Novel SrO-Containing Glass-Ceramic Sealants for Solid Oxide Electrolysis Cells (SOEC): Their Design and Characterization under Relevant Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
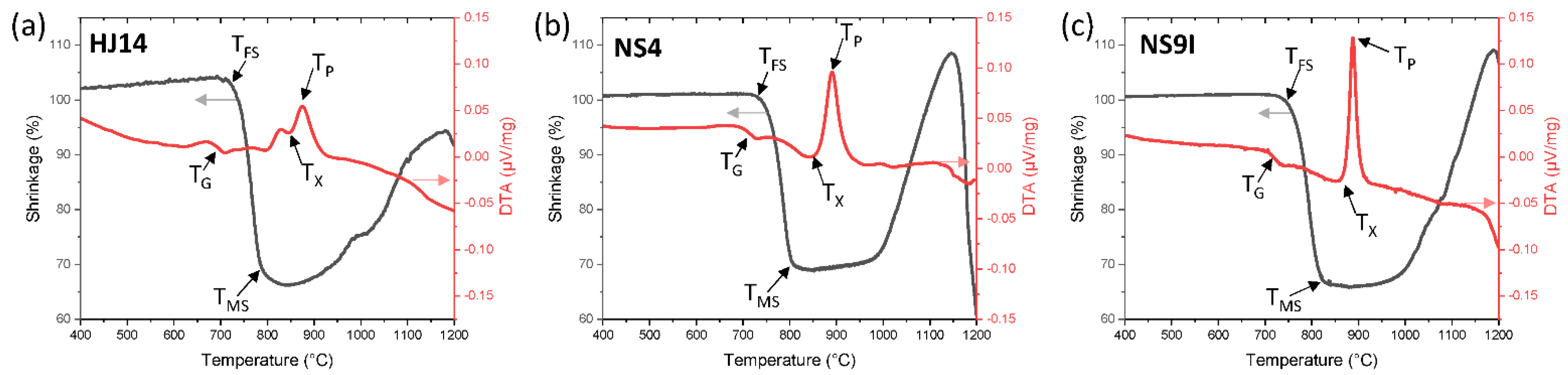
3.1. Thermal Analysis
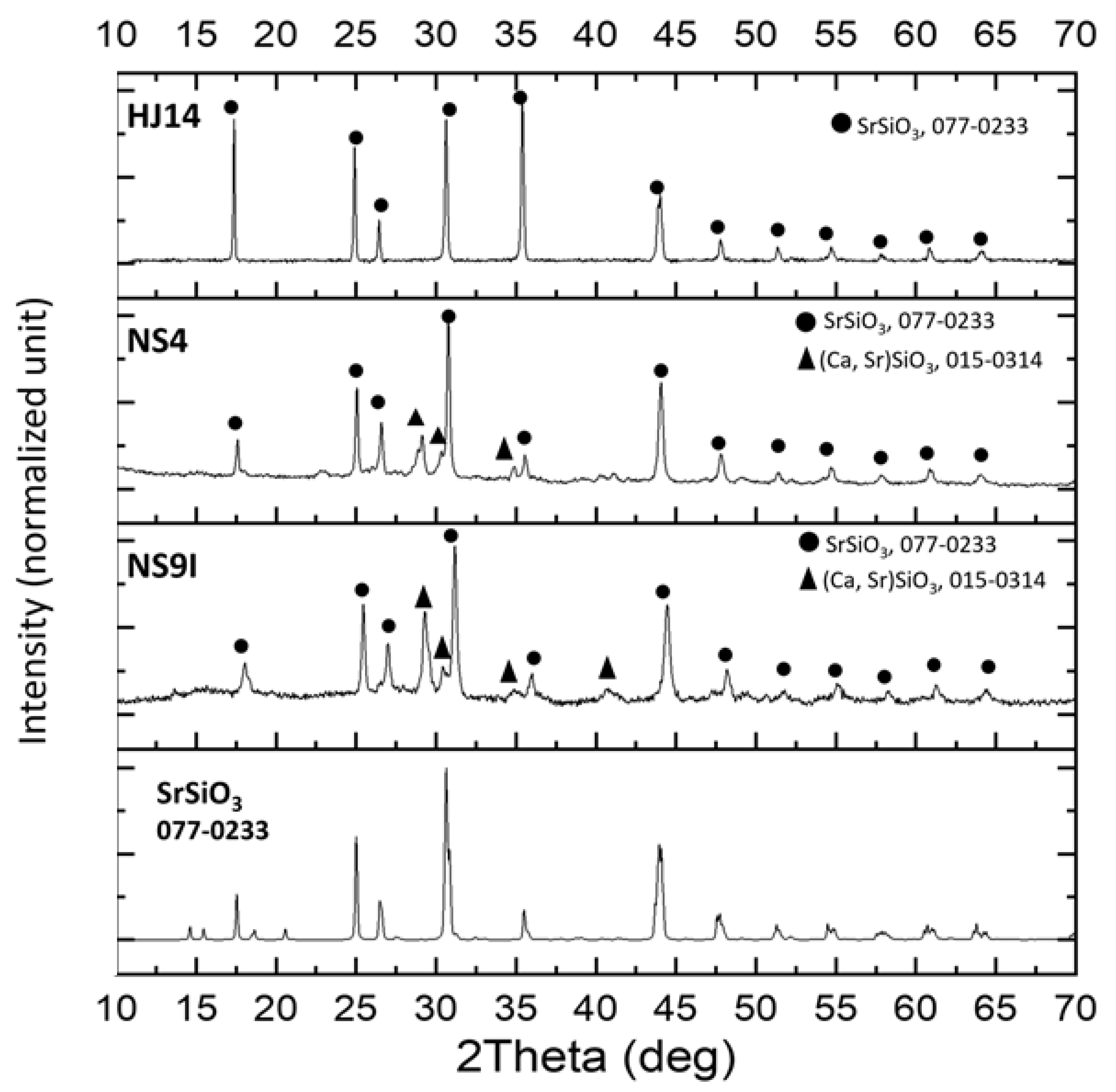
3.2. XRD Analysis of the Crystalline Phases
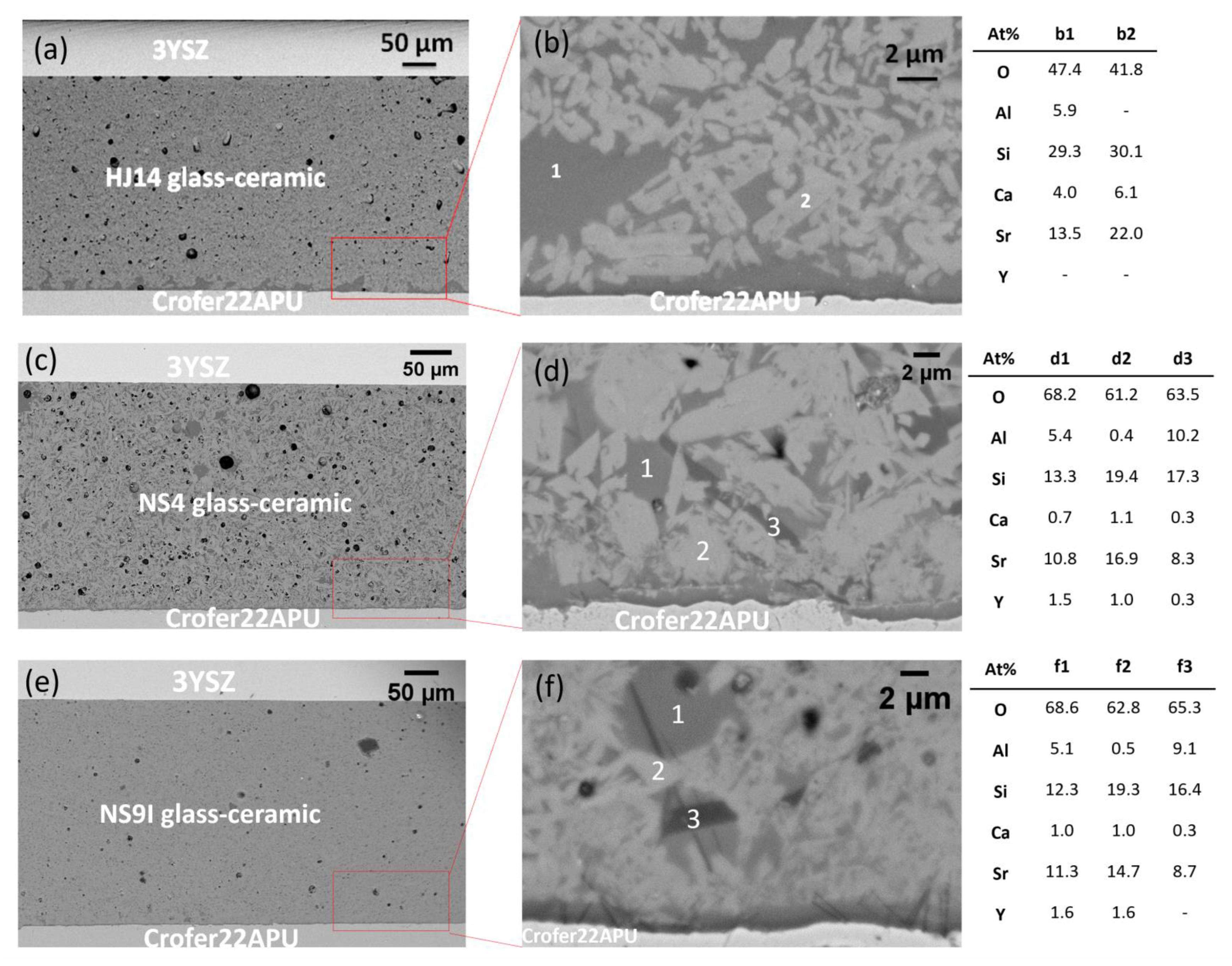
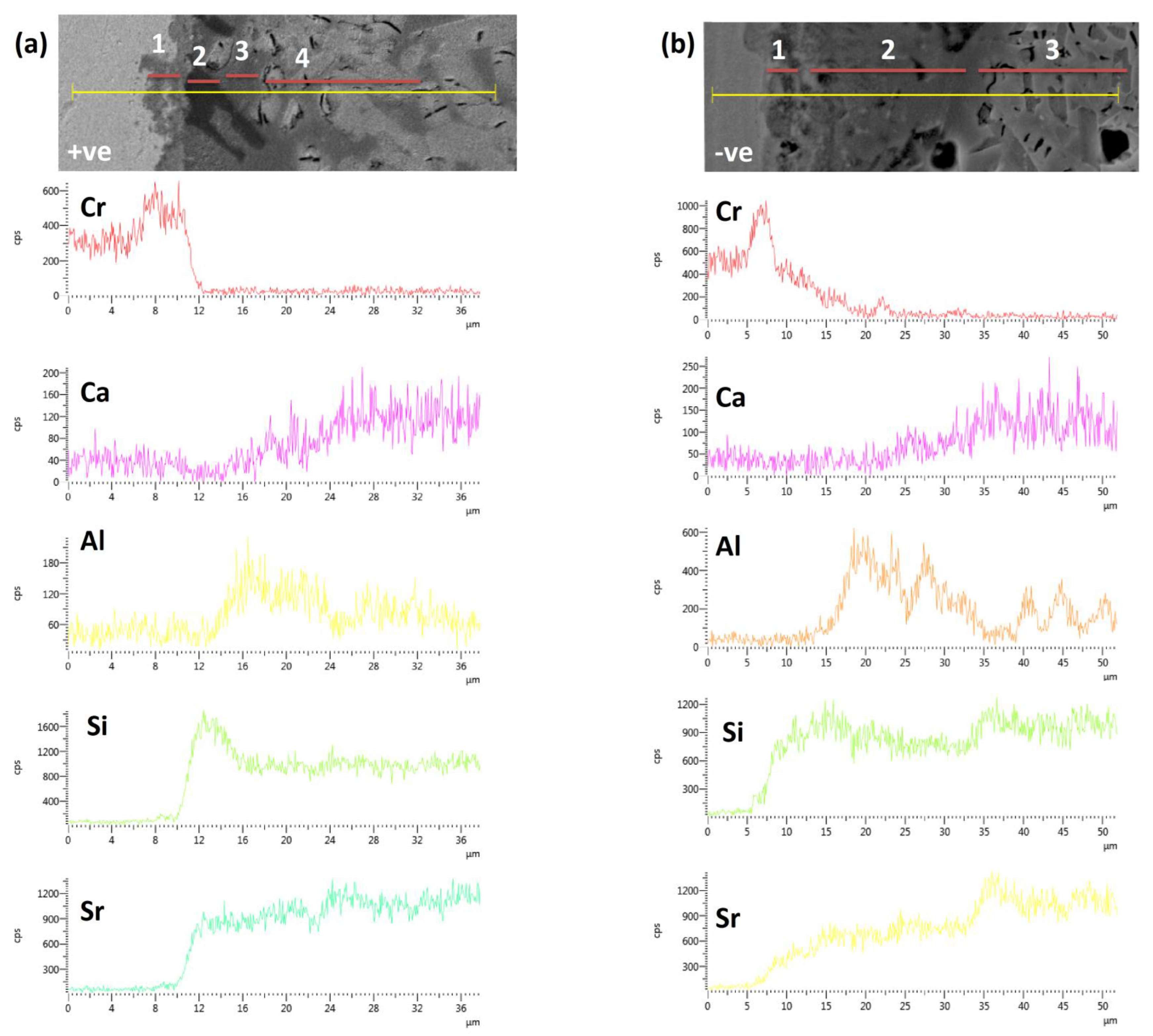
3.3. Morphological and Compositional Analysis
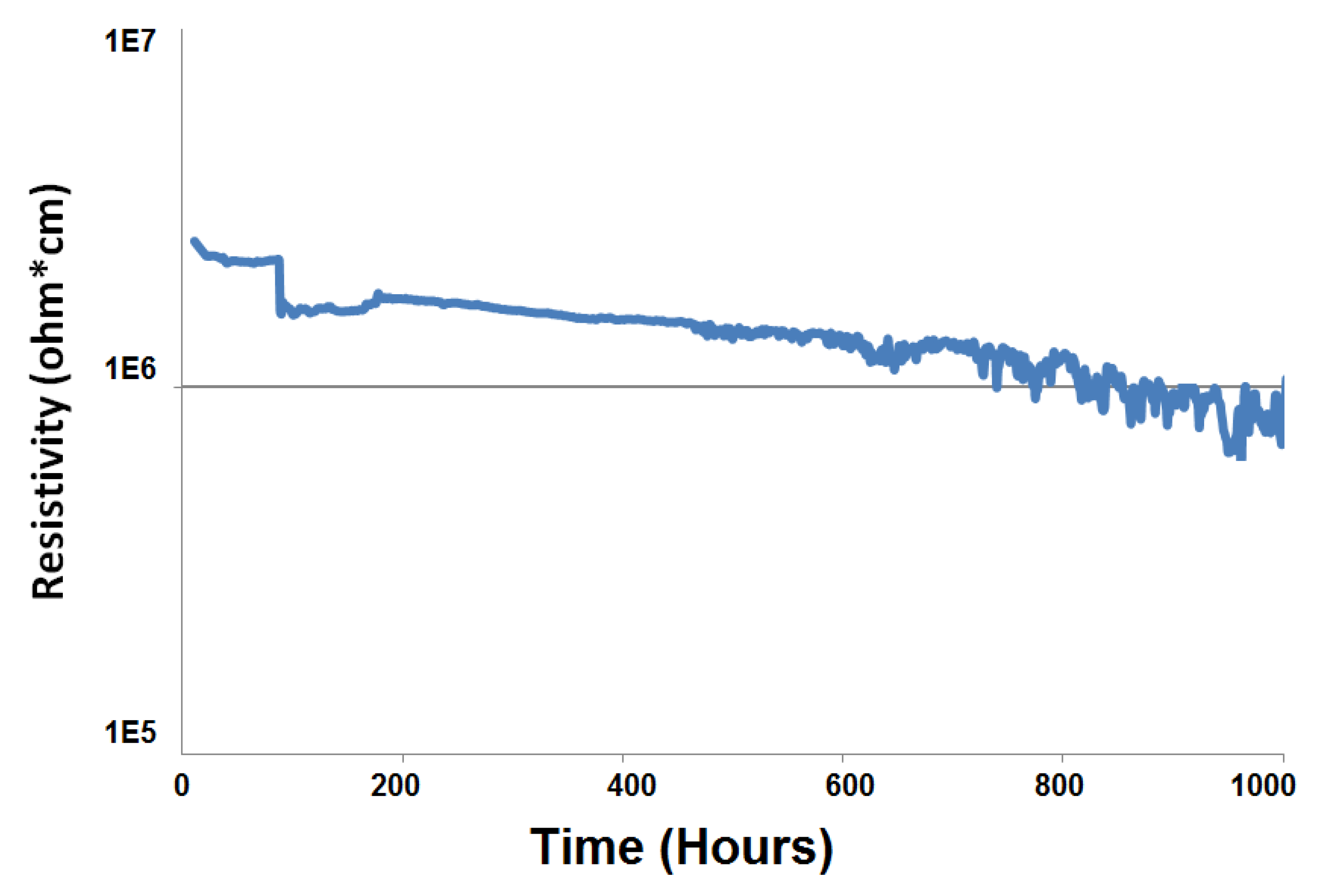
3.4. Electrical Resistivity Analyses in a Dual Atmosphere
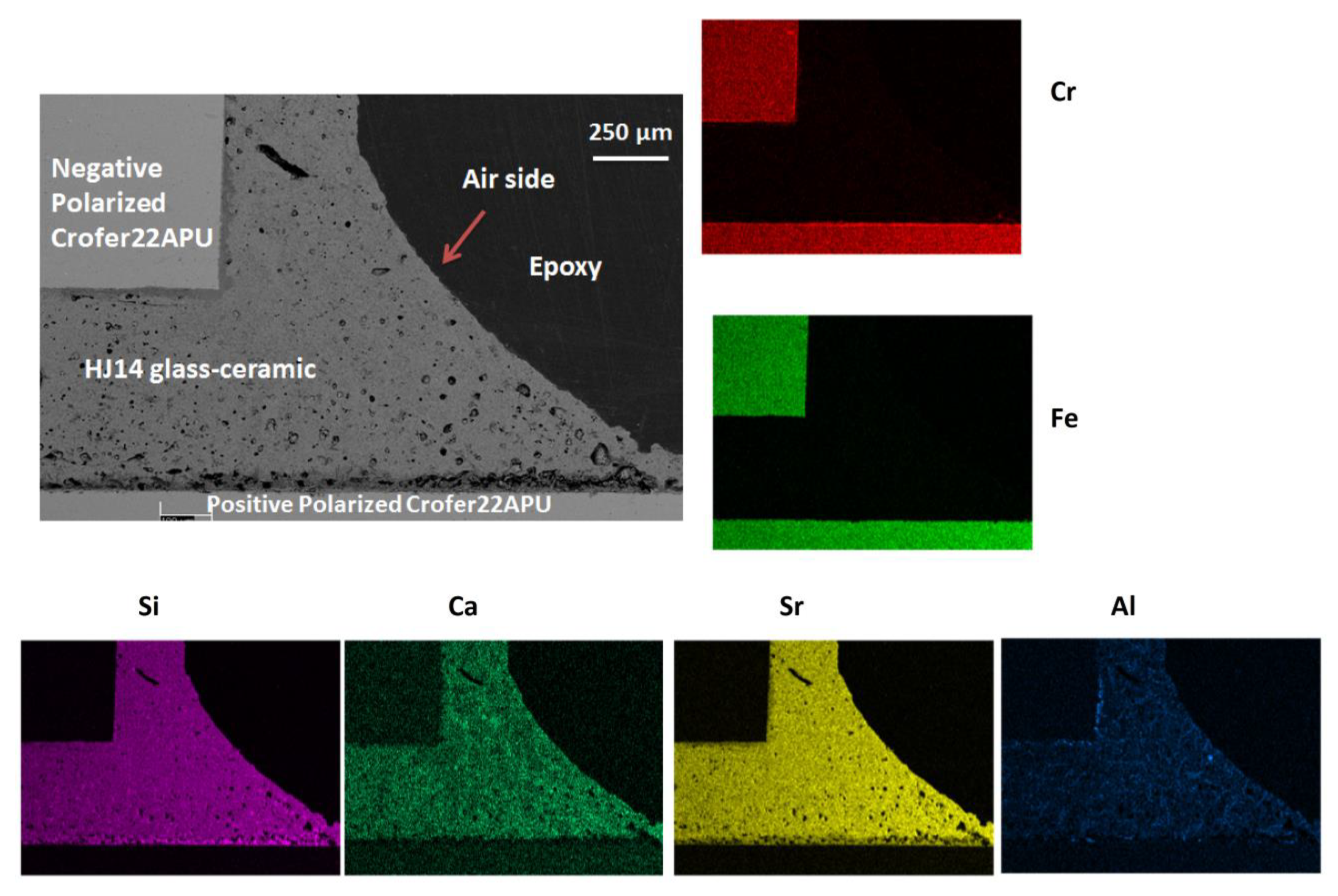
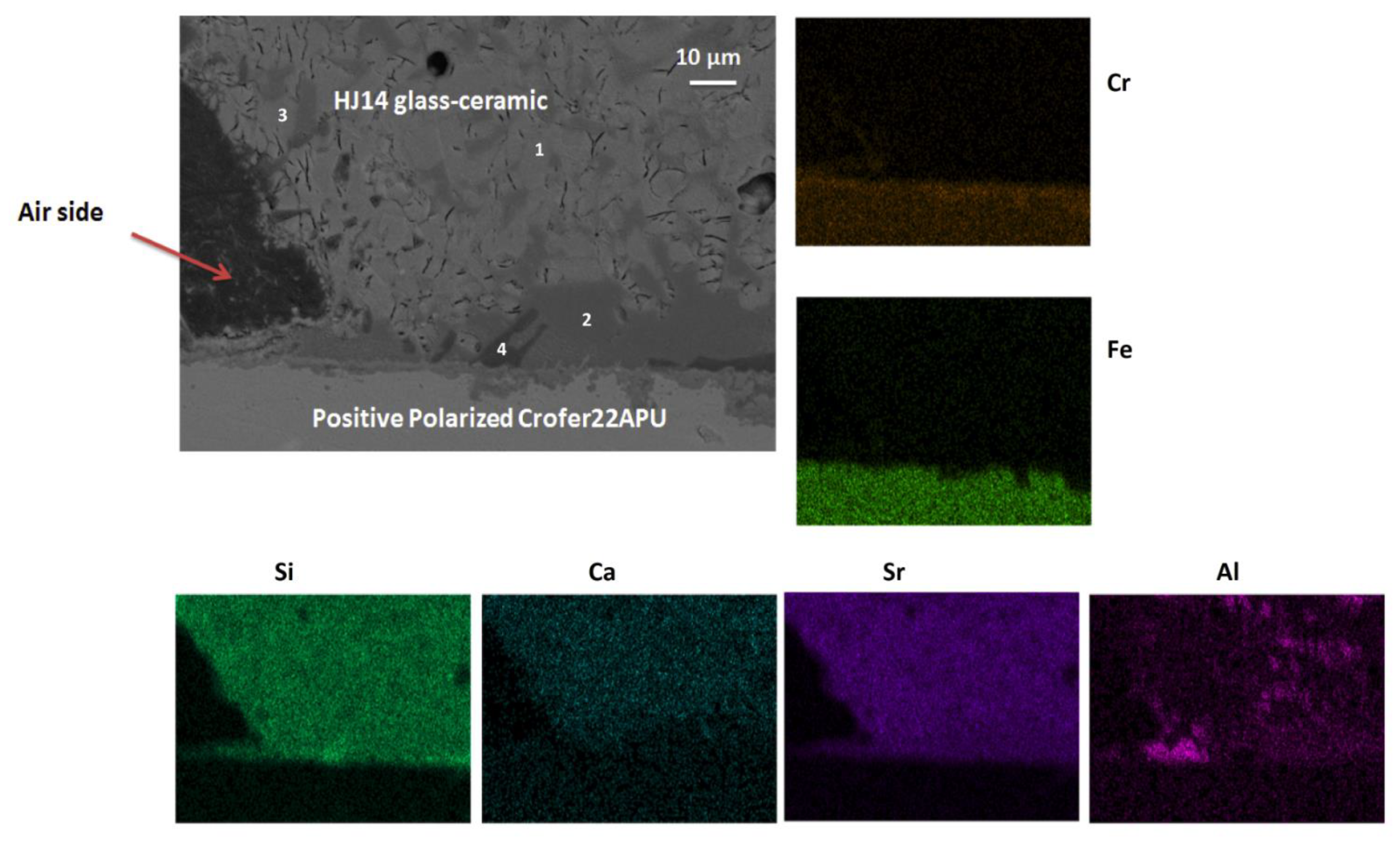
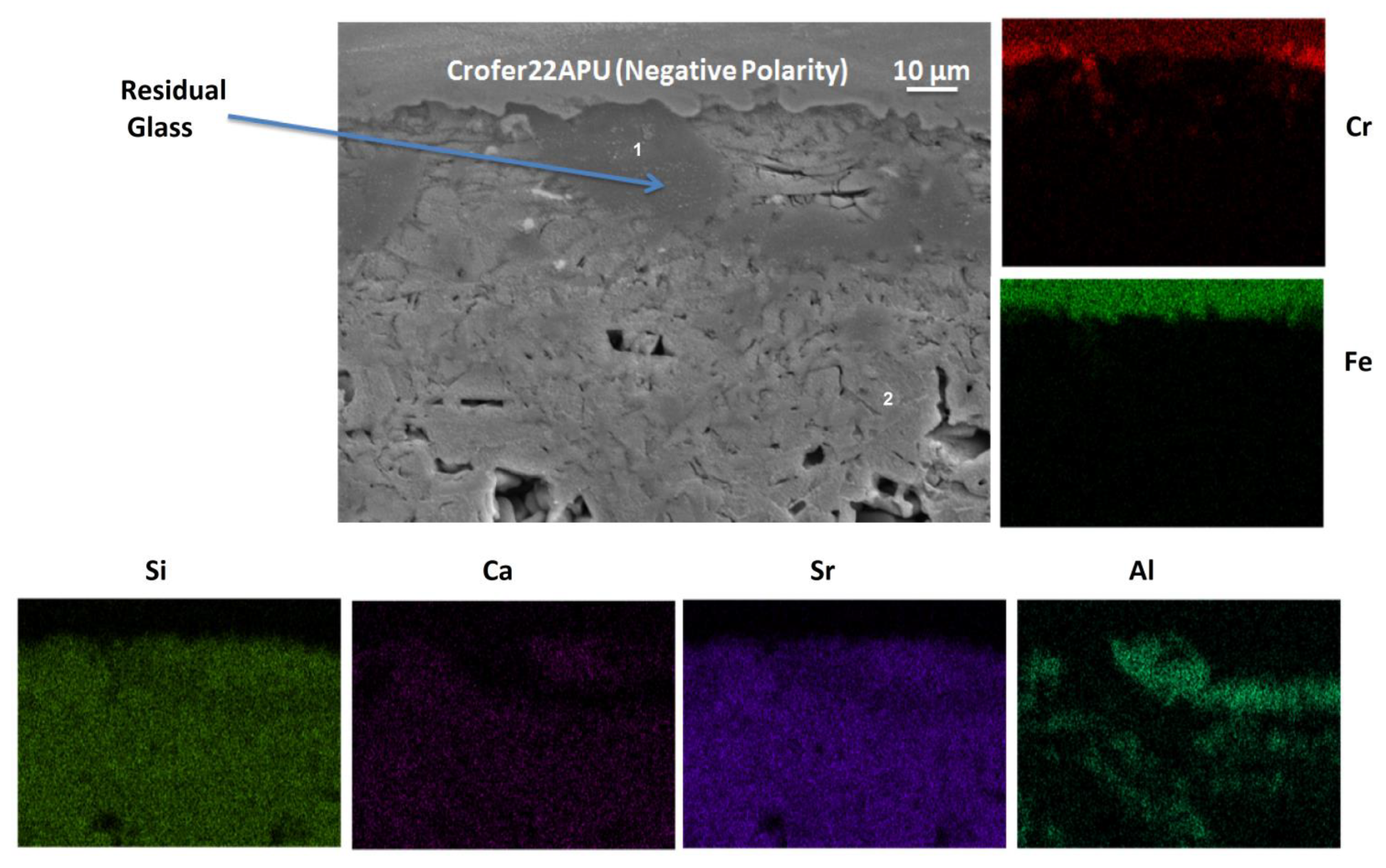
3.5. Post-Mortem Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
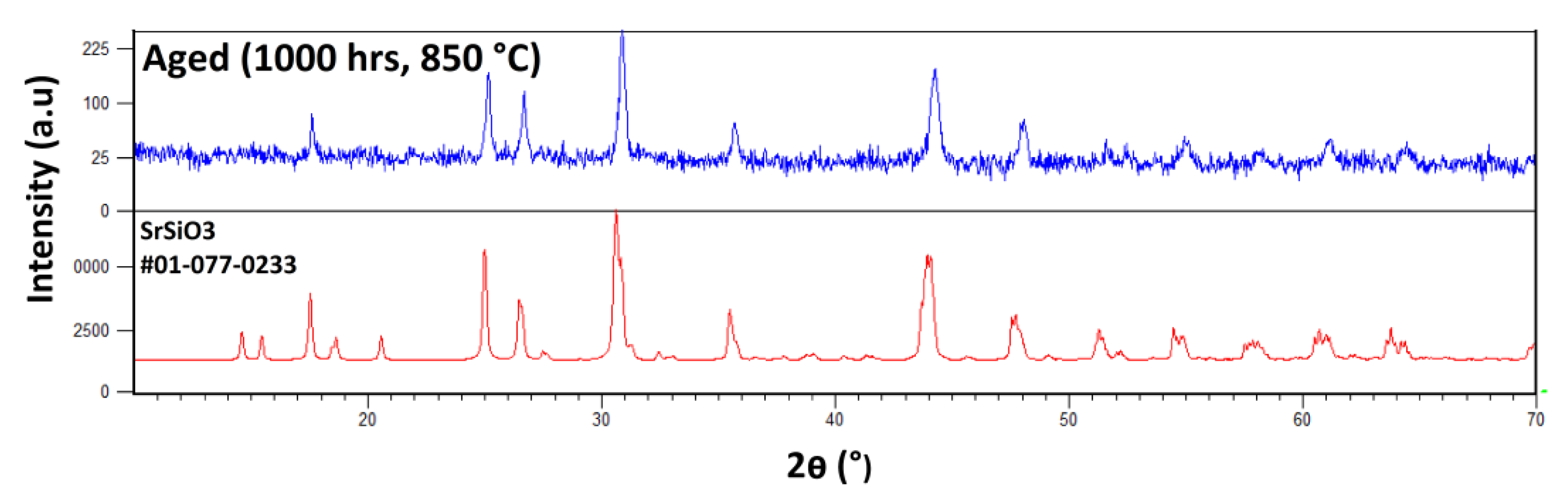
References
- Bi, L.; Boulfrad, S.; Traversa, E. Reversible solid oxide fuel cells (R-SOFCs) with chemically stable proton-conducting oxides. Solid State Ion. 2015, 275, 101–105. [Google Scholar] [CrossRef]
- Elsayed, H.; Javed, H.; Sabato, A.G.; Smeacetto, F.; Bernardo, E. Novel glass-ceramic SOFC sealants from glass powders and a reactive silicone binder. J. Eur. Ceram. Soc. 2018, 38, 4245–4251. [Google Scholar] [CrossRef]
- Rotureau, D.; Viricelle, J.P.; Pijolat, C.; Caillol, N.; Pijolat, M. Development of a planar SOFC device using screen-printing technology. J. Eur. Ceram. Soc. 2005, 25, 2633–2636. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Mogensen, M. Electrolysis of carbon dioxide in solid oxide electrolysis cells. J. Power Sources 2009, 193, 349–358. [Google Scholar] [CrossRef]
- Fujiwara, S.; Kasai, S.; Yamauchi, H.; Yamada, K.; Makino, S.; Matsunaga, K.; Yoshino, M.; Kameda, T.; Ogawa, T.; Momma, S.; et al. Hydrogen production by high temperature electrolysis with nuclear reactor. Prog. Nucl. Energy 2008, 50, 422–426. [Google Scholar] [CrossRef]
