Graphene Nanostructures by Pulsed Laser Ablation in Liquids: A Review
Abstract
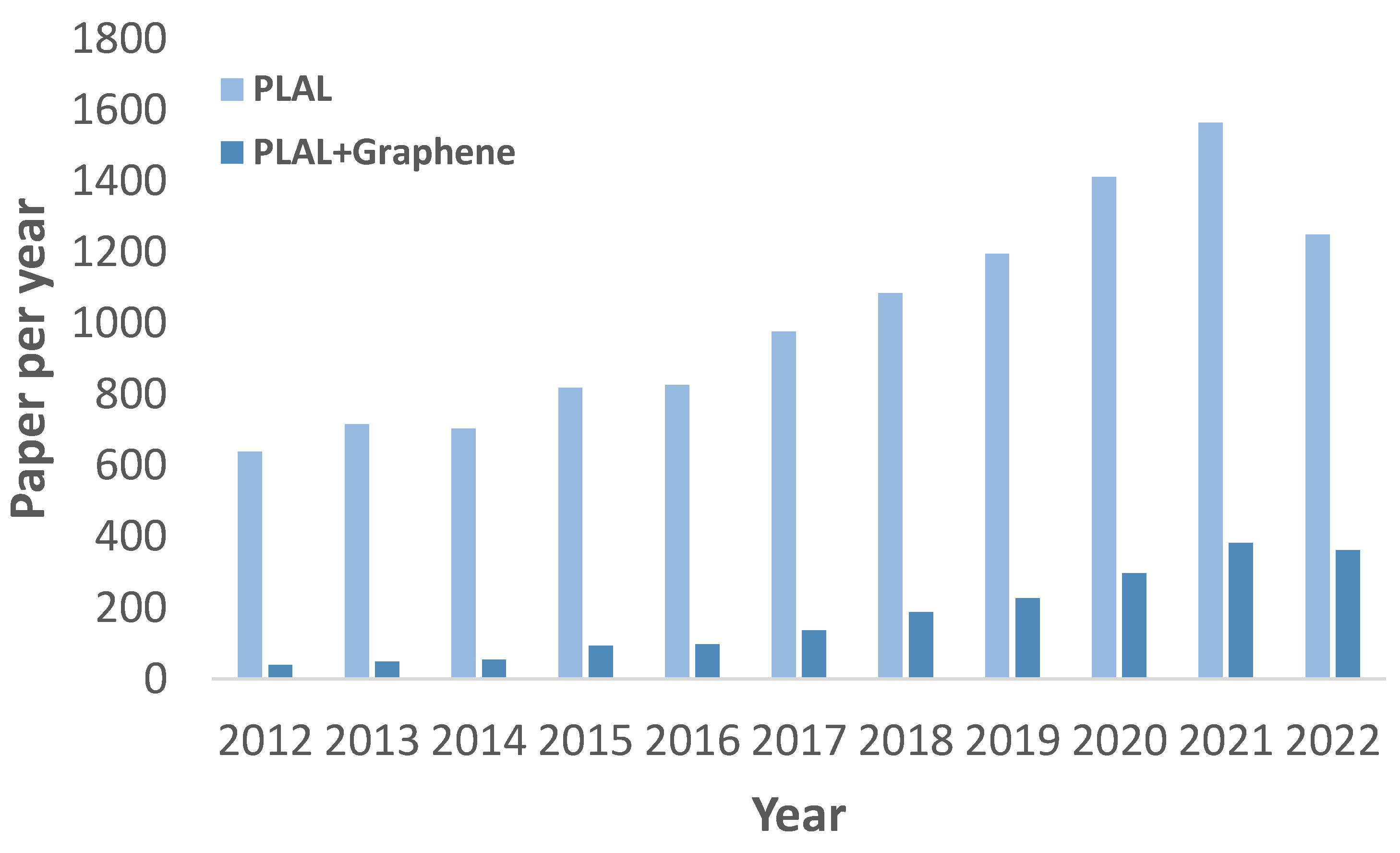
:1. Introduction
1.1. Why Graphene?
1.2. Methods of Synthesizing Graphene (Chemical/Physical)
1.3. Why PLAL?
2. Fundamentals of PLAL
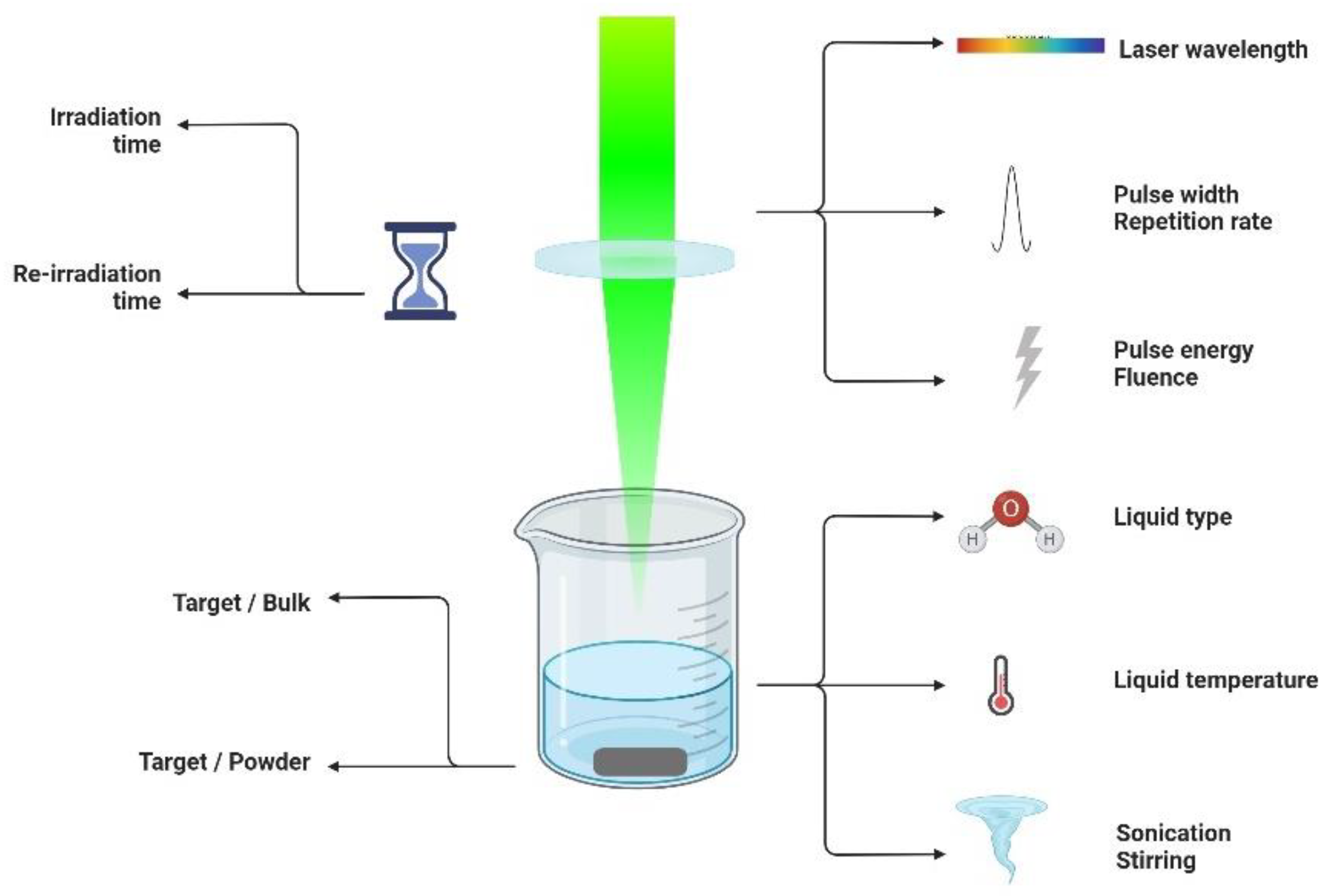
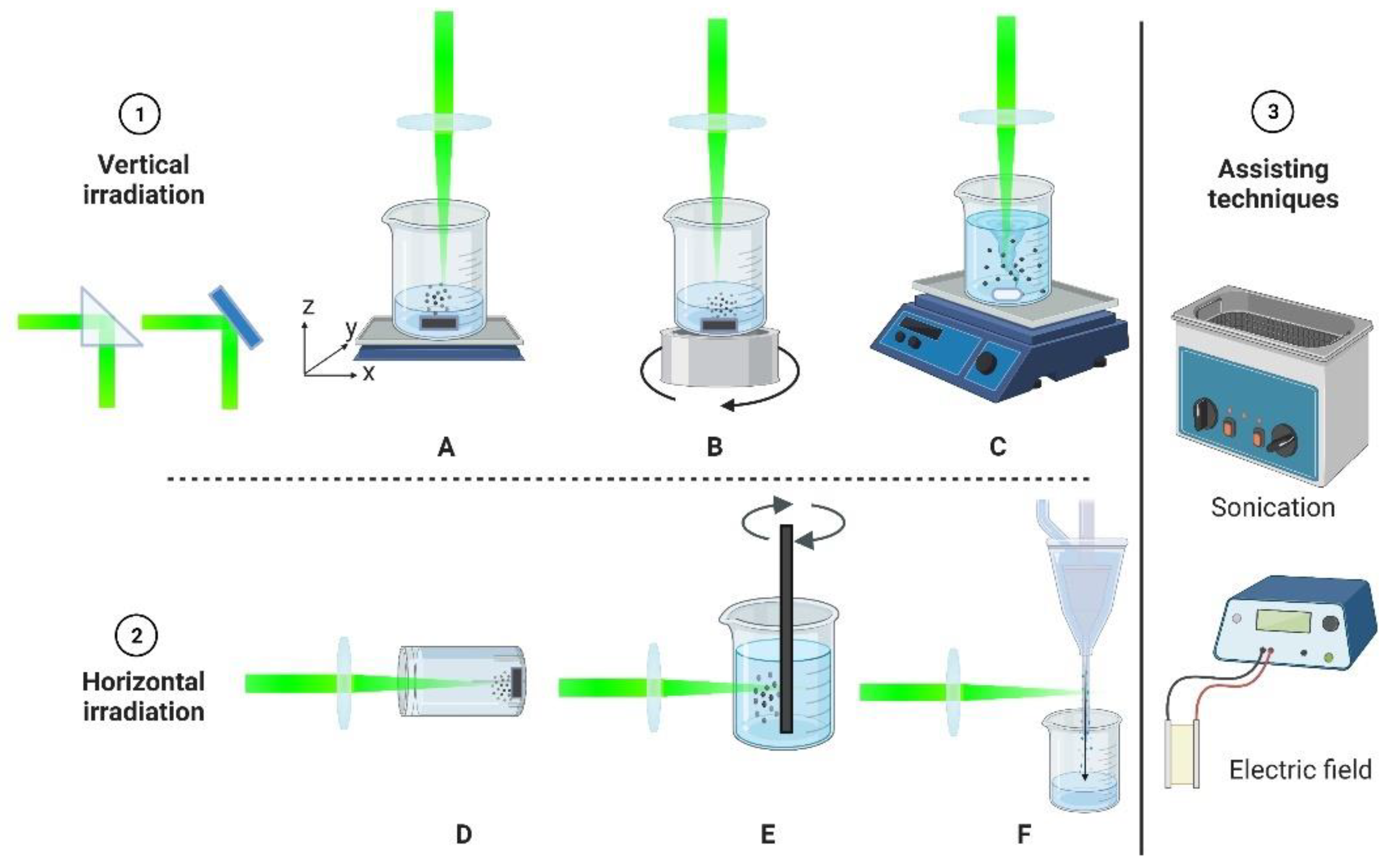
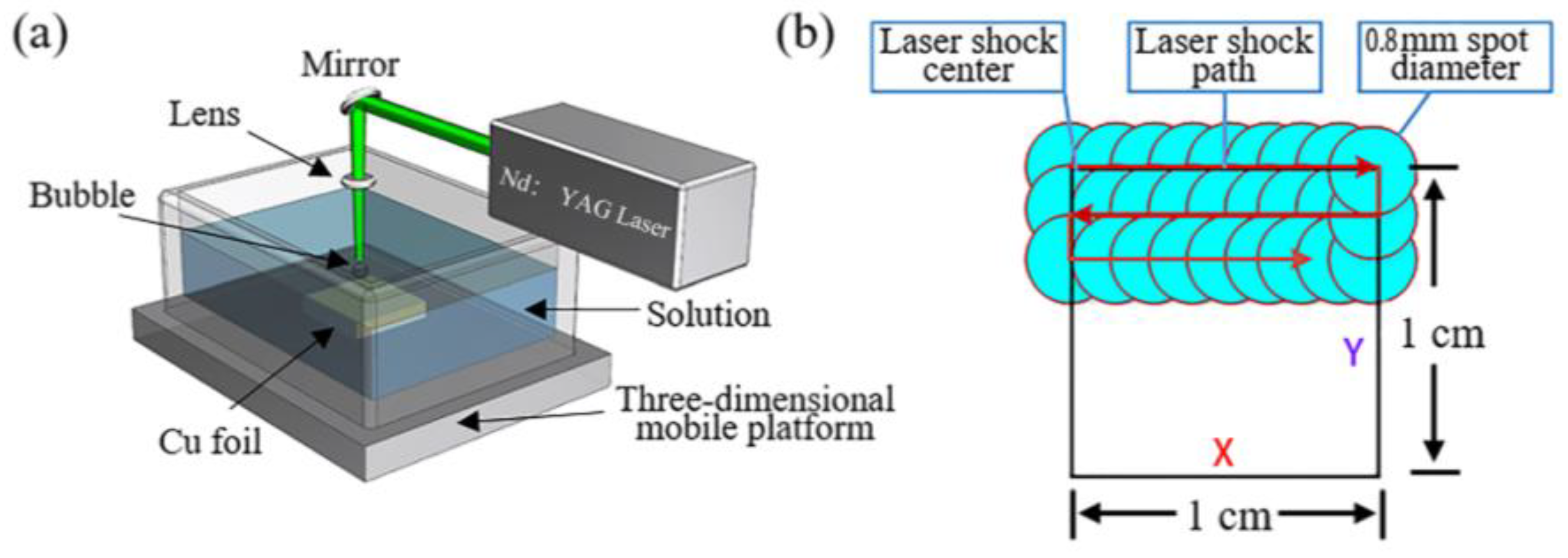
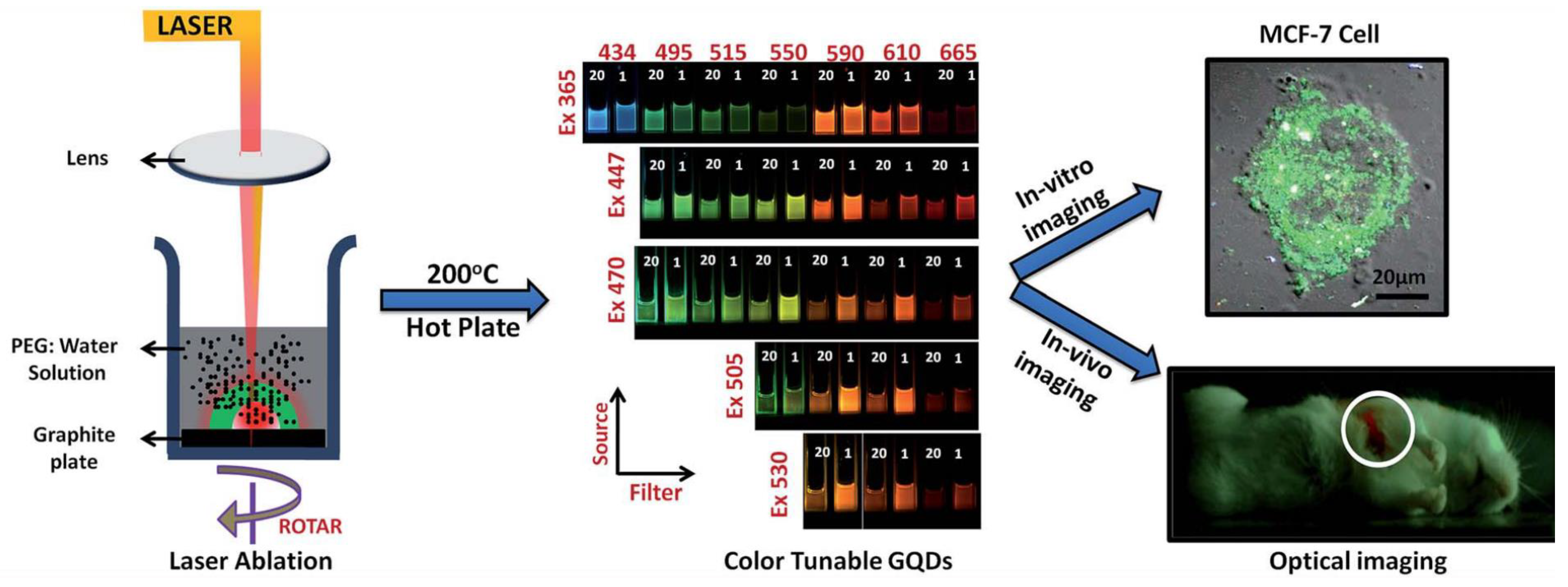
2.1. Experimental Setup for Nanostructure Formation via PLAL
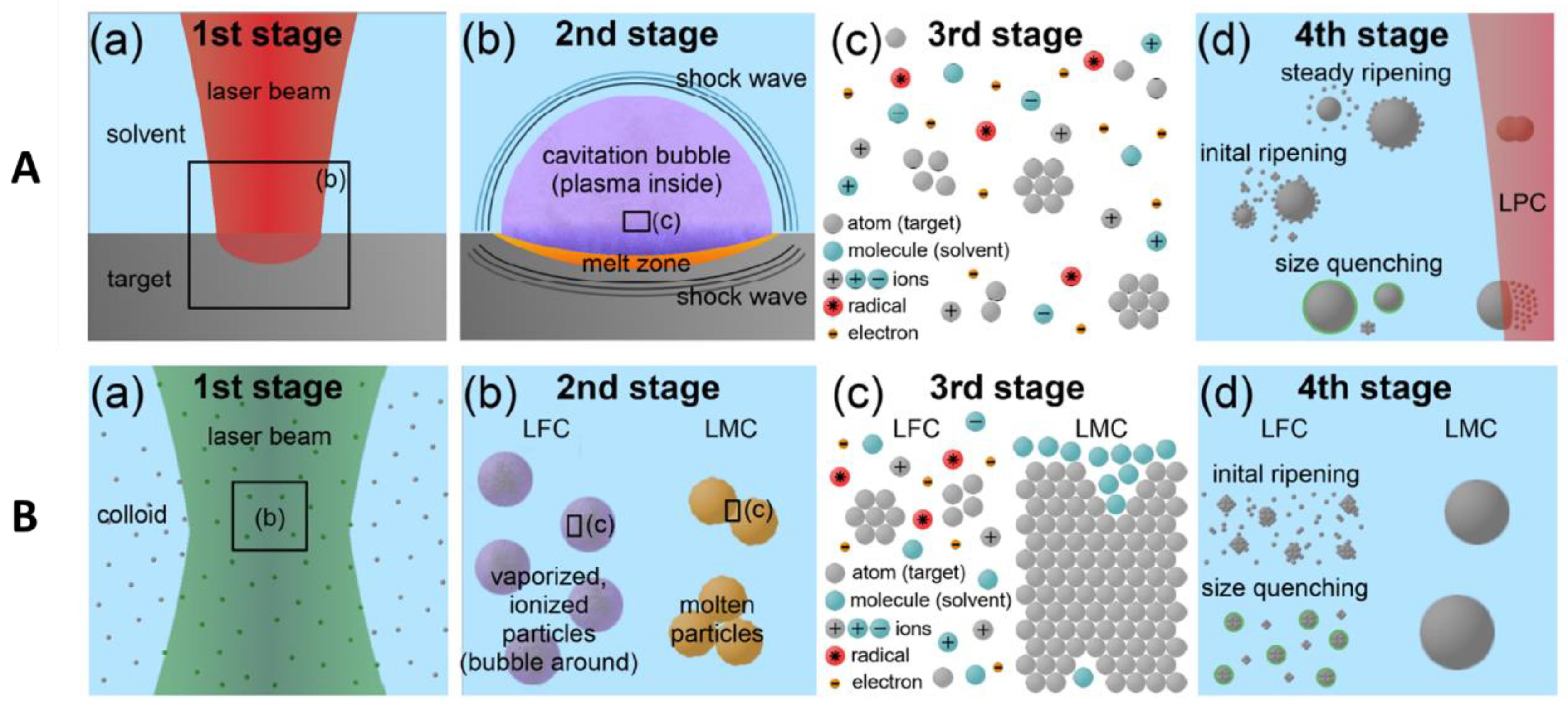
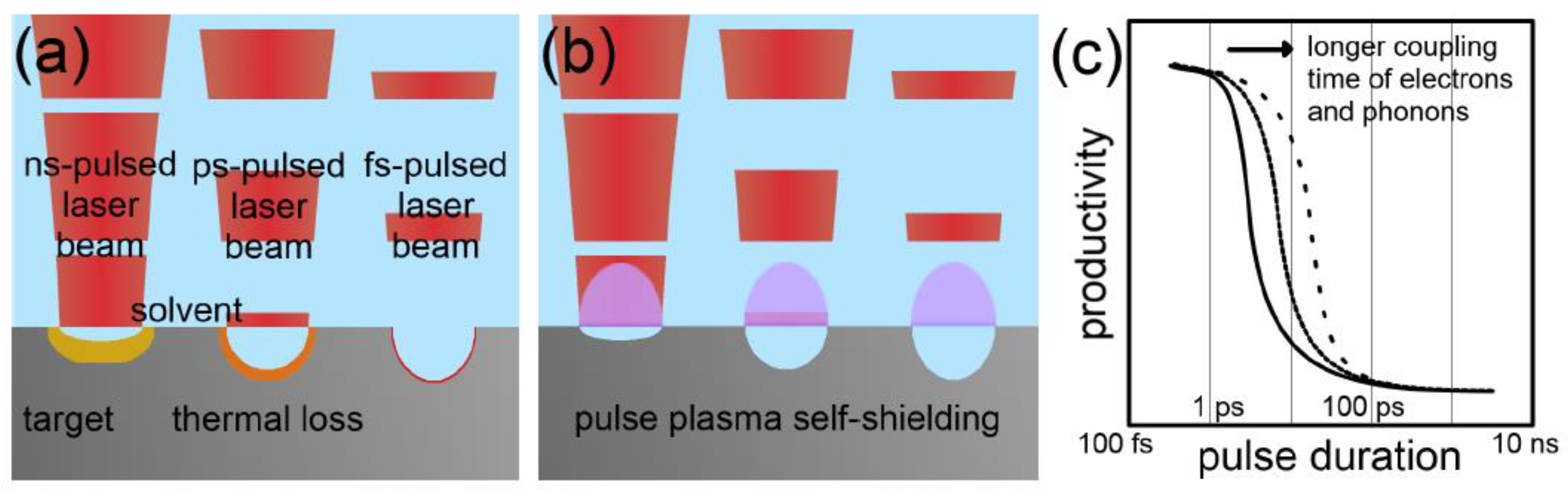
2.2. Laser–Matter Interaction
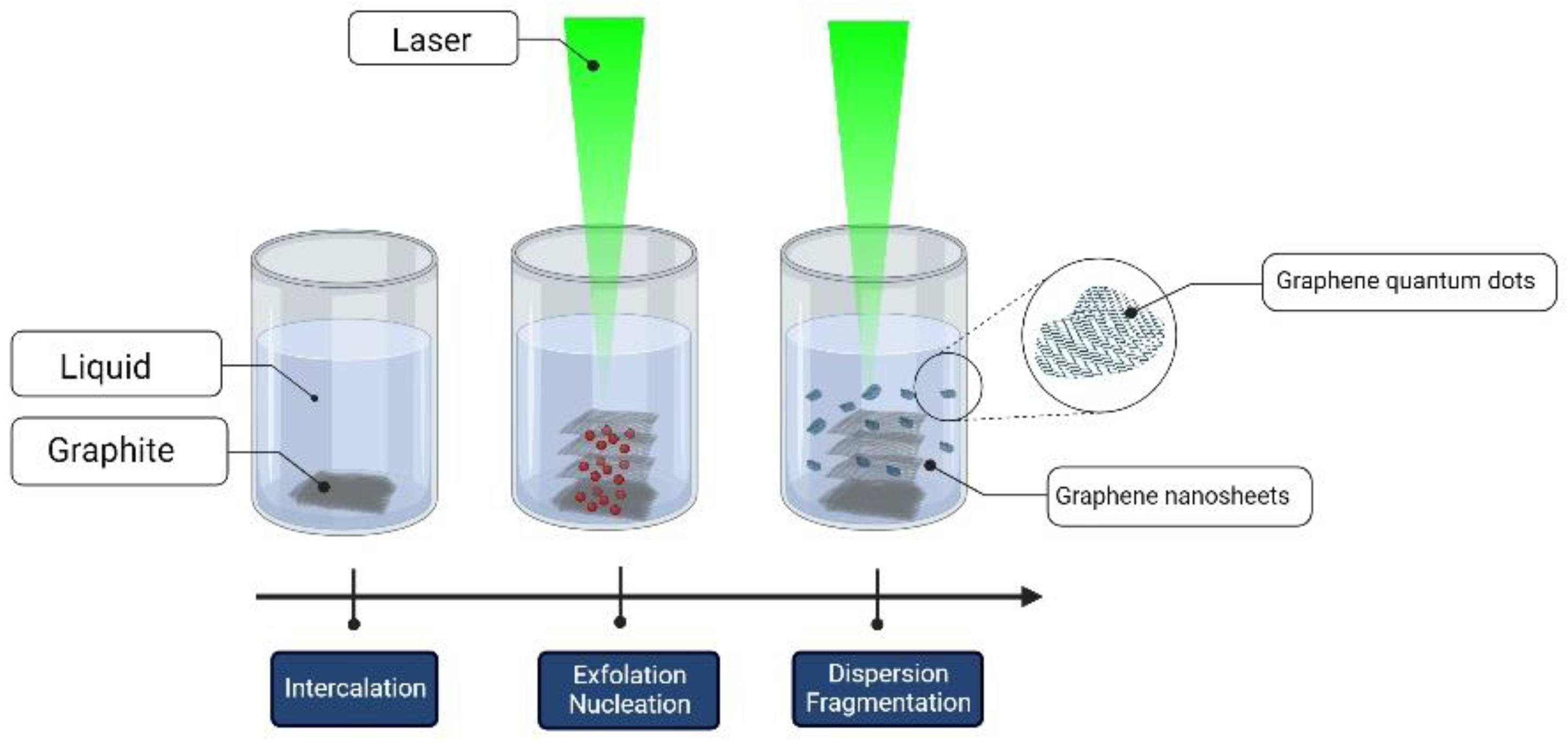
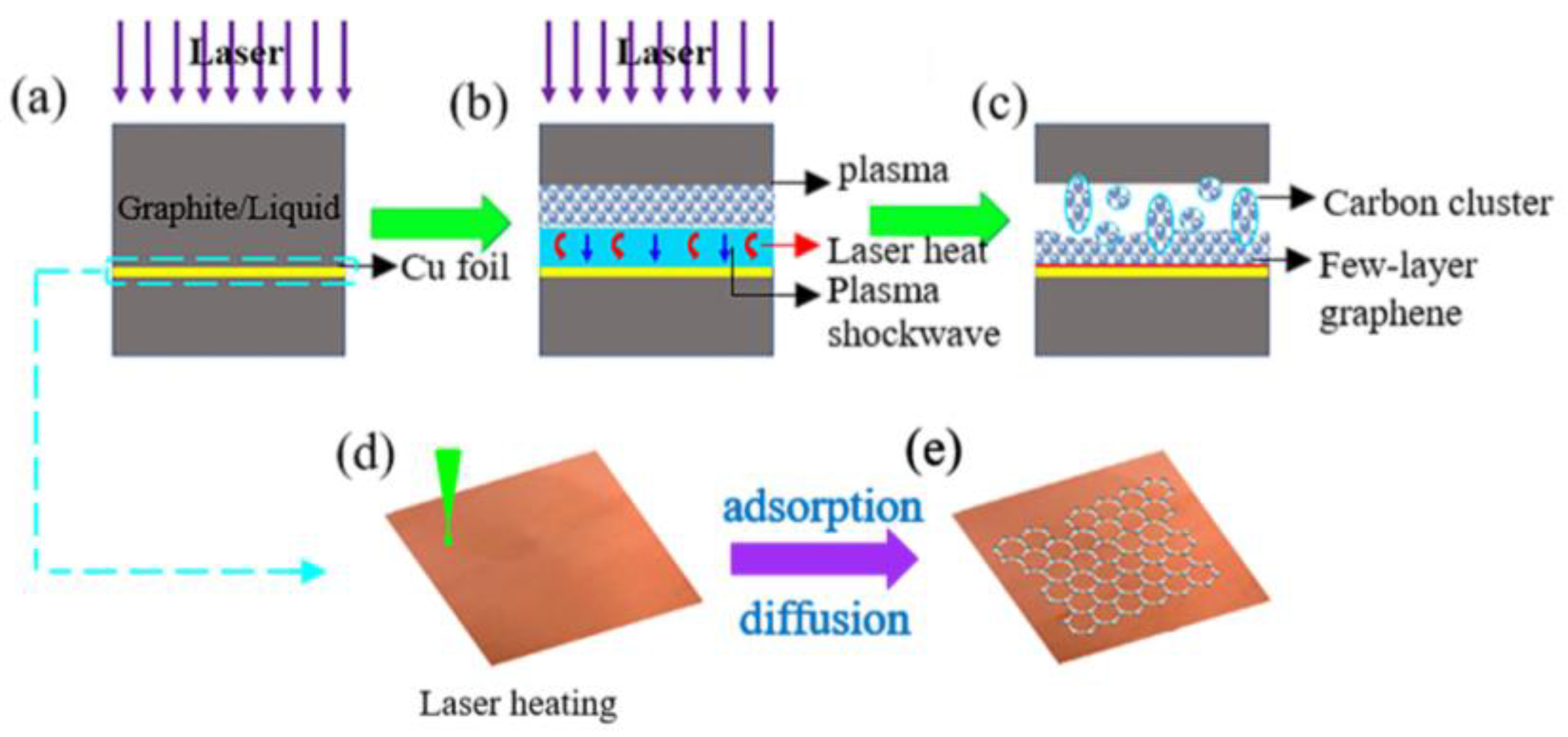
2.3. Graphene Formation Mechanisms
2.4. Characterizing Graphene Nanostructures
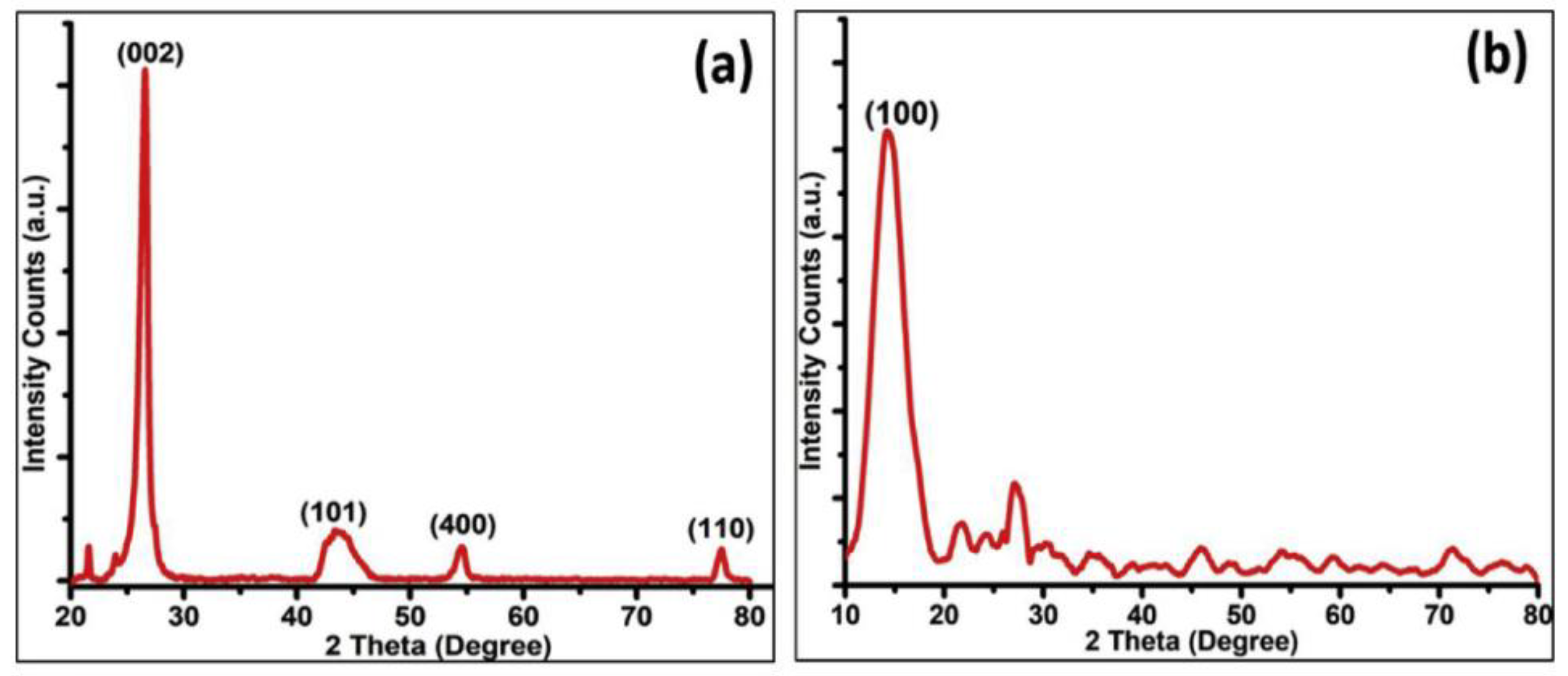
2.4.1. XRD Analysis
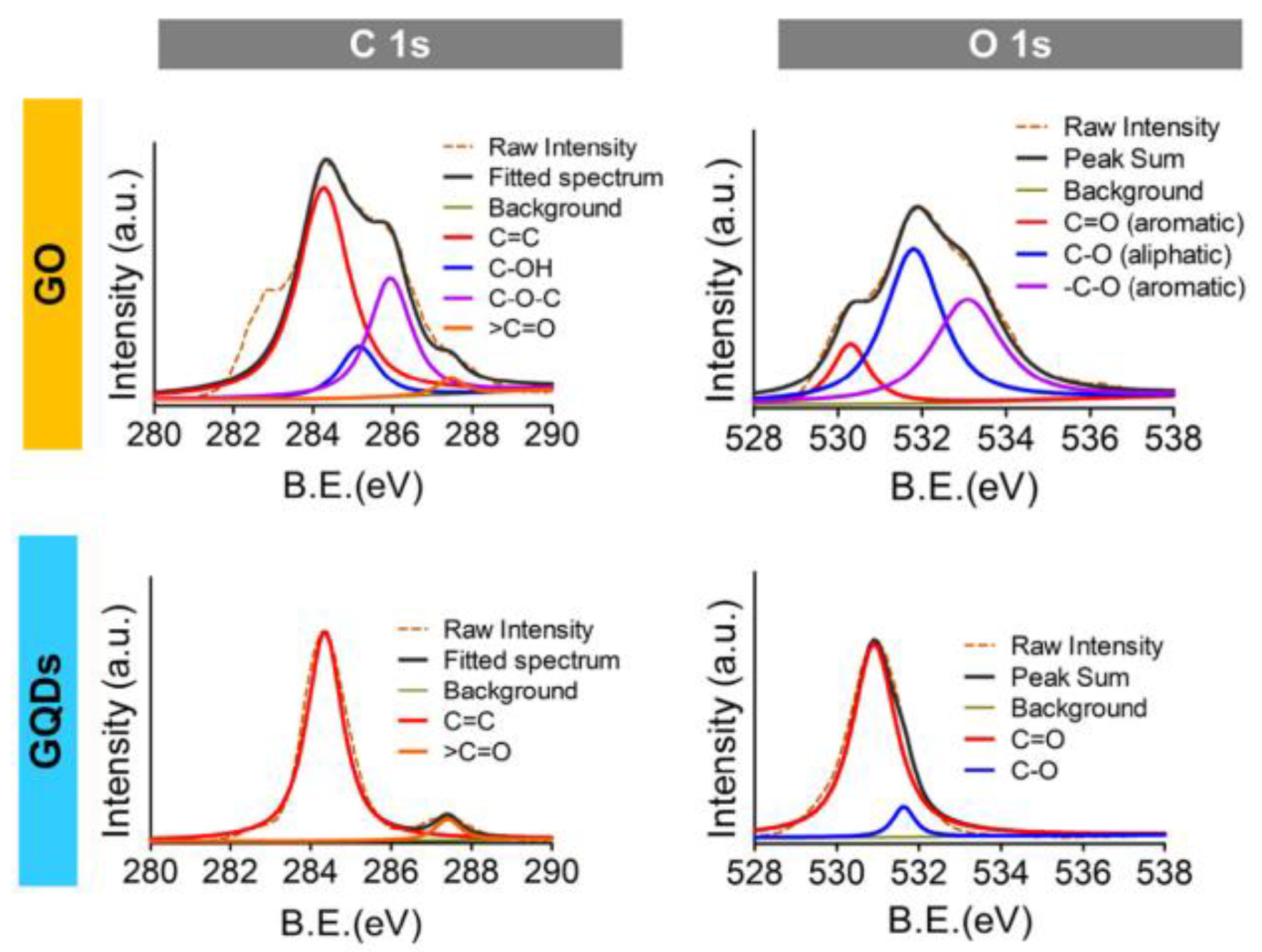
2.4.2. XPS Spectroscopy
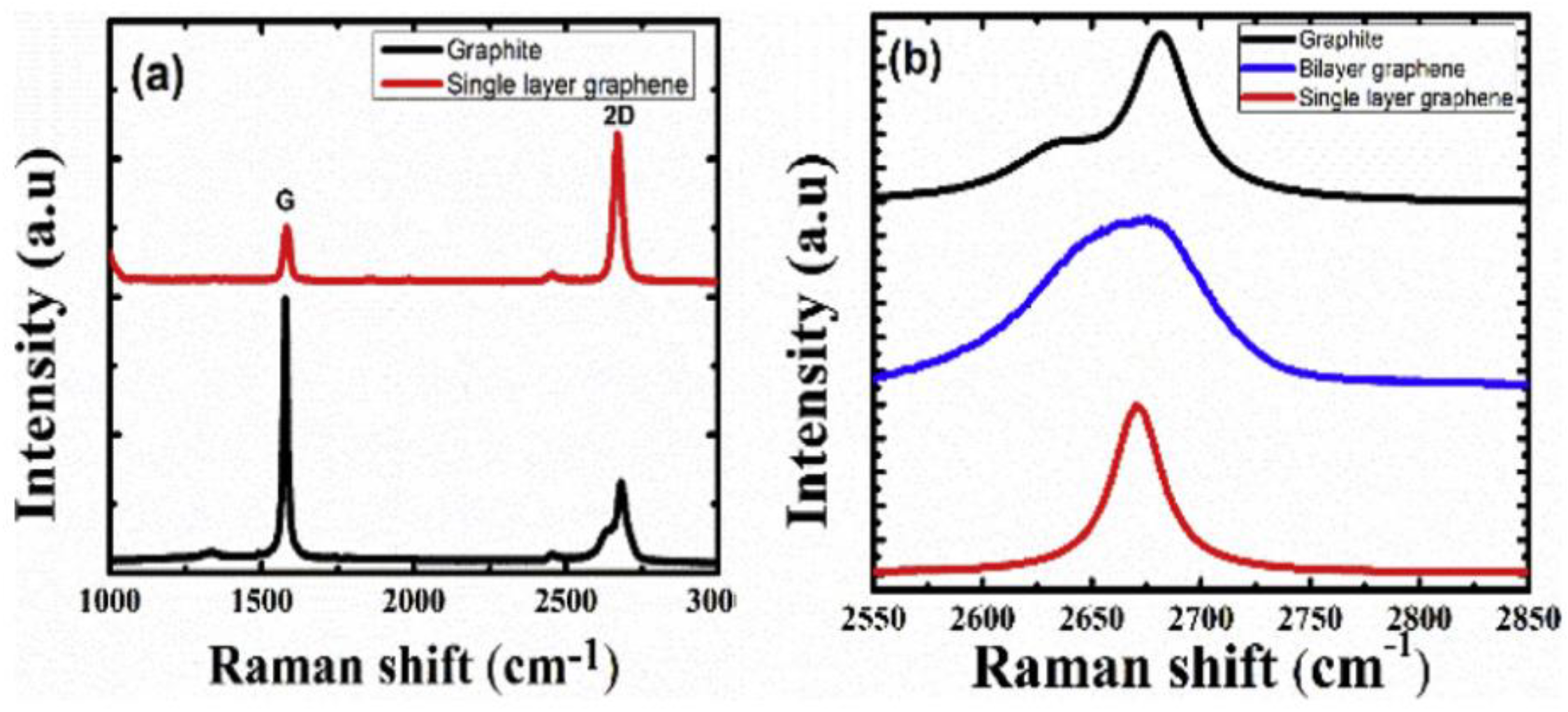
2.4.3. Raman Spectroscopy
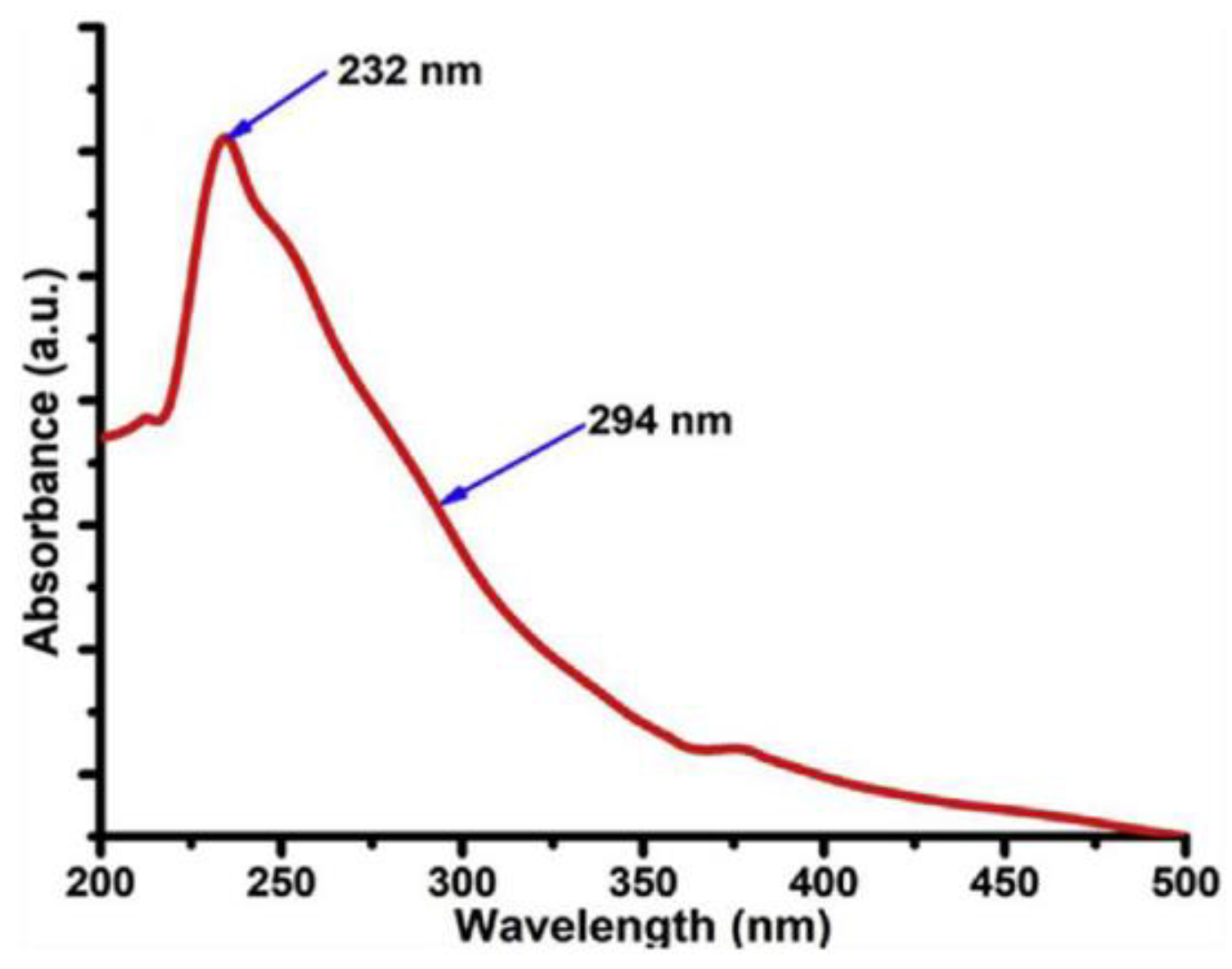
2.4.4. UV-Vis Absorbance Spectroscopy
2.4.5. Photoluminescence Emission (PL)
2.4.6. Fourier Transform Infrared Spectroscopy (FT-IR)
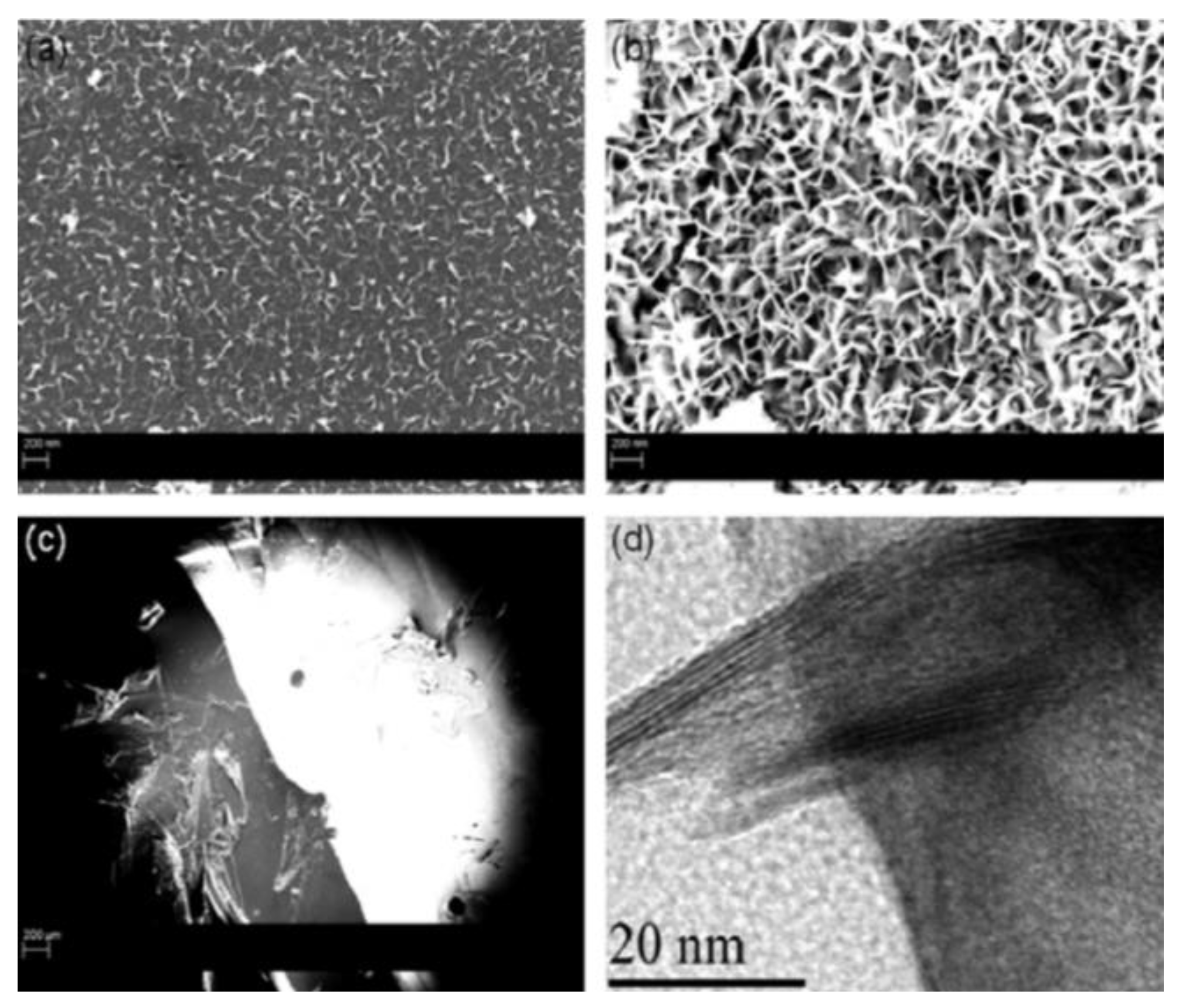
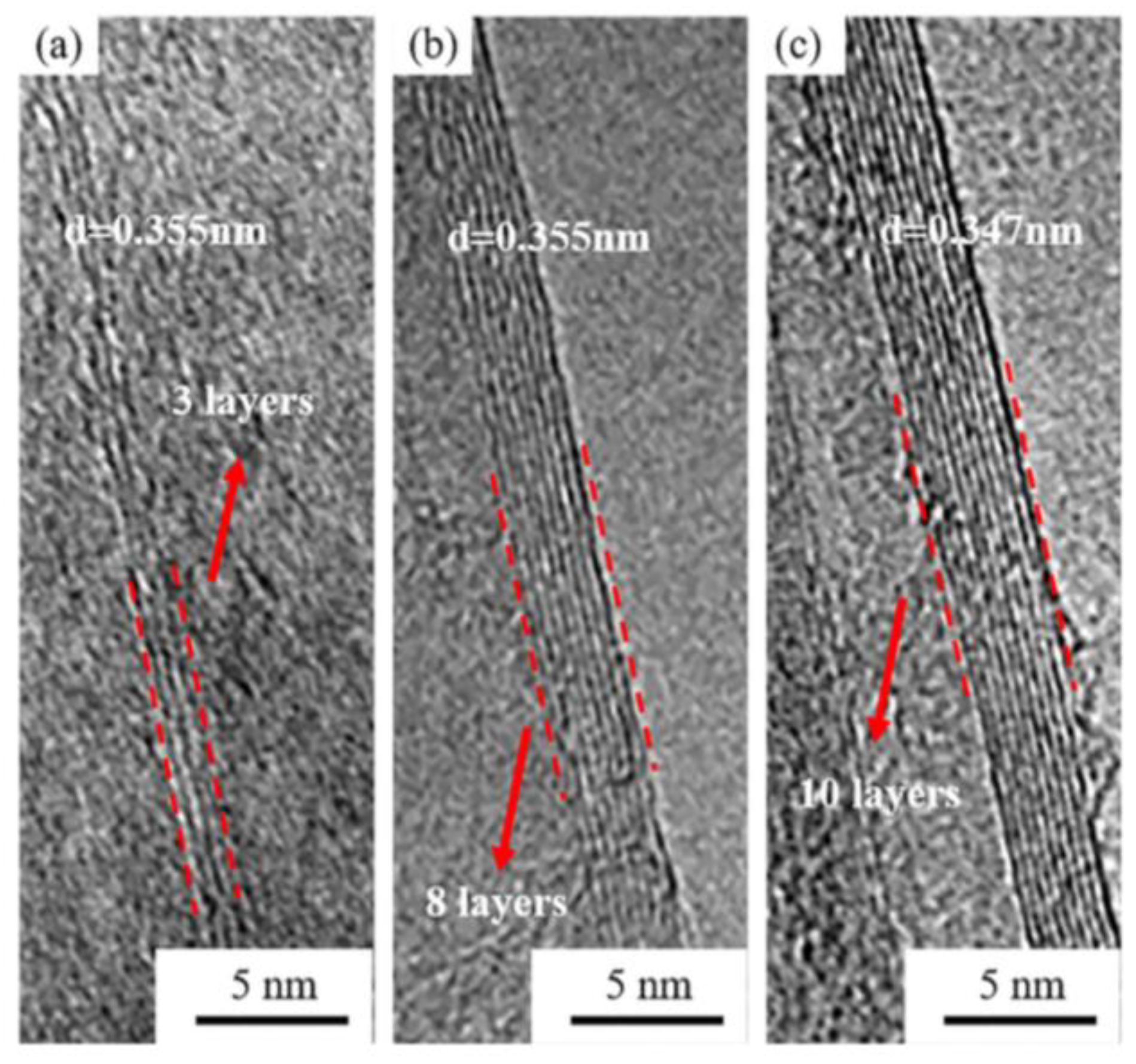
3. Graphene Nanostructures Prepared by PLAL
3.1. Graphene and Graphene-Oxide Nanosheets
3.1.1. Liquid Medium
3.1.2. Liquid Temperature
3.1.3. Laser Wavelength
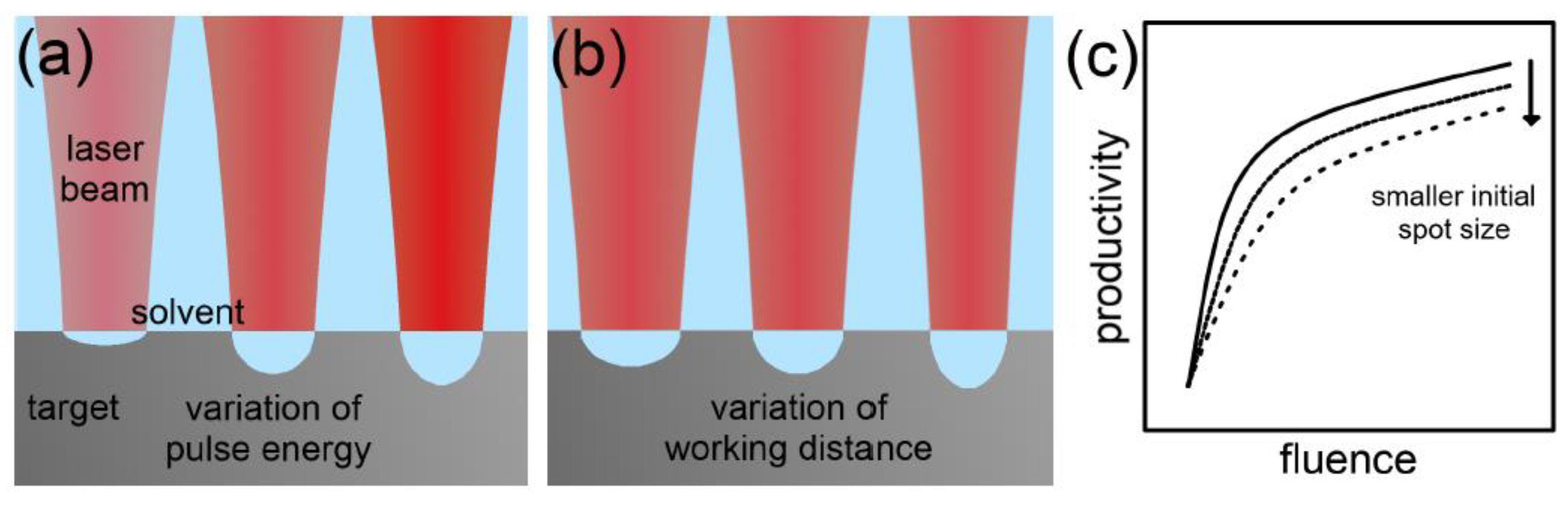
3.1.4. Laser Fluence
3.1.5. Irradiation Time/Pulse Duration/Repetition Rate
3.1.6. Target Material
3.1.7. Extending PLAL Technique
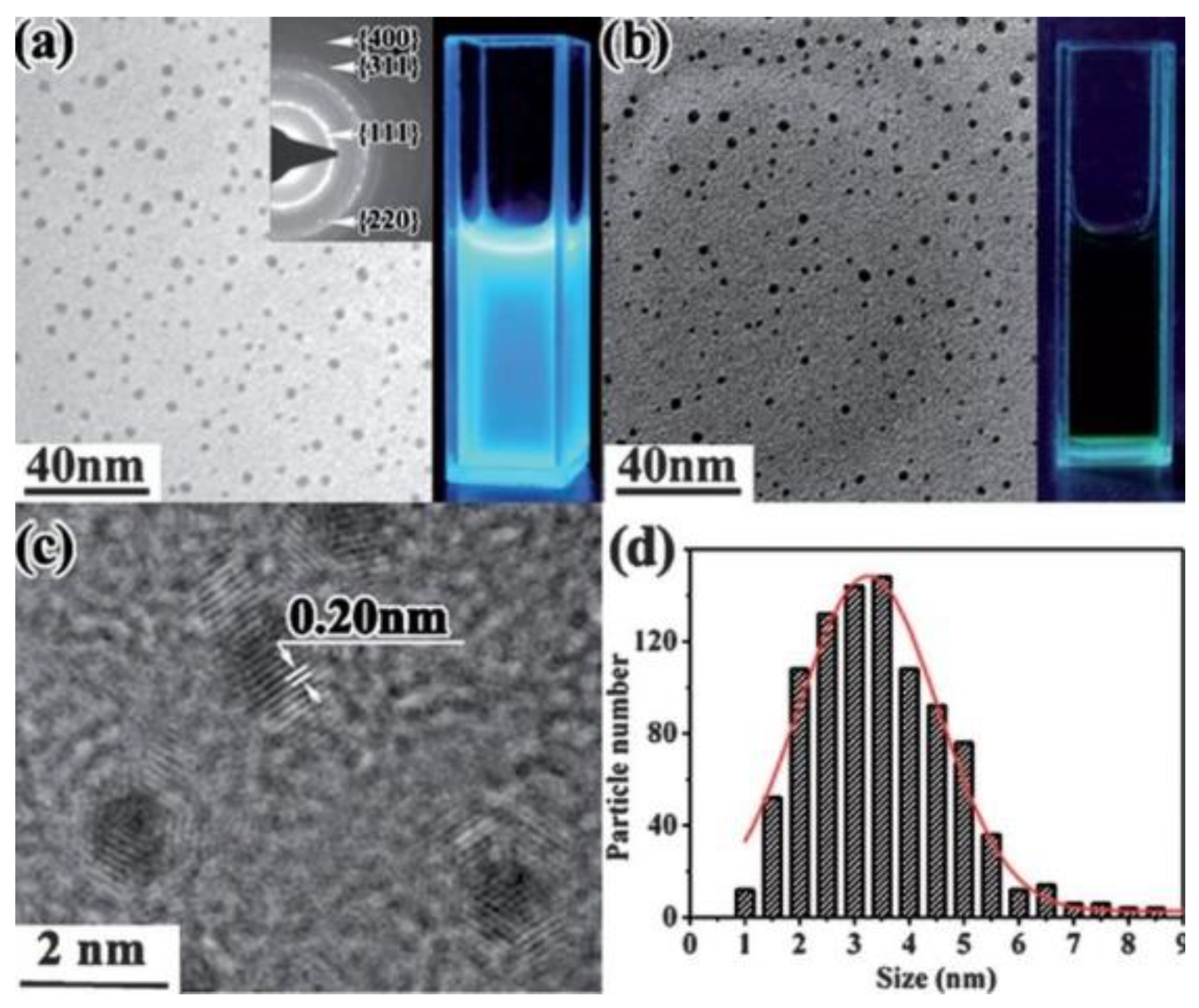
3.2. Graphene and Graphene-Oxide Quantum Dots
3.2.1. Liquid Medium
3.2.2. Laser Wavelength
3.2.3. Laser Fluence
3.2.4. Irradiation Time/Pulse Duration/Repetition Rate
3.2.5. Target Material
3.2.6. Extending PLAL Technique
4. Applications of PLAL Graphene Nanostructures
4.1. Bio-Applications
4.2. Catalysis
4.3. Energy-Relevant Applications
5. Summary and Outlook
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hirsch, A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-Scale Ballistic Transport in Encapsulated Graphene at Room Temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef]
- Moser, J.; Barreiro, A.; Bachtold, A. Current-induced cleaning of graphene. Appl. Phys. Lett. 2007, 91, 163513. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Cao, Y.; Fatemi, V.; Fang, S.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Jarillo-Herrero, P. Unconventional superconductivity in magic-angle graphene superlattices. Nature 2018, 556, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.A.; Mohsin, M.H.; Ali, A.K.; Hassoon, K.I.; Erten-Ela, S. Preparation and characterization of carbon nanotubes by pulsed laser ablation in water for optoelectronic application. Phys. E Low Dimens. Syst. Nanostruct. 2020, 119, 113997. [Google Scholar] [CrossRef]
- Ghosh, D.; Sarkar, K.; Devi, P.; Kim, K.-H.; Kumar, P. Current and future perspectives of carbon and graphene quantum dots: From synthesis to strategy for building optoelectronic and energy devices. Renew. Sustain. Energy Rev. 2020, 135, 110391. [Google Scholar] [CrossRef]
- Ahmed, M.; El-Naggar, M.E.; Aldalbahi, A.; El-Newehy, M.H.; Menazea, A. Methylene blue degradation under visible light of metallic nanoparticles scattered into graphene oxide using laser ablation technique in aqueous solutions. J. Mol. Liq. 2020, 315, 113794. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shuaib, E.P.; Roopmani, P.; Gumpu, M.B.; Krishnan, U.M.; Sastikumar, D. Fluorescent carbon nanoparticles from la-ser-ablated Bougainvillea alba flower extract for bioimaging applications. Appl. Phys. A 2019, 125, 379. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shuaib, E.; Roopmani, P.; Gumpu, M.B.; Krishnan, U.M.; Sastikumar, D. Synthesis, characterization and bioimaging application of laser-ablated graphene-oxide nanoparticles (nGOs). Diam. Relat. Mater. 2020, 104, 107733. [Google Scholar] [CrossRef]
