Amine-Modified Carbon Xerogels as Effective Carbon-Based Adsorbents of Anionic Dye from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Samples Characterization
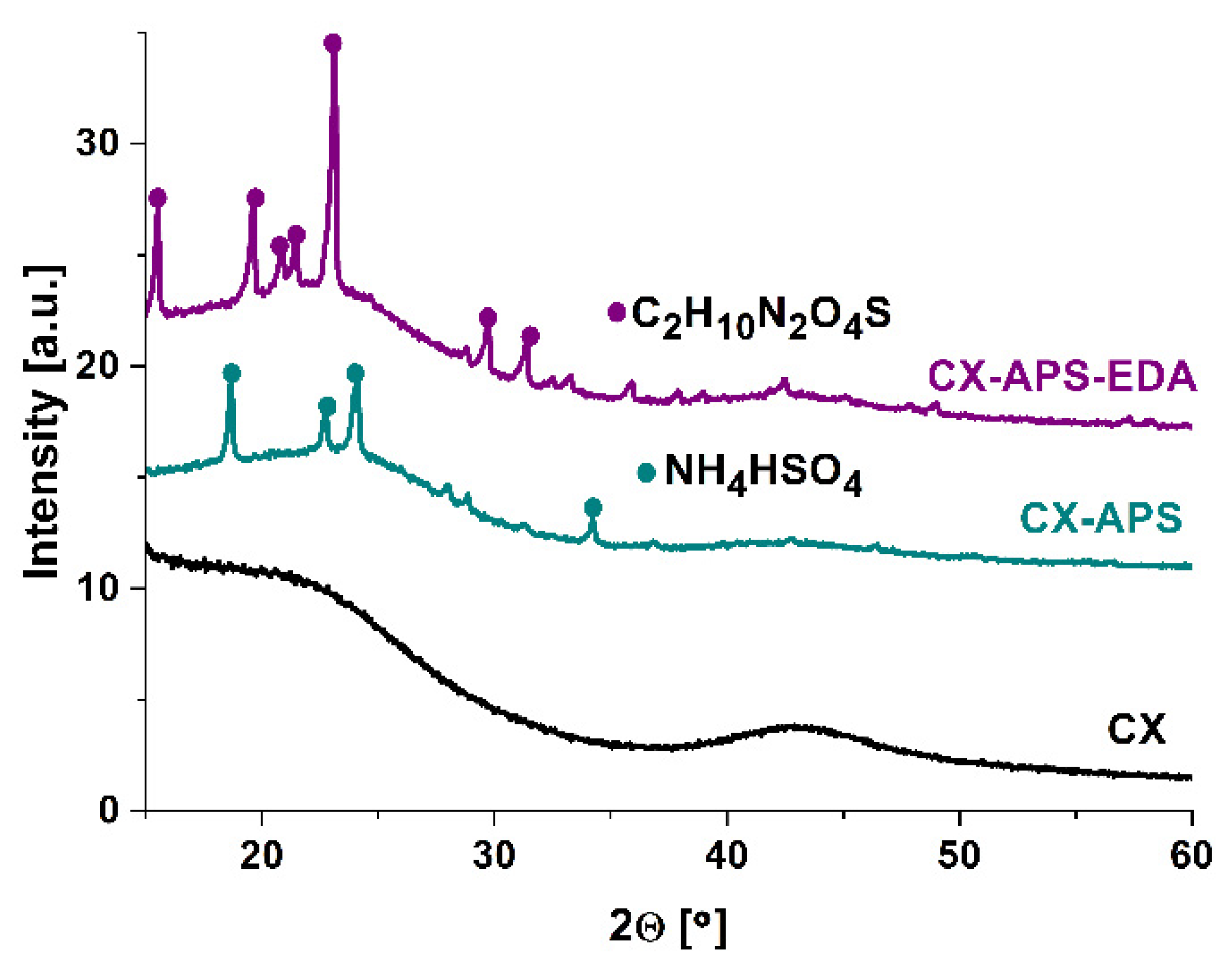
2.2.1. XRD
2.2.2. Nitrogen Sorption, Elemental Analysis and Acid–Base Properties
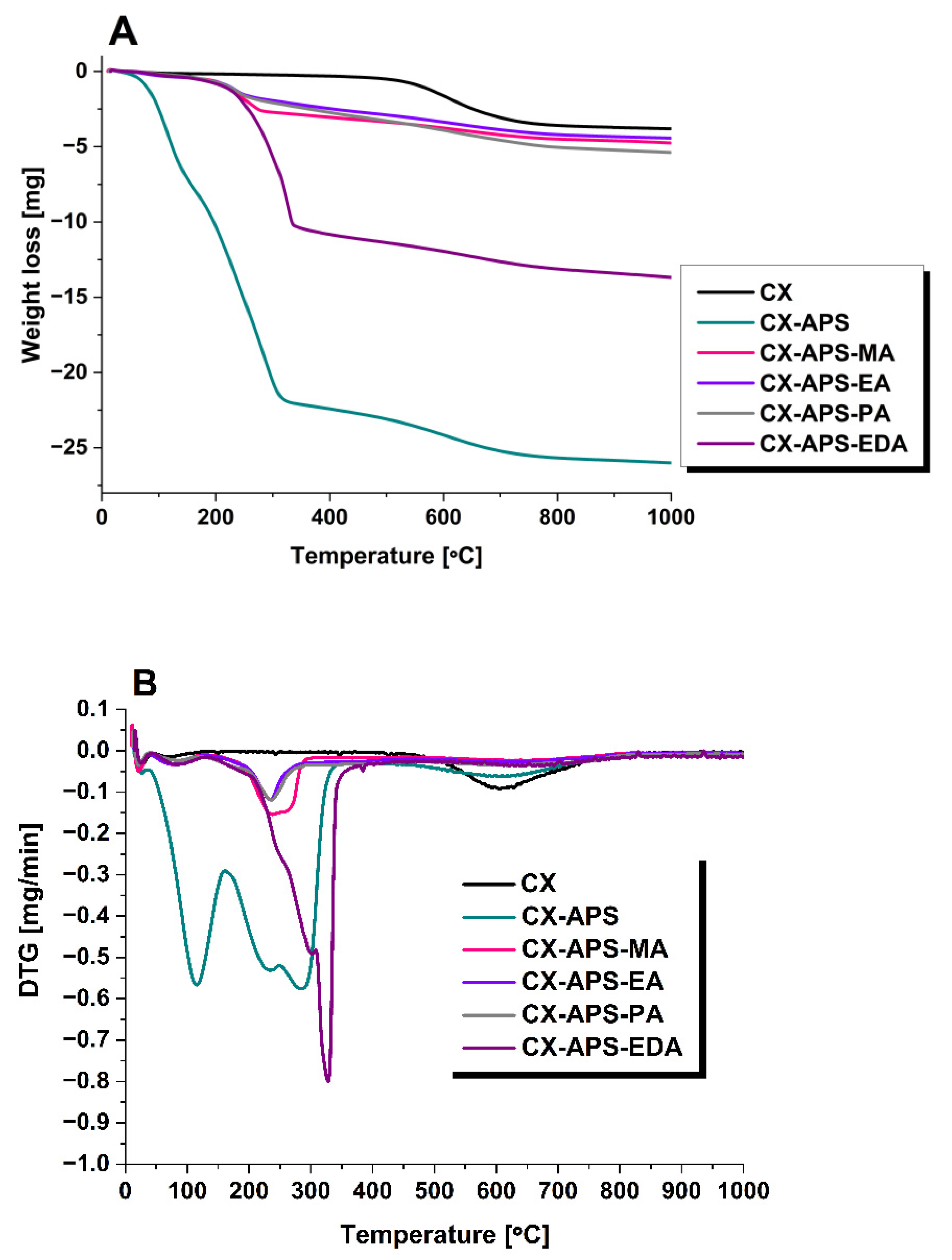
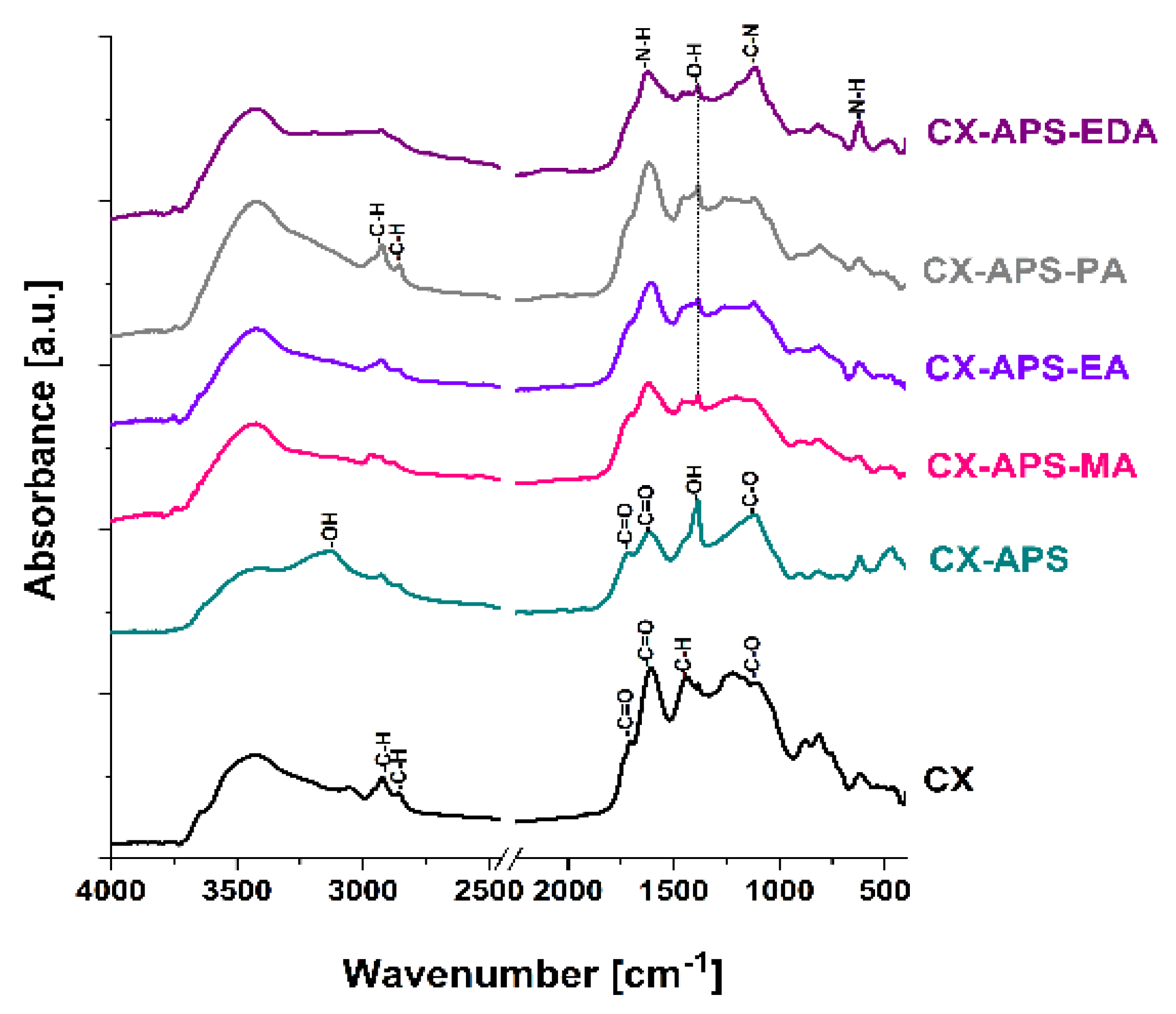
2.2.3. Thermogravimetric Analysis and Infrared Spectroscopy
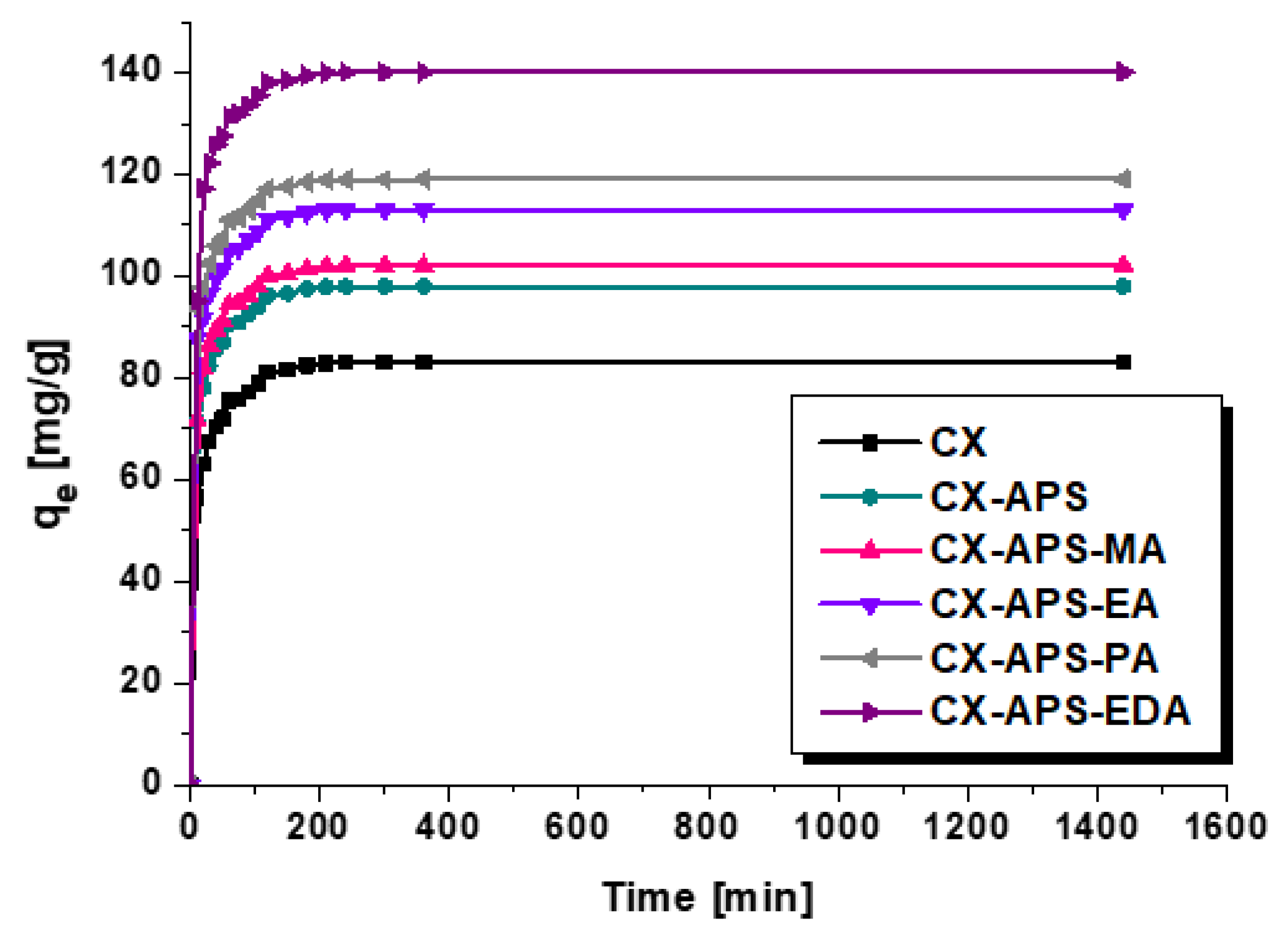
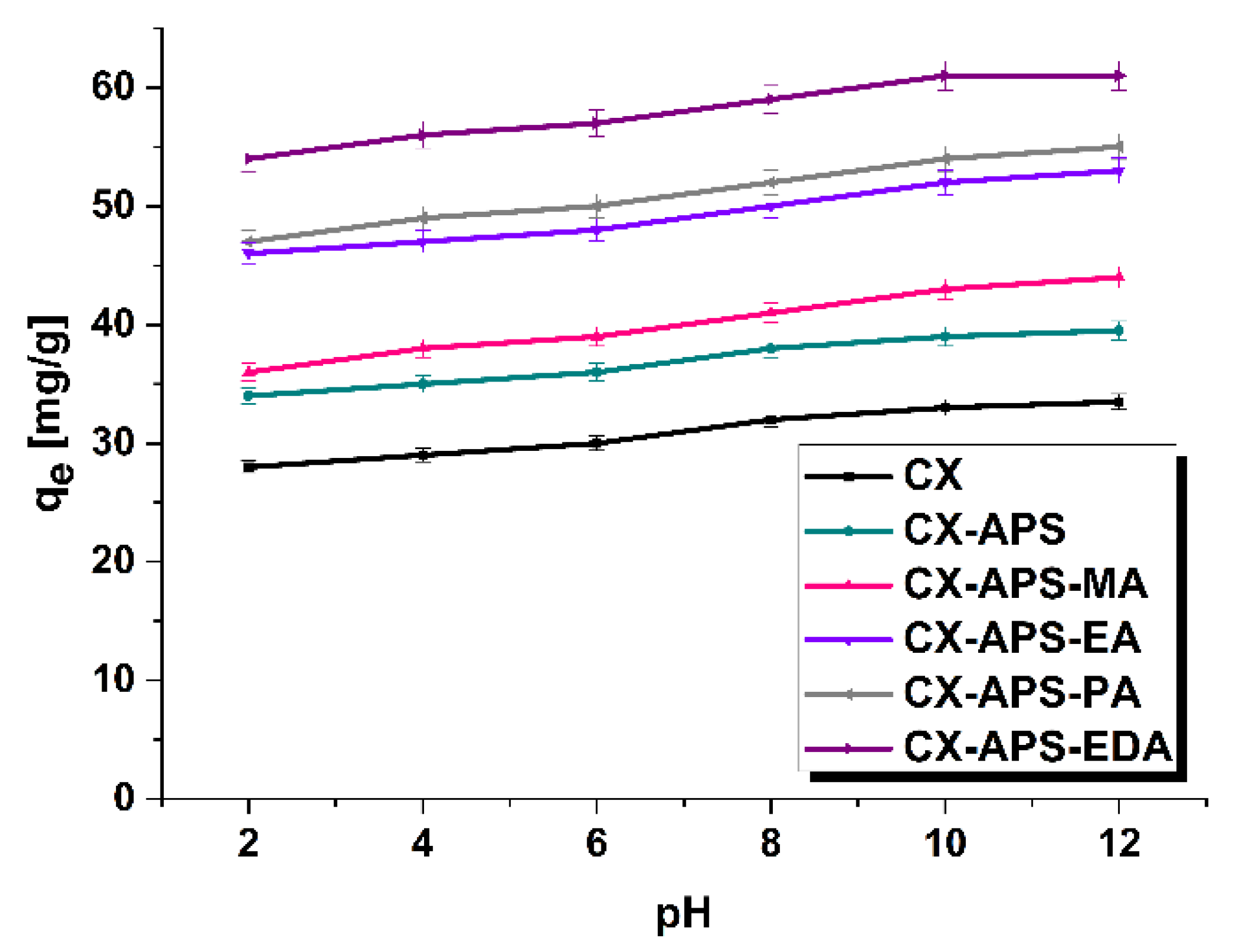
2.3. Adsorption of Dyes
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taka, A.L.; Pillay, K.; Mbianda, X.Y. Nanospongecyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from wastewater: A review. Carbohydr. Polym. 2017, 159, 94–104. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Interface Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef]
- Lan, T.; Cao, F.; Cao, L.; Wang, T.; Yu, C.; Wang, F. A comparative study on the adsorption behavior and mechanism of pesticides on agricultural film microplastics and straw degradation products. Chemosphere 2022, 303, 135058. [Google Scholar] [CrossRef]
- Goscianska, J.; Fathy, N.A.; Aboelenin, R.M.M. Adsorption of solophenyl red 3BL polyazo dye onto amine-functionalized mesoporous carbons. J. Colloid Interface Sci. 2017, 505, 593–604. [Google Scholar] [CrossRef]
- Afshari, M.; Dinari, M. Synthesis of new imine-linked covalent organic framework as high efficient absorbent and monitoring the removal of direct fast scarlet 4BS textile dye based on mobile phone colorimetric platform. J. Hazard. Mater. 2020, 385, 121514. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska, M.; Pietrzak, R. Equilibrium and kinetic studies of chromotrope 2R adsorption onto ordered mesoporous carbons modified with lanthanum. Chem. Eng. J. 2015, 270, 140–149. [Google Scholar] [CrossRef]
