Insight into the Structural, Electronic, Elastic, Optical, and Magnetic Properties of Cubic Fluoroperovskites ABF3 (A = Tl, B = Nb, V) Compounds: Probed by DFT
Abstract
:1. Introduction
2. Computational Methodology
3. Results and Discussion
3.1. Structural Properties
3.2. Elastic Properties
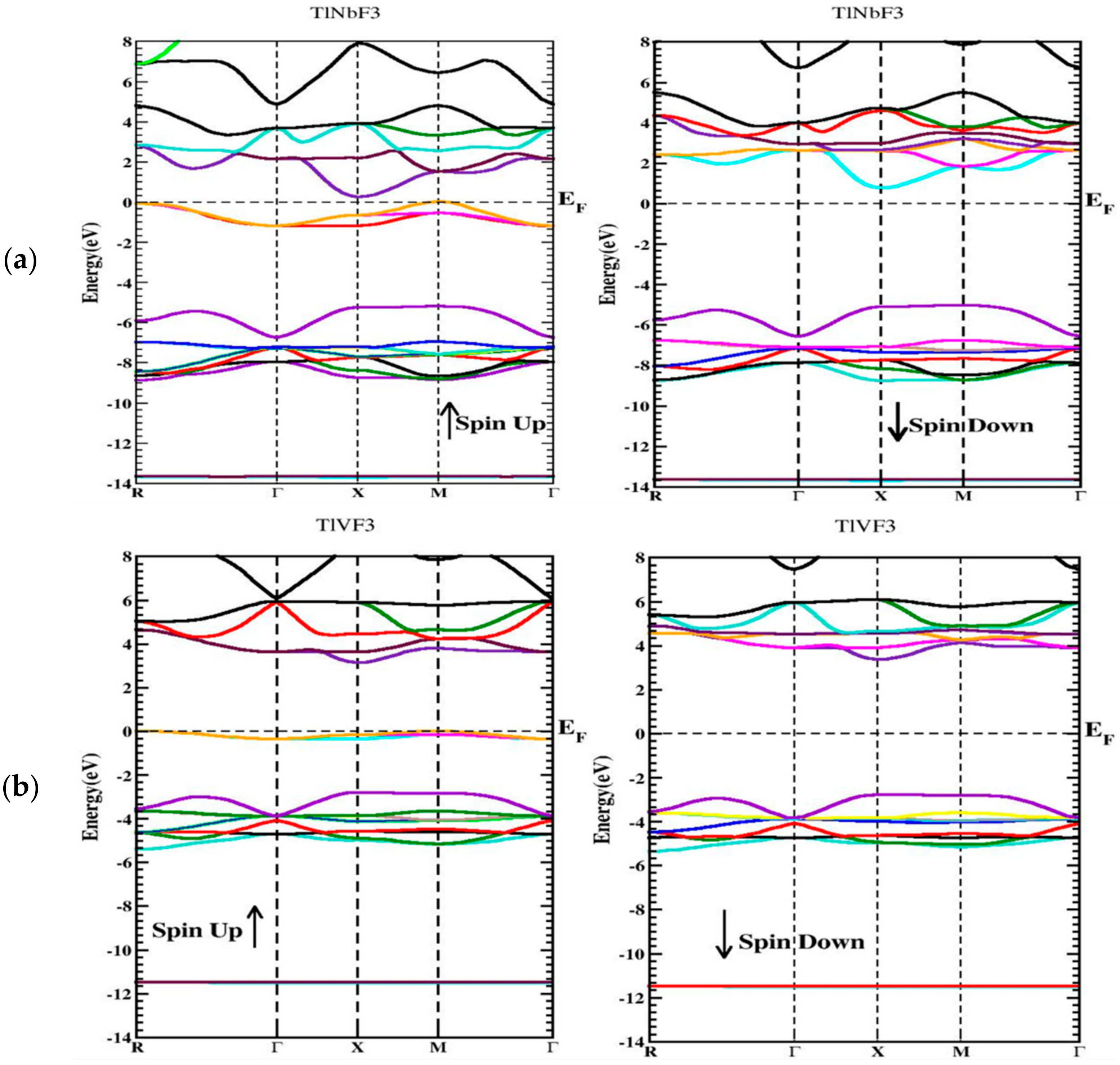
3.3. Electronic Properties
3.4. Magnetic Properties
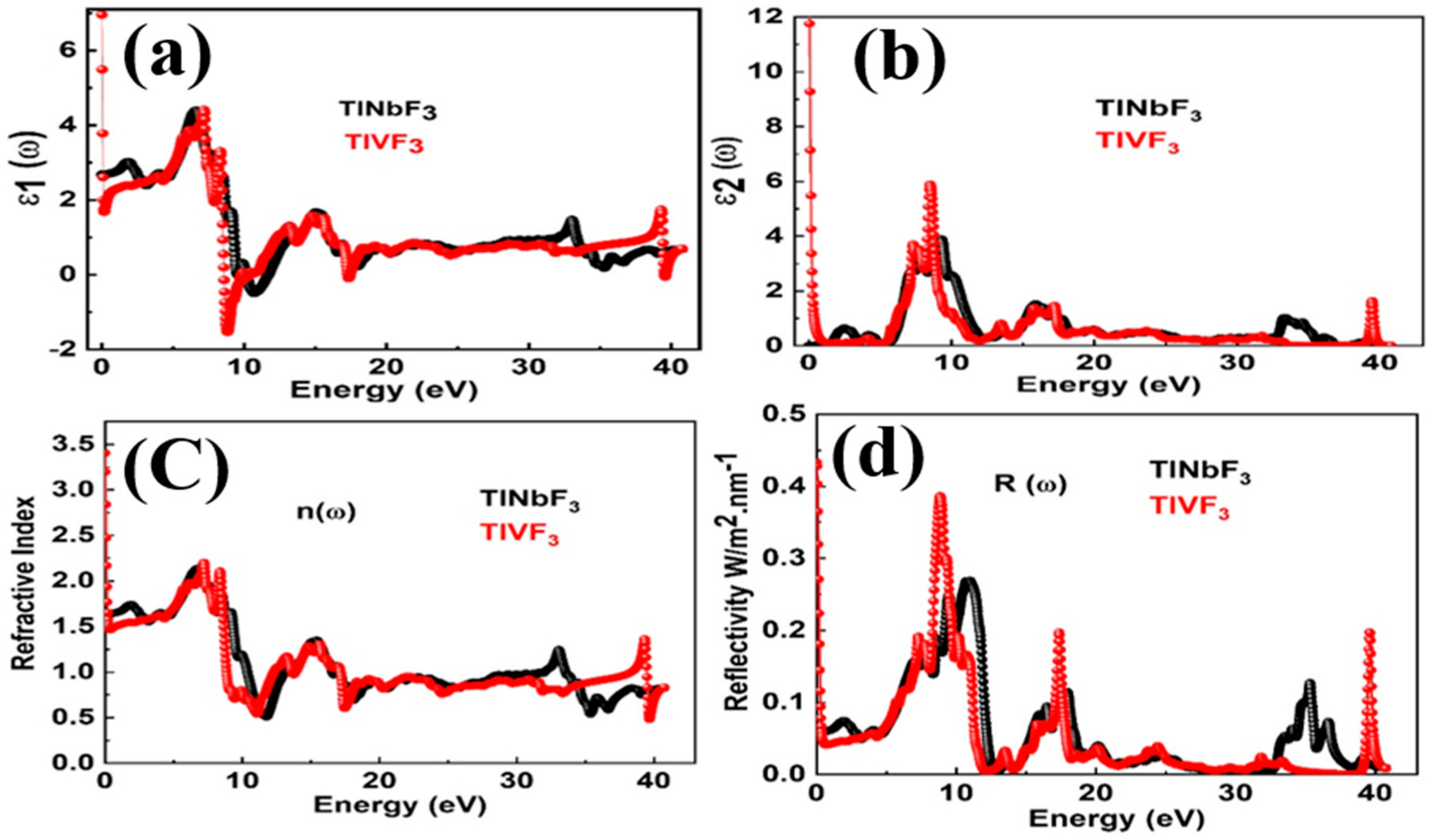
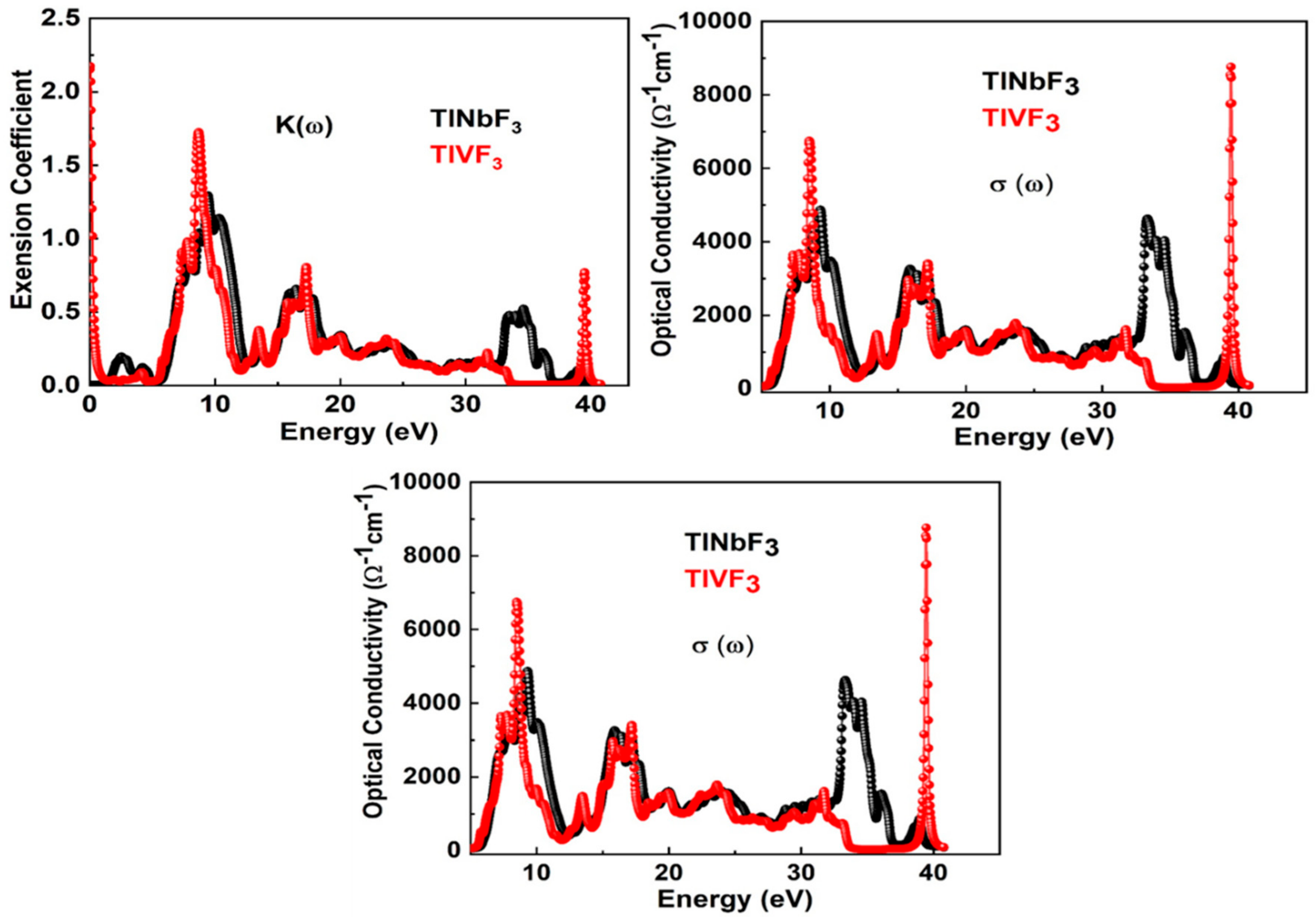
3.5. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, D.A.A.; Bağcı, S.; Karaca, E.; Tütüncü, H.M. Elastic properties of ABF3 (A: Ag, K and B: Mg, Zn) perovskites. In Proceedings of the AIP Conference Proceedings, Maharashtra, India, 5–6 July 2018; Volume 2042, p. 20035. [Google Scholar]
- Boyer, L.L.; Edwardson, P.J. Perovskite to antiperovskite in abf3 compounds. Ferroelectrics 1990, 104, 417–422. [Google Scholar] [CrossRef]
- Bouich, A.; Marí-Guaita, J.; Sahraoui, B.; Palacios, P.; Marí, B. Tetrabutylammonium (TBA)-Doped Methylammonium Lead Iodide: High Quality and Stable Perovskite Thin Films. Front. Energy Res. 2022, 10, 840817. [Google Scholar] [CrossRef]
- Arora, G.; Ahuja, G.; Ahuja, U. Electronic and elastic properties of ternary fluoro-perovskite RbCaF3. J. Phys. Conf. Ser. 2021, 1849, 012032. [Google Scholar] [CrossRef]
