Chemistry and Production Technology of Hallstatt Period Glass Beads from Bohemia
Abstract
:1. Introduction
2. Prehistoric Glass
3. Methods
4. Results and Discussion
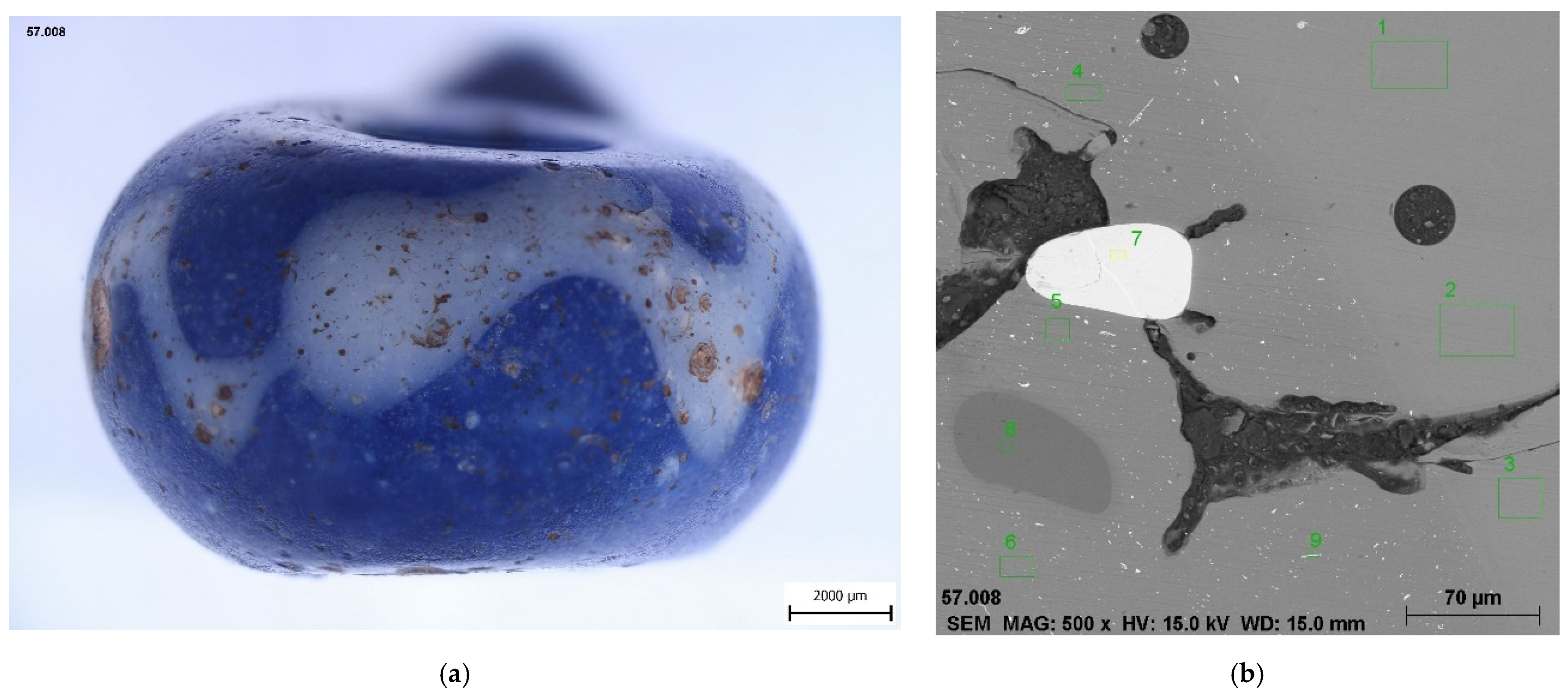
4.1. Optical Properties, Production Technology
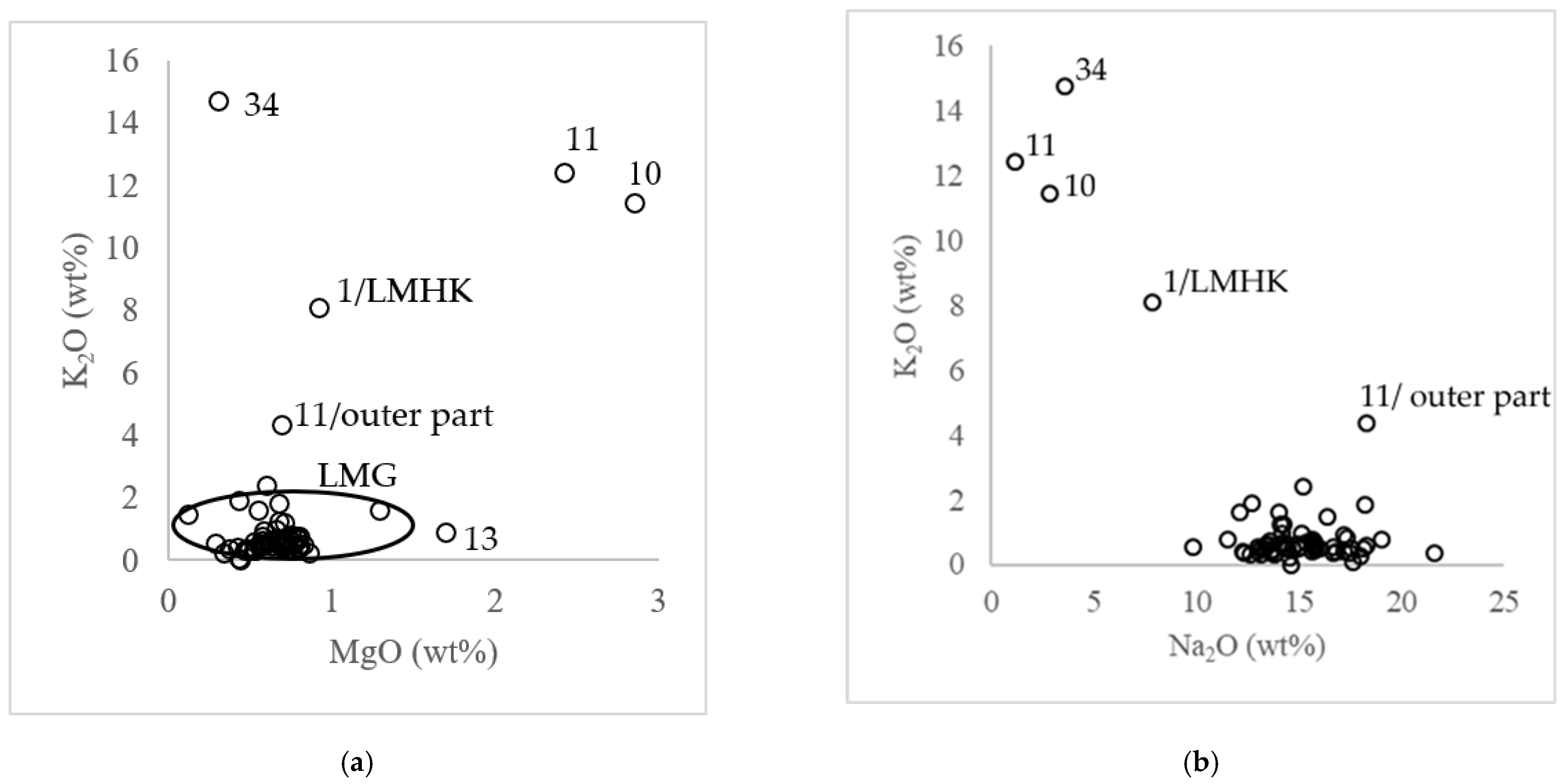
4.2. Chemical Composition of the Glass
| Sample | Na2O | MgO | Al2O3 | SiO2 | P2O5 | K2O | CaO | Note |
|---|---|---|---|---|---|---|---|---|
| 10 | 2.88 | 2.85 | 2.68 | 53.58 | 1.63 | 11.43 | 7.66 | This study |
| 11 | 1.17 | 2.42 | 2.26 | 54.39 | 4 | 12.42 | 6.09 | This study |
| Figure 4 | 1.18 | 0.68 | n.d | 71.08 | n.d | 16.39 | 2.81/8.95 | 14th–9th c. BC [50] |
| Table 2, RC 6g | 1.01 | 1.29 | 1.61 | 68.6 | 0.72 | 18.2 | 4.02 | 12th–10th c. BC [36] |
| Table 2 | 0.1–1 | 2–5.3 | 1.3–37 | 56–61 | 1.7–4.6 | 10.2–14.2 | 5.3–10.2 | 7th–5th c. BC/Chotín [36] |
| Table 2, LN 3 | 1.3 | 3.3 | 3.0 | 53.6 | 3.4 | 6.8 | 23.3 | 1st BC–2nd c. AD/La Négade [31] |
4.2.1. Non-Opaque Glasses
4.2.2. Opaque Glasses
White-Colored Glasses
Yellow-Colored Glasses
Red-Colored Glasses
Dark-Colored Glass
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sklenář, K. Pravěká a raně středověká archeologie v dějinách Národního muzea—Prehistoric and Early Medieval Archaeology in History of National Museum. Fontes Archaeol. Pragenses 2014, 40, 1–330. [Google Scholar]
- Podborský, V. Jihomoravská halštatská sídliště II. Sborník Pr. Filoz. Fak. Brněnské Univerzity E 1972, 17, 5–54. [Google Scholar]
- Frána, J.; Maštalka, A.; Venclová, N. Neutron activation analysis of some ancient glasses from Bohemia. Archaeometry 1987, 29, 69–89. [Google Scholar] [CrossRef]
- Venclová, N. Prehistoric Glass in Bohemia; Archeologický ústav CSAV: Prague, Czech Republic, 1990. [Google Scholar]
- Venclová, N.; Hulínský, V.; Henderson, J.; Chenery, S.; Šulová, L.; Hložek, J. Late Bronze Age mixed-alkali glasses from Bohemia. Archeol. Rozhl. 2011, 63, 559–585. [Google Scholar]
- Haevernick, T.E. Urnenfelderzeitliche Glasperlen. Z. Für Schweiz. Archäologie Und Kunstgesch. 1978, 35, 145–157. [Google Scholar]
- Venclová, N. Němčice and Staré Hradisko. Iron Age Glass and Glass-Working in Central Europe; Archeologický ústav AV CR: Prague, Czech Republic, 2016. [Google Scholar]
- Haevernick, T.E. Glasperlen der vorrömischen eisenzeit I. In Marburger Studien zur Vor- und Frühgeschichte; v. Zabern: Marburg, Germany, 1983; Band 5. [Google Scholar]