- Mahata, A.; Datta, P.; Basu, R.N. Microstructural and chemical changes after high temperature electrolysis in solid oxide electrolysis cell. J. Alloys Compd. 2015, 627, 244–250. [Google Scholar] [CrossRef]
- Jensen, S.H.; Graves, C.; Mogensen, M.; Wendel, C.; Braun, R.; Hughes, G.; Gao, Z.; Barnett, S.A. Large-scale electricity storage utilizing reversible solid oxide cells combined with underground storage of CO2 and CH4. Energy Environ. Sci. 2015, 8, 2471–2479. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K. Glass-based seals for solid oxide fuel and electrolyzer cells—A review. Mater. Sci. Eng. R Rep. 2010, 67, 65–85. [Google Scholar] [CrossRef]
- Drewniak, A.; Koszelow, D.; Błaszczak, P.; Górnicka, K.; Jurak, K.; Javed, H.; Sabato, A.G.; Jasiński, P.; Molin, S.; Smeacetto, F. Glass-ceramic sealants and steel interconnects: Accelerated interfacial stability and reactivity tests at high temperature. Mater. Des. 2021, 212, 110259. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K. Effect of atmosphere on interconnect-seal glass interaction for solid oxide fuel/electrolyzer cells. J. Am. Ceram. Soc. 2011, 94, 875–885. [Google Scholar] [CrossRef]
- Kim, S.D.; Seo, D.W.; Dorai, A.K.; Woo, S.K. The effect of gas compositions on the performance and durability of solid oxide electrolysis cells. Int. J. Hydrogen Energy 2013, 38, 6569–6576. [Google Scholar] [CrossRef]
- Lawlor, V. Review of the micro-tubular solid oxide fuel cell (Part II: Cell design issues and research activities). J. Power Sources 2013, 240, 421–441. [Google Scholar] [CrossRef]
- Javed, H.; Sabato, A.G.; Herbrig, K.; Ferrero, D.; Walter, C.; Salvo, M.; Smeacetto, F. Design and characterization of novel glass-ceramic sealants for solid oxide electrolysis cell (SOEC) applications. Int. J. Appl. Ceram. Technol. 2018, 15, 999–1010. [Google Scholar] [CrossRef]
