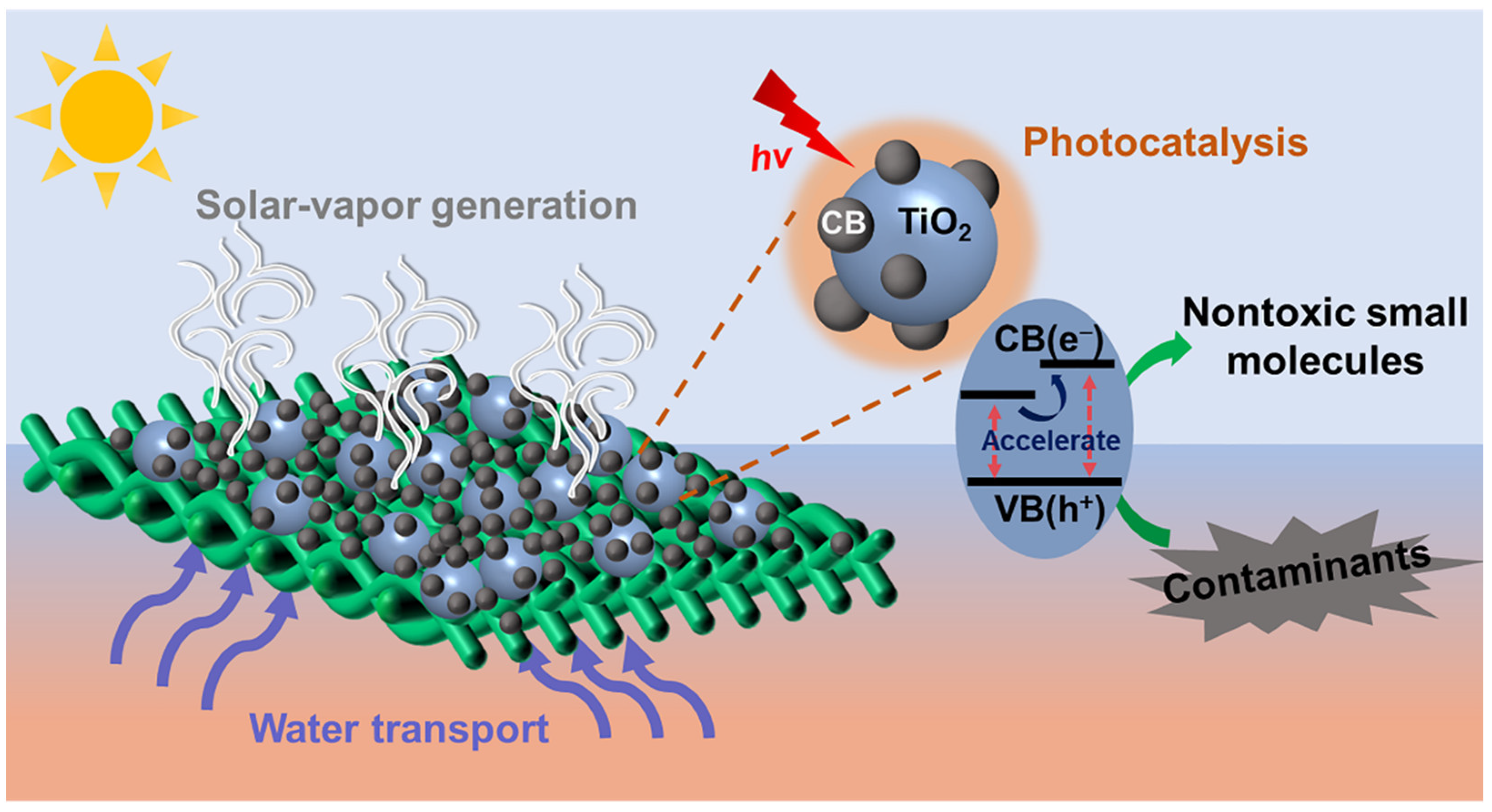
A Composite Fabric with Dual Functions for High-Performance Water Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanoparticles
2.3. Synthesis of TiO2@CB Nanoparticles
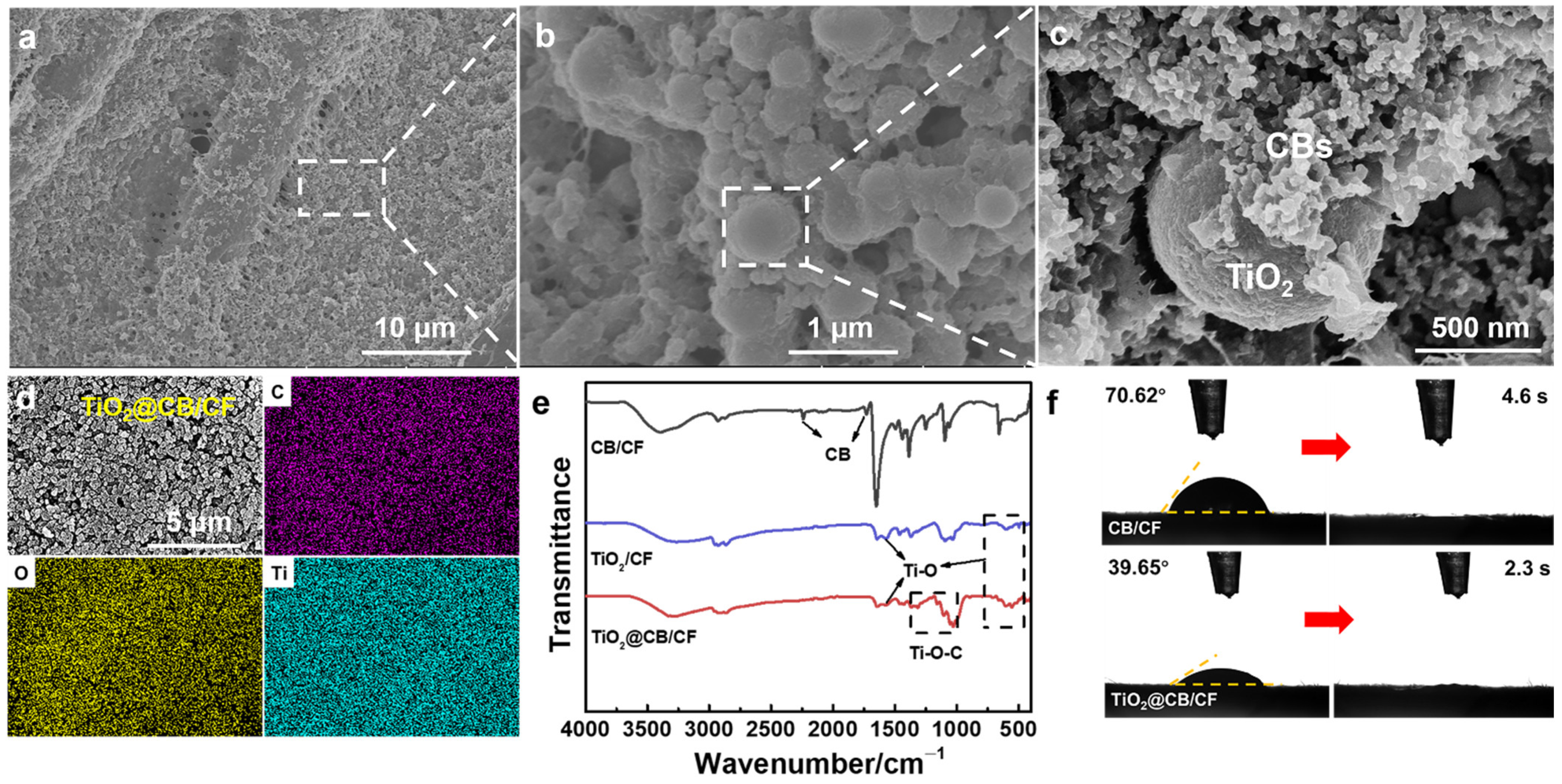
2.4. Preparation of TiO2@CB/CF
2.5. Characterization
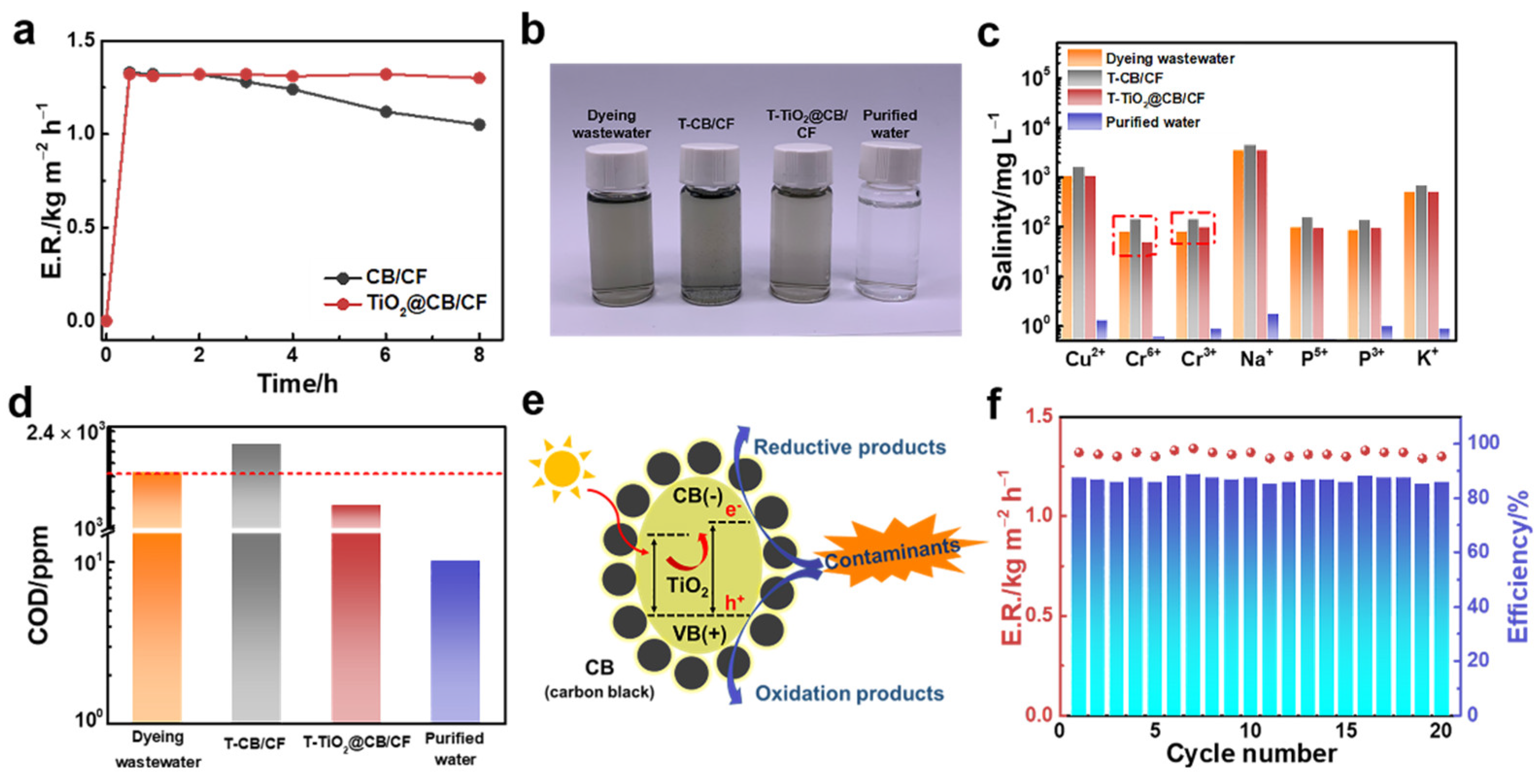
2.6. Evaporation under Simulated Solar Irradiation
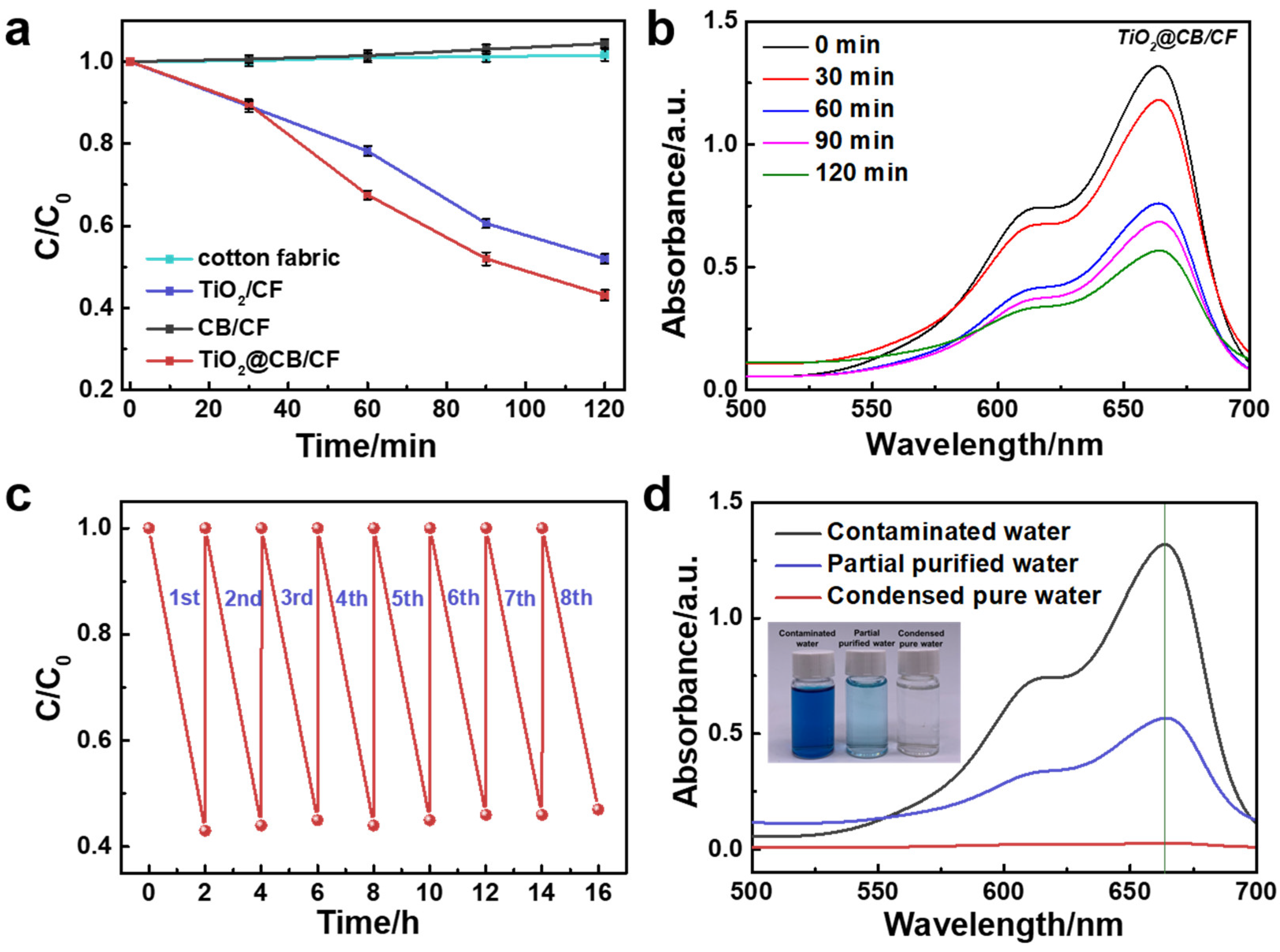
2.7. Photocatalytic Activity Test
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spellman, C.D.; Smyntek, P.M.; Cravotta, C.A.; Tasker, T.L.; Strosnider, W.H.J. Pollutant co-attenuation via in-stream interactions between mine drainage and municipal wastewater. Water Res. 2022, 214, 118173. [Google Scholar] [CrossRef]
- Wu, P.; Wu, X.; Wang, Y.; Xu, H.; Owens, G. Towards sustainable saline agriculture: Interfacial solar evaporation for simultaneous seawater desalination and saline soil remediation. Water Res. 2022, 212, 118099. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, G.; Lu, J.; Shen, C.; Dong, Z.; Yin, S.; Li, F. Spatio-vertical distribution of riverine microplastics: Impact of the textile industry. Environ. Res. 2022, 211, 112789. [Google Scholar] [CrossRef]
- Sun, T.; Yang, L.; Tang, J.; Li, N.; Chen, J.; Shen, A.; Shao, Y.; Zhang, Y.; Liu, H.; Xue, G. Flocculating-filtration-processed mesoporous structure in laminar ion-selective membrane for osmosis energy conversion and desalination. Chem. Eng. J. 2022, 437, 135484. [Google Scholar] [CrossRef]
- Du, C.; Du, J.R.; Feng, X.; Du, F.; Cheng, F.; Ali, M.E.A. Pervaporation-assisted desalination of seawater reverse osmosis brine. Sep. Purif. Technol. 2022, 290, 120820. [Google Scholar] [CrossRef]