- Afshari, M.; Dinari, M.; Zargoosh, K.; Moradi, H. Novel triazine-based covalent organic framework as a superadsorbent for the removal of mercury(II) from aqueous solutions. Ind. Eng. Chem. Res. 2020, 59, 9116–9126. [Google Scholar] [CrossRef]
- Goscianska, J.; Marciniak, M.; Pietrzak, R. Ordered mesoporous carbons modified with cerium as effective adsorbents for azo dyes removal. Sep. Purif. Technol. 2015, 154, 236–245. [Google Scholar] [CrossRef]
- Afshari, M.; Dinari, M.; Moradi, H.; Noori, Z. Polyaniline/sulfonated-covalent organic polymer nanocomposite: Structural and dye adsorption properties. Polym. Adv. Technol. 2020, 31, 2433–2442. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Mashkoor, F.; Nasar, A. Preparation, characterization and adsorption studies of the chemically modified luffa aegypticapeel as a potential adsorbent for the removal of malachite green from aqueous solution. J. Mol. Liq. 2019, 274, 315–327. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; Wang, Z.; Wu, A.; Zhang, Y. Synthesis of hollow mesoporous manganese dioxide nanoadsorbents with strong negative charge and their ultra-efficient adsorption for cationic dyes. Sep. Purif. Technol. 2022, 29, 121241. [Google Scholar] [CrossRef]
- Zhou, G.; Tian, H.; Sun, H.; Wang, S.; Buckley, C.E. Synthesis of carbon xerogels at varying sol–gel pHs, dye adsorption and chemical regeneration. Chem. Eng. J. 2011, 171, 1399–1405. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Fathy, N.A.; Attia, A.A.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Activated carbon xerogels for the removal of the anionic azo dyes Orange II and Chromotrope 2R by adsorption and catalytic wet peroxide oxidation. Chem. Eng. J. 2012, 195–196, 112–121. [Google Scholar] [CrossRef]