- Tian, L.; Hu, Z.; Liu, X.; Liu, Z.; Guo, P.; Xu, B.; Xue, Q.; Yip, H.-L.; Huang, F.; Cao, Y. Fluoro-and amino-functionalized conjugated polymers as electron transport materials for perovskite solar cells with improved efficiency and stability. ACS Appl. Mater. Interfaces 2019, 11, 5289–5297. [Google Scholar] [CrossRef]
- Turner, G. Global Renewable Energy Market Outlook 2013. Bloom. New Energy Financ. 2013, 26, 506. [Google Scholar]
- Mahmoud, N.T.; Khalifeh, J.M.; Mousa, A.A. Effects of rare earth element Eu on structural, electronic, magnetic, and optical properties of fluoroperovskite compounds SrLiF3: First principles calculations. Phys. B Condens. Matter 2019, 564, 37–44. [Google Scholar] [CrossRef]
- Reddy, B.V.R.; Ravikumar, R.; Nithya, C.; Gopukumar, S. High performance Nax CoO2 as a cathode material for rechargeable sodium batteries. J. Mater. Chem. A 2015, 3, 18059–18063. [Google Scholar] [CrossRef]
- Rahman, N.; Husain, M.; Yang, J.; Sajjad, M.; Murtaza, G.; Ul Haq, M.; Habib, A.; Zulfiqar; Rauf, A.; Karim, A.; et al. First principle study of structural, electronic, optical and mechanical properties of cubic fluoro-perovskites: (CdXF3, X = Y, Bi). Eur. Phys. J. Plus 2021, 136, 347. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Woodward, P.M. Prediction of the crystal structures of perovskites using the software program SPuDS. Acta Crystallogr. Sect. B Struct. Sci. 2001, 57, 725–738. [Google Scholar] [CrossRef]
- Mubarak, A.; Al-Omari, S. First-principles calculations of two cubic fluoropervskite compounds: RbFeF3 and RbNiF3. J. Magn. Magn. Mater. 2015, 382, 211–218. [Google Scholar] [CrossRef]
- Samanta, S.; Saini, S.M. Full-Potential Study of the Electronic and Optical Properties of the Transparent Oxide ZnCo2 O4 by Use of PBE and TB-mBJ Potentials. J. Electron. Mater. 2014, 43, 3659–3665. [Google Scholar] [CrossRef]
- Husain, M.; Rahman, N.; Reshak, A.H.; Habib, A.; Ali, S.; Laref, A.; Al Bakri, A.M.; Bila, J. Insight into the physical properties of the inter-metallic titanium-based binary compounds. Eur. Phys. J. Plus 2021, 136, 1–10. [Google Scholar] [CrossRef]
- Katsura, T.; Tange, Y. A simple derivation of the Birch–Murnaghan equations of state (EOSs) and comparison with EOSs derived from other definitions of finite strain. Minerals 2019, 9, 745. [Google Scholar] [CrossRef]