- Djellabi, R.; Noureen, L.; Dao, V.D.; Meroni, D.; Falletta, E.; Dionysiou, D.D.; Bianchi, C.L. Recent advances and challenges of emerging solar-driven steam and the contribution of photocatalytic effect. Chem. Eng. J. 2022, 431, 134024. [Google Scholar] [CrossRef]
- Mallek, R.; Miqueu, C.; Jacob, M.; Dicharry, C. Experimental evaluation of the partial thermal energy compensation of hydrate crystallization from cyclopentane-loaded porous activated carbon particles immersed in brine. Desalination 2022, 530, 115662. [Google Scholar] [CrossRef]
- Sathish, K.T.R.; Jegadheeswaran, S. Experimental Investigation on finned solar still with enhanced thermal energy storage. J. Therm. Sci. Eng. Appl. 2022, 14, 091001. [Google Scholar] [CrossRef]
- Dhivagar, R.; Deepanraj, B.; Mohanraj, M.; Prakash, A. Thermal performance, cost effectiveness and environmental analysis of a heat pump assisted regenerative solar still using slack wax as heat storage material. Sustain. Eng. Technol. Assess. 2022, 52, 102090. [Google Scholar] [CrossRef]
- Sharshir, S.W.; Joseph, A.; Kandeal, A.W.; Hussien, A.A. Performance improvement of tubular solar still using nano-coated hanging wick thin film, ultrasonic atomizers, and cover cooling. Sustain. Eng. Technol. Assess. 2022, 52, 102127. [Google Scholar] [CrossRef]
- Sheng, M.; Yang, Y.; Bin, X.; Zhao, S.; Pan, C.; Nawaz, F.; Que, W. Recent advanced self-propelling salt-blocking technologies for passive solar-driven interfacial evaporation desalination systems. Nano Energy 2021, 89, 106468. [Google Scholar] [CrossRef]
- Li, F.; Li, N.; Wang, S.; Qiao, L.; Yu, L.; Murto, P.; Xu, X. Self-repairing and damage-tolerant hydrogels for efficient solar-powered water purification and desalination. Adv. Funct. Mater. 2021, 31, 2104464. [Google Scholar] [CrossRef]