- Venclová, N. Skleněný náhrdelník z Platěnic. In Vita archaeologica. Sborník Víta Vokolka; Sedláček, R., Sigl, J., Vencl, S., Eds.; Muzeum východních Čech v Hradci Králové: Hradec Králové, Czech Republic, 2006; pp. 365–370. [Google Scholar]
- Tomková, K.; Venclová, N. Glasschmuck in Böhmen von der Bronzezeit bis ins Frühmittelalter: Archäologie und Archäometrie. In Glasarchäologie in Europa. Regionen—Produkte—Analysen; Černá, E., Steppuhn, P., Eds.; ASEP Repozitář AV ČR: Praha, Czech Republic, 2014; pp. 221–237. [Google Scholar]
- Nekvasil, J. Pohřebiště lužické kultury v Moravičanech. In Fontes archaeologiae Moravicae 14; Archeologický ústav ČSAV: Brno, Czech Republic, 1982. [Google Scholar]
- Kos, P. Pohřby žen z doby halštatské v Modřicích u Brna. In Popelnicová Pole a Doba Halštatská, Archeologické Výzkumy v Jižních Čechách; Jihočeské muzeum v Českých Budějovicích: České Budějovice, Czech Republic, 2004; pp. 271–292. [Google Scholar]
- Stabrava, P. Žárové pohřebiště na katastru města Příbora a jeho místo v regionálním vývoji kultury lužických popelnicových polí. Přehled Výzkumů 2011, 52, 75–110. [Google Scholar]
- Haevernick, T.E. Die Glasperlen des Grabhügels Magdalenenberg bei Villingen. In Magdalenenberg V, Der Hallstattzeitliche Fürstengrabhügel bei Villingen im Schwarzwald 5; Spindler: Villingen, Germany, 1977; pp. 137–139. [Google Scholar]
- Drda, P.; Rybová, A. Akropole na Hradišti Závist v 6.–4. stol. př. Kr; Archeologický ústav AV ČR: Praha, Czech Republic, 2008. [Google Scholar]
- Chytráček, M.; Metlička, M. Die Höhensiedlungen der Hallstatt—Und Latènezeit in Westböhmen; Archeologický ústav AV ČR: Prague, Czech Republic, 2004. [Google Scholar]
- Venclová, N. Prehistoric eye beads in Central Europe. J. Glass Stud. 1983, 25, 11–17. [Google Scholar]
- Holzer, V. Sechs Späthallstatt-/Frühlatènezeitliche Glasperlen Aus Vicenice, Böhmen. In Annalen Des Naturhistorischen Museums in Wien. Serie A Für Mineralogie Und Petrographie, Geologie Und Paläontologie, Anthropologie Und Prähistorie; Naturhistorisches Museum: Vienna, Austria, 1999; Volume 101, pp. 81–96. [Google Scholar]
- Haevernick, T.E. Die Glasperlen. In Die Býčí skála-Höhle. Einhallstattzeitlicher Höhlenopferplatz in Mähren; Parzinger, H., Nekvasil, J., Barth, F.E., Eds.; Römisch-Germanische Forschungen 54: Mainz am Rhein, Germany, 1995; pp. 93–97. [Google Scholar]
- Sedláček, Z.; Venclová, N. Nález pozdně halštatského skla na sídlišti v Hradeníně. Archeol. Rozhl. 1983, 35, 158–171. [Google Scholar]