- Lara, C.; Pascual, M.J.; Keding, R.; Durán, A. Electrical behaviour of glass–ceramics in the systems RO–BaO–SiO2 (R = Mg, Zn) for sealing SOFCs. J. Power Sources 2006, 157, 377–384. [Google Scholar] [CrossRef]
- Menzler, N.H.; Sebold, D.; Zahid, M.; Gross, S.M.; Koppitz, T. Interaction of metallic SOFC interconnect materials with glass–ceramic sealant in various atmospheres. J. Power Sources 2005, 152, 156–167. [Google Scholar] [CrossRef]
- Batfalsky, P.; Haanappel, V.A.C.; Malzbender, J.; Menzler, N.H.; Shemet, V.; Vinke, I.C.; Steinbrech, R.W. Chemical interaction between glass–ceramic sealants and interconnect steels in SOFC stacks. J. Power Sources 2006, 155, 128–137. [Google Scholar] [CrossRef]
- Kaur, G. Solid Oxide Fuel Cell Components: Seal Glass for Solid Oxide Fuel Cells; Springer: Cham, Switzerland, 2006; Volume 58, ISBN 978-3-319-25596-5. [Google Scholar]
- Jin, T.; Lu, K. Thermal stability of a new solid oxide fuel/electrolyzer cell seal glass. J. Power Sources 2010, 195, 195–203. [Google Scholar] [CrossRef]
- Greven, B.C.; Gross-Barsnick, S.; Koppitz, T.; Conradt, R.; Smeacetto, F.; Ventrella, A.; Ferraris, M. Torsional shear strength of novel glass-ceramic composite sealants for solid oxide fuel cell stacks. Int. J. Appl. Ceram. Technol. 2018, 15, 286–295. [Google Scholar] [CrossRef]
- Malzbender, J.; Mönch, J.; Steinbrech, R.W.; Koppitz, T.; Gross, S.M.; Remmel, J. Symmetric shear test of glass-ceramic sealants at SOFC operation temperature. J. Mater. Sci. 2007, 42, 6297–6301. [Google Scholar] [CrossRef]
- Smeacetto, F.; Salvo, M.; Ferraris, M.; Cho, J.; Boccaccini, A.R. Glass–ceramic seal to join Crofer 22 APU alloy to YSZ ceramic in planar SOFCs. J. Eur. Ceram. Soc. 2008, 28, 61–68. [Google Scholar] [CrossRef]