- Li, N.; Qiao, L.; He, J.; Wang, S.; Yu, L.; Murto, P.; Li, X.; Xu, X. Solar-driven interfacial evaporation and self-powered water wave detection based on an all-cellulose monolithic design. Adv. Funct. Mater. 2020, 31, 2008681. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Ni, G.; Zandavi, S.H.; Javid, S.M.; Boriskina, S.V.; Cooper, T.A.; Chen, G. A salt-rejecting floating solar still for low-cost desalination. Energy Environ. Sci. 2018, 11, 1510–1519. [Google Scholar] [CrossRef]
- Liu, F.; Lai, Y.; Zhao, B.; Bradley, R.; Wu, W. Photothermal materials for efficient solar powered steam generation. Front. Chem. Sci. Eng. 2019, 13, 636–653. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Z.; Xiang, W.; Shen, C.; Wang, Z.; Jia, X.; Sun, J.; Liu, C.J. Plasmonic wooden flower for highly efficient solar vapor generation. Nano Energy 2020, 76, 104998. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Chen, F.; Zhu, X.; Dai, J.; Li, Y.; Yang, Z.; Yan, X.; Song, J.; Wang, Y.; et al. Plasmonic wood for high-efficiency solar steam generation. Adv. Energy Mater. 2018, 8, 1701028. [Google Scholar] [CrossRef]
- Gao, T.; Li, Y.; Chen, C.; Yang, Z.; Kuang, Y.; Jia, C.; Song, J.; Hitz, E.M.; Liu, B.; Huang, H.; et al. Architecting a floatable, durable, and scalable Steam generator: Hydrophobic/hydrophilic bifunctional structure for solar evaporation enhancement. Small Methods. 2019, 3, 1800176. [Google Scholar] [CrossRef]
- Li, Y.; Gao, T.; Yang, Z.; Chen, C.; Kuang, Y.; Song, J.; Jia, C.; Hitz, E.M.; Yang, B.; Hu, L. Graphene oxide-based evaporator with one-dimensional water transport enabling high-efficiency solar desalination. Nano Energy 2017, 41, 201–209. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, D.; Xiang, H.; Ren, S.; Zhu, Z.; Liu, D.; Xu, H.; Cui, F.; Wang, W. Easily scaled-up photothermal membrane with structure-dependent auto-cleaning feature for high-efficient solar desalination. J. Membr. Sci. 2019, 586, 222–230. [Google Scholar] [CrossRef]
