Development of Environmentally Clean Construction Materials Using Industrial Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Mixing Combinations
2.3. Methods
3. Results and Discussion
3.1. Raw Materials Characterization
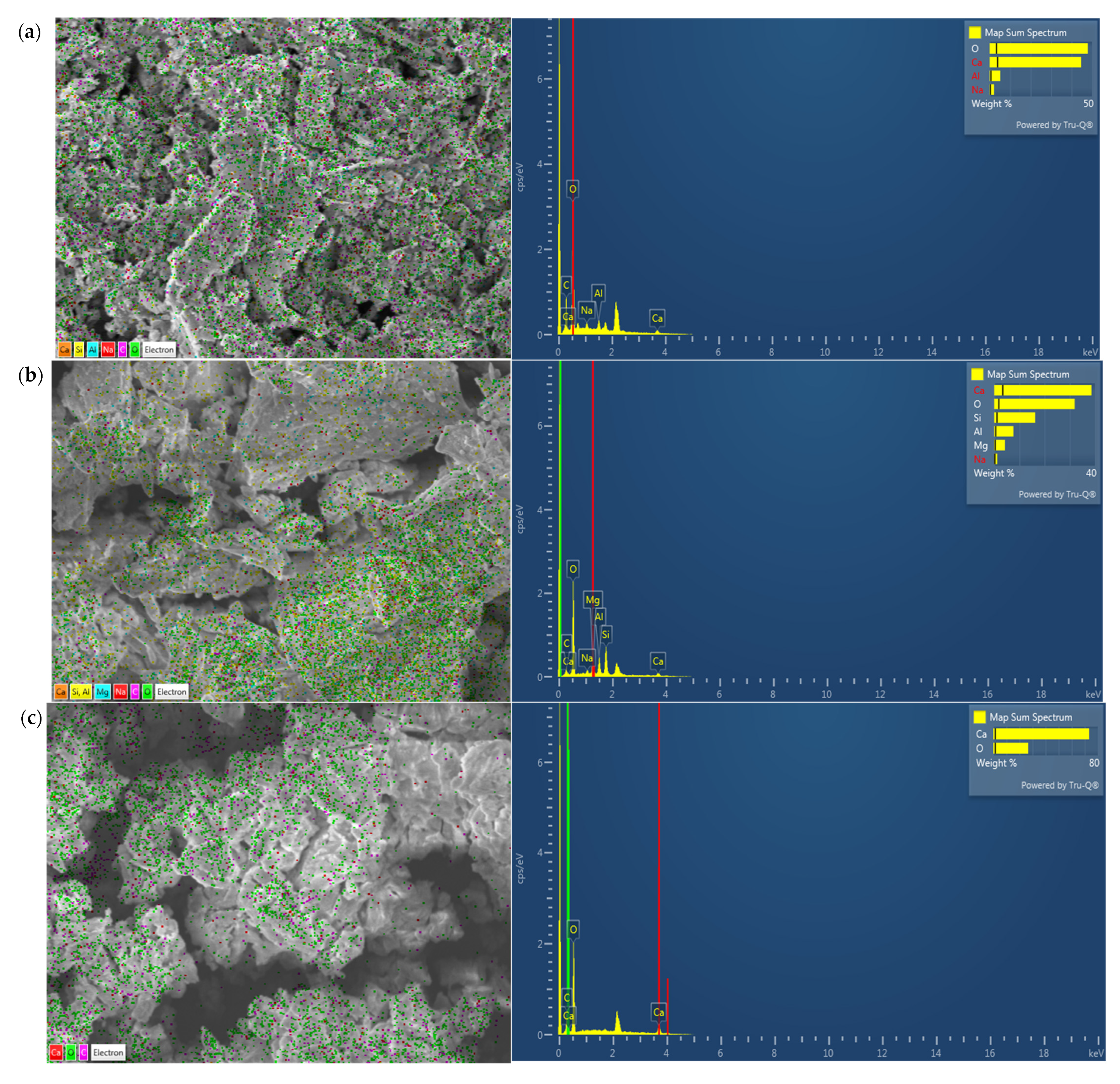
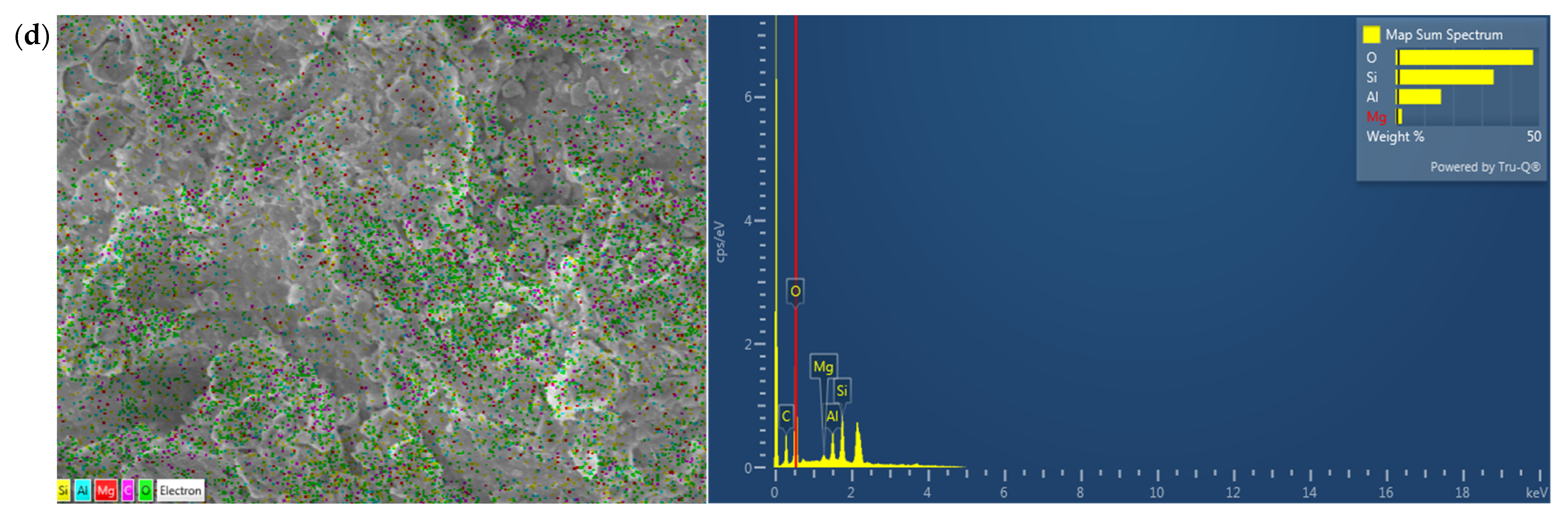
3.1.1. Chemical Composition
3.1.2. Structure of the Components
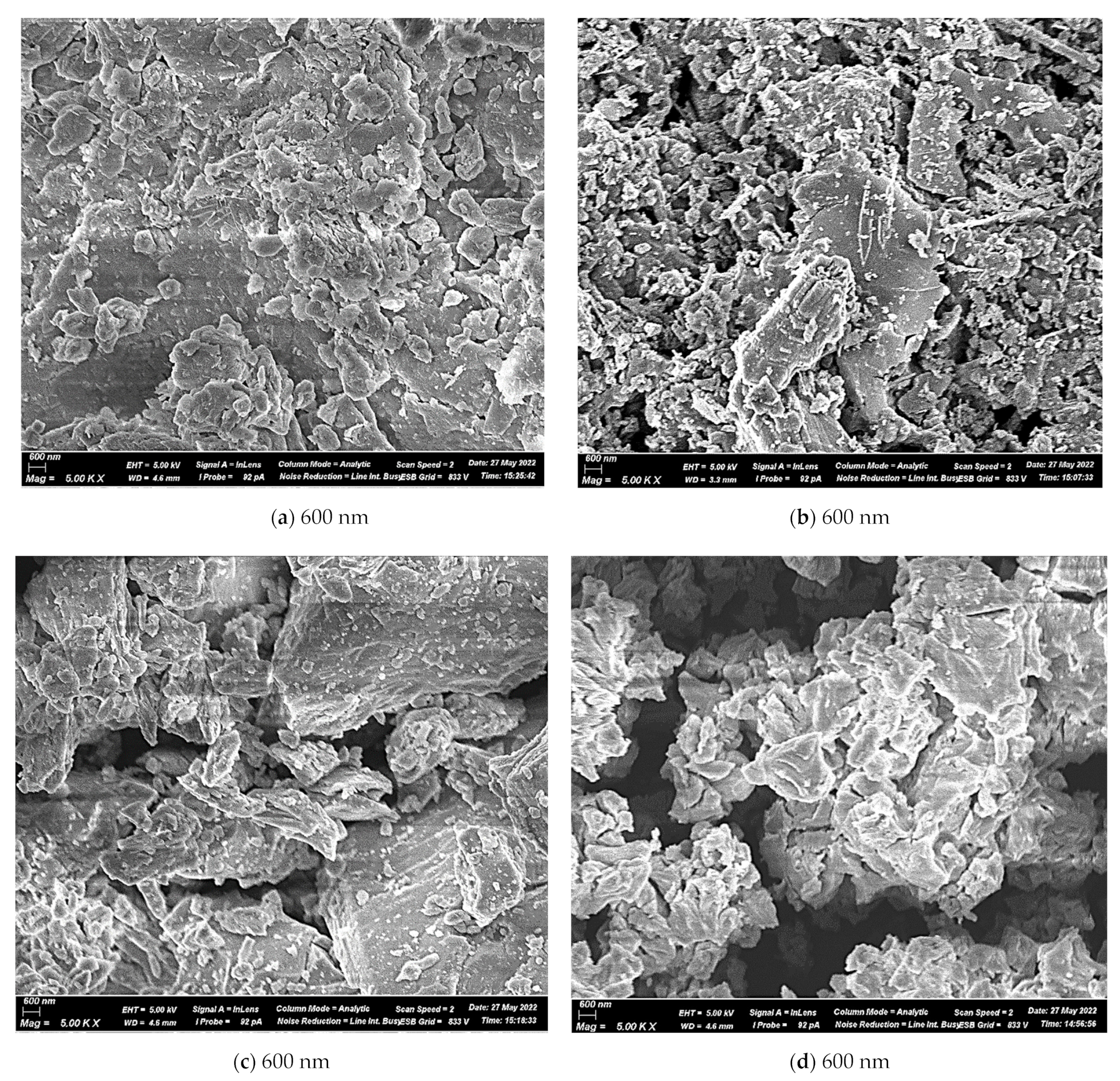
3.1.3. The Morphological Structure of the Components
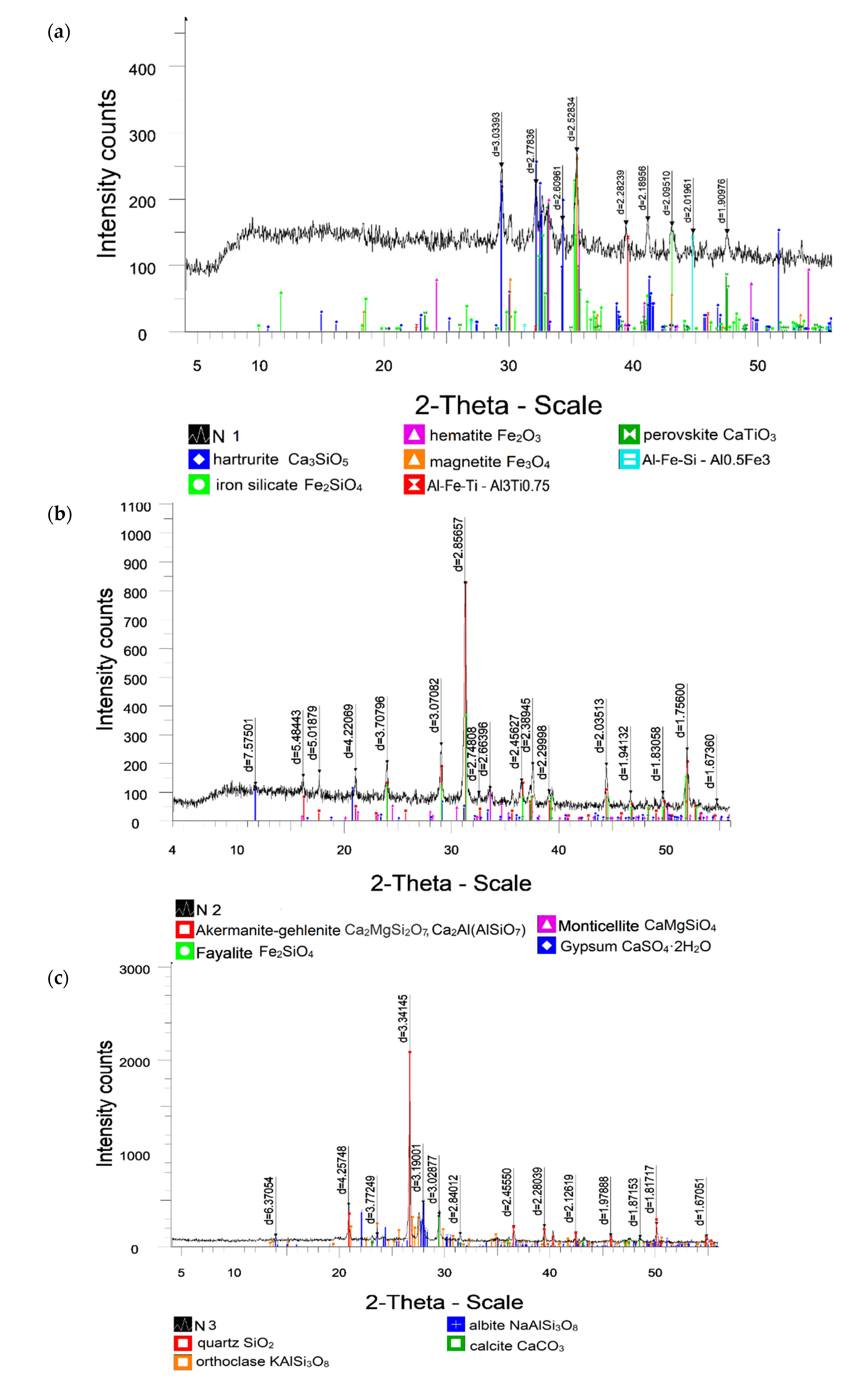
3.1.4. Mineral Composition
3.2. Physical and Mechanical Properties of the Developed Materials
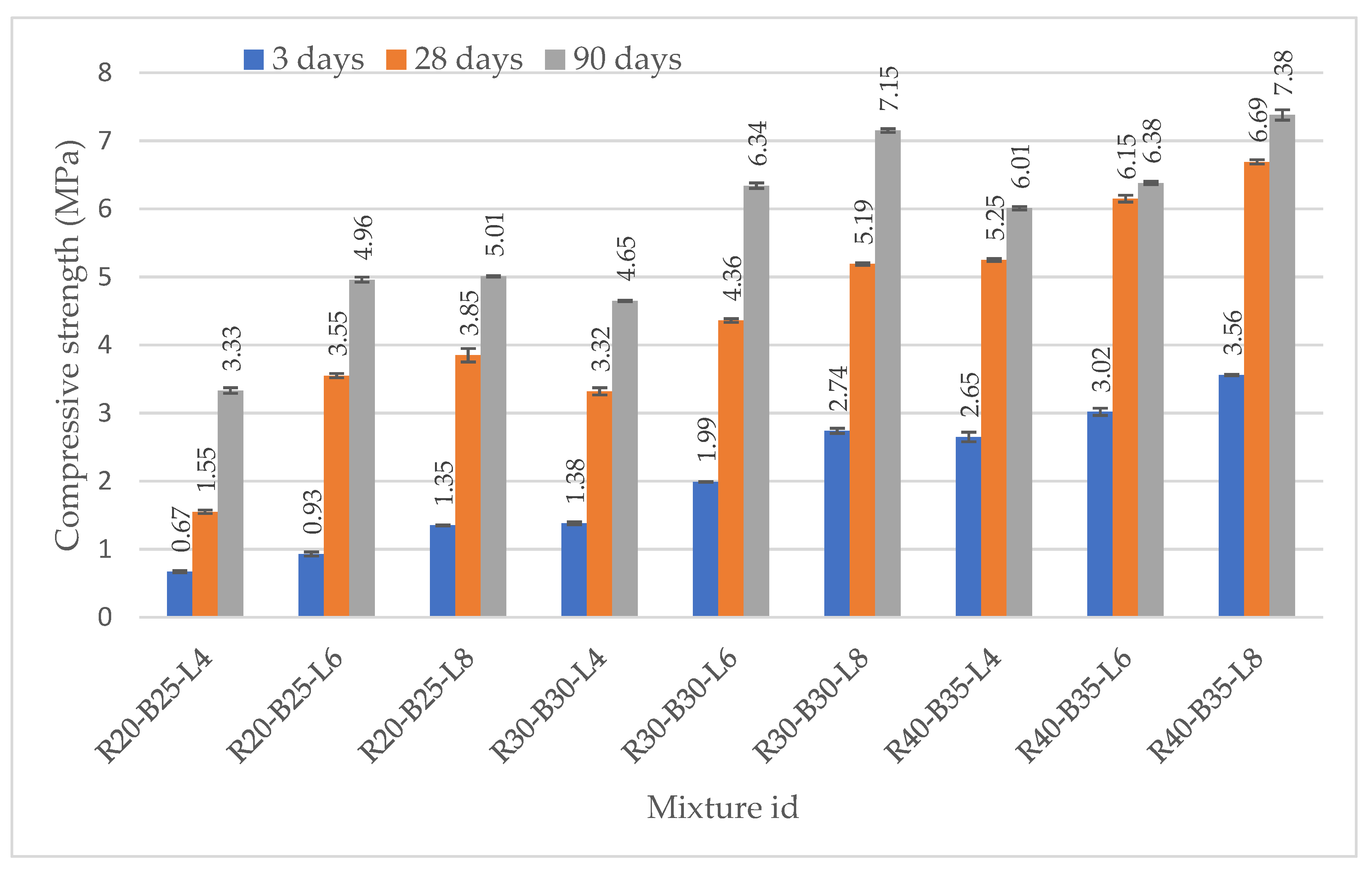
3.2.1. Axial Resistance of Test Specimens
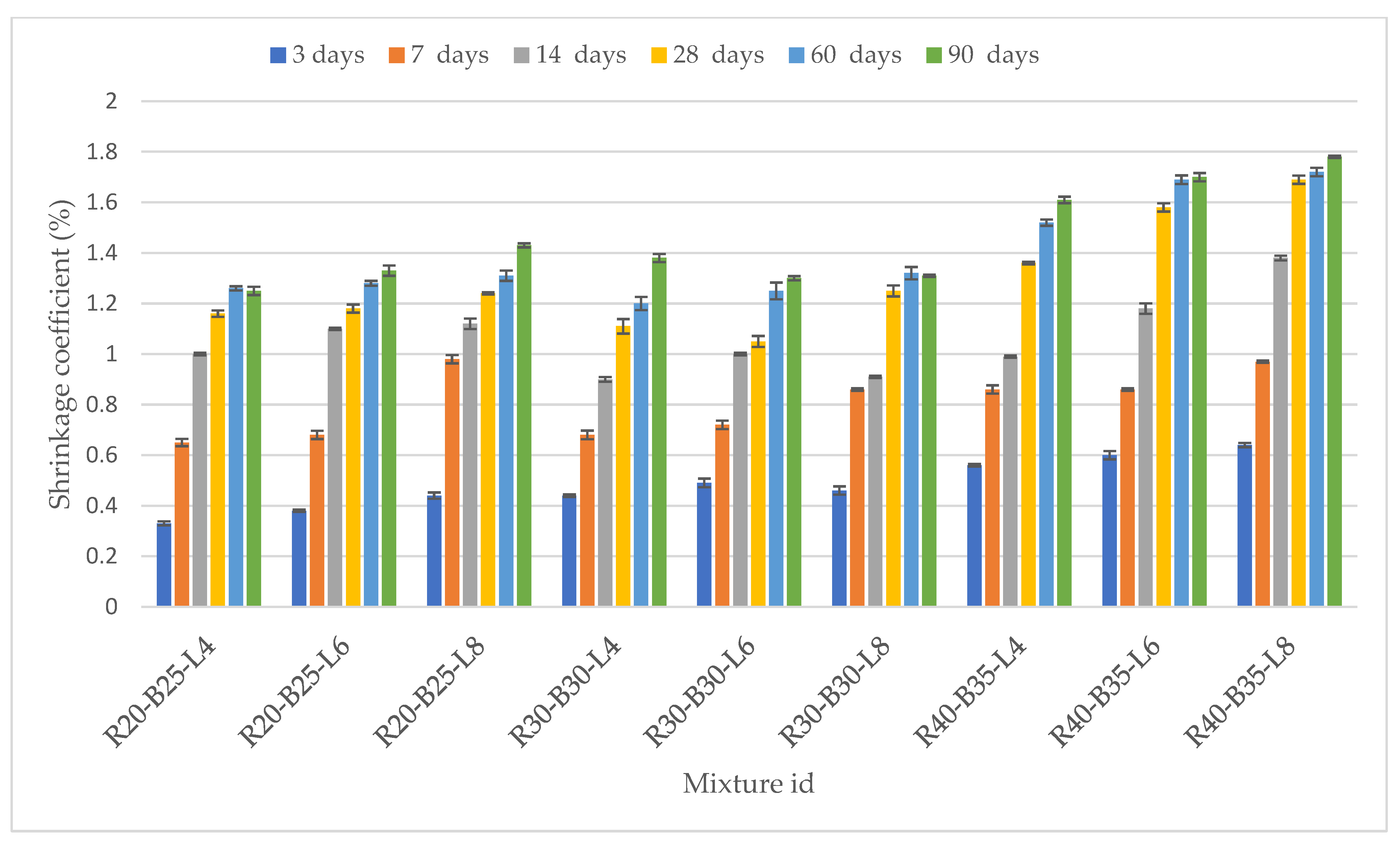
3.2.2. Coefficient of Linear Dilatation and Shrinkage of Samples
3.2.3. Water and Frost Resistance of the Materials
3.2.4. Changes in the Carbonate Content of Materials during Hydration of Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RM | Red mud |
| BFS | Blast furnace slag |
| SS | Steel slag |
| NL | Natural loam |
| LPW | Lime production waste |
| SEM | Scanning electron microscope |
| XRD | X-ray Diffraction |
| XRF | X-ray fluorescence |
| EDS | Energy dispersive spectroscopy |
| AAS | Atomic absorption spectroscopy |
References
- Kehagia, F. Construction of an Unpaved Road Using Industrial By-Products (Bauxite Residue). WSEAS Trans. Environ. Dev. 2014, 10, 160–168. [Google Scholar]
- Mohammadhosseini, H.; Ngian, S.P.; Alyousef, R.; Tahir, M.M. Synergistic Effects of Waste Plastic Food Tray as Low-Cost Fibrous Materials and Palm Oil Fuel Ash on Transport Properties and Drying Shrinkage of Concrete. J. Build. Eng. 2021, 42, 102826. [Google Scholar] [CrossRef]
- Senff, L.; Hotza, D.; Labrincha, J.A. Effect of Red Mud Addition on the Rheological Behaviour and on Hardened State Characteristics of Cement Mortars. Constr. Build. Mater. 2011, 25, 163–170. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Guan, X. An Active Dealkalization of Red Mud with Roasting and Water Leaching. J. Hazard. Mater. 2015, 286, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The Composition, Recycling and Utilisation of Bayer Red Mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Sabri, M.M.; Shashkin, K.G. Improvement of the Soil Deformation Modulus Using an Expandable Polyurethane Resin. Mag. Civ. Eng. 2018, 83, 222–234. [Google Scholar] [CrossRef]