- Smeacetto, F.; De Miranda, A.; Chrysanthou, A.; Bernardo, E.; Secco, M.; Bindi, M.; Salvo, M.; Sabato, A.G.; Ferraris, M. Novel glass-ceramic composition as sealant for SOFCs. J. Am. Ceram. Soc. 2014, 97, 3835–3842. [Google Scholar] [CrossRef]
- Chou, Y.S.; Stevenson, J.W.; Singh, P. Effect of pre-oxidation and environmental aging on the seal strength of a novel high-temperature solid oxide fuel cell (SOFC) sealing glass with metallic interconnect. J. Power Sources 2008, 184, 238–244. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K. Seal glass for solid oxide fuel cells. J. Power Sources 2010, 195, 7129–7139. [Google Scholar] [CrossRef]
- Lin, S.E.; Cheng, Y.R.; Wei, W.C.J. BaO–B2O3–SiO2–Al2O3 sealing glass for intermediate temperature solid oxide fuel cell. J. Non-Cryst. Solids 2012, 358, 174–181. [Google Scholar] [CrossRef]
- Kothiyal, G.P.; Goswami, M.; Tiwari, B.; Sharma, K.; Ananthanarayanan, A.; Montagne, L. Some recent studies on glass/glass-ceramics for use as sealants with special emphasis for high temperature applications. J. Adv. Ceram. 2012, 1, 110–129. [Google Scholar] [CrossRef]
- Fergus, J.W. Sealants for solid oxide fuel cells. J. Power Sources 2005, 147, 46–57. [Google Scholar] [CrossRef]
- Sohn, S.-B.; Choi, S.-Y.; Kim, G.-H.; Song, H.-S.; Kim, G.-D. Suitable glass-ceramic sealant for planar solid-oxide fuel cells. J. Am. Ceram. Soc. 2004, 87, 254–260. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K.; Bodnar, R.J. Network structure and thermal property of a novel high temperature seal glass. Appl. Phys. A 2009, 95, 493–500. [Google Scholar] [CrossRef]
- Lara, C.; Pascual, M.J.; Durán, A. Glass-forming ability, sinterability and thermal properties in the systems RO-BaO-SiO2(R = Mg, Zn). J. Non-Cryst. Solids 2004, 348, 149–155. [Google Scholar] [CrossRef]