- Xiao, P.; Gu, J.; Zhang, C.; Ni, F.; Liang, Y.; He, J.; Zhang, L.; Ouyang, J.; Kuo, S.W.; Chen, T. A scalable, low-cost and robust photothermal fabric with tunable and programmable 2D/3D structures towards environmentally adaptable liquid/solid-medium water extraction. Nano Energy 2019, 65, 104002. [Google Scholar] [CrossRef]
- Li, X.; Lin, R.; Ni, G.; Xu, N.; Hu, X.; Zhu, B.; Lv, G.; Li, J.; Zhu, S.; Zhu, J. Three-dimensional artificial transpiration for efficient solar wastewater treatment. Natl. Sci. Rev. 2018, 5, 70–77. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Q.; Fan, J.; Wang, W.; Yu, D. Simple and robust MXene/carbon nanotubes/cotton fabrics for textile wastewater purification via solar-driven interfacial water evaporation. Sep. Purif. Technol. 2021, 254, 117615. [Google Scholar] [CrossRef]
- Sugita, T.; Mori, M.; Mase, A.; Noguchi, S.N.; Tokutome, T.; Fujii, K.; Hara, C.; Katayama, K.; Iwamoto, S.; Itabashi, H. Evaluation of the water-treatment ability of silica-doped titanium dioxide-coated glass plates using a cationic coupling reagent based on a flow analytical system. Anal. Sci. 2015, 31, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.W.; Shakeel, R.A.R.; Devarapalli, R.R.; Shelke, M.V.; Jha, N. Carbon fabric based solar steam generation for waste water treatment. Sol. Energy 2018, 159, 800–810. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, L.; Yu, J.; Wang, Y.; Nie, S.; Zhu, S.; Zhu, J. Dual functional asymmetric plasmonic structures for solar water purification and pollution detection. Nano Energy 2018, 51, 451–456. [Google Scholar] [CrossRef]
