Thermomechanical and Alkaline Peroxide Mechanical Pulping of Lignocellulose Residue Obtained from the 2-Furaldehyde Production Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Samples
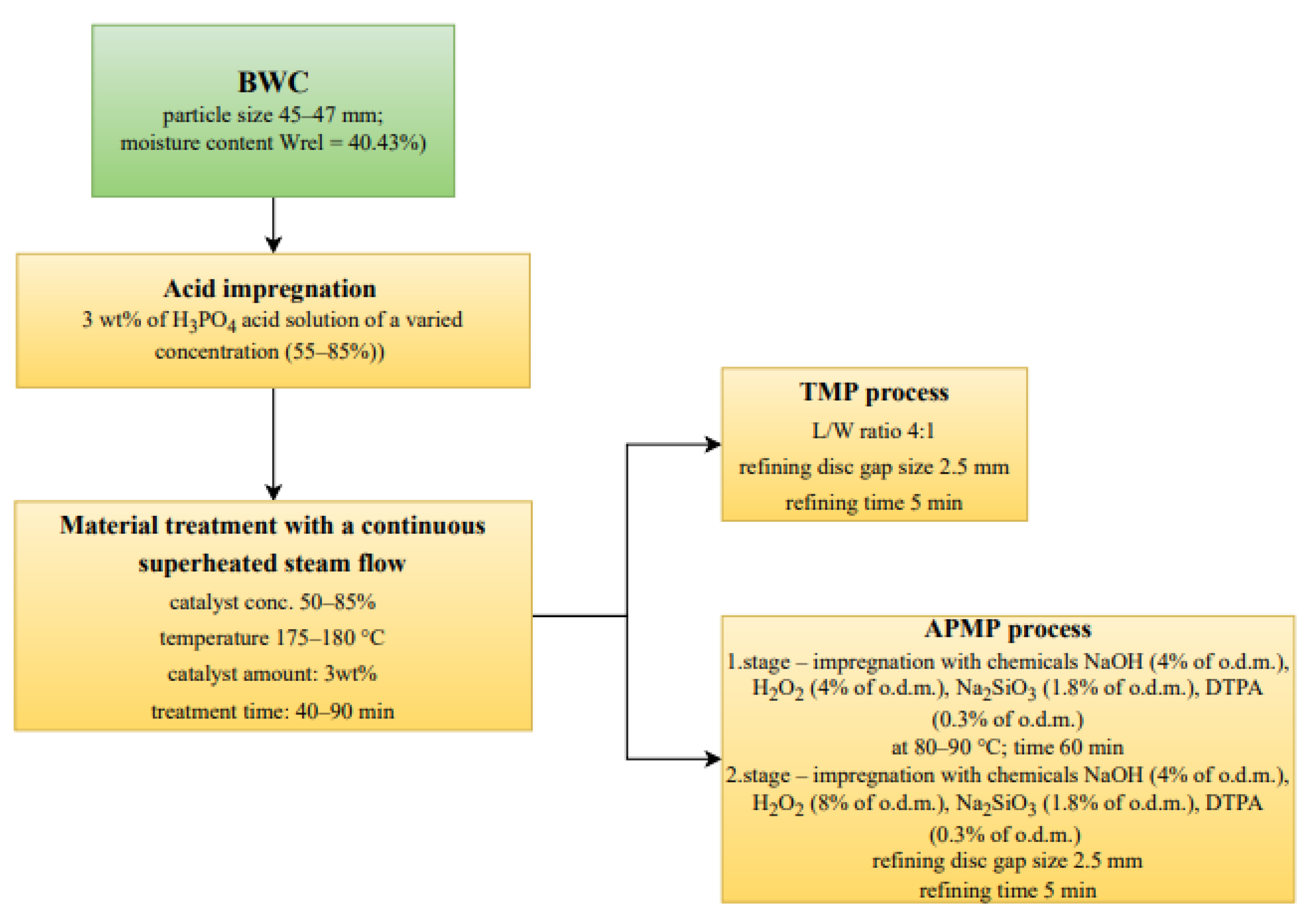
2.3. Catalyzed Pre-Treatment of BWC
2.4. HPLC Analysis
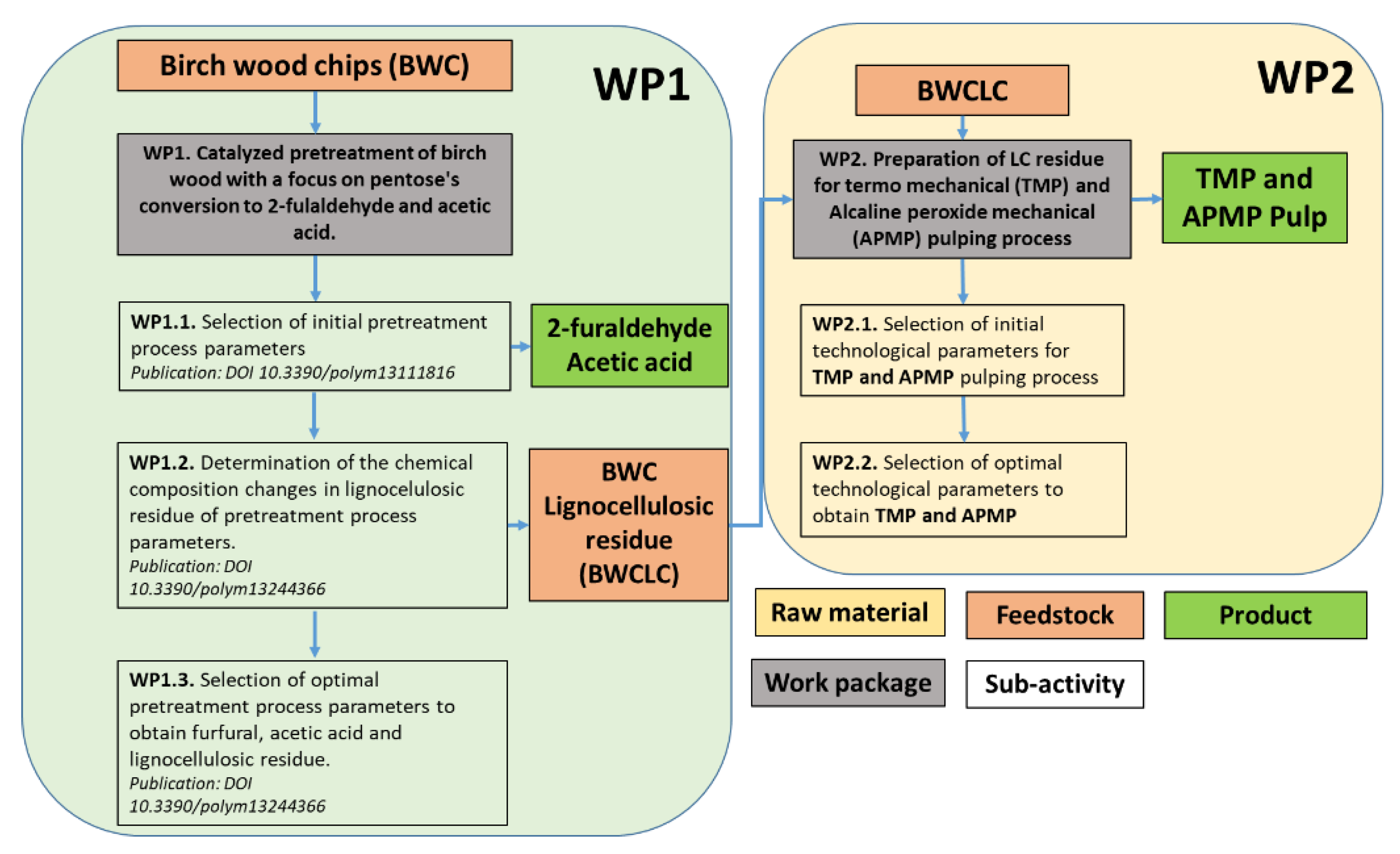
2.5. Pulping Process of LC Residue Obtained after BWC Pre-Treatment
2.6. Tensile Strength Determination
2.7. SEM Analysis
3. Results
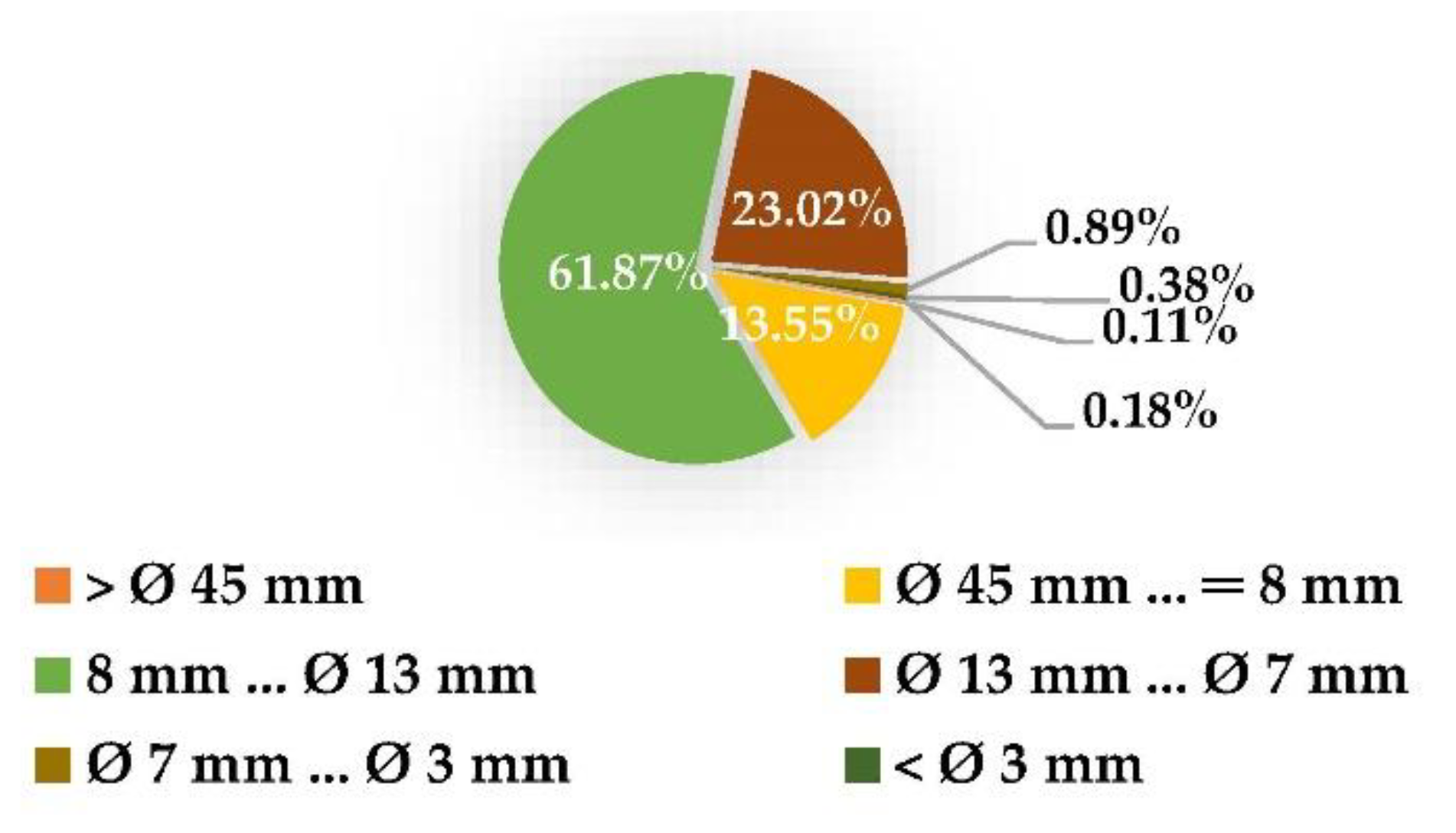
3.1. Analysis of the Raw Material