- Pascual, M.J.; Guillet, A.; Durán, A. Optimization of glass–ceramic sealant compositions in the system MgO-BaO-SiO2 for solid oxide fuel cells (SOFC). J. Power Sources 2007, 169, 40–46. [Google Scholar] [CrossRef]
- Silva, M.J.D.; Bartolomé, J.F.; Aza, A.H.D.; Mello-Castanh, S. Glass ceramic sealants belonging to BAS ( BaO-Al2O3-SiO2) ternary system modified with B2O3 addition: A different approach to access the SOFC seal issue. J. Eur. Ceram. Soc. 2016, 36, 631–644. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, Q. Thermal cycle stability of BaO-B2O3-SiO2 sealing glass. J. Power Sources 2009, 194, 880–885. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, Q. The Development of thermally stable sealing glass in the BaO-B2O3-SiO2 system for planar SOFC applications. J. Fuel Cell Sci. Technol. 2008, 5, 031210. [Google Scholar] [CrossRef]
- Kerstan, M.; Müller, M.; Rüssel, C. High temperature thermal expansion of BaAl2Si2O8, CaAl2Si2O8, and Ca2Al2SiO7 studied by high-temperature X-ray diffraction (HT-XRD). Solid State Sci. 2014, 38, 119–123. [Google Scholar] [CrossRef]
- Bansal, N.P.; Gamble, E.A. Crystallization kinetics of a solid oxide fuel cell seal glass by differential thermal analysis. J. Power Sources 2005, 147, 107–115. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K.; Reynolds, W.T. Thermophysical properties and devitrification of SrO-La2O3-Al2O3-B2O3-SiO2-based glass sealant for solid oxide fuel/electrolyzer cells. J. Power Sources 2008, 179, 106–112. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, H.; Zeng, F.; Wang, S.; Tang, D.; Zhang, T. Development of the CaO-SrO-ZrO2-B2O3-SiO2 sealing glasses for solid oxide fuel cell applications: Structure. RSC Adv. 2015, 5, 41772–41779. [Google Scholar] [CrossRef]
- Wang, X.; Ou, D.R.; Zhao, Z.; Cheng, M. Stability of SrO-La2O3-Al2O3-SiO2 glass sealants in high-temperature air and steam. Ceram. Int. 2016, 42, 7514–7523. [Google Scholar] [CrossRef]