- Chytráček, M.; Chvojka, O.; John, J.; Michálek, J.; Stránská, P.; Šálková, T. Lidská oběť z pozdní doby halštatské v jižních Čechách? K interpretaci nálezů pod výšinnou lokalitou starší doby železné na Vraném vrchu u Spolí, okr. Český Krumlov. Archeol. Rozhl. 2017, 69, 583–628. [Google Scholar]
- Černá, E.; Tomková, K.; Hulínský, V. Proměny skel od 11. do konce 13. století v Čechách. Archeol. Rozhl. 2015, 67, 79–108. [Google Scholar]
- Koch, U. Das Alamannisch Fränkische Gräberfeld bei Pleidelsheim; Landesdenkmalamt Baden-Wüttenberg: Stuttgart, Germany, 2001. [Google Scholar]
- Pásztor, A. Perlenfunde aus dem frühawarenzeitlichen Gräberfeld von Keszthely-Fenékpuszta, Ödenkirche-Flur. In Die Gräberfelder von Keszthely-Fenékpuszta S; Müller, R., Ed.; Castellum Pannonicum Pelsonense, Vol.5; VML Verlag Marie Leidorf GmbH: Rahden, Germany, 2014; pp. 257–310. [Google Scholar]
- Venclová, N.; Hulínský, V.; Jonášová, Š. Merovingian Glass Beads from Holubice in Moravia: Chemical and Technological View. In Moravské Křižovatky. Střední Podunají Mezi Pravěkem A Historií; Čižmářová, J., Venclová, N., Březinová, G., Eds.; Moravské zemské muzeum: Brno, Czech Republic, 2014; pp. 815–826. [Google Scholar]
- Gutsmiedl-Schümann, D. Das frühmittelalterliche Gräberfeld Aschheim-Bajuwarenring; Materialhefte zur Bayerischen Vorgeschichte A 94. Kallmünz/Of; 2010; Available online: https://www.researchgate.net/publication/338297635_Das_fruhmittelalterliche_Graberfeld_Aschheim-Bajuwarenring (accessed on 22 February 2022).
- Čižmář, M. Das Gräberfeld von Holubice. In Langobardische Gräberfelde in Mähren I.; Tejral, J., Ed.; Archäologisches Institut der Akademie der Wissenschaften der Tschechischen Republik: Brno, Czech Republic, 2011; pp. 129–224. [Google Scholar]
- Svoboda, B. Šperky z XXXII. hrobu ve Smolíně. Památky Archeol. 1957, 48, 463–494. [Google Scholar]
- Motyková, K.; Drda, P.; Rybová, A. Keltské hradiště Závist—Dosavadní výzkum a jeho perspektivy. Památky Archeol. 1982, 73, 432–454. [Google Scholar]
- Angelini, I.; Gratuze, B.; Artioli, G. Glass and other vitreous materials through history. In EMU 20—The Contribution of Mineralogy to Cultural Heritage; Artioli, G., Oberti, R., Eds.; European Mineralogical Union: Middlesex, UK, 2019; Volume 20, pp. 87–150. [Google Scholar]
- Henderson, J. Electron probe microanalysis of mixed-alkali glasses. Archaeometry 1988, 20, 77–91. [Google Scholar] [CrossRef]