- Paéz, C.A.; Contreras, M.S.; Léonard, A.; Blacher, S.; Olivera-Fuentes, C.-G.; Pirard, J.P.; Job, N. Effect of CO2 activation of carbon xerogels on the adsorption of methylene blue. Adsorption 2012, 18, 199–211. [Google Scholar] [CrossRef]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Removal of rhodamine B from water by modified carbon xerogels. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 109–117. [Google Scholar] [CrossRef]
- Girgis, B.S.; Attia, A.A.; Fathy, N.A. Potential of nano-carbon xerogels in the remediation of dye-contaminated water discharges. Desalination 2011, 265, 169–176. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Sousa, J.P.S.; Orge, C.A.; Pereira, M.F.R.; Órfão, J.J.M. Adsorption of dyes on carbon xerogels and templated carbons: Influence of surface chemistry. Adsorption 2011, 17, 431–441. [Google Scholar] [CrossRef]
- Zaggout, F.R.; El-Nahhal, I.M.; Qaraman, A.E.A.; Al-Dahoudi, N. Behavior of thymol blue analytical pH-indicator entrapped into sol–gel matrix. Mater. Lett. 2006, 60, 3463–3467. [Google Scholar] [CrossRef]
- Hopkins, A.E.; Sell, K.S.; Soli, A.L.; Byrne, R.H. In-situ spectrophotometric pH measurements: The effect of pressure on thymolblue protonation and absorbance characteristics. Mar. Chem. 2000, 71, 103–109. [Google Scholar] [CrossRef]
- Hankare, P.P.; Patil, R.P.; Jadhav, A.V.; Garadkar, K.M.; Sasikala, R. Enhanced photocatalytic degradation of methyl red and thymolblue using titania–alumina–zinc ferrite nanocomposite. Appl. Catal. B Environ. 2011, 107, 333–339. [Google Scholar] [CrossRef]
- Sousa, J.P.S.; Pereira, M.F.R.; Figueiredo, J.L. Carbon Xerogel Catalyst for NO Oxidation. Catalysts 2012, 2, 447–465. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Adsorption of dyes on the surface of polymer nanocomposites modified with methylamine and copper(II) chloride. J. Colloid Interface Sci. 2017, 504, 549–560. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, W.; Huang, H.; Liu, Y.; Yang, Q.; Li, L. Facile synthesis of N,S-Codoped hierarchically porous carbon with high volumetric pseudocapacitance. ACS Sustain. Chem. Eng. 2019, 7, 16710–16719. [Google Scholar] [CrossRef]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Synthesis of carbon xerogels modified with amine groups and copper for efficient adsorption of caffeine. Chem. Eng. J. 2018, 345, 13–21. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A.; Nowak, I.; Marciniak, M.; Pietrzak, R. Stability analysis of functionalized mesoporous carbon materials in aqueous solution. Chem. Eng. J. 2016, 290, 209–219. [Google Scholar] [CrossRef]
- Moreno, A.H.; Arenillas, A.; Calvo, E.G.; Bermúdez, J.M.; Menéndez, J.A. Carbonisation of resorcinol–formaldehyde organic xerogels: Effect of temperature, particle size and heating rate on the porosity of carbon xerogels. J. Anal. Appl. Pyrol. 2013, 100, 111–116. [Google Scholar] [CrossRef]
- Álvarez, S.; Ribeiro, R.S.; Gomes, H.T.; Sotelo, J.L.; García, J. Synthesis of carbon xerogels and their application in adsorption studies of caffeine and diclofenac as emerging contaminants. Chem. Eng. Res. Des. 2015, 95, 229–238. [Google Scholar] [CrossRef]
- Laginhas, C.; Nabais, J.M.V.; Titirici, M.M. Activated carbons with high nitrogen content by a combination of hydrothermal carbonization with activation. Micropor. Mesopor. Mater. 2016, 226, 125–132. [Google Scholar] [CrossRef]
- Allahbakhsh, A.; Khodabadi, F.N.; Hosseini, F.S.; Haghighi, A.H. 3-Aminopropyl-triethoxysilane-functionalized rice husk and rice husk ash reinforced polyamide 6/graphene oxide sustainable nanocomposites. Eur. Polym. J. 2017, 94, 417–430. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A. Dispersion stability of the aminosilane-grafted mesoporous carbons in different solvents. Microporous Mesopor. Mater. 2018, 265, 149–161. [Google Scholar] [CrossRef]
- Goscianska, J.; Marciniak, M.; Pietrzak, R. Mesoporous carbons modified with lanthanum(III) chloride for methyl orange adsorption. Chem. Eng. J. 2014, 247, 258–264. [Google Scholar] [CrossRef]