- Rodríguez-López, S.; Wei, J.; Laurenti, K.C.; Mathias, I.; Justo, V.M.; Serbena, F.C.; Baudín, C.; Malzbender, J.; Pascual, M.J. Mechanical properties of solid oxide fuel cell glass-ceramic sealants in the system BaO/SrO-MgO-B2O3-SiO2. J. Eur. Ceram. Soc. 2017, 37, 3579–3594. [Google Scholar] [CrossRef]
- Ojha, P.K.; Chongdar, T.K.; Gokhale, N.M.; Kulkarni, A.R. Investigation of crystallization kinetic of SrO-La2O3-Al2O3-B2O3-SiO2 glass and its suitability for SOFC sealant. Int. J. Hydrog. Energy 2011, 36, 14996–15001. [Google Scholar] [CrossRef]
- Reddy, A.A.; Goel, A.; Tulyaganov, D.U.; Sardo, M.; Mafra, L.; Pascual, M.J.; Kharton, V.V.; Tsipis, E.V.; Kolotygin, V.A.; Ferreira, J.M.F. Thermal and mechanical stability of lanthanide-containing glass–ceramic sealants for solid oxide fuel cells. J. Mater. Chem. A 2014, 2, 1834. [Google Scholar] [CrossRef]
- Javed, H.; Sabato, A.G.; Dlouhy, I.; Halasova, M.; Bernardo, E.; Salvo, M.; Herbrig, K.; Walter, C.; Smeacetto, F. Shear performance at room and high temperatures of glass–ceramic sealants for solid oxide electrolysis cell technology. Materials 2019, 12, 298. [Google Scholar] [CrossRef]
- Javed, H.; Herbrig, K.; Sabato, A.G.; Ferrero, D.; Santarelli, M.; Walter, C.; Smeacetto, F. Electrical characterization of glass-ceramic sealant-metallic interconnect joined samples under solid oxide electrolysis cell conditions; influence on the microstructure and composition at the different polarized interfaces. Ceram. Int. 2021, 47, 8184–8190. [Google Scholar] [CrossRef]
- Chou, Y.S.; Stevenson, J.W.; Xia, G.G.; Yang, Z.G. Electrical stability of a novel sealing glass with (Mn, Co)-spinel coated Crofer22APU in a simulated SOFC dual environment. J. Power Sources 2010, 195, 5666–5673. [Google Scholar] [CrossRef]