- Purowski, T.; Kępa, L.; Wagner, B. Glass on the Amber Road: The chemical composition of glassbeads from the Bronze Age in Poland. Archaeol. Anthropol. Sci. 2018, 10, 1283–1302. [Google Scholar] [CrossRef]
- Angelini, I.; Artioli, G.; Bellintani, P.; Diella, V.; Gemmi, M.; Polla, A.; Rossi, A. Chemical analyses of Bronze Age glasses from Frattesina di Rovigo, Northern Italy. J. Archaeol. Sci. 2004, 31, 1175–1184. [Google Scholar] [CrossRef]
- Arletti, R.; Maiorano, C.; Ferrari, D.; Vezzalini, G.; Quartieri, S. The first archaeometric data on polychrome Iron Age glass from sites located in northern Italy. J. Archaeol. Sci. 2010, 37, 703–712. [Google Scholar] [CrossRef]
- de Ferri, L.; Mezzadri, F.; Falcone, R.; Quagliani, V.; Milazzo, F.; Pojana, G. A non-destructive approach for the characterization of glass artifacts: The case of glass beads from the Iron Age Picene necropolises of Novilara and Crocefisso-Matelica (Italy). J. Archaeol. Sci. Rep. 2020, 29, 102124. [Google Scholar] [CrossRef]
- Conte, S.; Matarese, I.; Vezzalini, G.; Pacciarelli, M.; Scarano, T.; Vanzetti, A.; Gratuze, B.; Arletti, R. How much is known about glassy materials in Bronze and Iron Age Italy? New data and general overview. Archaeol. Anthropol. Sci. 2019, 11, 1813–1841. [Google Scholar] [CrossRef]
- Purowski, T.; Dzieranowski, P.; Bulska, E.; Wagner, B.; Nowak, A. A study of glass beads from the hallstatt C-D from southwestern Poland: Implications for glass technology and provenance. Archaeometry 2012, 54, 144–166. [Google Scholar] [CrossRef]
- Purowski, T.; Wagner, B.; Bulska, E.; Syta, O.; Dzieranowski, P. Glassy faience from the Hallstatt C period in Poland: A chemico-physical study. J. Archaeol. Sci. 2014, 50, 288–304. [Google Scholar] [CrossRef]
- Hodgkinson, A.K. Using Spatial Analysis for Understanding the Manufacture and Manipulation, of Late Bronze Age Egyptian and Ancient Near Eastern Glass. In Approaches to the Analysis of Production Activity at Archaeological Sites; Archaeopress: Oxford, UK, 2020; pp. 84–106. [Google Scholar]
- Hodgkinson, A.K.; Bertram, M. Working with fire: Making glass beads at Amarna using methods from metallurgical scenes. J. Archaeol. Sci. 2020, 33, 102488. [Google Scholar] [CrossRef]
- Heck, M.; Rehren, T.; Hoffmann, P. The production of lead-tin yellow at Merovegian Schlewitzheim (Schwitzerland). Archaeometry 2003, 45, 33–44. [Google Scholar] [CrossRef]
- Ngan-Tillard, D.J.M.; Huisman, D.J.; Corbell, F.; Van Nass, A. Over the rainbow? Micro-CT scanning to non-destructively study Roman and early medieval glass bead manufacture. J. Archaeol. Sci. 2018, 98, 7–21. [Google Scholar] [CrossRef]