- Sabri, M.M.; Shashkin, K.G.; Zakharin, E.; Ulybin, A.V. Soil Stabilization and Foundation Restoration Using an Expandable Polyurethane Resin. Mag. Civ. Eng. 2018, 82, 68–80. [Google Scholar] [CrossRef]
- Sabri, M.; Bugrov, A.; Panov, S.; Davidenko, V. Ground Improvement Using an Expandable Polyurethane Resin. In Proceedings of the MATEC Web of Conferences, Saint-Petersburg, Russia, 19–20 November 2018; Volume 245. [Google Scholar]
- Sabri, M.M.; Shashkin, K.G. The Mechanical Properties of the Expandable Polyurethane Resin Based on Its Volumetric Expansion Nature. Mag. Civ. Eng. 2020, 98, 9811. [Google Scholar] [CrossRef]
- Sabri, M.M.S.; Vatin, N.I.; Alsaffar, K.A.M. Soil Injection Technology Using an Expandable Polyurethane Resin: A Review. Polymers 2021, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yao, W.; Zheng, K.; Cui, N.; Xie, N. Synergistically Using Bauxite Residue (Red Mud) and Other Solid Wastes to Manufacture Eco-Friendly Cementitious Materials. Buildings 2022, 12, 117. [Google Scholar] [CrossRef]
- Kong, X.; Guo, Y.; Xue, S.; Hartley, W.; Wu, C.; Ye, Y.; Cheng, Q. Natural Evolution of Alkaline Characteristics in Bauxite Residue. J. Clean. Prod. 2017, 143, 224–230. [Google Scholar] [CrossRef]
- Somlai, J.; Jobbágy, V.; Kovács, J.; Tarján, S.; Kovács, T. Radiological Aspects of the Usability of Red Mud as Building Material Additive. J. Hazard. Mater. 2008, 150, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wang, N.; Liu, S. Radiological Restrictions of Using Red Mud as Building Material Additive. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2012, 30, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y. The Neutralization and Recycling of Red Mud-a Review. J. Phys. Conf. Ser. 2021, 1759, 012004. [Google Scholar] [CrossRef]
- Paramguru, R.K.; Rath, P.C.; Misra, V.N. Trends in Red Mud Utilization—A Review. Miner. Process. Extr. Metall. Rev. 2005, 26, 1–29. [Google Scholar] [CrossRef]
- Deelwal, K.; Dharavath, K.; Kulshreshtha, M. Stabilization of Red Mud by Lime, Gypsum and Investigating Its Possible Use As a Geotechnical Material in the Civil Construction. Int. J. Adv. Eng. Technol. 2014, 7, 1238–1244. [Google Scholar]
- Zhang, J.; Yao, Z.; Wang, K.; Wang, F.; Jiang, H.; Liang, M.; Wei, J.; Airey, G. Sustainable Utilization of Bauxite Residue (Red Mud) as a Road Material in Pavements: A Critical Review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Jitsangiam, P.; Nikraz, H. Sustainable Use of Coarse Bauxite Residue for Alternative Roadway Construction Materials. Aust. J. Civ. Eng. 2013, 11, 1–12. [Google Scholar] [CrossRef]