- Mitra, S.; Aravindh, A.; Das, G.; Pak, Y.; Ajia, I.; Loganathan, K.; Di Fabrizio, E.; Roqan, I.S. High-performance solar-blind flexible deep-UV photodetectors based on quantum dots synthesized by femtosecond-laser ablation. Nano Energy 2018, 48, 551–559. [Google Scholar] [CrossRef]
- Novoa-De León, I.C.; Johny, J.; Vázquez-Rodríguez, S.; García-Gómez, N.; Carranza-Bernal, S.; Mendivil, I.; Shaji, S.; Sepúlve-da-Guzmán, S. Tuning the luminescence of nitrogen-doped graphene quantum dots synthesized by pulsed laser ablation in liquid and their use as a selective photoluminescence on–off–on probe for ascorbic acid detection. Carbon 2019, 150, 455–464. [Google Scholar] [CrossRef]
- Dhar, S.; Barman, A.R.; Ni, G.X.; Wang, X.; Xu, X.F.; Zheng, Y.; Tripathy, S.; Ariando; Rusydi, A.; Loh, K.P.; et al. A new route to graphene layers by selective laser ablation. AIP Adv. 2011, 1, 022109. [Google Scholar] [CrossRef]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant Intrinsic Carrier Mobilities in Graphene and Its Bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [Green Version]
- Castro, E.; Novoselov, K.; Morozov, S.; Peres, N.M.R.; dos Santos, J.M.B.L.; Nilsson, J.; Guinea, F.; Geim, A.K.; Neto, A.H.C. Biased Bilayer Graphene: Semiconductor with a Gap Tunable by the Electric Field Effect. Phys. Rev. Lett. 2007, 99, 216802. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Barkan, T. The Graphene Report 2022: The Graphene Council. 2022. Available online: https://www.thegraphenecouncil.org/ (accessed on 14 July 2022).
- Saha, J.K.; Dutta, A. A Review of Graphene: Material Synthesis from Biomass Sources. Waste Biomass Valoriz. 2021, 13, 1385–1429. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Botlhoko, O.J.; Setshedi, K.; Scriba, M.; Masukume, M.; Ray, S.S. Top-down synthesis of graphene: A comprehensive review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for car-bon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Liu, W.W.; Chai, S.-P.; Mohamed, A.R.; Hashim, U. Synthesis and characterization of graphene and carbon nanotubes: A review on the past and recent developments. J. Ind. Eng. Chem. 2014, 20, 1171–1185. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Aziz, A.; Khe, C.-S.; Hidayah, N.M.S.; Hashim, U. Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Adv. 2017, 7, 15644–15693. [Google Scholar] [CrossRef]
- Liu, P.; Cui, H.; Wang, C.X.; Yang, G.W. From nanocrystal synthesis to functional nanostructure fabrication: Laser ablation in liquid. Phys. Chem. Chem. Phys. 2010, 12, 3942–3952. [Google Scholar] [CrossRef]
- Amans, D.; Diouf, M.; Lam, J.; Ledoux, G.; Dujardin, C. Origin of the nano-carbon allotropes in pulsed laser ablation in liquids synthesis. J. Colloid Interface Sci. 2017, 489, 114–125. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shukla, S.; Sastikumar, D.; Koinkar, P. Progress in pulsed laser ablation in liquid (PLAL) technique for the synthesis of carbon nanomaterials: A review. Appl. Phys. A 2021, 127, 1–40. [Google Scholar] [CrossRef]
- Ma, H.; Shen, Z. Exfoliation of graphene nanosheets in aqueous media. Ceram. Int. 2020, 46, 21873–21887. [Google Scholar] [CrossRef]
- Ren, X.; Ma, F.; Wang, R.; Qian, L.; Ma, W. Morphology-selective preparation and formation mechanism of few-layer graphene on Cu substrate by liquid-phase pulsed laser ablation. AIP Adv. 2019, 9, 125004. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 33–56. [Google Scholar] [CrossRef]
- Semaltianos, N.G. Nanoparticles by Laser Ablation. Crit. Rev. Solid State Mater. Sci. 2010, 35, 105–124. [Google Scholar] [CrossRef]
- Yan, Z.; Chrisey, D.B. Pulsed laser ablation in liquid for micro-/nanostructure generation. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 204–223. [Google Scholar] [CrossRef]
