Synthesis and Evaluation of LaBaCo2−xMoxO5+δ Cathode for Intermediate-Temperature Solid Oxide Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Structure
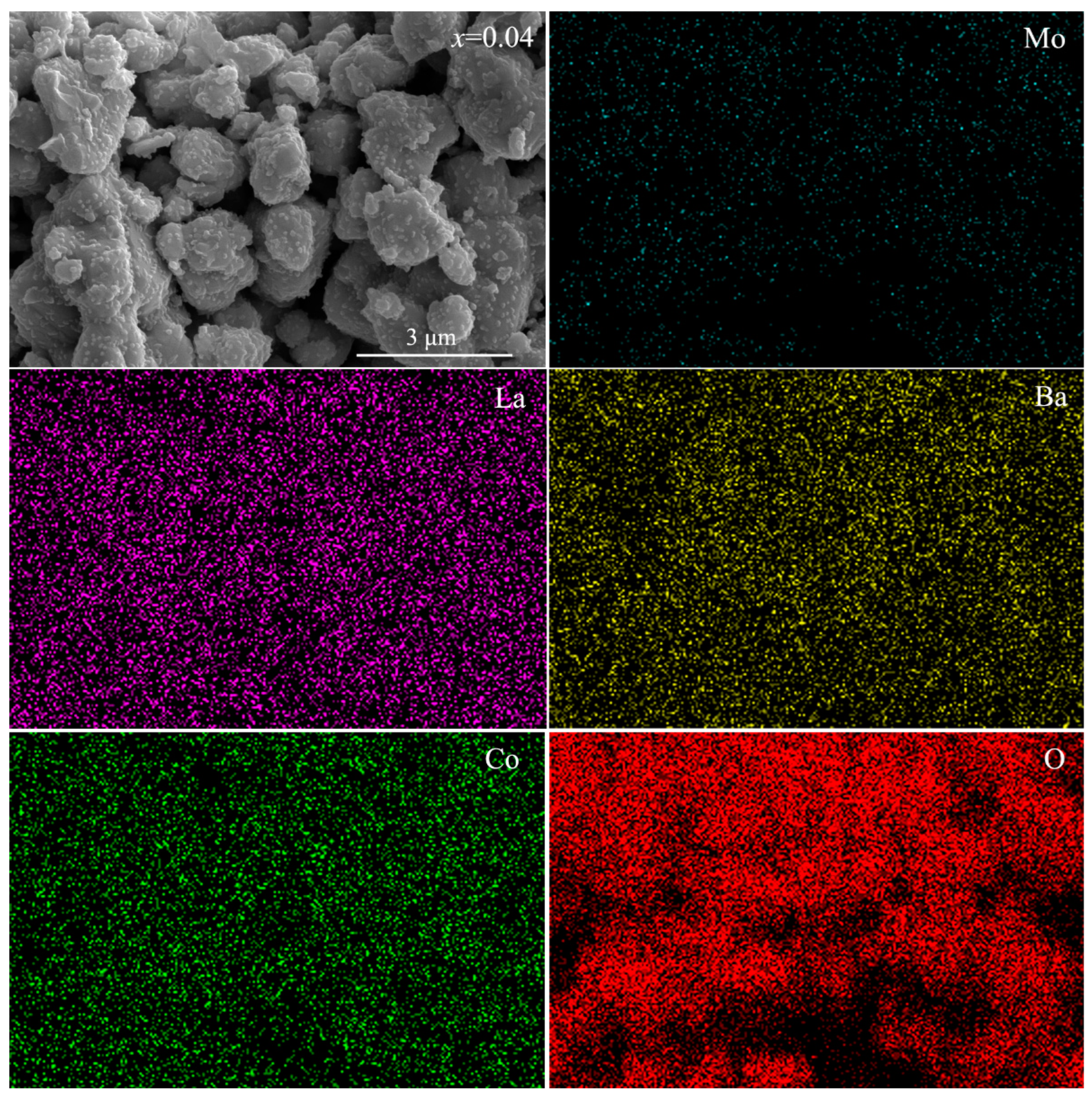
3.2. Microstructure
3.3. Thermal Expansion
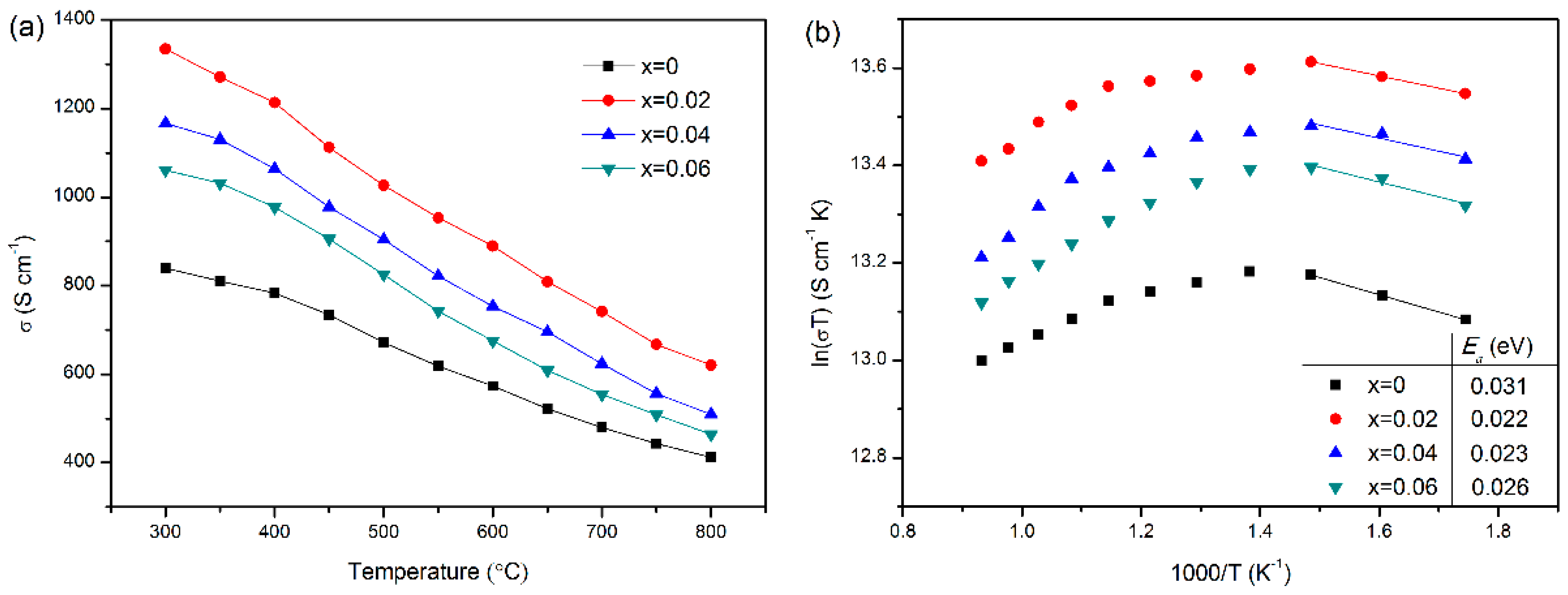
3.4. Electrical Conductivity
3.5. Electrochemical Impedance
3.6. Single-Cell Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klyndyuk, A.I.; Chizhova, E.A.; Kharytonau, D.S.; Medvedev, D.A. Layered Oxygen-Deficient Double Perovskites as Promising Cathode Materials for Solid Oxide Fuel Cells. Materials 2021, 15, 141. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.; Lai, S.Y.; Gerdes, K.; Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zhu, Z. Perovskite Cathode Materials for Low-Temperature Solid Oxide Fuel Cells: Fundamentals to Optimization. Electrochem. Energy Rev. 2022, 5, 263–311. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium cobalt ferrite perovskite electrodes of solid oxide fuel cells—A review. Int. J. Hydrogen Energy 2019, 44, 7448–7493. [Google Scholar] [CrossRef]
- Cai, C.; Xie, M.; Xue, K.; Shi, Y.; Li, S.; Liu, Y.; An, S.; Yang, H. Enhanced electrochemical performance of La0.6Sr0.4Co0.2Fe0.8O3-δ cathode via Ba-doping for intermediate-temperature solid oxide fuel cells. Nano Res. 2022, 15, 3264–3272. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, Y.; Huang, K.; Xiong, X.; Wang, J.; Liu, M.; Ding, D.; Chen, Y.; Liu, T. Surface enhanced performance of La0.6Sr0.4Co0.2Fe0.8O3-δ cathodes by infiltration Pr-Ni-Mn-O progress. J. Alloys Compd. 2022, 902, 163337. [Google Scholar] [CrossRef]
- Safian, S.D.; Abd Malek, N.I.; Jamil, Z.; Lee, S.-W.; Tseng, C.-J.; Osman, N. Study on the surface segregation of mixed ionic-electronic conductor lanthanum-based perovskite oxide La1−xSrxCo1−yFeyO3−δ materials. Int. J. Energy Res. 2022, 46, 7101–7117. [Google Scholar] [CrossRef]
- Žužić, A.; Ressler, A.; Macan, J. Perovskite oxides as active materials in novel alternatives to well-known technologies: A review. Ceram. Int. 2022, 48, 27240–27261. [Google Scholar] [CrossRef]