- Mukiza, E.; Liu, X.; Zhang, L.; Zhang, N. Preparation and Characterization of a Red Mud-Based Road Base Material: Strength Formation Mechanism and Leaching Characteristics. Constr. Build. Mater. 2019, 220, 297–307. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Xu, Y.; Tang, B.; Wang, Y.; Mukiza, E. Synergic Effects of Electrolytic Manganese Residue-Red Mud-Carbide Slag on the Road Base Strength and Durability Properties. Constr. Build. Mater. 2019, 220, 364–374. [Google Scholar] [CrossRef]
- O’Connor, J.; Nguyen, T.B.T.; Honeyands, T.; Monaghan, B.; O’Dea, D.; Rinklebe, J.; Vinu, A.; Hoang, S.A.; Singh, G.; Kirkham, M.B.; et al. Production, Characterisation, Utilisation, and Beneficial Soil Application of Steel Slag: A Review. J. Hazard. Mater. 2021, 419, 126478. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hou, H.; Zhu, S. Characteristics and Mechanisms of Phosphate Adsorption onto Basic Oxygen Furnace Slag. J. Hazard. Mater. 2009, 162, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and Environmental Aspects of Slag: A Review; Elsevier: Amsterdam, The Netherlands, 2015; Volume 57, ISBN 7036486252. [Google Scholar]
- Bayless, E.R.; Bullen, T.D.; Fitzpatrick, J.A. Use of 87Sr/86Sr and Δ11B to Identify Slag-Affected Sediment in Southern Lake Michigan. Environ. Sci. Technol. 2004, 38, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Yan, X.; Tu, C.; Yu, Z. Environmental Risks for Application of Iron and Steel Slags in Soils in China: A Review. Pedosphere 2021, 31, 28–42. [Google Scholar] [CrossRef]
- Wang, D.L.; Chen, M.L.; Tsang, D.D.C.W. Chapter 5—Green Remediation by Using Low-Carbon Cement-Based Stabilization/Solidification Approaches. In Sustainable Remediation of Contaminated Soil and Groundwater; Hou, D., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 93–118. ISBN 978-0-12-817982-6. [Google Scholar]
- Kambole, C.; Paige-Green, P.; Kupolati, W.K.; Ndambuki, J.M.; Adeboje, A.O. Basic Oxygen Furnace Slag for Road Pavements: A Review of Material Characteristics and Performance for Effective Utilisation in Southern Africa. Constr. Build. Mater. 2017, 148, 618–631. [Google Scholar] [CrossRef]
- Jamshidi, A.; White, G. Evaluation of Performance and Challenges of Use of Waste Materials in Pavement Construction: A Critical Review. Appl. Sci. 2020, 10, 226. [Google Scholar] [CrossRef]