- Zhang, J.; Claverie, J.; Chaker, M.; Ma, D. Colloidal Metal Nanoparticles Prepared by Laser Ablation and their Applications. ChemPhysChem 2017, 18, 986–1006. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jung, K.H.; Mhin, S.; Son, Y.; Lee, K.; Kim, W.R.; Choi, H.; Ryu, J.H.; Han, H.; Kim, K.M. Fundamental Understanding of the Formation Mechanism for Graphene Quantum Dots Fabricated by Pulsed Laser Fragmentation in Liquid: Experimental and Theoretical Insight. Small 2020, 16, e2003538. [Google Scholar] [CrossRef]
- Barcikowski, S.; Compagnini, G. Advanced nanoparticle generation and excitation by lasers in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3022–3026. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shuaib, E.P.; Kalai Priya, A.; Rohini, P.; Anandhan, S.V.; Krishnan, U.M.; Kalyanavalli, V.; Shukla, S.; Sastikumar, D. Syn-thesis of water-soluble fluorescent carbon nanoparticles (CNPs) from nanosecond pulsed laser ablation in ethanol. Opt. Laser Technol. 2021, 135, 106717. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Yeh, C.-S. Laser ablation method: Use of surfactants to form the dispersed Ag nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2002, 197, 133–139. [Google Scholar] [CrossRef]
- Lau, M.; Barcikowski, S. Quantification of mass-specific laser energy input converted into particle properties during picosecond pulsed laser fragmentation of zinc oxide and boron carbide in liquids. Appl. Surf. Sci. 2015, 348, 22–29. [Google Scholar] [CrossRef]
- Hupfeld, T.; Sommereyns, A.; Riahi, F.; Doñate-Buendía, C.; Gann, S.; Schmidt, M.; Gökce, B.; Barcikowski, S. Analysis of the Nano-particle Dispersion and Its Effect on the Crystalline Microstructure in Carbon-Additivated PA12 Feedstock Material for Laser Powder Bed Fusion. Materials 2020, 13, 3312. [Google Scholar] [CrossRef]
- Fabbro, R.; Fournier, J.; Ballard, P.; Devaux, D.; Virmont, J. Physical study of laser-produced plasma in confined geometry. J. Appl. Phys. 1990, 68, 775–784. [Google Scholar] [CrossRef]
- Waag, F. Laser Synthesis of Metallic and Oxidic Transition Metal, Multi Element Nanoparticles for Catalytic Applications: Institute for Technical Chemistry-University of Duisburg-Essen. Ph.D. Thesis, University of Duisburg-Essen, Duisburg, Germany, 2019. [Google Scholar]
- De Giacomo, A.; Dell’Aglio, M.; Santagata, A.; Gaudiuso, R.; De Pascale, O.; Wagener, P.; Messina, G.C.; Compagnini, G.; Barcikowski, S. Cavitation dynamics of laser ablation of bulk and wire-shaped metals in water during nanoparticles production. Phys. Chem. Chem. Phys. 2013, 15, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J. The effect of recoil pressure in the ablation of polycrystalline graphite by a nanosecond laser pulse. J. Phys. D Appl. Phys. 2015, 48, 235201. [Google Scholar] [CrossRef]
- Reich, S.; Schönfeld, P.; Wagener, P.; Letzel, A.; Ibrahimkutty, S.; Gökce, B.; Barcikowski, S.; Menzel, A.; Rolo, T.D.S.; Plech, A. Pulsed laser ablation in liquids: Impact of the bubble dynamics on particle formation. J. Colloid Interface Sci. 2017, 489, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Tomko, J.; O’Malley, S.; Trout, C.; Naddeo, J.; Jimenez, R.; Griepenburg, J.C.; Soliman, W.; Bubb, D. Cavitation bubble dynamics and nanoparticle size distributions in laser ablation in liquids. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 368–372. [Google Scholar] [CrossRef]
- Chichkov, B.N.; Momma, C.; Nolte, S.; von Alvensleben, F.; Tünnermann, A. Femtosecond, picosecond and nanosecond laser ab-lation of solids. Appl. Phys. A Mater. Sci. Process. 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Dittrich, S.; Barcikowski, S.; Gökce, B. Plasma and nanoparticle shielding during pulsed laser ablation in liquids cause ablation efficiency decrease. Opto-Electron. Adv. 2021, 4, 200072. [Google Scholar] [CrossRef]