3.2. Chemical Composition of Hydrolysis Condensate
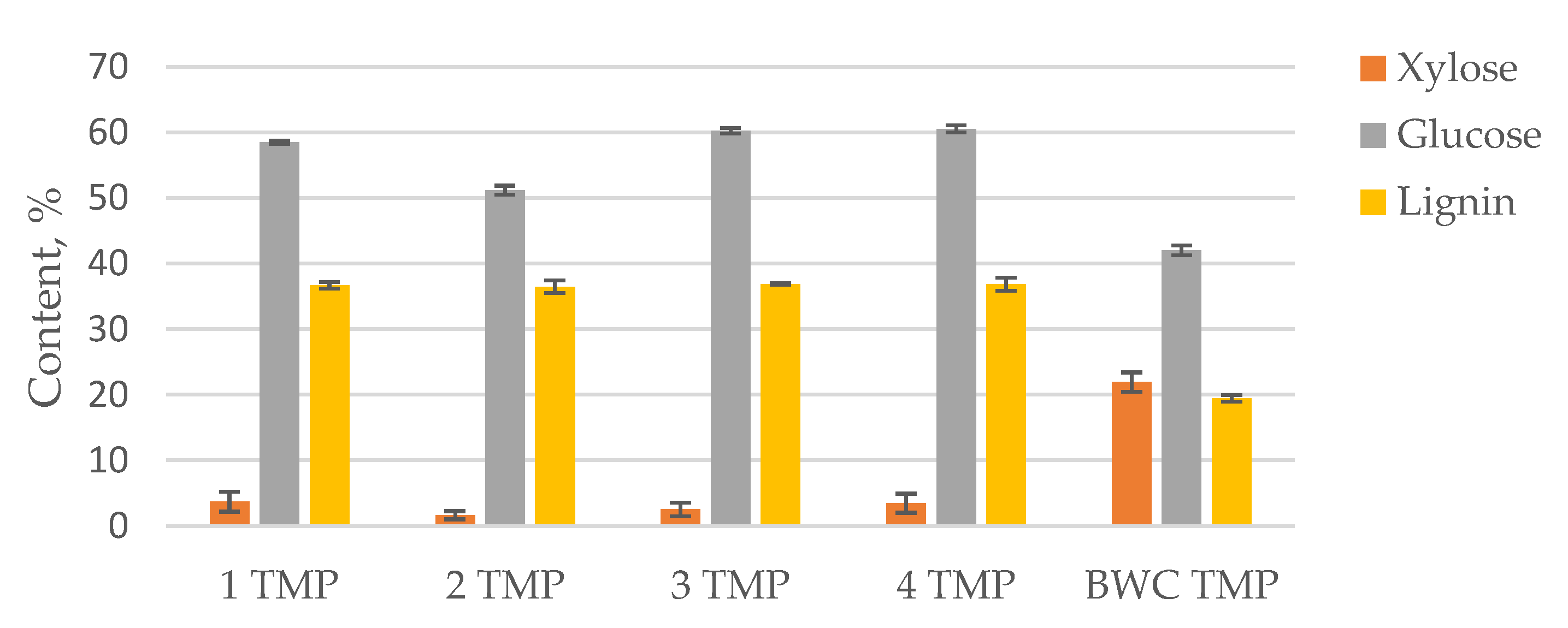
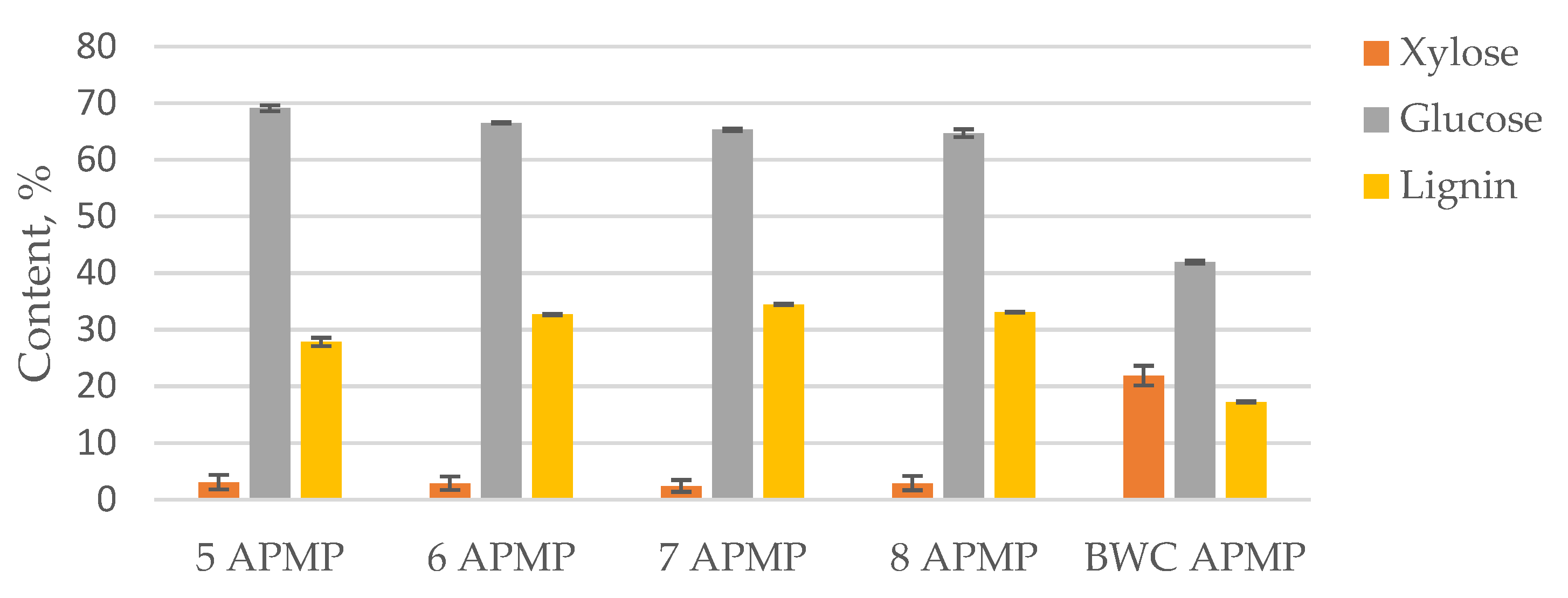
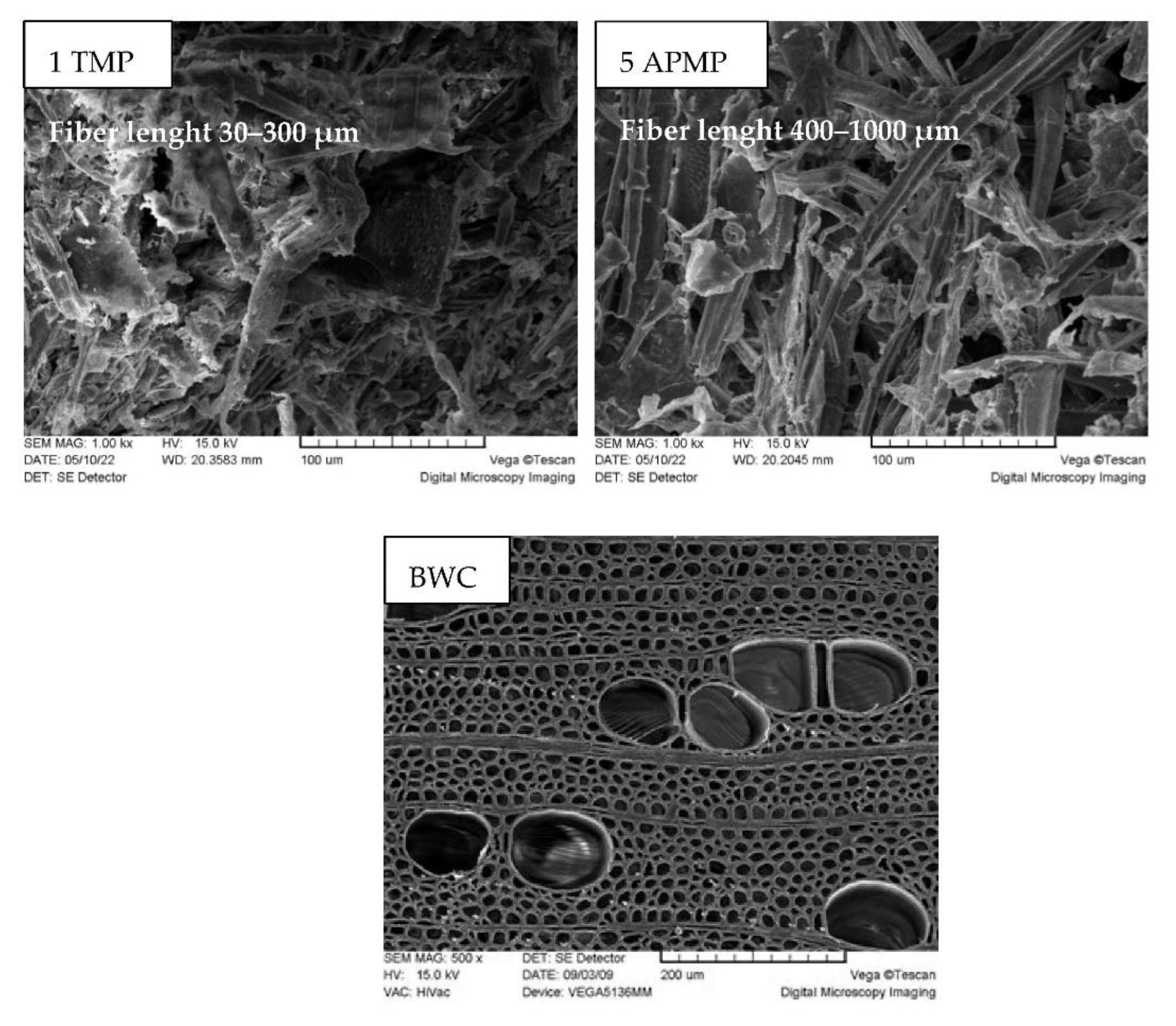
3.3. LC Residue Pulping Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muzyka, C.; Monbaliu, J.-C.M. Perspectives for the Upgrading of Bio-Based Vicinal Diols within the Developing European Bioeconomy. ChemSusChem 2022, 15, e202102391. [Google Scholar] [CrossRef] [PubMed]
- European Council. Fit for 55. Available online: https://www.consilium.europa.eu/lv/policies/green-deal/fit-for-55-the-eu-plan-for-a-green-transition/ (accessed on 5 June 2022).