- Zhang, Y.; Ong, Y.J.; Yi, Y. Comparison between CaO- and MgO-Activated Ground Granulated Blast-Furnace Slag (GGBS) for Stabilization/Solidification of Zn-Contaminated Clay Slurry. Chemosphere 2022, 286, 131860. [Google Scholar] [CrossRef]
- Smirnova, O.M.; Potyomkin, D.A. Influence of Ground Granulated Blast Furnace Slag Properties on the Superplasticizers Effect. Int. J. Civ. Eng. Technol. 2018, 9, 874–880. [Google Scholar]
- Wang, Q.; Yan, P.; Mi, G. Effect of Blended Steel Slag-GBFS Mineral Admixture on Hydration and Strength of Cement. Constr. Build. Mater. 2012, 35, 8–14. [Google Scholar] [CrossRef]
- Oti, J.; Kinuthia, J.; Bai, J. Compressive Strength and Microstructural Analysis of Unfired Clay Masonry Bricks. Eng. Geol. 2009, 109, 230–240. [Google Scholar] [CrossRef]
- Samadi, M.; Huseien, G.F.; Lim, N.H.A.S.; Mohammadhosseini, H.; Alyousef, R.; Mirza, J.; Rahman, A.B.A. Enhanced Performance of Nano-Palm Oil Ash-Based Green Mortar against Sulphate Environment. J. Build. Eng. 2020, 32, 101640. [Google Scholar] [CrossRef]
- Benjeddou, O.; Alyousef, R.; Mohammadhosseini, H.; Soussi, C.; Khadimallah, M.A.; Alabduljabbar, H.; Tahir, M.M. Utilisation of Waste Marble Powder as Low-Cost Cementing Materials in the Production of Mortar. J. Build. Eng. 2020, 32, 101642. [Google Scholar] [CrossRef]
- Mymrin, V.; Aibuldinov, E.K.; Alekseev, K.; Petukhov, V.; Avanci, M.A.; Kholodov, A.; Taskin, A.; Catai, R.E.; Iarozinski, A. Efficient Road Base Material from Kazakhstan’s Natural Loam Strengthened by Ground Cooled Ferrous Slag Activated by Lime Production Waste. J. Clean. Prod. 2019, 231, 1428–1436. [Google Scholar] [CrossRef]
- Asaad, M.A.; Huseien, G.F.; Memon, R.P.; Ghoshal, S.K.; Mohammadhosseini, H.; Alyousef, R. Enduring Performance of Alkali-Activated Mortars with Metakaolin as Granulated Blast Furnace Slag Replacement. Case Stud. Constr. Mater. 2022, 16, e00845. [Google Scholar] [CrossRef]
- Obuzor, G.N.; Kinuthia, J.M.; Robinson, R.B. Enhancing the Durability of Flooded Low-Capacity Soils by Utilizing Lime-Activated Ground Granulated Blastfurnace Slag (GGBS). Eng. Geol. 2011, 123, 179–186. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C. Experimental Study on Lime and Fly Ash–Stabilized Sintered Red Mud in Road Base. J. Test. Eval. 2018, 46, 2022. [Google Scholar] [CrossRef]