- Hoffman, J.; Chrzanowska, J.; Kucharski, S.; Mościcki, T.; Mihailescu, I.N.; Ristoscu, C.; Szymański, Z. The effect of laser wavelength on the ablation rate of carbon. Appl. Phys. A 2014, 117, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nano-particles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Compagnini, G.; Sinatra, M.; Russo, P.; Messina, G.C.; Puglisi, O.; Scalese, S. Deposition of few layer graphene nanowalls at the electrodes during electric field-assisted laser ablation of carbon in water. Carbon 2012, 50, 2362–2365. [Google Scholar] [CrossRef]
- Escobar-Alarcón, L.; Espinosa-Pesqueira, M.E.; Solis-Casados, D.A.; Gonzalo, J.; Solis, J.; Martinez-Orts, M.; Haro-Poniatowski, E. Two-dimensional carbon nanostructures obtained by laser ablation in liquid: Effect of an ultrasonic field. Appl. Phys. A 2018, 124, 141. [Google Scholar] [CrossRef]
- Vaghri, E.; Dorranian, D. Effects of green laser fluence on the characteristics of graphene nanosheets synthesized by laser ablation method in liquid nitrogen medium. Opt. Quantum Electron. 2018, 50, 110. [Google Scholar] [CrossRef]
- Mahdian Asl, P.; Dorranian, D. Effect of liquid medium temperature on the production rate and quality of graphene nanosheets produced by laser ablation. Opt. Quantum Electron. 2016, 48, 535. [Google Scholar] [CrossRef]
- Barberio, M.; Antici, P. Laser-Plasma Driven Synthesis of Carbon-Based Nanomaterials. Sci. Rep. 2017, 7, 12009. [Google Scholar] [CrossRef]
- Li, L.; Zhou, M.; Jin, L.; Liu, L.; Mo, Y.; Li, X.; Mo, Z.; Liu, Z.; You, S.; Zhu, H. Research Progress of the Liquid-Phase Exfoliation and Stable Dispersion Mechanism and Method of Graphene. Front. Mater. 2019, 6, 325. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef]
- Zamiranvari, A.; Solati, E.; Dorranian, D. Effect of CTAB concentration on the properties of graphene nanosheet produced by laser ablation. Opt. Laser Technol. 2017, 97, 209–218. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Gumpu, M.B.; Shuaib, E.P.; Sastikumar, D. Laser-induced transformation of graphene into graphene oxide nanospheres (GONs). Mater. Res. Bull. 2019, 115, 227–234. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Bahadur, R.; Kaku, T.; Prabhuraj, R.S.; Ninawe, A.; Srivastava, R. Preparation of graphene oxide-graphene quantum dots hybrid and its application in cancer theranostics. Mater. Sci. Eng. C 2019, 103, 109774. [Google Scholar] [CrossRef] [PubMed]
- Vaghri, E.; Dorranian, G. Effect of ablation on environment on the characteristics of graphene nanosheets produced by laser ablation. Studia Ubb Chem. 2016, 4, 227–284. [Google Scholar]
- Juvaid, M.M.; Kumar, D.; Ramachandra, R. Realization of good quality bilayer graphene by single step laser ablation process. Mater. Res. Bull. 2020, 126, 110840. [Google Scholar] [CrossRef]
- Duque Buitrago, J.S.; Mesa Yandy, A.M.; Riascos Landázuri, H. Investigation of laser wavelength effect on optical properties of graphene oxide colloidal nanostructures prepared by pulsed laser ablation. J. Phys. Conf. Ser. 2019, 1247, 012025. [Google Scholar] [CrossRef]
- Kang, S.; Ryu, J.H.; Lee, B.; Jung, K.H.; Shim, K.B.; Han, H.; Kim, K.M. Laser wavelength modulated pulsed laser ablation for selective and efficient production of graphene quantum dots. RSC Adv. 2019, 9, 13658–13663. [Google Scholar] [CrossRef]
- Choudhary, R.P.; Shukla, S.; Vaibhav, K.; Pawar, P.B.; Saxena, S. Optical properties of few layered graphene quantum dots. Mater. Res. Express 2015, 2, 095024. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Liu, J.; Chen, Q.; Zhu, X.; Liang, C. Carbon-Encapsulated Metal/Metal Carbide/Metal Oxide Core–Shell Nanostructures Generated by Laser Ablation of Metals in Organic Solvents. ACS Appl. Nano Mater. 2018, 2, 28–39. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Thomas, A.; Kotov, N.A.; Haag, R. Functional Graphene Nanomaterials Based Architectures: Biointeractions, Fabrications, and Emerging Biological Applications. Chem. Rev. 2017, 117, 1826–1914. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Ma, H.; Ma, M.; Zeng, J.; Guoa, X.; Ma, Y. Hydrothermal synthesis of graphene nanosheets and its application in electrically conductive adhesives. Mater. Lett. 2016, 178, 181–184. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Sugioka, K. Laser ablation in liquids for nanomaterial synthesis: Diversities of targets and liquids. J. Phys. Photonics 2021, 3, 042002. [Google Scholar] [CrossRef]