- Balan, V.; Bals, B.; Chundawat, S.P.S.; Marshall, D.; Dale, B.E. Lignocellulosic Biomass Pretreatment Using AFEX. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 581, pp. 61–77. [Google Scholar] [CrossRef]
- Adsul, M.G.; Singhvi, M.S.; Gaikaiwari, S.A.; Gokhale, D.V. Development of Biocatalysts for Production of Commodity Chemicals from Lignocellulosic Biomass. Bioresour. Technol. 2011, 102, 4304–4312. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Yahyazadeh, A. Extraction and Investigation of Furfural in Tea Leaves and Comparing with Furfural in Rice Hull. J. Pharm. Res. 2011, 4, 4338–4339. [Google Scholar]
- Huber, G.W.; Dumesic, J.A. An Overview of Aqueous-Phase Catalytic Processes for Production of Hydrogen and Alkanes in a Biorefinery. Catal. Today 2006, 111, 119–132. [Google Scholar] [CrossRef]
- Wojcik, B.H. Catalytic Hydrogenation of Furan Compounds. Ind. Eng. Chem. 1948, 40, 210–216. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated Furfural Production as a Renewable Fuel and Chemical Platform from Lignocellulosic Biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Vedernikovs, N.; Puke, M.; Kruma, I. Furfural and Bioethanol Production from Hardwood and Agricultural Waste. In Proceedings of the 5th UEAA General Assembly and the Associated Workshop on Renewable Energy Resources, Production and Technologies, Zinātne, Latvia, 28–31 May 2008; pp. 176–191. [Google Scholar]
- Forest Resources in Latvia. Available online: https://www.zm.gov.lv/mezi/statiskas-lapas/nozares-informacija/meza-resursi?nid=1086#jump (accessed on 12 June 2022).