- Sun, J.; Liu, L.; Yang, F. A visible-light-driven photocatalytic fuel cell/peroxymonosulfate (PFC/PMS) system using blue TiO2 nanotube arrays (TNA) anode and Cu-Co-WO3 cathode for enhanced oxidation of organic pollutant and ammonium nitrogen in real seawater. Appl. Catal. B 2022, 308, 121215. [Google Scholar] [CrossRef]
- Pan, J.; Yu, X.; Dong, J.; Zhao, L.; Liu, L.; Liu, J.; Zhao, X.; Liu, L. Diatom-inspired TiO2-PANi-decorated bilayer photothermal foam for solar-driven clean water generation. ACS Appl. Mater. Interfaces 2021, 13, 58124–58133. [Google Scholar] [CrossRef]
- Li, D.; Chen, S.; Huang, R.; Xue, C.; Li, P.; Li, Y.; Chang, Q.; Wang, H.; Li, N.; Jia, S.; et al. Photothermal, photocatalytic, and anti-bacterial Ti-Ag-O nanoporous powders for interfacial solar driven water evaporation. Ceram. Int. 2021, 47, 19800–19808. [Google Scholar] [CrossRef]
- Liu, Y.; Lou, J.; Ni, M.; Song, C.; Wu, J.; Dasgupta, N.P.; Tao, P.; Shang, W.; Deng, T. Bioinspired bifunctional membrane for efficient clean water generation. ACS Appl. Mater. Interfaces 2016, 8, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Yang, Y.; Xu, B.; Cai, Z. Bifunctional fabric with photothermal effect and photocatalysis for highly efficient clean water generation. ACS Sustain. Chem. Eng. 2018, 6, 10789–10797. [Google Scholar] [CrossRef]
- Lin, L.Y.; Nie, Y.; Kavadiya, S.; Soundappan, T.; Biswas, P. N-doped reduced graphene oxide promoted nano TiO2 as a bifunctional adsorbent/photocatalyst for CO2 photoreduction: Effect of N species. Chem. Eng. J. 2017, 316, 449–460. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Anpo, M. Use of visible light. Second-generation titanium oxide photocatalysts prepared by the application of an advanced metal ion-implantation method. Pure Appl. Chem. 2000, 72, 1787–1792. [Google Scholar] [CrossRef]
- Nosaka, Y.; Matsushita, M.; Nishino, J.; Nosaka, A. Nitrogen-doped titanium dioxide photocatalysts for visible response prepared by using organic compounds. Sci. Technol. Adv. Mater. 2005, 6, 143–148. [Google Scholar] [CrossRef]