- Žužić, A.; Ressler, A.; Šantić, A.; Macan, J.; Gajović, A. The effect of synthesis method on oxygen nonstoichiometry and electrical conductivity of Sr-doped lanthanum manganites. J. Alloys Compd. 2022, 907, 164456. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Manthiram, A.; Kim, J.-H.; Kim, Y.N.; Lee, K.-T. Crystal chemistry and properties of mixed ionic-electronic conductors. J. Electroceram. 2011, 27, 93–107. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Rioja-Monllor, L.; Nakamura, T.; Ricote, S.; O’Hayre, R.; Amezawa, K.; Einarsrud, M.A.; Grande, T. Effect of Cation Ordering on the Performance and Chemical Stability of Layered Double Perovskite Cathodes. Materials 2018, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zan, J.; Qiu, W.; Zheng, D.; Li, F.; Chen, W.; Pei, Q.; Jiang, L. Evaluation of perovskite oxides LnBaCo2O5+δ (Ln = La, Pr, Nd and Sm) as cathode materials for IT-SOFC. J. Electroanal. Chem. 2021, 886, 115144. [Google Scholar] [CrossRef]
- Singh, K.; Baral, A.K.; Thangadurai, V. Electrochemical studies of Gd0.5Pr0.5BaCo2O5+δ (GPBC) cathode for oxide ion and proton conducting solid oxide fuel cells. Solid State Ion. 2016, 288, 351–356. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. Layered LnBaCo2O5+δ perovskite cathodes for solid oxide fuel cells: An overview and perspective. J. Mater. Chem. A 2015, 3, 24195–24210. [Google Scholar] [CrossRef]
- Joung, Y.-H.; Kang, H.I.; Choi, W.S.; Kim, J.H. Investigation of X-ray photoelectron spectroscopy and electrical conductivity properties of the layered perovskite LnBaCo2O5+δ (Ln = Pr, Nd, Sm, and Gd) for IT-SOFC. Electron. Mater. Lett. 2013, 9, 463–465. [Google Scholar] [CrossRef]
- Fu, D.; Jin, F.; He, T. A-site calcium-doped Pr1−xCaxBaCo2O5+δ double perovskites as cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 2016, 313, 134–141. [Google Scholar] [CrossRef]
- Baek, S.-W.; Azad, A.K.; Irvine, J.T.S.; Choi, W.S.; Kang, H.; Kim, J.H. Electrochemical properties of composite cathodes using Sm doped layered perovskite for intermediate temperature-operating solid oxide fuel cell. Appl. Surf. Sci. 2018, 432, 272–277. [Google Scholar] [CrossRef]
- Seymour, I.D.; Tarancon, A.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W. Anisotropic oxygen diffusion in PrBaCo2O5.5 double perovskites. Solid State Ion. 2012, 216, 41–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, L.; Wang, Y.; Du, Z.; Zhang, B.; Ciucci, F.; Zhao, H. Enhanced oxygen reduction kinetics of IT-SOFC cathode with PrBaCo2O5+δ/Gd0.1Ce1.9O2−δ coherent interface. J. Mater. Chem. A 2022, 10, 3495–3505. [Google Scholar]