- Kumar Sharma, N. Utilization of Fly Ash, Lime Sludge and Polypropylene Fiber as Stabilizers to Enhance Soil Properties. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Siddika, A.; Al Mamun, M.A.; Alyousef, R.; Mohammadhosseini, H. State-of-the-Art-Review on Rice Husk Ash: A Supplementary Cementitious Material in Concrete. J. King Saud Univ. Eng. Sci. 2021, 33, 294–307. [Google Scholar] [CrossRef]
- Mymrin, V.; Alekseev, K.; Fortini, O.M.; Aibuldinov, Y.K.; Pedroso, C.L.; Nagalli, A.; Winter, E.; Catai, R.E.; Costa, E.B.C. Environmentally Clean Materials from Hazardous Red Mud, Ground Cooled Ferrous Slag and Lime Production Waste. J. Clean. Prod. 2017, 161, 376–381. [Google Scholar] [CrossRef]
- Mymrin, V.; Alekseev, K.; Catai, R.E.; Nagalli, A.; Aibuldinov, Y.K.; Bekturganov, N.S.; Rose, J.L.; Izzo, R.L.S. Red Ceramics from Composites of Hazardous Sludge with Foundry Sand, Glass Waste and Acid Neutralization Salts. J. Environ. Chem. Eng. 2016, 4, 753–761. [Google Scholar] [CrossRef]
- Collins, R.N.; Clark, M.W.; Payne, T.E. Solid Phases Responsible for MnII, CrIII, CoII, Ni, CuII and Zn Immobilization by a Modified Bauxite Refinery Residue (Red Mud) at PH 7.5. Chem. Eng. J. 2014, 236, 419–429. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Li, Z.; Gao, Y.; Liu, C. Feasibility Study of Red Mud-Blast Furnace Slag Based Geopolymeric Grouting Material: Effect of Superplasticizers. Constr. Build. Mater. 2021, 267, 120910. [Google Scholar] [CrossRef]
- Akıncı, A.; Artir, R. Characterization of Trace Elements and Radionuclides and Their Risk Assessment in Red Mud. Mater. Charact. 2008, 59, 417–421. [Google Scholar] [CrossRef]
- Qi, X.; Wang, H.; Huang, C.; Zhang, L.; Zhang, J.; Xu, B.; Li, F.; Araruna, J.T. Analysis of Bauxite Residue Components Responsible for Copper Removal and Related Reaction Products. Chemosphere 2018, 207, 209–217. [Google Scholar] [CrossRef]
- Pu, H.; Mastoi, A.K.; Chen, X.; Song, D.; Qiu, J.; Yang, P. An Integrated Method for the Rapid Dewatering and Solidification/Stabilization of Dredged Contaminated Sediment with a High Water Content. Front. Environ. Sci. Eng. 2021, 15, 67. [Google Scholar] [CrossRef]
- Duan, W.; Wang, D.; Wang, Z.; Zhan, Y.; Mu, T.; Yu, Q. A Novel Synergistic Method on Potential Green and High Value-Added Utilization of Blast Furnace Slag. J. Clean. Prod. 2021, 329, 129804. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Guan, Y.; Wang, C.; Ge, Z.; Sun, R.; Ling, Y.; Šavija, B. Statistical Mixture Design for Carbide Residue Activated Blast Furnace Slag Foamed Lightweight Concrete. SSRN Electron. J. 2022, 342, 127840. [Google Scholar] [CrossRef]