- Venclová, N.; Kozáková, R.; Křížová, Š. Prstencové korále: Vrchol nebo úpadek latténského sklářství? Kraj. Archeol. Kraj. Skla 2020, 197–205. [Google Scholar]
- Kücükerman, Ö. Glass Beads, Anatolian Glass Beads Making. In Tourkish Touring and Automobile Association; Turkish Touring and Automobile Association: Istanbul, Turkey, 1998. [Google Scholar]
- Šmit, Ž.; Laharnar, B.; Turk, P. Analysis of prehistoric glass from Slovenia. J. Archaeol. Sci. Rep. 2020, 29, 10114. [Google Scholar] [CrossRef]
- Freestone, I.C. Glass Production in the First Millennium CE: A Compositional Perspective. In Vom Künstlichen Stein zum durchsichtigen Massenprodukt/from Artificial Stone to Translucent Mass-Product; Klimscha, F., Karlsen, H.J., Hansen, S., Renn, J., Eds.; Studies of the Ancient World, Edition TOPOI: Berlin, Germany, 2021; Volume 67, pp. 245–263. [Google Scholar]
- Zlámalová Cílová, Z.; Gelnar, M.; Randáková, S. Trends in Colouring Blue Glass in Central Europe in Relation to Changes in Chemical Composition of Glass from the Middle Ages to Modern Age. Minerals 2021, 11, 1001. [Google Scholar] [CrossRef]
- Jackson, C.M.; Booth, C.A.; Smedley, J.W. Glass by design? Raw materials, recipes and compositional data. Archaeometry 2005, 47, 781–795. [Google Scholar] [CrossRef]
- Adlington, L.W.; Freestone, I.C.; Kunicki-Goldfinger, J.J.; Ayers, T.; Gilderdale Scott, H.; Eavis, A. Regional patterns in medieval European glass composition as a provenancing tool. J. Archaeol. Sci. 2019, 110, 104991. [Google Scholar] [CrossRef]
- Mildner, S.; Schüssler, U.; Falkenstein, F.; Brätz, H. Bronze zeitliches Glas im westlichen Mitteleuropa—Funde, Zusammensetzung und die Frage nach seiner Herkunft. In Ressourcen und Rohstoff ein der Bronzezeit. Nutzung—Distribution—Kontrolle; Nessel, B., Heske, I., Brandherm, D., Eds.; Brandenburgisches Landesamt für Denkmalpflege und Archäologisches Landesmuseum: Wünsdorf, Germany, 2014; pp. 100–108. [Google Scholar]
- Biron, I.; Chopinet, M.H. Colouring, Decolouring and Opacifying of Glass. In Modern Methods for Analysing Archaeological and Historical Glass; Janssens, K., Ed.; Wiley: West Sussex, UK, 2013; Volume 1, pp. 49–65. [Google Scholar]
- Purowski, T.; Syta, O.; Wagner, B. Between east and west: Glass beads from the eighth to third centuries BCE from Poland. Archaeometry 2020, 62, 752–773. [Google Scholar] [CrossRef]