- Jin, F.; Xu, H.; Long, W.; Shen, Y.; He, T. Characterization and evaluation of double perovskites LnBaCoFeO5+δ (Ln = Pr and Nd) as intermediate-temperature solid oxide fuel cell cathodes. J. Power Sources 2013, 243, 10–18. [Google Scholar] [CrossRef]
- Xia, L.-N.; He, Z.-P.; Huang, X.W.; Yu, Y. Synthesis and properties of SmBaCo2−xNixO5+δ perovskite oxide for IT-SOFC cathodes. Ceram. Int. 2016, 42, 1272–1280. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, J.H.; Manthiram, A. Effect of Fe substitution on the structure and properties of LnBaCo2−xFexO5+δ (Ln=Nd and Gd) cathodes. J. Power Sources 2010, 195, 6411–6419. [Google Scholar] [CrossRef]
- Xu, J.; Cai, H.; Hao, G.; Zhang, L.; Song, Z.; Long, W.; Zhang, L.; Wang, L. Characterization of high–valence Mo–doped PrBaCo2O5+δ cathodes for IT–SOFCs. J. Alloys Compd. 2020, 842, 155600. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Dai, Z.; Tan, S.; Chen, D. Promoting hydrogen-evolution activity and stability of perovskite oxides via effectively lattice doping of molybdenum. Electrochim. Acta 2019, 312, 128–136. [Google Scholar] [CrossRef]
- Gu, H.; Chen, H.; Gao, L.; Guo, L. Electrochemical properties of LaBaCo2O5+δ–Sm0.2Ce0.8O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2009, 54, 7094–7098. [Google Scholar] [CrossRef]
- Wei, B.; Schroeder, M.; Martin, M. Surface Cation Segregation and Chromium Deposition on the Double-Perovskite Oxide PrBaCo2O5+δ. ACS Appl. Mater. Interfaces 2018, 10, 8621–8629. [Google Scholar] [CrossRef]
- Anjum, U.; Khan, T.S.; Agarwal, M.; Haider, M.A. Identifying the Origin of the Limiting Process in a Double Perovskite PrBa0.5Sr0.5Co1.5Fe0.5O5+δ Thin-Film Electrode for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 25243–25253. [Google Scholar] [CrossRef]
- Huang, K.; Lee, H.Y.; Goodenough, J.B. Sr- and Ni-Doped LaCoO3 and LaFeO3 Perovskites. J. Electrochem. Soc. 1998, 145, 3220–3227. [Google Scholar] [CrossRef]
- Kostogloudis, G.C.; Vasilakos, N.; Ftikos, C. Crystal structure, thermal and electrical properties of Pr1−xSrxCoO3−δ (x=0, 0.15, 0.3, 0.4, 0.5) perovskite oxides. Solid State Ion. 1998, 106, 207–218. [Google Scholar] [CrossRef]