- Latvian Forest Sector in Facts and Figures. NGO Zalas Majas, Riga, 2021. Available online: https://www.zm.gov.lv/public/ck/files/ZM/mezhi/buklets/skaitlifakti_ENG_2021.pdf (accessed on 15 June 2022).
- Muguet, M.C.D.S.; Colodette, L.J.; Jääskeläinen, S.A. Effect of Eucalyptus Wood Characteristics on Alkaline Peroxide Mechanical Pulping (APMP) Energy Consumption and Pulp Properties. In Proceedings of the 5th International Colloquium on Eucalyptus Pulp, Porto Seguro, Brazil, 9–12 May 2011; pp. 1–8. [Google Scholar]
- Puke, M.; Godina, D.; Kirpluks, M.; Rizikovs, J.; Brazdausks, P. Characterization of Birch Wood Residue after 2-Furaldehyde Obtaining, for Further Integration in Biorefinery Processing. Polymers 2021, 13, 4366. [Google Scholar] [CrossRef] [PubMed]
- Puke, M.; Godina, D.; Kirpluks, M.; Rizikovs, J.; Brazdausks, P. Residual Birch Wood Lignocellulose after 2-Furaldehyde Production as a Potential Feedstock for Obtaining Fiber. Polymers 2021, 13, 1816. [Google Scholar] [CrossRef] [PubMed]
- Vedernikovs, N. Method and Device Used for Producing Furfural and Acetate. LV Patent CN102336727A, 20 June 2010. [Google Scholar]
- Technical Association of Pulp and Paper Industry. T204 Solvent Extractives of Wood and Pulp; Lin, T., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-420-7. [Google Scholar]