- Thieme, C.; Rüssel, C. Thermal expansion behavior of SrSiO3 and Sr2SiO4 determined by high-temperature X-ray diffraction and dilatometry. J. Mater. Sci. 2015, 50, 5533–5539. [Google Scholar] [CrossRef]
- Romero-Serrano, A.; Cruz-Ramirez, A.; Zeifert, B.; Hallen-Lopez, M.; Hernandez-Ramirez, A. Thermodynamic modeling of the BaO-SiO2 and SrO-SiO2 binary melts. Glass Phys. Chem. 2010, 36, 171–178. [Google Scholar] [CrossRef]
- Beals, M.D.; Zerfoss, S. Volume change attending low-to-high inversion of cristobalite. J. Am. Ceram. Soc. 1944, 27, 285–292. [Google Scholar] [CrossRef]
- Javed, H.; Sabato, A.G.; Mansourkiaei, M.; Ferrero, D.; Santarelli, M.; Herbrig, K.; Walter, C.; Smeacetto, F. Glass-ceramic sealants for SOEC: Thermal characterization and electrical resistivity in dual atmosphere. Energies 2020, 13, 3682. [Google Scholar] [CrossRef]
- Sabato, A.G.; Cempura, G.; Montinaro, D.; Chrysanthou, A.; Salvo, M.; Bernardo, E.; Secco, M.; Smeacetto, F. Glass-ceramic sealant for solid oxide fuel cells application: Characterization and performance in dual atmosphere. J. Power Sources 2016, 328, 262–270. [Google Scholar] [CrossRef]
- Ghosh, S.; Sharma, A.D.; Kundu, P.; Basu, R.N. Glass-ceramic sealants for planar IT-SOFC: A bilayered approach for joining electrolyte and metallic interconnect. J. Electrochem. Soc. 2008, 155, B473–B478. [Google Scholar] [CrossRef]
- Rost, A.; Schilm, J.; Suffner, J.; Kusnezoff, M.; Michaelis, A. Development and testing of sealing glasses for SOFCs based on CFY-interconnects. In Proceedings of the 11th European SOFC and SOE Forum 2014, Luzern, Switzerland, 1–4 July 2014; pp. 1–9. [Google Scholar]
| Glass ID | ||||
|---|---|---|---|---|
| HJ14 | 1.3 | 1.5 | 3 | 4 |
| NS4 | 1.5 | 1.4 | 2 | 12 |
| NS9I | 1.4 | 1.3 | 3 | 12 |
| Glass ID | Glass Transition Temperature Tg (°C) | First Shrinkage Temperature TFS (°C) | Maximum Shrinkage Temperature TMS (°C) | Onset Crystallization Temperature Tx (°C) | Peak Crystallization Temperature Tp (°C) |