- Lu, C.; Niu, B.; Yu, S.; Yi, W.; Luo, S.; Xu, B.; Ji, Y. Efficient and stable symmetrical electrode La0.6Sr0.4Co0.2Fe0.7Mo0.1O3−δ for direct hydrocarbon solid oxide fuel cells. Electrochim. Acta 2019, 323, 134857. [Google Scholar] [CrossRef]
- Yao, C.; Yang, J.; Zhang, H.; Chen, S.; Lang, X.; Meng, J.; Cai, K. Evaluation of A-site Ba-deficient PrBa0.5−xSr0.5Co2O5+δ (x = 0, 0.04 and 0.08) as cathode materials for solid oxide fuel cells. J. Alloys Compd. 2021, 883, 160759. [Google Scholar] [CrossRef]
- Wang, S.-F.; Hsu, Y.-F.; Liao, Y.-L.; Huang, S.-T.; Jasinski, P. High-performance NdSrCo2O5+δ–Ce0.8Gd0.2O2−δ composite cathodes for electrolyte-supported microtubular solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 31778–31787. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, F.; Hao, X.; Niu, B.; Lyu, P.; He, T. B-site-ordered Co-based double perovskites Sr2Co1−xNbxFeO5+δ as active and stable cathodes for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2020, 829, 154470. [Google Scholar] [CrossRef]
- He, S.; Dai, H.; Chen, X.; Shi, Q.; Tao, Z. Investigation of La0.75Sr0.25Cr0.5Mn0.5O3−δ–Ag composite anodes for solid oxide fuel cells obtained via a low-temperature sintering process. Ionics 2020, 26, 6225–6232. [Google Scholar] [CrossRef]
- Huang, S.; Lu, Q.; Feng, S.; Li, G.; Wang, C. Ba0.9Co0.7Fe0.2Mo0.1O3−δ: A Promising Single-Phase Cathode for Low Temperature Solid Oxide Fuel Cells. Adv. Energy Mater. 2011, 1, 1094–1096. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, J.; He, B.; Chen, F.; Xia, C. Synthesis, characterization and evaluation of PrBaCo2−xFexO5+δ as cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 3658–3665. [Google Scholar] [CrossRef]
- Kim, J.H.; Irvine, J.T.S. Characterization of layered perovskite oxides NdBa1−xSrxCo2O5+δ (x = 0 and 0.5) as cathode materials for IT-SOFC. Int. J. Hydrogen Energy 2012, 37, 5920–5929. [Google Scholar] [CrossRef]
- Yang, Q.; Tian, D.; Liu, R.; Wu, H.; Chen, Y.; Ding, Y.; Lu, X.; Lin, B. Exploiting rare-earth-abundant layered perovskite cathodes of LnBa0.5Sr0.5Co1.5Fe0.5O5+δ (Ln=La and Nd) for SOFCs. Int. J. Hydrogen Energy 2021, 46, 5630–5641. [Google Scholar] [CrossRef]