- Vandini, M.; Fiorentino, S. From crystals to color: A compendium of multi/analytical data on mineralogical phases in opaque colored glass mosaic tesserae. Minerals 2020, 10, 609. [Google Scholar] [CrossRef]
- Basso, E.; Invernizzi, C.; Malagodi, M.; La Russa, M.F.; Bersani, D.; Lottici, P.P. Characterization of colorants and opacifiers in roman glass mosaic tesserae through spectroscopic and spectrometric techniques. J. Raman Spectrosc. 2014, 45, 238–245. [Google Scholar] [CrossRef]
- Lahlil, S.; Biron, I.; Susini, J.; Menguy, N. Synthesis of calcium antimonate nano-crystals by the 18th dynasty Egyptian glassmakers. Appl. Phys. A 2010, 98, 1. [Google Scholar] [CrossRef]
- Shortland, A.J. The use and origin of antimonate colorants in early egyptian glass. Archaeometry 2002, 44, 517–530. [Google Scholar] [CrossRef]
- Boschetti, C.; Gratuze, B.; Schibille, N. Commercial and social significance of glass beads in migration-period Italy: The cemetery of Campo Marchione. Oxf. J. Archaeol. 2020, 39, 319–342. [Google Scholar] [CrossRef]
- Foy, D.; Picon, M.; Vichy, M.; Thirion-Merle, V. Caractérisation des verres de la fin de l‘Antiquité en Méditerranée occidentale: L’émergence de nouveaux courants commerciaux. In Échanges et Commerce du Verre Dans le Monde Antique: Actes du Colloque de l‘Association Française Pour L’archéologie du Verre; Foy, D., Nenna, M.-D., Eds.; Editions Mergol: Dremil-Lafage, France, 2003; pp. 41–85. [Google Scholar]
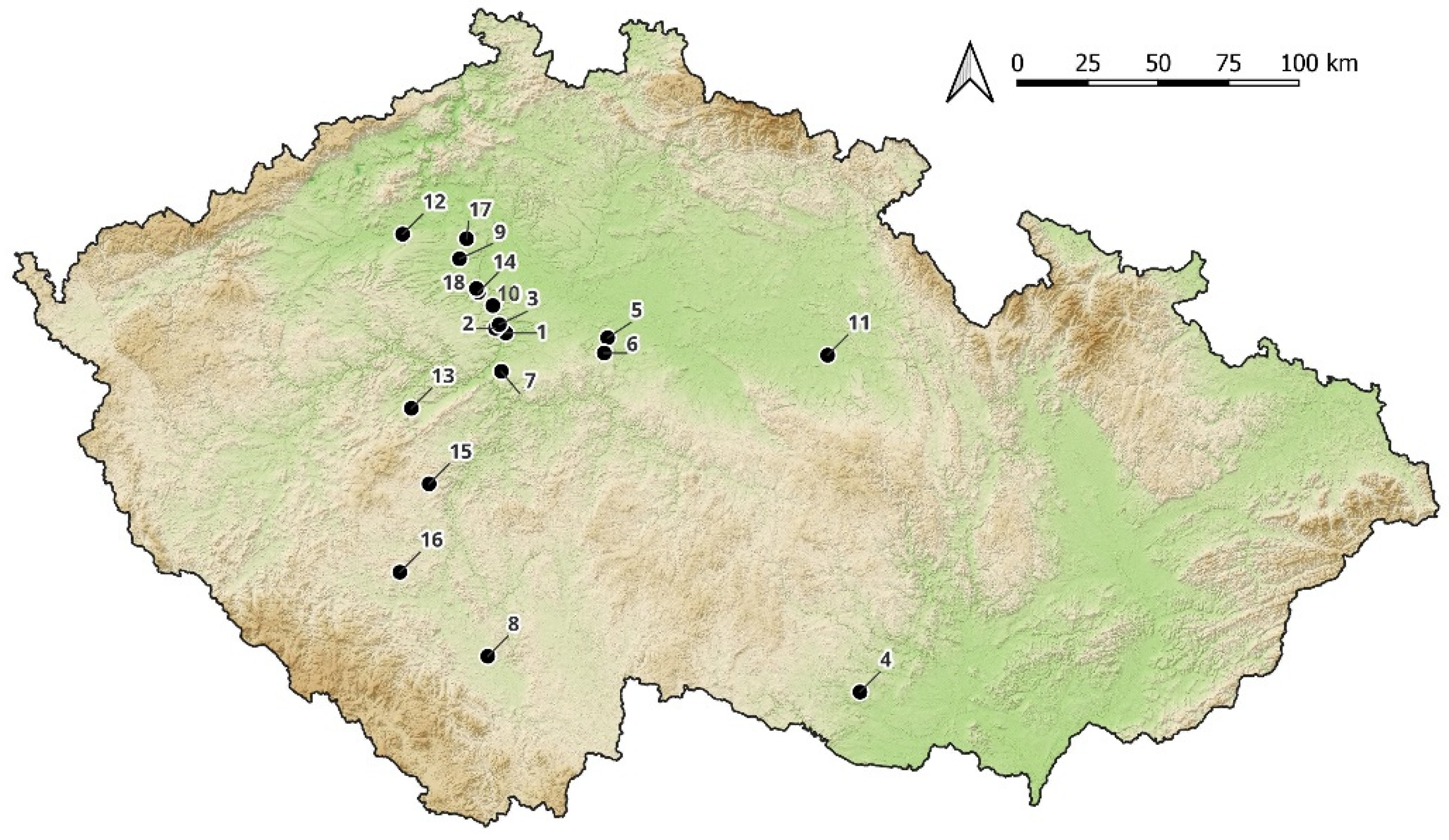
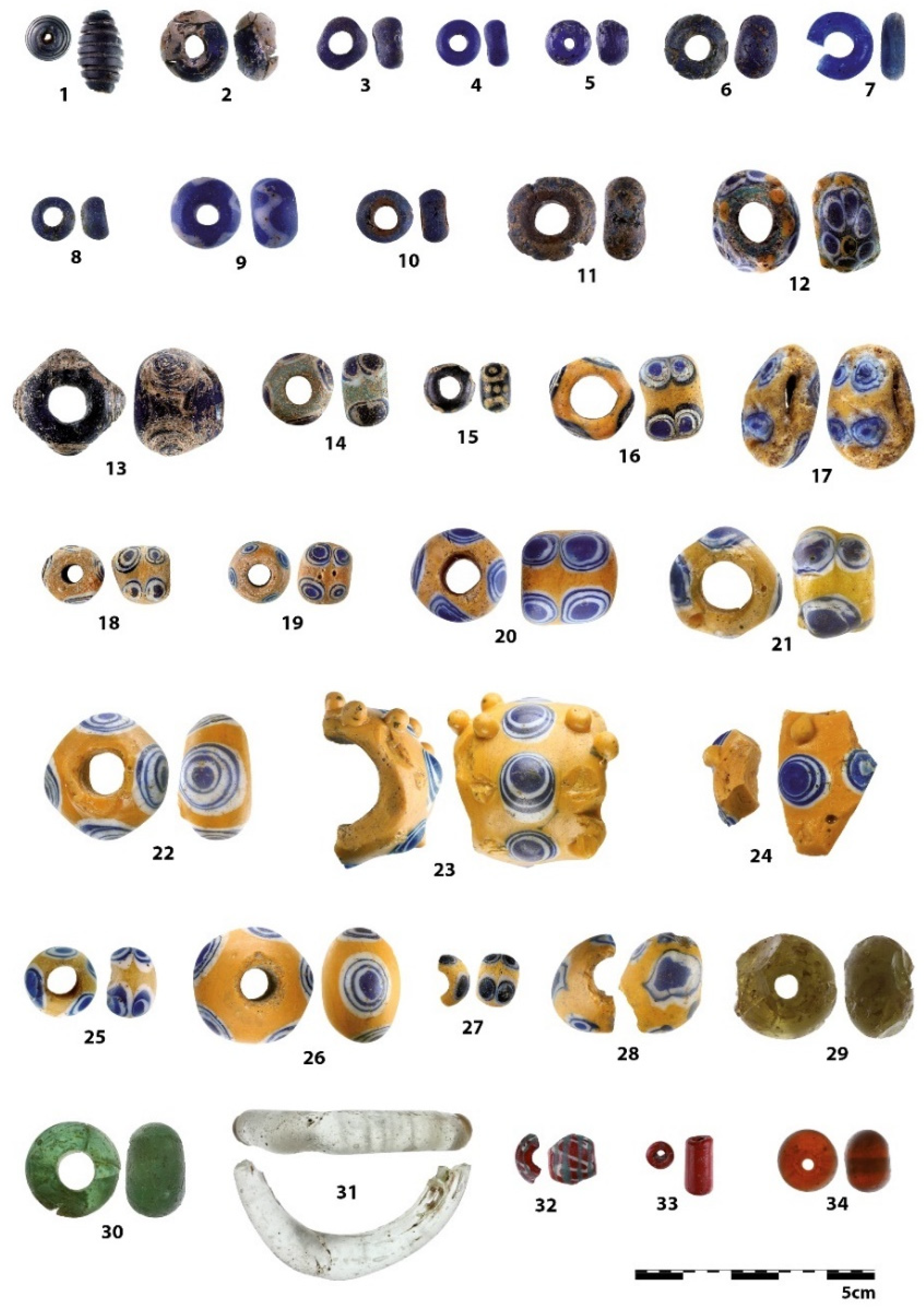



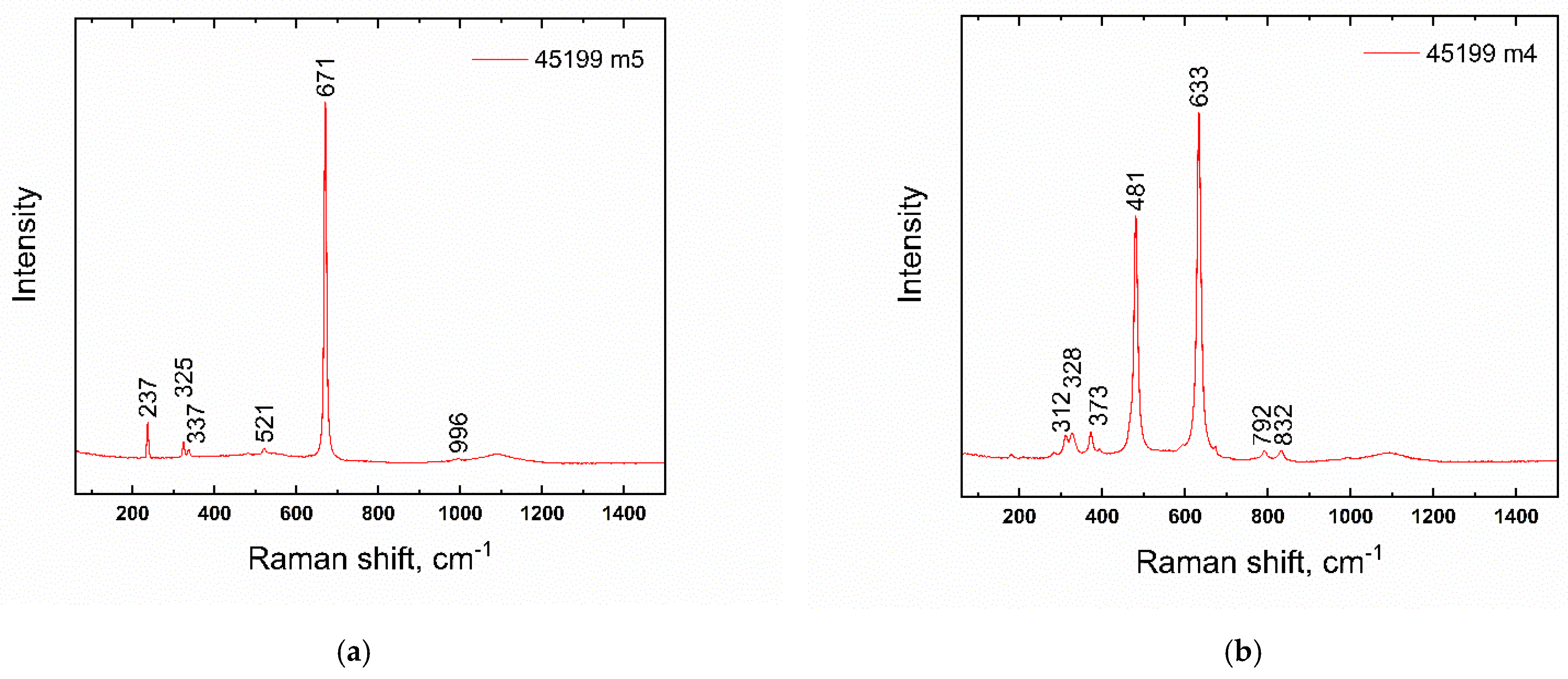

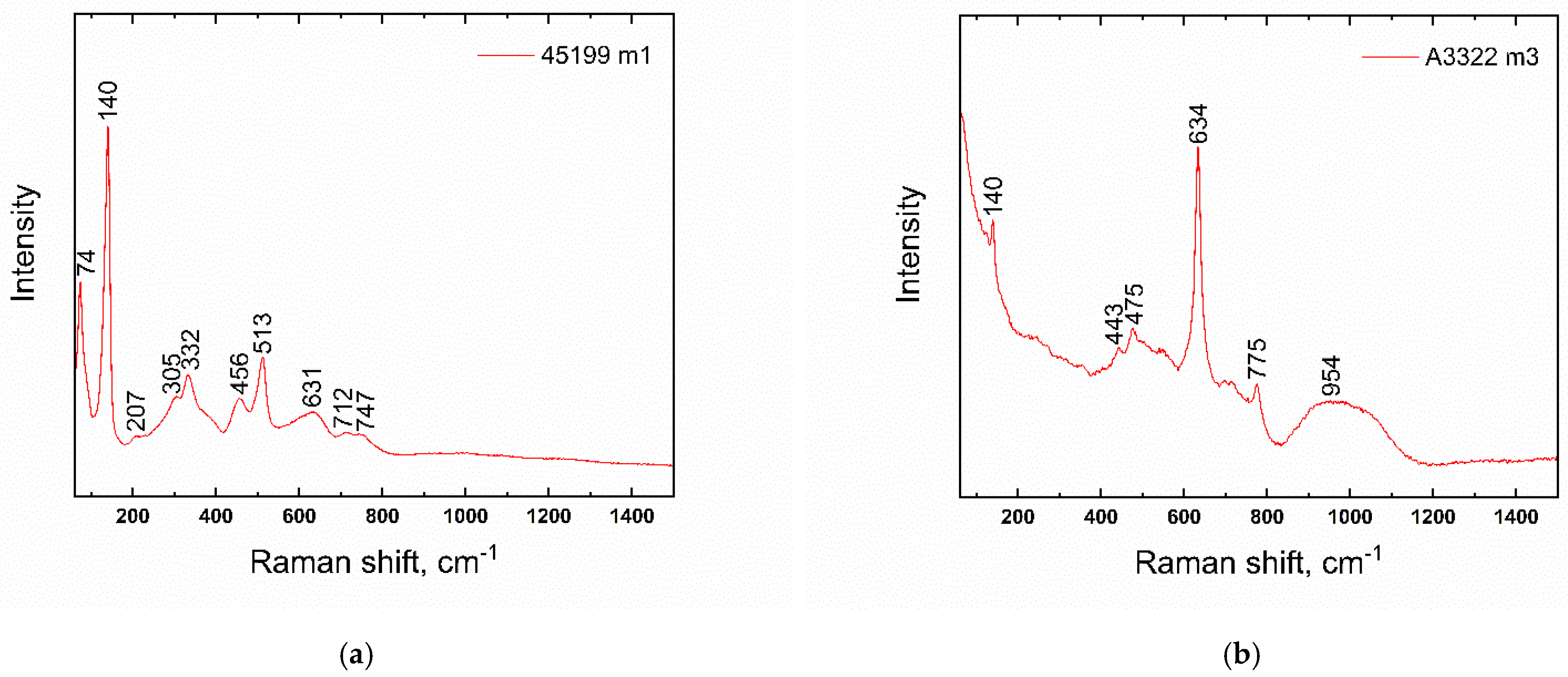
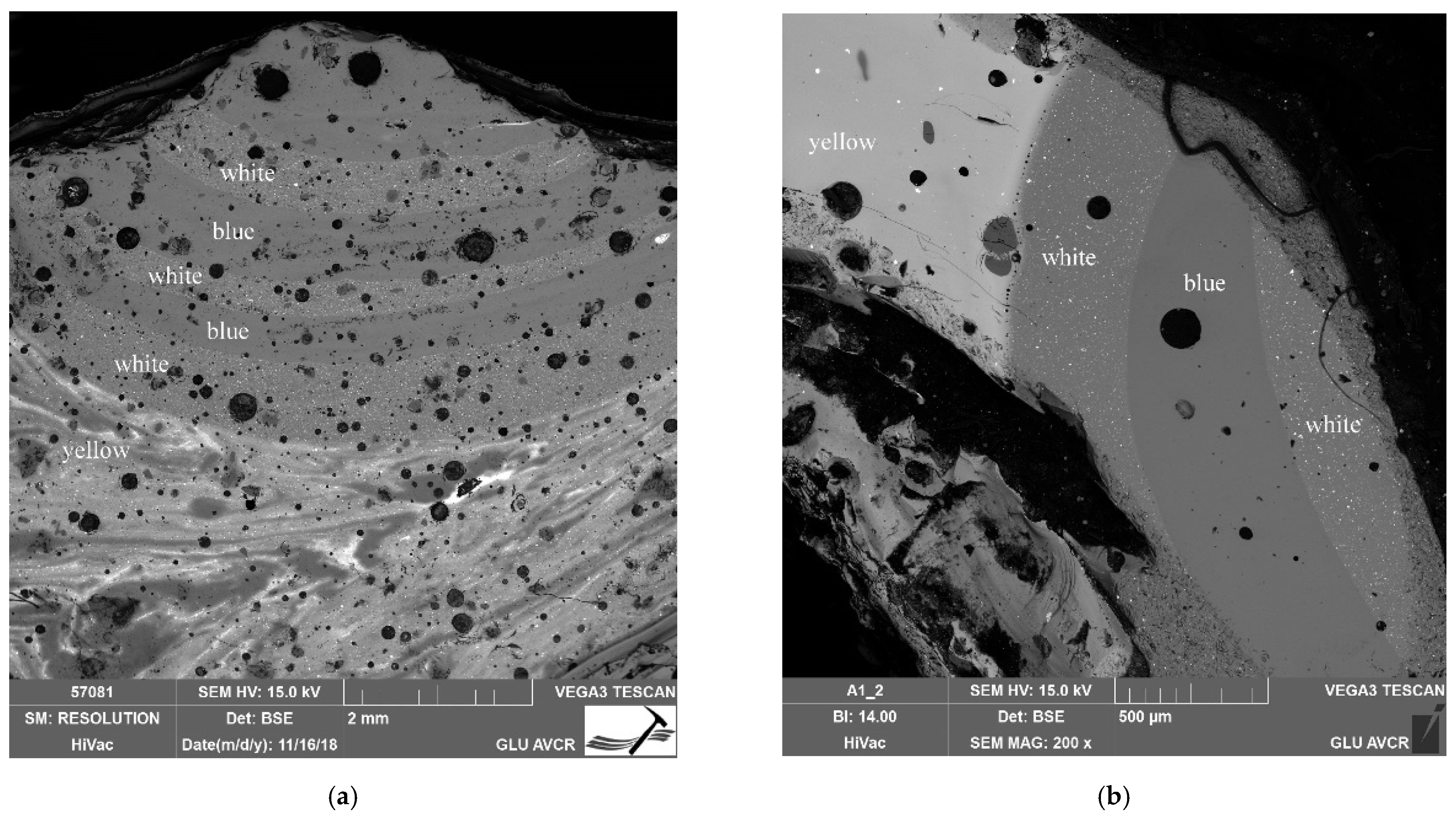
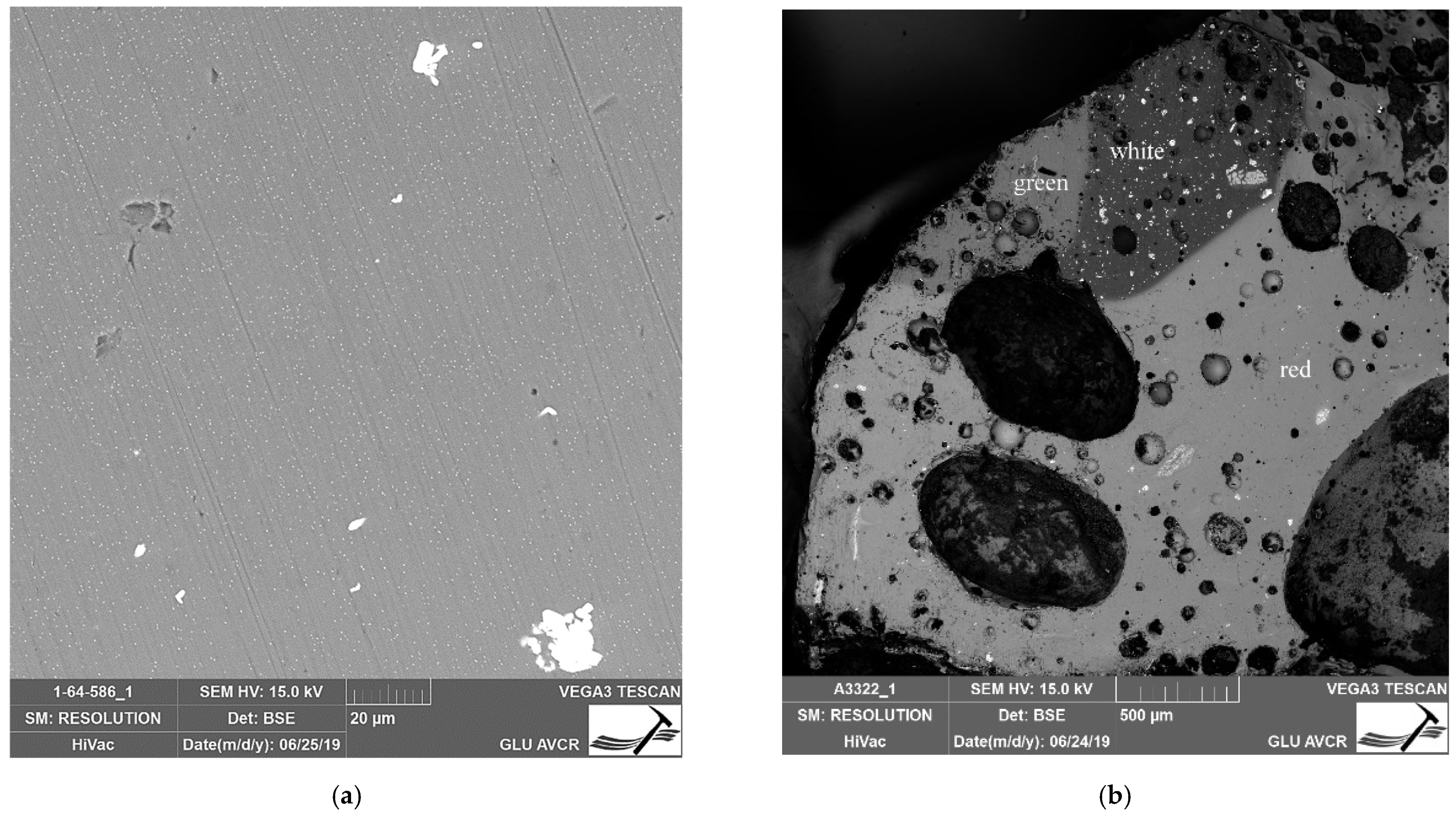
| Number | Inventory/Museum Number | Site | Basic Description | Find Context | Chronology | Type |
|---|---|---|---|---|---|---|
| 1 | H1–111324 | Křepice hillfort | fusiform/cylindrical bead with spiral decoration | settlement, accidental find | Ha B (1000–800 BC) | pfahlbauperle/type 4 after Venclová 1990 |
| 2 | H1–46623 | Prague, Střešovice | blue rounded bead | necropolis, inhumation grave | Ha C (800–600 BC) | type 119 after Venclová 1990 |
| 3 | H1–110925 | Bylany | blue rounded bead | necropolis, grave №16/1897 | Ha C (800–600 BC) | type 121 after Venclová 1990 |
| 4 | H1–45060 | Prague, Bubeneč | blue annular | necropolis, cremation grave №1/31 | Ha D (600–450 BC) | type 155 after Venclová 1990 |
| 5 | H1–111320 | Křepice hillfort | blue rounded | settlement, accidental find | Ha C–LT (800–50 BC)? | type 119 after Venclová 1990 |