- Sun, L.; Shi, Y.; Li, B.; Li, X.; Wang, Y. Preparation and characterization of polypyrrole/TiO2 nanocomposites by reverse microemulsion polymerization and its photocatalytic activity for the degradation of methyl orange under natural light. Polym. Compos. 2013, 34, 1076–1080. [Google Scholar] [CrossRef]
- Karimi, L.; Zohoori, S.; Yazdanshenas, M.E. Photocatalytic degradation of azo dyes in aqueous solutions under UV irradiation using nano-strontium titanate as the nano photocatalyst. J. Saudi Chem. Soc. 2014, 18, 581–588. [Google Scholar] [CrossRef]
- Yang, P.; Lu, C.; Hua, N.; Du, Y. Titanium dioxide nanoparticles co-doped with Fe3+ and Eu3+ ions for photocatalysis. Mater. Lett. 2002, 57, 794–801. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, P.; Liang, L.; Hu, H.; Peng, Y.; Li, X.; Liu, C. Facile preparation of superhydrophobic cotton fabric with a photothermal conversion effect via polypyrrole deposition for oil/water separation. J. Environ. Chem. Eng. 2022, 10, 106915. [Google Scholar] [CrossRef]
- Pakdel, E.; Xie, W.; Wang, J.; Kashi, S.; Sharp, J.; Zhang, Q.; Varley, R.J.; Sun, L.; Wang, X. Superhydrophobic natural melanin-coated cotton with excellent UV protection and personal thermal management functionality. Chem. Eng. J. 2022, 433, 133688. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Wen, H.; Zhu, Z.; Huang, W.; Liu, C. Photothermal conversion-assisted oil Water separation by superhydrophobic Cotton yarn prepared via the silver mirror reaction. Colloids Surf. A 2021, 610, 125684. [Google Scholar] [CrossRef]
- Liang, J.; Liu, H.; Yu, J.; Zhou, L.; Zhu, J. Plasmon-enhanced solar vapor generation. Nanophotonics 2019, 8, 771–786. [Google Scholar] [CrossRef]