- Ganash, E.A.; Al-Jabarti, G.A.; Altuwirqi, R.M. The synthesis of carbon-based nanomaterials by pulsed laser ablation in water. Mater. Res. Express 2019, 7, 015002. [Google Scholar] [CrossRef]
- Qian, M.; Zhou, Y.S.; Gao, Y.; Feng, T.; Sun, Z.; Jiang, L.; Lu, Y.F. Production of few-layer graphene through liquid-phase pulsed laser exfoliation of highly ordered pyrolytic graphite. Appl. Surf. Sci. 2012, 258, 9092–9095. [Google Scholar] [CrossRef]
- Ghavidel, E.; Sari, A.H.; Dorranian, D. Experimental investigation of the effects of different liquid environments on the graphene oxide produced by laser ablation method. Opt. Laser Technol. 2018, 103, 155–162. [Google Scholar] [CrossRef]
- Vaghri, E.; Dorranian, D.; Ghoranneviss, M. Effects of CTAB concentration on the quality of graphene oxide nanosheets produced by green laser ablation. Mater. Chem. Phys. 2018, 203, 235–242. [Google Scholar] [CrossRef]
- Vaghri, E.; Khalaj, Z.; Dorranian, D. Investigating the Effects of Different Liquid Environments on the Characteristics of Multilayer Graphene and Graphene Oxide Nanosheets Synthesized by Green Laser Ablation Method. Diam. Relat. Mater. 2020, 103, 107697. [Google Scholar] [CrossRef]
- Woo, W.K.; Hung, Y.M.; Wang, X. Anomalously enhanced thermal conductivity of graphite-oxide nanofluids synthesized via liquid-phase pulsed laser ablation. Case Stud. Therm. Eng. 2021, 25, 100993. [Google Scholar] [CrossRef]
- Russo, P.; Hu, A.; Compagnini, G.; Duley, W.W.; Zhou, N.Y. Femtosecond laser ablation of highly oriented pyrolytic graphite: A green route for large-scale production of porous graphene and graphene quantum dots. Nanoscale 2013, 6, 2381–2389. [Google Scholar] [CrossRef]
- Mortazavi, S.Z.; Parvin, P.; Reyhani, A. Fabrication of graphene based on Q-switched Nd:YAG laser ablation of graphite target in liquid nitrogen. Laser Phys. Lett. 2012, 9, 547–552. [Google Scholar] [CrossRef]
- Sadeghi, H.; Dorranian, D. Influence of size and morphology on the optical properties of carbon nanostructures. J. Theor. Appl. Phys. 2015, 10, 7–13. [Google Scholar] [CrossRef]
- Sadeghi, H.; Solati, E.; Dorranian, D. Producing graphene nanosheets by pulsed laser ablation: Effects of liquid environment. J. Laser Appl. 2019, 31, 042003. [Google Scholar] [CrossRef]
- Tabatabaie, N.; Dorranian, D. Effect of fluence on carbon nanostructures produced by laser ablation in liquid nitrogen. Appl. Phys. A 2016, 122, 558. [Google Scholar] [CrossRef]
- Hameed, R.; Khashan, K.S.; Sulaiman, G.M. Preparation and characterization of graphene sheet prepared by laser ablation in liquid. Mater. Today Proc. 2019, 20, 535–539. [Google Scholar] [CrossRef]
- Pramanik, A.; Karmakar, S.; Kumbhakar, P.; Biswas, S.; Sarkar, R.; Kumbhakar, P. Synthesis of bilayer graphene nanosheets by pulsed laser ablation in liquid and observation of its tunable nonlinearity. Appl. Surf. Sci. 2019, 499, 143902. [Google Scholar] [CrossRef]
- Jalili, M.; Ghanbari, H.; Bellah, S.M.; Malekfar, R. High-quality liquid phase-pulsed laser ablation graphene synthesis by flexible graphite exfoliation. J. Mater. Sci. Technol. 2018, 35, 292–299. [Google Scholar] [CrossRef]
- Solati, E.; Vaghri, E.; Dorranian, D. Effects of wavelength and fluence on the graphene nanosheets produced by pulsed laser ablation. Appl. Phys. A 2018, 124, 749. [Google Scholar] [CrossRef]