- Reddy, P.S.; Reddy, N.G.; Serjun, V.Z.; Mohanty, B.; Das, S.K.; Reddy, K.R.; Rao, B.H. Properties and Assessment of Applications of Red Mud (Bauxite Residue): Current Status and Research Needs; Springer: Dordrecht, The Netherlands, 2021; Volume 12, ISBN 0123456789. [Google Scholar]
- Kannan, P.; Banat, F.; Hasan, S.W.; Abu Haija, M. Neutralization of Bayer Bauxite Residue (Red Mud) by Various Brines: A Review of Chemistry and Engineering Processes. Hydrometallurgy 2021, 206, 105758. [Google Scholar] [CrossRef]
- Swain, B.; Goswami, S.; Das, M. Impact of Mining on Soil Quality: A Case Study from Hingula Opencast Coal Mine, Angul District, Orissa. Geol. Res. 2011, 10, 77–81. [Google Scholar]
- Kempl, J.; Çopuroǧlu, O. EH-PH- and Main Element Analyses of Blast Furnace Slag Cement Paste Pore Solutions Activated with Sodium Monofluorophosphate—Implications for Carbonation and Self-Healing. Cem. Concr. Compos. 2016, 71, 63–76. [Google Scholar] [CrossRef]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite Residue Issues: III. Alkalinity and Associated Chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Long, G.; Li, L.; Li, W.; Ma, K.; Dong, W.; Bai, C.; Zhou, J.L. Enhanced Mechanical Properties and Durability of Coal Gangue Reinforced Cement-Soil Mixture for Foundation Treatments. J. Clean. Prod. 2019, 231, 468–482. [Google Scholar] [CrossRef]
- Gonzalez, J.; Sargent, P.; Ennis, C. Sewage Treatment Sludge Biochar Activated Blast Furnace Slag as a Low Carbon Binder for Soft Soil Stabilisation. J. Clean. Prod. 2021, 311, 127553. [Google Scholar] [CrossRef]
- Vladić Kancir, I.; Serdar, M. Contribution to Understanding of Synergy between Red Mud and Common Supplementary Cementitious Materials. Materials 2022, 15, 1968. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Naidu, R.; Ming, H. Red Mud as an Amendment for Pollutants in Solid and Liquid Phases. Geoderma 2011, 163, 1–12. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Tayeh, B.A.; Isleem, H.F.; de Azevedo, A.R.G.; Ahmed, H.U.; Emad, W. Sustainable Utilization of Red Mud Waste (Bauxite Residue) and Slag for the Production of Geopolymer Composites: A Review. Case Stud. Constr. Mater. 2022, 16, e00994. [Google Scholar] [CrossRef]