- Jain, M.; Khan, S.A.; Pandey, A.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.T. Instructive analysis of engineered carbon materials for potential application in water and wastewater treatment. Sci. Total Environ. 2021, 793, 148583. [Google Scholar] [CrossRef]
- Liu, Y.; Song, H.; Bei, Z.; Zhou, L.; Zhao, C.; Ooi, B.S.; Gan, Q. Ultra-thin dark amorphous TiOX hollow nanotubes for full spectrum solar energy harvesting and conversion. Nano Energy 2021, 84, 105872. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, M.; Hou, Y.; Wang, D.; Zhang, L.; Wang, D.; Fu, S. Mussel-inspired photothermal synergetic system for clean water production using full-spectrum solar energy. Chem. Eng. J. 2021, 423, 129099. [Google Scholar]
- Yang, Y.; Zhao, H.; Yin, Z.; Zhao, J.; Yin, X.; Li, N.; Yin, D.; Li, Y.; Lei, B.; Du, Y.; et al. A general salt-resistant hydrophilic/hydrophobic nanoporous double layer design for efficient and stable solar water evaporation distillation. Mater. Horiz. 2018, 5, 1143–1150. [Google Scholar] [CrossRef]
- Fang, Q.; Li, T.; Lin, H.; Jiang, R.; Liu, F. Highly efficient solar steam generation from activated carbon fiber cloth with matching water supply and durable fouling resistance. ACS Appl. Energy Mater. 2019, 2, 4354–4361. [Google Scholar] [CrossRef]
- Ancy, K.; Bindhu, M.R.; Bai, J.S.; Gatasheh, M.K.; Hatamleh, A.A.; Ilavenil, S. Photocatalytic degradation of organic synthetic dyes and textile dyeing waste water by Al and F co-doped TiO2 nanoparticles. Environ. Res. 2022, 206, 112492. [Google Scholar] [CrossRef]