- Ravindran, P.; Fast, L.; Korzhavyi, P.A.; Johansson, B.; Wills, J.; Eriksson, O. Density functional theory for calculation of elastic properties of orthorhombic crystals: Application to TiSi2. J. Appl. Phys. 1998, 84, 4891–4904. [Google Scholar] [CrossRef]
- Bockstedte, M.; Kley, A.; Neugebauer, J.; Scheffler, M. Density-functional theory calculations for poly-atomic systems: Electronic structure, static and elastic properties and ab initio molecular dynamics. Comput. Phys. Commun. 1997, 107, 187–222. [Google Scholar] [CrossRef]
- Luo, X.; Wang, B. Structural and elastic properties of LaAlO3 from first-principles calculations. J. Appl. Phys. 2008, 104, 073518. [Google Scholar] [CrossRef]
- Güler, E.; Güler, M. Elastic and mechanical properties of cubic diamond under pressure. Chin. J. Phys. 2015, 53, 195–205. [Google Scholar]
- Okeke, O.U. Computational Study of Oxynitride Based Strong Materials. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2010. [Google Scholar]
- Bandyopadhyay, K. Seismic Anisotropy: Geological Causes and its Implications to Reservoir Geophysics; Stanford University: Stanford, CA, USA, 2009; Available online: https://www.worldcat.org/title/seismic-anisotropy-geological-causes-and-its-implications-to-reservoir-geophysics/oclc/745998864 (accessed on 14 June 2022).
