J. New two-dimensional wide band gap hydrocarbon insulator by hydrogenation of a biphenylene sheet. J. Phys. Chem. Lett. 2021, 12, 8889–8896. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y. Density functional theory study of the silicene-like SiX and XSi3 (X = B, C, N, Al, P) honeycomb lattices: The various buckled structures and versatile electronic properties. J. Phys. Chem. C 2013, 117, 18266–18278. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Jena, P. ψ-Graphene: A new metallic allotrope of planar carbon with potential applications as anode materials for Lithium-Ion batteries. J. Phys. Chem. Lett. 2017, 3234–3241. [Google Scholar] [CrossRef]
- Popov, V.N.; Van Doren, V.E.; Balkanski, M. Elastic properties of single-walled carbon nanotubes. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 114, 395–399. [Google Scholar]
- Sun, T.; Yao, X.; Fabris, S. Effects of thermal electronic excitations on the diffusion of oxygen adatoms on graphene. J. Phys. Chem. A 2016, 120, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Bonn, D.; Eggers, J.; Indekeu, J.; Meunier, J.; Rolley, E. Wetting and spreading. Rev. Mod. Phys. 2009, 81, 739–805. [Google Scholar] [CrossRef]
- Raj, R.; Maroo, S.C.; Wang, E.N. Wettability of graphene. Nano Lett. 2013, 13, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettability and surface free energy of graphene films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef]
- Rafiee, J.; Mi, X.; Gullapalli, H.; Thomas, A.V.; Yavari, F.; Shi, Y.; Ajayan, P.M.; Koratkcar, N.A. Wetting transparency of graphene. Nat. Mater. 2012, 11, 217–222. [Google Scholar] [CrossRef]
- Han, T.; Liu, E.; Li, J.; Zhao, N.; He, C. A bottom-up strategy toward metal nano-particles modified graphene nanoplates for fabricating aluminum matrix composites and interface study. J. Mater. Sci. Technol. 2020, 42, 21–32. [Google Scholar] [CrossRef]


| Ci/O | C1 | C2 | C3 | C4 | C5 | C6 | O |
|---|---|---|---|---|---|---|---|
| e | −0.141 | −0.083 | −0.031 | −0.095 | +0.147 | +0.061 | +0.477 |
| Ci/O | C1 | C2 | C3 | C4 | C5 | C6 | O |
|---|---|---|---|---|---|---|---|
| e | +0.001 | −0.191 | −0.161 | −0.001 | −0.042 | 0.021 | +0.420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, K.; Meng, K.; Rong, J.; Yu, X. Biphenylene: A Two−Dimensional Graphene−Based Coating with Superior Anti−Corrosion Performance. Materials 2022, 15, 5675. https://doi.org/10.3390/ma15165675
Ke K, Meng K, Rong J, Yu X. Biphenylene: A Two−Dimensional Graphene−Based Coating with Superior Anti−Corrosion Performance. Materials. 2022; 15(16):5675. https://doi.org/10.3390/ma15165675
Chicago/Turabian StyleKe, Ke, Kun Meng, Ju Rong, and Xiaohua Yu. 2022. "Biphenylene: A Two−Dimensional Graphene−Based Coating with Superior Anti−Corrosion Performance" Materials 15, no. 16: 5675. https://doi.org/10.3390/ma15165675
APA StyleKe, K., Meng, K., Rong, J., & Yu, X. (2022). Biphenylene: A Two−Dimensional Graphene−Based Coating with Superior Anti−Corrosion Performance. Materials, 15(16), 5675. https://doi.org/10.3390/ma15165675