- Hernández-Morales, V.; Nava, R.; Acosta-Silva, Y.J.; Macías-Sánchez, S.A.; Pérez-Bueno, J.J.; Pawelec, B. Adsorption of lead (II) on SBA-15 mesoporous molecular sieve functionalized with –NH2 groups. Micropor. Mesopor. Mater. 2012, 160, 133–142. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, Y.; Zhang, X.; Jia, M.; Yao, J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionicdyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; An, C.; Zhao, S.; Zhang, P. Adsorption of anionic azo dyes from aqueous solution on cationic gemini surfactant-modified flax shives: Synchrotron infrared, optimization and modeling studies. J. Clean. Prod. 2018, 172, 1986–1997. [Google Scholar] [CrossRef]
- Jung, K.W.; Choi, B.H.; Hwang, M.J.; Jeong, T.U.; Ahn, K.H. Fabrication of granular activated carbons derived from spent coffee grounds by entrapment in calcium alginate beads for adsorption of acid orange 7 and methylene blue. Bioresour. Technol. 2016, 219, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Vucurović, V.M.; Razmovski, R.N.; Miljić, U.D.; Puskas, V.S. Removal of cationic and anionic azo dyes from aqueous solutions by adsorption on maize stem tissue. J. Taiwan Inst. Chem. Eng. 2014, 45, 1700–1708. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, Q.; Ren, X.; Yin, M.; Xue, Z. Adsorption and removal of sulfonic dyes from aqueous solution onto a coordination polymeric xerogel with amino groups. Colloids Surf. A Physicochem. Eng. Asp. 2015, 485, 125–135. [Google Scholar] [CrossRef]
- Bakhsh, E.M.; Bilal, M.; Ali, M.; Ali, J.; Wahab, A.; Akhtar, K.; Fagieh, T.M.; Danish, E.Y.; Asiri, A.M.; Khan, S.B. Synthesis of Activated Carbon from Trachycarpusfortunei Seeds for the Removal of Cationic and Anionic Dyes. Materials 2022, 15, 1986. [Google Scholar] [CrossRef]