- Bilgic, A. Fabrication of monoBODIPY-functionalized Fe3O4@SiO2@TiO2 nanoparticles for the photocatalytic degradation of rhodamine B under UV irradiation and the detection and removal of Cu (II) ions in aqueous solutions. J. Alloy. Compd. 2022, 899, 163360. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Zhang, W.; Xiao, Y.; Xu, H.; Liu, Y.; Liu, Z.; Zhang, J.; Jiang, Y. Visible-light-driven double-shell SnIn4S8/TiO2 heterostructure with enhanced photocatalytic activity for MO removal and Cr (VI) cleanup. Appl. Surf. Sci. 2022, 587, 152867. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Jing, L.; Xie, M.; Song, Y.; Xu, H.; Li, H.; Xie, J. In situ construction efficient visible-light-driven three-dimensional Polypyrrole/Zn3In2S6 nanoflower to systematically explore the photoreduction of Cr (VI): Performance, factors and mechanism. J. Hazard. Mater. 2020, 384, 121480. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.T.T.; Tran, A.T.K.; Hoang, M.H.; Nguyen, T.T.H.; Bui, X.T. Synergistic effect of TiO2 /chitosan/glycerol photocatalyst on color and COD removal from a dyeing and textile secondary effluent. Environ. Technol. Innov. 2021, 21, 101255. [Google Scholar] [CrossRef]
- Pitcher, M.W.; Emin, S.M.; Valant, M. A simple demonstration of photocatalysis using sunlight. J. Chem. Educ. 2012, 89, 1439–1441. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Yang, X.; Xu, L.; Wang, X.; Yu, J.; Wu, D.; Li, F.; Gao, T. A Composite Fabric with Dual Functions for High-Performance Water Purification. Materials 2022, 15, 5917. https://doi.org/10.3390/ma15175917
Tian Y, Yang X, Xu L, Wang X, Yu J, Wu D, Li F, Gao T. A Composite Fabric with Dual Functions for High-Performance Water Purification. Materials. 2022; 15(17):5917. https://doi.org/10.3390/ma15175917
Chicago/Turabian StyleTian, Yankuan, Xin Yang, Long Xu, Xueli Wang, Jianyong Yu, Dequn Wu, Faxue Li, and Tingting Gao. 2022. "A Composite Fabric with Dual Functions for High-Performance Water Purification" Materials 15, no. 17: 5917. https://doi.org/10.3390/ma15175917