- Milenov, T.; Nikolov, A.; Avdeev, G.; Avramova, I.; Russev, S.; Karashanova, D.; Konstadinov, I.; Georgieva, B.; Mladenoff, J.; Balchev, I.; et al. Synthesis of graphene-like phases in a water colloid by laser ablation of graphite. Mater. Sci. Eng. B 2019, 247, 114379. [Google Scholar] [CrossRef]
- Calabro, R.L.; Yang, D.-S.; Kim, D.Y. Liquid-phase laser ablation synthesis of graphene quantum dots from carbon nano-onions: Comparison with chemical oxidation. J. Colloid Interface Sci. 2018, 527, 132–140. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Sousa, H.; Martins, C.; Prior, J. You Don’t Learn That in School: An Updated Practical Guide to Carbon Quantum Dots. Nanomaterials 2021, 11, 611. [Google Scholar] [CrossRef]
- Kang, S.; Kim, K.M.; Jung, K.; Son, Y.; Mhin, S.; Ryu, J.H.; Shim, K.B.; Lee, B.; Han, H.; Song, T. Graphene Oxide Quantum Dots Derived from Coal for Bioimaging: Facile and Green Approach. Sci. Rep. 2019, 9, 4101. [Google Scholar] [CrossRef]
- Kang, S.; Jeong, Y.K.; Jung, K.H.; Son, Y.; Choi, S.-C.; An, G.S.; Han, H.; Kim, K.M. Simple preparation of graphene quantum dots with controllable surface states from graphite. RSC Adv. 2019, 9, 38447–38453. [Google Scholar] [CrossRef]
- Kang, S.; Jeong, Y.K.; Ryu, J.H.; Son, Y.; Kim, W.R.; Lee, B.; Jung, K.H.; Kim, K.M. Pulsed laser ablation based synthetic route for nitrogen-doped graphene quantum dots using graphite flakes. Appl. Surf. Sci. 2020, 506, 144998. [Google Scholar] [CrossRef]
- Ibrahim, Y.O.; Gondal, M.; Alaswad, A.; Moqbel, R.; Hassan, M.; Cevik, E.; Qahtan, T.; Dastageer, M.; Bozkurt, A. Laser-induced anchoring of WO3 nanoparticles on reduced graphene oxide sheets for photocatalytic water decontamination and energy storage. Ceram. Int. 2019, 46, 444–451. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, M.; Zhou, Q.-F.; Li, Y.-S.; Fu, Z.-W. Platinum nanoparticle–graphene hybrids synthesized by liquid phase pulsed laser ablation as cathode catalysts for Li-air batteries. Electrochem. Commun. 2012, 20, 11–14. [Google Scholar] [CrossRef]
- Chen, G.X.; Hong, M.H.; Chong, T.C.; Elim, H.I.; Ma, G.H.; Ji, W. Preparation of carbon nanoparticles with strong optical limiting properties by laser ablation in water. J. Appl. Phys. 2004, 95, 1455–1459. [Google Scholar] [CrossRef]
- Chen, G.X.; Hong, M.H.; Tan, L.S.; Chong, T.C.; Elim, H.I.; Chen, W.Z.; Ji, W. Optical limiting phenomena of carbon nanoparticles prepared by laser ablation in liquids. J. Phys. Conf. Ser. 2007, 59, 289–292. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shuaib, E.; Sastikumar, D. Photoluminescence properties of carbon nanoparticles synthesized from activated carbon powder (4% ash) by laser ablation in solution. Mater. Res. Bull. 2017, 91, 220–226. [Google Scholar] [CrossRef]
- Calabro, R.L.; Yang, D.-S.; Kim, D.Y. Controlled Nitrogen Doping of Graphene Quantum Dots through Laser Ablation in Aqueous Solutions for Photoluminescence and Electrocatalytic Applications. ACS Appl. Nano Mater. 2019, 2, 6948–6959. [Google Scholar] [CrossRef]
- Nguyen, V.; Yan, L.; Si, J.; Hou, X. Femtosecond laser-induced size reduction of carbon nanodots in solution: Effect of laser fluence, spot size, and irradiation time. J. Appl. Phys. 2015, 117, 084304. [Google Scholar] [CrossRef]
- Xu, H.; Yan, L.; Nguyen, V.; Yu, Y.; Xu, Y. One-step synthesis of nitrogen-doped carbon nanodots for ratiometric pH sensing by femtosecond laser ablation method. Appl. Surf. Sci. 2017, 414, 238–243. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, S.; Huang, F.; Zhou, H.; Zhang, H.; Wang, S.; Zhou, S. Nitrogen-doped graphene quantum dots synthesized by femtosecond laser ablation in liquid from laser induced graphene. Nanotechnology 2021, 33, 115602. [Google Scholar] [CrossRef]