- Man, C.-S.; Huang, M. A simple explicit formula for the Voigt-Reuss-Hill average of elastic polycrystals with arbitrary crystal and texture symmetries. J. Elast. 2011, 105, 29–48, Erratum in J. Elast. 2012, 106, 105. [Google Scholar] [CrossRef]
- Ballato, A. Voigt-Reuss-Hill moduli for ferroelectric aggregates. In Proceedings of the 2006 15th IEEE International Symposium on the Applications of Ferroelectrics, Sunset Beach, NC, USA, 30 July–2 August 2006; pp. 208–211. [Google Scholar]
- Gittes, F.; MacKintosh, F. Dynamic shear modulus of a semiflexible polymer network. Phys. Rev. E 1998, 58, R1241. [Google Scholar] [CrossRef]
- Li, C.-X.; Duan, Y.-H.; Hu, W.-C. Electronic structure, elastic anisotropy, thermal conductivity and optical properties of calcium apatite Ca5 (PO4) 3X (X = F, Cl or Br). J. Alloys Compd. 2015, 619, 66–77. [Google Scholar] [CrossRef]
- Haines, J.; Leger, J.; Bocquillon, G. Synthesis and design of superhard materials. Annu. Rev. Mater. Res. 2001, 31, 1–23. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Adachi, H.; Takakura, N. Effects of plastic strain and strain path on youngs modulus of sheet metals. Met. Mater. 1998, 4, 420–425. [Google Scholar] [CrossRef]



| Compounds | ao | B (GPa) | B′ (GPa) | Vo (a.u)3 |
|---|---|---|---|---|
| TlVF3 | 4.638 | 63.429 | 4.272 | 513.37 |
| TlNbF3 | 4.372 | 109.561 | 5.544 | 616.26 |
| Compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TlVF3 | 131.3907 | 27.3113 | −3.8737 | 63.429 | −0.0744 | 5.8491 | 17.0243 | 0.6725 | 10.8441 |
| TlNbF3 | 168.0201 | 77.5591 | −17.2098 | 109.561 | −0.3804 | −15.332 | −48.2495 | 0.8816 | 7.1454 |
| Site | TlNbF3 | TlVF3 |
|---|---|---|
| Tl | 0.076 | 0.083 |
| F | 0.087 | 0.068 |
| V | 0 | 2.47 |
| Nb | 3.063 | 0 |
| Interstitial site | 0.894 | 0.673 |
| Total | 4.12 | 3.294 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.A.; Husain, M.; Rahman, N.; Sohail, M.; Khan, R.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O.; Abu El Maati, L.; Yessoufou, K.; et al. Insight into the Structural, Electronic, Elastic, Optical, and Magnetic Properties of Cubic Fluoroperovskites ABF3 (A = Tl, B = Nb, V) Compounds: Probed by DFT. Materials 2022, 15, 5684. https://doi.org/10.3390/ma15165684
Shah SA, Husain M, Rahman N, Sohail M, Khan R, Alataway A, Dewidar AZ, Elansary HO, Abu El Maati L, Yessoufou K, et al. Insight into the Structural, Electronic, Elastic, Optical, and Magnetic Properties of Cubic Fluoroperovskites ABF3 (A = Tl, B = Nb, V) Compounds: Probed by DFT. Materials. 2022; 15(16):5684. https://doi.org/10.3390/ma15165684
Chicago/Turabian StyleShah, Saima Ahmad, Mudasser Husain, Nasir Rahman, Mohammad Sohail, Rajwali Khan, Abed Alataway, Ahmed Z. Dewidar, Hosam O. Elansary, Lamia Abu El Maati, Kowiyou Yessoufou, and et al. 2022. "Insight into the Structural, Electronic, Elastic, Optical, and Magnetic Properties of Cubic Fluoroperovskites ABF3 (A = Tl, B = Nb, V) Compounds: Probed by DFT" Materials 15, no. 16: 5684. https://doi.org/10.3390/ma15165684