| 6 | H1–111321 | Křepice hillfort | blue with wavy line | settlement, accidental find | Ha C–LT (800–50 BC) | type 708–710 after Venclová 1990 |
| 7 | UAPPSČ | Chotýš | blue annular | settlement | Ha D2–LT A (550–380 BC) | type 155 after Venclová 1990 |
| 8 | A 4471 a | Lhota–Závist hillfort | blue rounded | settlement, acropolis | Ha D2–LT A (550–380 BC) | type 119 after Venclová 1990 |
| 9 | H1–57008 | Mydlovary | blue with white wavy line | ? | Ha C–LT (800–50 BC)? | type 707 after Venclová 1990 |
| 10 | A 4471 b | Lhota–Závist hillfort | blue rounded, double layer | settlement, acropolis | Ha D2–LT A (550–380 BC) | type 119 after Venclová 1990 |
| 11 | H1–15394 | Černuc | blue rounded, double layer | cremation grave ? | Ha D (600–450 BC)? | type 119 after Venclová 1990 |
| 12 | C 214 | Lhota–Závist hillfort | blue-green with compound eyes | settlement, acropolis | Ha D2–LT A (550–380 BC) | type 549 after Venclová 1990 |
| 13 | F 390 | Lhota–Závist hillfort, gate D | translucent blue flattened globular bead with spiral decoration | settlement | Ha D2–LT D (550–380 BC) | type 414 after Venclová 1990 |
| 14 | H1–26276 | Žalov | blue with blue-white eyes | ? | Ha C–D (800–450 BC) | type 520 after Venclová 1990 |
| 15 | H1–225671 | Platěnice | blue with yellow circules | necropolis, cremation grave №60 | Ha C (800–600 BC) | type 552 after Venclová 1990 |
| 16 | H1–26286 | Žalov (u Prahy) | yellow with blue-white eyes | ? | Ha C–D (800–450 BC) | type 533 after Venclová 1990 |
| 17 | H1–26773 | Prague, Nové Město | yellow with blue-white eyes, deformed | ? | Ha C–D (800–450 BC) | type 533 after Venclová 1990 |
| 18 | H1–26922 | Pátek | yellow with blue-white eyes | inhumation graves? | Ha C–D (800–450 BC) | type 519 after Venclová 1990 |
| 19 | H1–26923 | Pátek | yellow with blue-white eyes | inhumation graves? | Ha C–D (800–450 BC) | type 519 after Venclová 1990 |
| 20 | H1–45196 | Prague, Bubeneč | yellow with blue-white eyes | necropolis, inhumation grave | Ha D (600–450 BC) | type 533 after Venclová 1990 |
| 21 | H1–45199 | Prague, Bubeneč | yellow with blue-white eyes | necropolis, inhumation grave | Ha D (600–450 BC) | type 533 after Venclová 1990 |
| 22 | H1–45200 | Prague, Bubeneč | yellow with blue-white eyes | necropolis, inhumation grave | Ha D (600–450 BC) | type 539 after Venclová 1990 |
| 23 | H1–57001 | Lochovice | yellow with blue-white eyes and yellow prunts | burial mound | Ha D2–LT A (550–380 BC) | type 548 after Venclová 1990 |