- Thongpool, V.; Asanithi, P.; Limsuwan, P. Synthesis of Carbon Particles using Laser Ablation in Ethanol. Procedia Eng. 2012, 32, 1054–1060. [Google Scholar] [CrossRef]
- Thongpool, V.; Phunpueok, A.; Piriyawong, V.; Limsuwan, S.; Limsuwan, P. Pulsed Laser Ablation of Graphite Target in Dimethyformamide. Energy Procedia 2013, 34, 610–616. [Google Scholar] [CrossRef]
- Narasimhan, A.K.; Lakshmi, B.S.; Santra, T.S.; Rao, M.S.R.; Krishnamurthi, G. Oxygenated graphene quantum dots (GQDs) synthesized using laser ablation for long-term real-time tracking and imaging. RSC Adv. 2017, 7, 53822–53829. [Google Scholar] [CrossRef]
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Dudek, M.; Rosowski, A.; Koperkiewicz, A.; Grobelny, J.; Wach, R.; Sharp, M.; French, P.; Janasz, L.; Kozanecki, M. Carbon nanoparticles fabricated by infrared laser ablation of graphite and polycrystalline diamond targets. Phys. Status Solidi (A) 2016, 214, 1600318. [Google Scholar] [CrossRef]
- Reyes-Contreras, D.; Camacho-López, M.; Camacho-López, M.A.; Camacho-López, S.; Rodríguez-Beltrán, R.I.; Mayorga-Rojas, M. Influence of the per pulse laser fluence on the optical properties of carbon nanoparticles synthesized by laser ablation of solids in liquids. Opt. Laser Technol. 2015, 74, 48–52. [Google Scholar] [CrossRef]
- Al-Hamaoy, A.; Chikarakara, E.; Jawad, H.; Gupta, K.; Kumar, D.; Rao, M.R.; Krishnamurthy, S.; Morshed, M.; Fox, E.; Brougham, D.; et al. Liquid Phase—Pulsed Laser Ablation: A route to fabricate different carbon nanostructures. Appl. Surf. Sci. 2014, 302, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Habiba, K.; Makarov, V.I.; Avalos, J.; Guinel, M.J.; Weiner, B.R.; Morell, G. Luminescent graphene quantum dots fabricated by pulsed laser synthesis. Carbon 2013, 64, 341–350. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Denis, P.; Krajewski, M.; Moscicki, T.; Malolepszy, A.; Hoffman, J. Improved Laser Ablation Method for the Production of Luminescent Carbon Particles in Liquids. Materials 2021, 14, 2365. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Guo, B.; Gao, N.; Zhang, X. Synthesis of N-Doped Micropore Carbon Quantum Dots with High Quantum Yield and Dual-Wavelength Photoluminescence Emission from Biomass for Cellular Imaging. Nanomaterials 2019, 9, 495. [Google Scholar] [CrossRef]
- Castro, H.P.S.; Pereira, M.K.; Ferreira, V.C.; Hickmann, J.M.; Correia, R.R.B. Optical characterization of carbon quantum dots in colloidal suspensions. Opt. Mater. Express 2017, 7, 401. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.; Yang, D.-S.; Yang, F. Soybean-derived blue photoluminescent carbon dots. Beilstein J. Nanotechnol. 2020, 11, 606–619. [Google Scholar] [CrossRef]
- Kalkal, A.; Kadian, S.; Pradhan, R.; Manik, G.; Packirisamy, G. Recent advances in graphene quantum dot-based optical and electrochemical (bio)analytical sensors. Mater. Adv. 2021, 2, 5513–5541. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P.; Joanni, E.; Yadav, R.M.; Moshkalev, S.A. Laser-assisted synthesis, reduction and micro-patterning of graphene: Recent progress and applications. Coord. Chem. Rev. 2017, 342, 34–79. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Tite, T.; Loir, A.-S.; Maddi, C.; Donnet, C.; Garrelie, F. Review of Graphene Growth From a Solid Carbon Source by Pulsed Laser Deposition (PLD). Front. Chem. 2018, 6, 572. [Google Scholar] [CrossRef]
- Wan, Z.; Streed, E.W.; Lobino, M.; Wang, S.; Sang, R.T.; Cole, I.S.; Thiel, D.V.; Li, Q. Laser-Reduced Graphene: Synthesis, Properties, and Applications. Adv. Mater. Technol. 2018, 3, 1700315. [Google Scholar] [CrossRef]
- Kaushik, V.; Pathak, S.; Sharma, H.; Shukla, A.; Vankar, V. Growth of hydrophilic graphene oxide layers using continuous laser ablation. Vacuum 2020, 182, 109721. [Google Scholar] [CrossRef]
- Rode, A.; Elliman, R.; Gamaly, E.; Veinger, A.; Christy, A.; Hyde, S.; Luther-Davies, B. Electronic and magnetic properties of carbon nanofoam produced by high-repetition-rate laser ablation. Appl. Surf. Sci. 2002, 197–198, 644–649. [Google Scholar] [CrossRef]