- Manjunath, K.V.; Shekhar, H.; Kumar, M.; Kumar, P.; Kumar, R. Stabilization of Black Cotton Soil Using Ground Granulated Blast Furnace Slag. Int. J. Recent Technol. Eng. 2012, 1, 387–390. [Google Scholar]
- Raja, K.; Venkatachalam, S.; Vishnuvardhan, K.; Krishnan, R.S.R.; Selvan, V.T.; Vetriselvan, N. Materials Today: Proceedings A Review on Soil Stabilization Using Rice Husk Ash and Lime Sludge. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Mymrin, V.A.; Alekseev, K.P.; Catai, R.E.; Izzo, R.L.S.; Rose, J.L.; Nagalli, A.; Romano, C.A. Construction Material from Construction and Demolition Debris and Lime Production Wastes. Constr. Build. Mater. 2015, 79, 207–213. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Wu, C.; Li, J.; Wu, S.; Jiang, W.; Wang, X.; Wang, W.; Feng, M. Investigation of Hierarchical Porous Cold Bonded Lightweight Aggregates Produced from Red Mud and Solid-Waste-Based Cementitious Material. Constr. Build. Mater. 2021, 308, 124990. [Google Scholar] [CrossRef]





| Samples | Mix Id | Compositions, wt.% | ||||
|---|---|---|---|---|---|---|
| Natural Loam | Red Mud | Blast Furnace Slag | Lime Production Waste | Moisture Content | ||
| 1 | R20-B25-L4 | 51 | 20 | 25 | 4 | 10–12 |
| 2 | R20-B25-L6 | 49 | 20 | 25 | 6 | 10–12 |
| 3 | R20-B25-L8 | 47 | 20 | 25 | 8 | 10–12 |
| 4 | R30-B30-L4 | 36 | 30 | 30 | 4 | 10–12 |
| 5 | R30-B30-L6 | 34 | 30 | 30 | 6 | 10–12 |
| 6 | R30-B30-L8 | 32 | 30 | 30 | 8 | 10–12 |
| 7 | R40-B35-L4 | 21 | 40 | 35 | 4 | 10–12 |
| 8 | R40-B35-L6 | 19 | 40 | 35 | 6 | 10–12 |
| 9 | R40-B35-L8 | 17 | 40 | 35 | 8 | 10–12 |
| Raw Materials | Elements, wt.% (Average Value) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Mg | Na | Ca | K | Si | S | Al | Ti | Mn | Fe | |
| RM | 40.53 | 0.18 | 1.84 | 28.58 | - | 7.35 | 0.24 | 2.12 | 1.52 | 0.17 | 17.47 |
| BFS | 40.57 | 5.01 | 0.30 | 26.51 | 0.68 | 14.64 | 1.05 | 5.22 | 0.38 | 0.52 | 5.12 |
| LPW | 42.25 | 0.41 | - | 57.07 | - | 0.15 | - | 0.11 | - | - | - |
| NL | 47.28 | 1.55 | 0.45 | 7.78 | 2.81 | 25.26 | - | 8.37 | 0.37 | 0.19 | 5.92 |
| Raw Materials | Oxides, wt.% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | Fe2O3 | CaO | K2O | MgO | MnO | Na2O | SiO2 | SO3 | TiO2 | C.L. * | CO2 ** | Σ | |
| RM | 4.57 | 25.39 | 45.15 | - | 0.33 | 0.26 | 2.81 | 17.59 | 0.67 | 2.90 | 0.33 | 0.86 | 100.00 |
| BFS | 10.02 | 6.70 | 37.75 | 0.84 | 8.42 | 0.68 | 0.41 | 31.86 | 2.67 | 0.64 | 0.65 | 5.65 | 100.00 |