- Baijnath; Tiwari, P.; Basu, S. Cobalt and molybdenum co-doped Ca2Fe2O5 cathode for solid oxide fuel cell. Int. J. Hydrogen Energy 2019, 44, 10059–10070. [Google Scholar] [CrossRef]





| Space Group | a (Å) | b (Å) | c (Å) | V (Å)3 | |
|---|---|---|---|---|---|
| x = 0 | Pm-3m | 5.4869 | 5.4869 | 5.4869 | 165.1890 |
| x = 0.02 | 5.4890 | 5.4890 | 5.4890 | 165.3787 | |
| x = 0.04 | 5.4913 | 5.4913 | 5.4913 | 165.5867 | |
| x = 0.06 | 5.4916 | 5.4916 | 5.4916 | 165.6139 | |
| x = 0.08 | 5.7123 | 5.7123 | 5.7123 | 186.3945 |
| x = 0 | x = 0.02 | x = 0.04 | x = 0.06 | |
|---|---|---|---|---|
| TEC (×10−6 K−1) | 20.87 | 19.61 | 19.30 | 18.47 |
| 700 °C | 750 °C | 800 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | RP | R1 | R2 | RP | R1 | R2 | RP | |
| x = 0 | 0.338 (93%) | 0.027 (7%) | 0.365 | 0.136 (96%) | 0.006 (4%) | 0.142 | 0.057 (95%) | 0.003 (5%) | 0.060 |
| x = 0.02 | 0.258 (91%) | 0.026 (9%) | 0.284 | 0.117 (94%) | 0.007 (6%) | 0.124 | 0.053 (93%) | 0.004 (7%) | 0.057 |
| x = 0.04 | 0.194 (90%) | 0.022 (10%) | 0.216 | 0.080 (91%) | 0.008 (9%) | 0.088 | 0.032 (89%) | 0.004 (11%) | 0.036 |
| x = 0.06 | 0.237 (93%) | 0.019 (7%) | 0.256 | 0.103 (95%) | 0.006 (5%) | 0.109 | 0.046 (94%) | 0.003 (6%) | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Zhang, L.; Cai, J.; Wu, X.; Sun, H.; Du, T. Synthesis and Evaluation of LaBaCo2−xMoxO5+δ Cathode for Intermediate-Temperature Solid Oxide Fuel Cells. Materials 2022, 15, 5858. https://doi.org/10.3390/ma15175858
He S, Zhang L, Cai J, Wu X, Sun H, Du T. Synthesis and Evaluation of LaBaCo2−xMoxO5+δ Cathode for Intermediate-Temperature Solid Oxide Fuel Cells. Materials. 2022; 15(17):5858. https://doi.org/10.3390/ma15175858
Chicago/Turabian StyleHe, Shoucheng, Lanqing Zhang, Jiantao Cai, Xingyu Wu, Hanxi Sun, and Tao Du. 2022. "Synthesis and Evaluation of LaBaCo2−xMoxO5+δ Cathode for Intermediate-Temperature Solid Oxide Fuel Cells" Materials 15, no. 17: 5858. https://doi.org/10.3390/ma15175858
APA StyleHe, S., Zhang, L., Cai, J., Wu, X., Sun, H., & Du, T. (2022). Synthesis and Evaluation of LaBaCo2−xMoxO5+δ Cathode for Intermediate-Temperature Solid Oxide Fuel Cells. Materials, 15(17), 5858. https://doi.org/10.3390/ma15175858