- Technical Association of Pulp and Paper Industry. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C. TAPPI Test Methods; Lin, T., Ed.; InTech: London, UK, 2009; Volume 1995, pp. 1–173. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 Analytical Procedure–Determination of Structural Carbohydrates and Lignin in Biomass. 2012. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 12 July 2022).
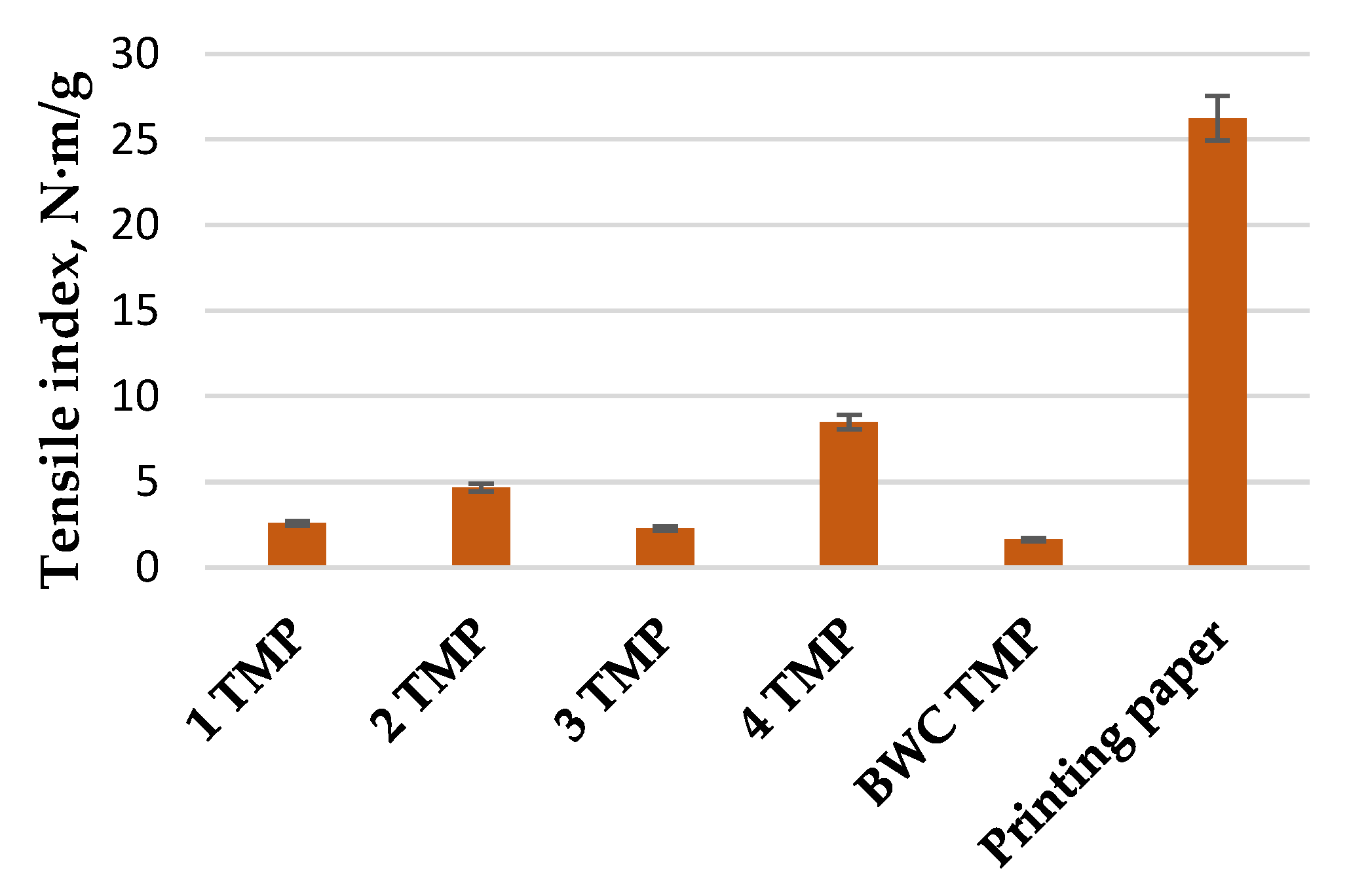
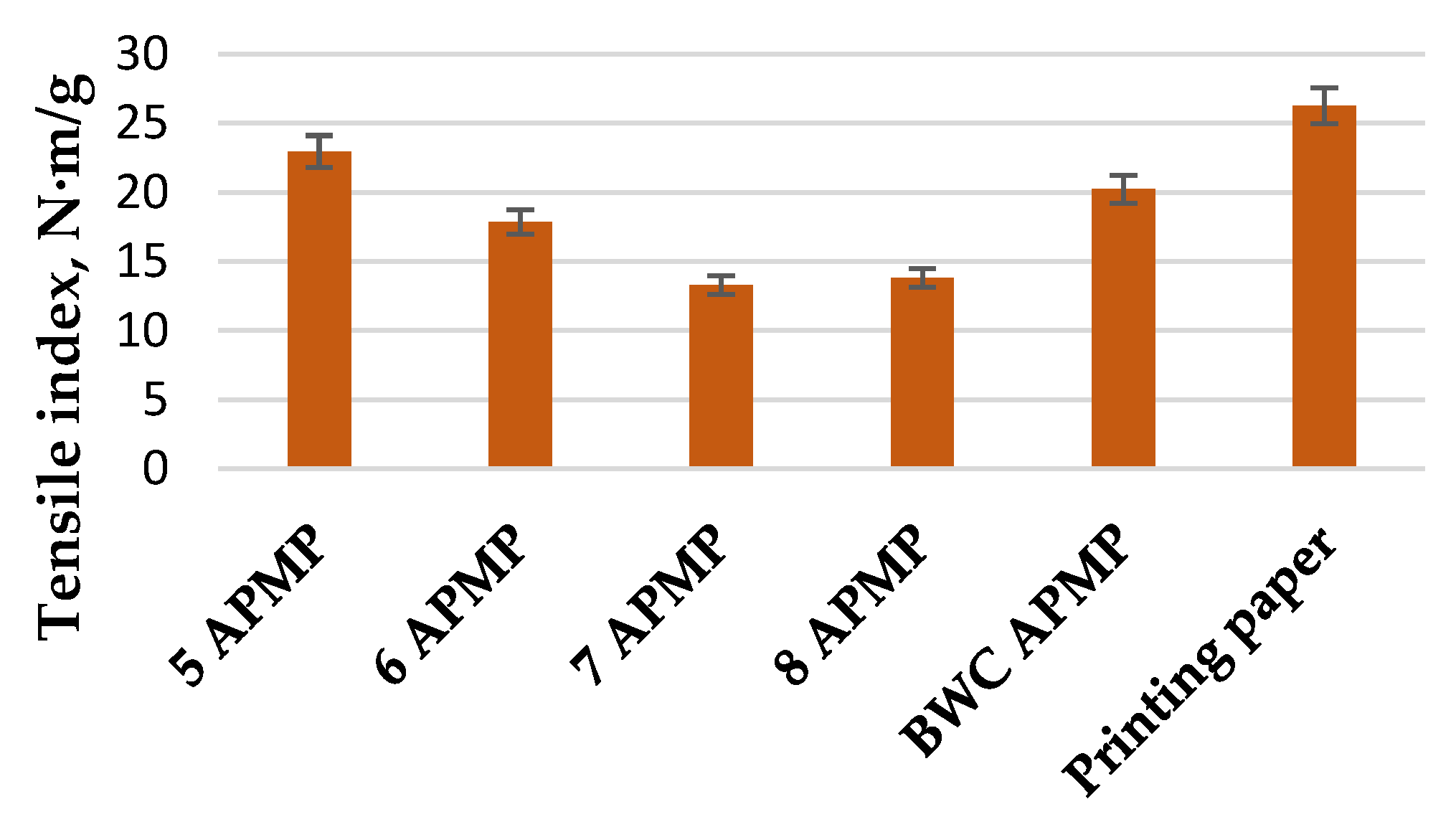
| No. | Catalyst Conc. (c) | Temperature (T) | Catalyst Amount (m) | Treatment Time (τ) |
|---|---|---|---|---|
| % | °C | wt.% | min | |
| 1 | 50 | 175 | 3 | 90 |
| 2 | 50 | 175 | 3 | 60 |
| 3 | 50 | 180 | 3 | 50 |
| 4 | 50 | 180 | 3 | 40 |
| 5 | 70 | 175 | 3 | 60 |
| 6 | 70 | 175 | 3 | 50 |
| 7 | 85 | 175 | 3 | 60 |
| 8 | 85 | 175 | 3 | 40 |
| Stage | Chemicals, % of o.d.m. | |||
|---|---|---|---|---|
| NaOH | H2O2 | Na2SiO3 | DTPA | |
| 1 | 4.0 | 4.0 | 1.8 | 0.3 |
| 2 | 4.0 | 8.0 | 1.8 | 0.3 |
| Total | 8.0 | 12.0 | 3.6 | 0.6 |
| No | Amount, % o.d.m. | |||||
|---|---|---|---|---|---|---|
| LC Residue | Formic Acid | Acetic Acid | Levulinic Acid | 5-HMF | 2-Furaldehyde | |
| 1 | 48.94 ± 0.45 | 0.19 ± 0.01 | 2.52 ± 0.01 | 0.03 ± 0.04 | 0.03 ± 0.01 | 10.31 ± 0.10 |
| 2 | 49.14 ± 0.32 | 0.21 ± 0.02 | 2.12 ± 0.03 | 0.02 ± 0.01 | <0.01 | 8.15 ± 0.04 |
| 3 | 46.70 ± 0.36 | 0.17 ± 0.02 | 2.23 ± 0.01 | 0.02 ± 0.01 | <0.01 | 8.32 ± 0.11 |
| 4 | 45.88 ± 0.45 | 0.02 ± 0.01 | 1.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 7.78 ± 0.04 |
| 5 | 41.72 ± 0.29 | 0.44 ± 0.11 | 4.45 ± 0.03 | <0.01 | <0.01 | 7.85 ± 0.19 |