| LPW | 0.26 | - | 98.52 | - | 0.84 | - | - | 0.41 | - | - | 0.16 | 7.77 | 100.00 |
| NL | 16.41 | 7.94 | 11.43 | 3.57 | 2.67 | 0.26 | - | 56.45 | - | 0.65 | 0.62 | 4.86 | 100.00 |
| Metals | RM | BFS | SanPiN * 2.1.7–2010 | ||
|---|---|---|---|---|---|
| Leaching, (mg/L) | Solubility, (mg/L) | Leaching, (mg/L) | Solubility, (mg/L) | ||
| As | 0.39 | <0.001 | 0.20 | <0.001 | 10 |
| Pb | <0.11 | <0.1 | <0.1 | <0.1 | 250 |
| Hg | <0.05 | <0.05 | <0.05 | <0.05 | 15 |
| Cr | <0.1 | <0.01 | <0.01 | <0.001 | 1000 |
| Ba | <0.05 | <0.05 | <0.05 | <0.005 | - |
| Cd | <0.01 | <0.002 | <0.01 | <0.002 | 15 |
| Al | 0.50 | 0.85 | <0.10 | <0.10 | - |
| Cu | <0.05 | <0.05 | <0.05 | <0.05 | 750 |
| Fe | 0.56 | 0.74 | 0.30 | 0.20 | - |
| Ni | <0.05 | <0.05 | <0.05 | <0.05 | 200 |
| Zn | <0.10 | <0.10 | <0.10 | <0.10 | 1750 |
| PH Value of Raw Materials | |||
|---|---|---|---|
| NL | BFS | RM | LPW |
| 7.3 | 8.5 | 9.6 | 12.10 |
| Raw Materials | Granulometric Fractions | |||||||
|---|---|---|---|---|---|---|---|---|
| Mkm | <1 | 1–5 | 6–10 | 11–50 | 51–100 | 101–250 | >250 | |
| RM | Weight % | 2 | 46 | 5 | 20 | 11 | 10 | 6 |
| FS | 10 | 9 | 10 | 42 | 11 | 8 | 10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzhanova, G.Z.; Aibuldinov, Y.K.; Iskakova, Z.B.; Khabidolda, S.M.; Abdiyussupov, G.G.; Omirzak, M.T.; Murali, G.; Vatin, N.I. Development of Environmentally Clean Construction Materials Using Industrial Waste. Materials 2022, 15, 5726. https://doi.org/10.3390/ma15165726
Alzhanova GZ, Aibuldinov YK, Iskakova ZB, Khabidolda SM, Abdiyussupov GG, Omirzak MT, Murali G, Vatin NI. Development of Environmentally Clean Construction Materials Using Industrial Waste. Materials. 2022; 15(16):5726. https://doi.org/10.3390/ma15165726
Chicago/Turabian StyleAlzhanova, Galiya Zhanzakovna, Yelaman Kanatovich Aibuldinov, Zhanar Baktybaevna Iskakova, Saniya Manarbekkyzy Khabidolda, Gaziz Galymovich Abdiyussupov, Madi Toktasynuly Omirzak, Gunasekaran Murali, and Nikolai Ivanovich Vatin. 2022. "Development of Environmentally Clean Construction Materials Using Industrial Waste" Materials 15, no. 16: 5726. https://doi.org/10.3390/ma15165726
APA StyleAlzhanova, G. Z., Aibuldinov, Y. K., Iskakova, Z. B., Khabidolda, S. M., Abdiyussupov, G. G., Omirzak, M. T., Murali, G., & Vatin, N. I. (2022). Development of Environmentally Clean Construction Materials Using Industrial Waste. Materials, 15(16), 5726. https://doi.org/10.3390/ma15165726