| 24 | AS 18/3/3 | Holubice | yellow with blue-white eyes and yellow prunts | settlement | Ha D2–LT A (550–380 BC) | type 548 after Venclová 1990 |
| 25 | H1–57005 | Rtišovice | yellow with blue-white eyes | ? | Ha C–D (800–450 BC) | type 519 after Venclová 1990 |
| 26 | H1–40054 | Láz | yellow with blue-white eyes | necropolis, mound IX/1922 | Ha D2/Ha D3 (550–450 BC) | type 531 after Venclová 1990 |
| 27 | A 3031 | Lhota–Závist hillfort | yellow with blue-white eyes | settlement, acropolis | Ha D2–LT A (550–380 BC) | type 519 after Venclová 1990 |
| 28 | A 4309 | Lhota–Závist hillfort | yellow with blue-white eyes, deformed | settlement, acropolis | LT A (450–380 BC) | ? |
| 29 | H1–111202 | Straškov | green rounded | inhumation grave | Ha C (800–600 BC) | type 132 after Venclová 1990 |
| 30 | H1–600070 | Minice hillfort | green rounded | settlement | Ha D (600–450 BC) | type 132 after Venclová 1990 |
| 31 | F 1762 | Lhota–Závist hillfort | ring colourless | settlement, gate D | Ha D (600–450 BC) | type 34 after Venclová 1990 |
| 32 | A 3322 | Lhota–Závist hillfort | larger rounded bead with line decoration | settlement, acropolis | Migration period (380–570 AD) | – |
| 33 | 1/64_586 | Lhota–Závist hillfort | cylindrical bead | settlement, acropolis, secondary position | Migration period (380–570 AD)? | type 147 after Venclová 1990 |
| 34 | H1–111322 | Křepice hillfort | orange rounded | settlement, accidental find | recent | type 124 after Venclová 1990_amber |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlámalová Cílová, Z.; Čisťakova, V.; Kozáková, R.; Lapčák, L. Chemistry and Production Technology of Hallstatt Period Glass Beads from Bohemia. Materials 2022, 15, 5740. https://doi.org/10.3390/ma15165740
Zlámalová Cílová Z, Čisťakova V, Kozáková R, Lapčák L. Chemistry and Production Technology of Hallstatt Period Glass Beads from Bohemia. Materials. 2022; 15(16):5740. https://doi.org/10.3390/ma15165740
Chicago/Turabian StyleZlámalová Cílová, Zuzana, Viktoria Čisťakova, Romana Kozáková, and Ladislav Lapčák. 2022. "Chemistry and Production Technology of Hallstatt Period Glass Beads from Bohemia" Materials 15, no. 16: 5740. https://doi.org/10.3390/ma15165740
APA StyleZlámalová Cílová, Z., Čisťakova, V., Kozáková, R., & Lapčák, L. (2022). Chemistry and Production Technology of Hallstatt Period Glass Beads from Bohemia. Materials, 15(16), 5740. https://doi.org/10.3390/ma15165740