- Shehata, H.M.; Ali, D.A.; Al-Akraa, I.M.; Elazab, H.A.; Elsawy, H.A. Development of novel adsorbent for industrial waste water treatment. Int. J. Adv. Trends Comput. Sci. Eng. 2020, 9, 704–712. [Google Scholar] [CrossRef]
- Song, W.; Gao, B.; Xu, X.; Xing, L.; Han, S.; Duan, P.; Song, W.; Jia, R. Adsorption-desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater. Bioresour. Technol. 2016, 210, 123–130. [Google Scholar] [CrossRef]
- Haddad, M.E.; Mamouni, R.; Saffaj, N.; Lazar, S. Evaluation of Performance of Animal Bone Meal as a new low cost adsorbent for the removal of a cationic dye RhodamineB from aqueous solutions. J. Saudi Chem. Soc. 2016, 20, S53–S59. [Google Scholar] [CrossRef]
- Hasan, H.M.I.; Alfutisi, H.M.M. Removing of thymol blue from aqueous solutions by pomegranate peel. Int. J. Appl. Sci. 2018, 1, 111–119. [Google Scholar]
- Koyuncu, H.; Aldemir, A.; Kul, A.R.; Canayaz, M. Removal of thymol blue from aqueous solution by natural and modified bentonite: Comparative analysis of ANN and ANFIS models for the prediction of removal percentage. Pollution 2021, 7, 959–980. [Google Scholar] [CrossRef]
- Kuete, I.-H.T.; Tchuifon, D.R.T.; Ndifor-Angwafor, G.N.; Kamdem, A.T.; Anagho, S.G. Kinetic, isotherm and thermodynamic studies of the adsorption of thymol blue onto powdered activated carbons from garcinia cola nut shells impregnated with H3PO4 and KOH: Non-linear regression analysis. J. Encapsul. Adsorpt. Sci. 2020, 10, 1–27. [Google Scholar] [CrossRef]



| Material | C | H | N | S | O * |
|---|---|---|---|---|---|
| CX | 78.77 | 2.60 | 0.00 | 0.00 | 18.63 |
| CX-APS | 31.18 | 3.35 | 1.86 | 19.32 | 44.29 |
| CX-APS-MA | 70.97 | 2.06 | 2.20 | 2.79 | 21.98 |
| CX-APS-EA | 77.23 | 1.96 | 1.16 | 1.21 | 18.44 |
| CX-APS-PA | 74.55 | 2.36 | 1.26 | 1.33 | 20.50 |
| CX-APS-EDA | 50.32 | 3.61 | 9.23 | 10.43 | 26.41 |
| Material | Total Surface Area 1 (BET) [m2/g] | Micropore Area [m2/g] | Total Pore Volume [cm3/g] | Micropore Volume [cm3/g] | Average Pore Diameter [nm] |