| 6 | 41.01 ± 0.34 | 0.40 ± 0.05 | 3.81 ± 0.04 | <0.01 | <0.01 | 6.40 ± 0.09 |
| 7 | 42.20 ± 0.47 | 0.45 ± 0.03 | 4.48 ± 0.11 | 0.03 ± 0.01 | <0.01 | 7.95 ± 0.07 |
| 8 | 42.65 ± 0.26 | 0.38 ± 0.01 | 3.98 ± 0.01 | 0.02 ± 0.01 | <0.01 | 6.68 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puke, M.; Godina, D.; Brazdausks, P.; Rizikovs, J.; Fridrihsone, V. Thermomechanical and Alkaline Peroxide Mechanical Pulping of Lignocellulose Residue Obtained from the 2-Furaldehyde Production Process. Materials 2022, 15, 5872. https://doi.org/10.3390/ma15175872
Puke M, Godina D, Brazdausks P, Rizikovs J, Fridrihsone V. Thermomechanical and Alkaline Peroxide Mechanical Pulping of Lignocellulose Residue Obtained from the 2-Furaldehyde Production Process. Materials. 2022; 15(17):5872. https://doi.org/10.3390/ma15175872
Chicago/Turabian StylePuke, Maris, Daniela Godina, Prans Brazdausks, Janis Rizikovs, and Velta Fridrihsone. 2022. "Thermomechanical and Alkaline Peroxide Mechanical Pulping of Lignocellulose Residue Obtained from the 2-Furaldehyde Production Process" Materials 15, no. 17: 5872. https://doi.org/10.3390/ma15175872
APA StylePuke, M., Godina, D., Brazdausks, P., Rizikovs, J., & Fridrihsone, V. (2022). Thermomechanical and Alkaline Peroxide Mechanical Pulping of Lignocellulose Residue Obtained from the 2-Furaldehyde Production Process. Materials, 15(17), 5872. https://doi.org/10.3390/ma15175872