|---|---|---|---|---|---|
| HJ14 | 695 ± 3 | 716 ± 2 | 820 ± 3 | 854 ± 5 | 876 ± 5 |
| NS4 | 702 ± 2 | 743 ±2 | 830 ± 3 | 858 ±3 | 890 ± 2 |
| NS9I | 707 ± 5 | 746 ± 2 | 832 ± 5 | 850 ± 5 | 887 ± 3 |
| Glass ID | CTEs of as-Joined Glass-Ceramic |
|---|---|
| HJ14 | (10.3 ± 0.2) × 10−6 K−1 |
| NS4 | (9.9 ± 0.2) × 10−6 K−1 |
| NS9I | (10.3 ± 0.1) × 10−6 K−1 |
| O | Al | Si | Ca | Cr | Sr | |
|---|---|---|---|---|---|---|
| Point 1 | 57.2 | --- | 21.9 | 4.3 | --- | 16.5 |
| Point 2 | 66.9 | 3.1 | 20.1 | 0.7 | 0.3 | 8.9 |
| Point 3 | 54.6 | --- | 24.0 | 4.4 | --- | 17.0 |
| Point 4 | 66.5 | --- | 33.5 | --- | --- | --- |
| O | Al | Si | Ca | Cr | Sr | |
|---|---|---|---|---|---|---|
| Point 1 | 58.5 | 11.8 | 20.1 | 0.7 | 1.2 | 7.4 |
| Point 2 | 56.8 | 0.0 | 23.0 | 4.6 | 0.0 | 15.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, H.; Zanchi, E.; D’Isanto, F.; Bert, C.; Ferrero, D.; Santarelli, M.; Smeacetto, F. Novel SrO-Containing Glass-Ceramic Sealants for Solid Oxide Electrolysis Cells (SOEC): Their Design and Characterization under Relevant Conditions. Materials 2022, 15, 5805. https://doi.org/10.3390/ma15175805
Javed H, Zanchi E, D’Isanto F, Bert C, Ferrero D, Santarelli M, Smeacetto F. Novel SrO-Containing Glass-Ceramic Sealants for Solid Oxide Electrolysis Cells (SOEC): Their Design and Characterization under Relevant Conditions. Materials. 2022; 15(17):5805. https://doi.org/10.3390/ma15175805
Chicago/Turabian StyleJaved, Hassan, Elisa Zanchi, Fabiana D’Isanto, Chiara Bert, Domenico Ferrero, Massimo Santarelli, and Federico Smeacetto. 2022. "Novel SrO-Containing Glass-Ceramic Sealants for Solid Oxide Electrolysis Cells (SOEC): Their Design and Characterization under Relevant Conditions" Materials 15, no. 17: 5805. https://doi.org/10.3390/ma15175805