|---|---|---|---|---|---|
| CX | 663 | 401 | 1.49 | 0.171 | 20.30 |
| CX-APS | 155 | 8 | 0.82 | 0.002 | 21.19 |
| CX-APS-MA | 450 | 229 | 1.28 | 0.123 | 33.65 |
| CX-APS-EA | 368 | 142 | 1.21 | 0.075 | 33.14 |
| CX-APS-PA | 340 | 113 | 1.14 | 0.058 | 26.65 |
| CX-APS-EDA | 172 | 13 | 0.88 | 0.005 | 20.46 |
| Material | Acidic Oxygen Functional Groups [mmol/g] | Basic Oxygen Functional Groups [mmol/g] |
|---|---|---|
| CX | 1.27 ± 0.02 | 0.49 ± 0.01 |
| CX-APS | 6.16 ± 0.05 | 0.00 ± 0.00 |
| CX-APS-MA | 0.91 ± 0.01 | 0.50 ± 0.01 |
| CX-APS-EA | 1.23 ± 0.02 | 0.74 ± 0.01 |
| CX-APS-PA | 0.91 ± 0.01 | 0.74 ± 0.01 |
| CX-APS-EDA | 0.73 ± 0.01 | 1.49 ± 0.02 |
| Material | qe(exp) [mg/g] | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe(cal) [mg/g] | k1 [1/min] | R2 | qe(cal) [mg/g] | k2 [g mg−1 × min−1] | R2 | ||
| CX | 83 ± 1.6 | 50.90 | 0.0524 | 0.9811 | 83.40 | 0.0025 | 0.9999 |
| CX-APS | 98 ± 1.9 | 52.21 | 0.0533 | 0.9784 | 98.43 | 0.0025 | 0.9999 |
| CX-APS-MA | 102 ± 2.0 | 54.60 | 0.0532 | 0.9707 | 102.46 | 0.0025 | 0.9999 |
| CX-APS-EA | 113 ± 2.3 | 95.64 | 0.0700 | 0.9392 | 113.51 | 0.0024 | 0.9999 |
| CX-APS-PA | 119 ± 2.4 | 82.57 | 0.0817 | 0.9197 | 119.47 | 0.0025 | 0.9999 |
| CX-APS-EDA | 140 ± 2.8 | 76.30 | 0.0734 | 0.9426 | 140.65 | 0.0022 | 0.9999 |
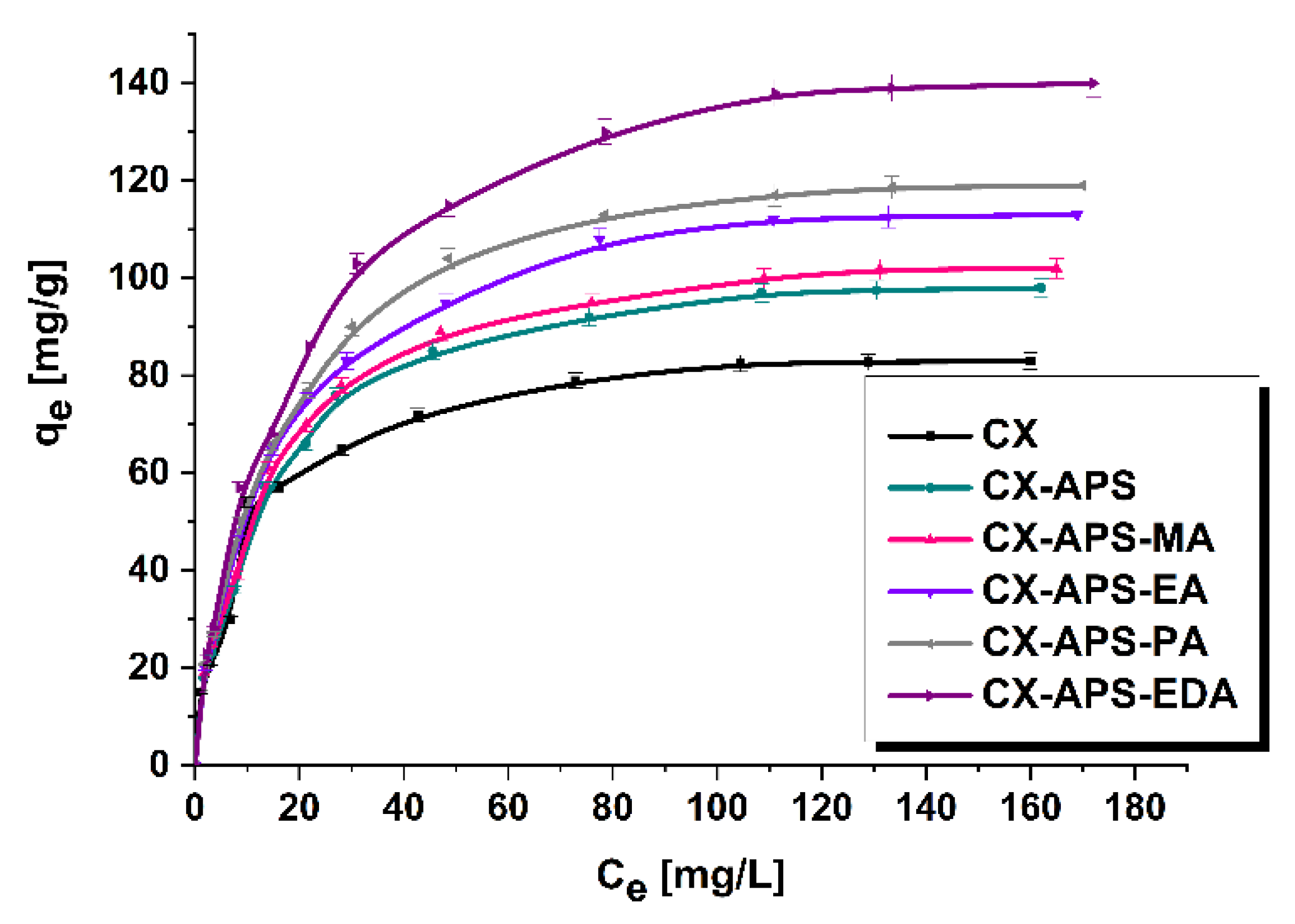
| Material | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm [mg/g] | KL [L/mg] | R2 | KF [mg/g (L/mg)1/n] | 1/n | R2 | |
| CX | 88.2 | 0.11 | 0.9991 | 16.90 | 0.348 | 0.8926 |
| CX-APS | 105.3 | 0.09 | 0.9993 | 19.16 | 0.351 | 0.9280 |
| CX-APS-MA | 109.8 | 0.09 | 0.9993 | 18.47 | 0.367 | 0.9306 |
| CX-APS-EA | 121.9 | 0.08 | 0.9994 | 19.54 | 0.377 | 0.9241 |
| CX-APS-PA | 128.4 | 0.08 | 0.9993 | 20.07 | 0.383 | 0.9188 |
| CX-APS-EDA | 153.4 | 0.07 | 0.9991 | 20.51 | 0.409 | 0.9332 |
| Material | Temperature [°C] | ∆G [kJ/mol] | ∆H [kJ/mol] | ∆S [J/mol K] |
|---|---|---|---|---|
| CX | 25 | −4.13 | 6.57 | 35.99 |
| 45 | −4.93 | |||
| 60 | −5.38 | |||
| CX-APS | 25 | −3.57 | 6.75 | 34.60 |
| 45 | −4.23 | |||
| 60 | −4.78 | |||
| CX-APS-MA | 25 | −3.65 | 6.64 | 34.62 |
| 45 | −4.46 | |||
| 60 | −4.84 | |||
| CX-APS-EA | 25 | −3.71 | 7.78 | 38.73 |
| 45 | −4.66 | |||
| 60 | −5.04 | |||
| CX-APS-PA | 25 | −3.72 | 8.38 | 40.80 |
| 45 | −4.73 | |||
| 60 | −5.11 | |||
| CX-APS-EDA | 25 | −3.78 | 9.40 | 44.28 |
| 45 | −4.41 | |||
| 60 | −4.76 |
| Adsorbent | qmax [mg/g] | References |
|---|---|---|
| CX-APS-EDA | 153.4 | This study |
| activated carbon from Trachycarpusfortunei seeds | 130.38 | [41] |
| pomegranate peel | 5.28 | [45] |
| bentonite | 117.6471 | [46] |
| activated carbon from garcinia cola nutshells | 396.04 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptaszkowska-Koniarz, M.; Goscianska, J.; Bazan-Wozniak, A.; Pietrzak, R. Amine-Modified Carbon Xerogels as Effective Carbon-Based Adsorbents of Anionic Dye from Aqueous Solutions. Materials 2022, 15, 5736. https://doi.org/10.3390/ma15165736
Ptaszkowska-Koniarz M, Goscianska J, Bazan-Wozniak A, Pietrzak R. Amine-Modified Carbon Xerogels as Effective Carbon-Based Adsorbents of Anionic Dye from Aqueous Solutions. Materials. 2022; 15(16):5736. https://doi.org/10.3390/ma15165736
Chicago/Turabian StylePtaszkowska-Koniarz, Magdalena, Joanna Goscianska, Aleksandra Bazan-Wozniak, and Robert Pietrzak. 2022. "Amine-Modified Carbon Xerogels as Effective Carbon-Based Adsorbents of Anionic Dye from Aqueous Solutions" Materials 15, no. 16: 5736. https://doi.org/10.3390/ma15165736
APA StylePtaszkowska-Koniarz, M., Goscianska, J., Bazan-Wozniak, A., & Pietrzak, R. (2022). Amine-Modified Carbon Xerogels as Effective Carbon-Based Adsorbents of Anionic Dye from Aqueous Solutions. Materials, 15(16), 5736. https://doi.org/10.3390/ma15165736