- Cutroneo, M.; Torrisi, L.; Havranek, V.; Mackova, A.; Malinsky, P.; Silipigni, L.; Fernandes, S.; Sofer, Z.; Stammers, J. Localized modification of graphene oxide properties by laser irradiation in vacuum. Vacuum 2019, 165, 134–138. [Google Scholar] [CrossRef]
- Guan, Y.C.; Fang, Y.W.; Lim, G.C.; Zheng, H.Y.; Hong, M.H. Fabrication of Laser-reduced Graphene Oxide in Liquid Nitrogen Environment. Sci. Rep. 2016, 6, 28913. [Google Scholar] [CrossRef]
- Trusovas, R.; Ratautas, K.; Raciukaitis, G.; Niaura, G. Graphene layer formation in pinewood by nanosecond and picosecond laser irradiation. Appl. Surf. Sci. 2018, 471, 154–161. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog. Energy Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Kurra, N.; Jiang, Q.; Nayak, P.; Alshareef, H.N. Laser-derived graphene: A three-dimensional printed graphene electrode and its emerging applications. Nano Today 2019, 24, 81–102. [Google Scholar] [CrossRef]
- Djuric, S.M.; Kitic, G.; Dubourg, G.; Gajic, R.; Tomasevic-Ilic, T.; Minic, V.; Spasenovic, M. Miniature graphene-based supercapacitors fabricated by laser ablation. Microelectron. Eng. 2017, 182, 1–7. [Google Scholar] [CrossRef]
- Kwon, S.; Yoon, Y.; Ahn, J.; Lim, H.; Kim, G.; Kim, J.-H.; Choi, K.-B.; Lee, J. Facile laser fabrication of high quality graphene-based microsupercapacitors with large capacitance. Carbon 2018, 137, 136–145. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Zhang, C.; Lin, J. Upgrading coal to multifunctional graphene-based materials by direct laser scribing. Carbon 2019, 153, 585–591. [Google Scholar] [CrossRef]
- Russo, P.; Xiao, M.; Zhou, N.Y. Carbon nanowalls: A new material for resistive switching memory devices. Carbon 2017, 120, 54–62. [Google Scholar] [CrossRef]
- Wang, C.-P.; Chou, C.-P.; Chang, T.-L.; Chou, C.-Y. Micromachining of graphene based micro-capacitor using picosecond laser ablation. Microelectron. Eng. 2018, 189, 69–73. [Google Scholar] [CrossRef]
- Wang, C.-P.; Chou, C.-P.; Wang, P.-C.; Chang, T.-L. Flexible graphene-based micro-capacitors using ultrafast laser ablation. Microelectron. Eng. 2019, 215, 111000. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Z.; Chen, J.; Zhai, Y.; Xu, Y.; Luo, S. Freestanding laser induced graphene paper based liquid sensors. Carbon 2019, 153, 472–480. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Lin, N.; Xie, Y.; Bai, S.; Lin, Z.; Lu, L.; Tang, Y. Facile fabrication of super-hydrophilic porous graphene with ultra-fast spreading feature and capillary effect by direct laser writing. Mater. Chem. Phys. 2020, 251, 123083. [Google Scholar] [CrossRef]
- Wu, W.; Liang, R.; Lu, L.; Wang, W.; Ran, X.; Yue, D. Preparation of superhydrophobic laser-induced graphene using taro leaf structure as templates. Surf. Coat. Technol. 2020, 393, 125744. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Wang, S.; Luo, S. Laser-induced graphene enabled 1D fiber electronics. Carbon 2020, 168, 308–318. [Google Scholar] [CrossRef]
- Stefanuk, B.; Sánchez-Aké, C.; Benítez, J.L.; Depablos-Rivera, O.; García-Fernández, T.; Negrete-Aragón, S.; Villagrán-Muniz, M. Laser-induced breakdown spectroscopy enhancement of glass by few-layer graphene coating. Spectrochim. Acta Part B At. Spectrosc. 2020, 167, 105823. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- de Menezes, F.D.; Dos Reis, S.R.R.; Pinto, S.R.; Portilho, F.L.; do Vale Chaves, E.M.F.; Helal-Neto, E.; da Silva de Barros, A.O.; Alencar, L.M.R.; de Menezes, A.S.; Dos Santos, C.C.; et al. Graphene quantum dots unraveling: Green synthesis, characterization, radiolabeling with 99mTc, in vivo behavior and mutagenicity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 405–514. [Google Scholar] [CrossRef]





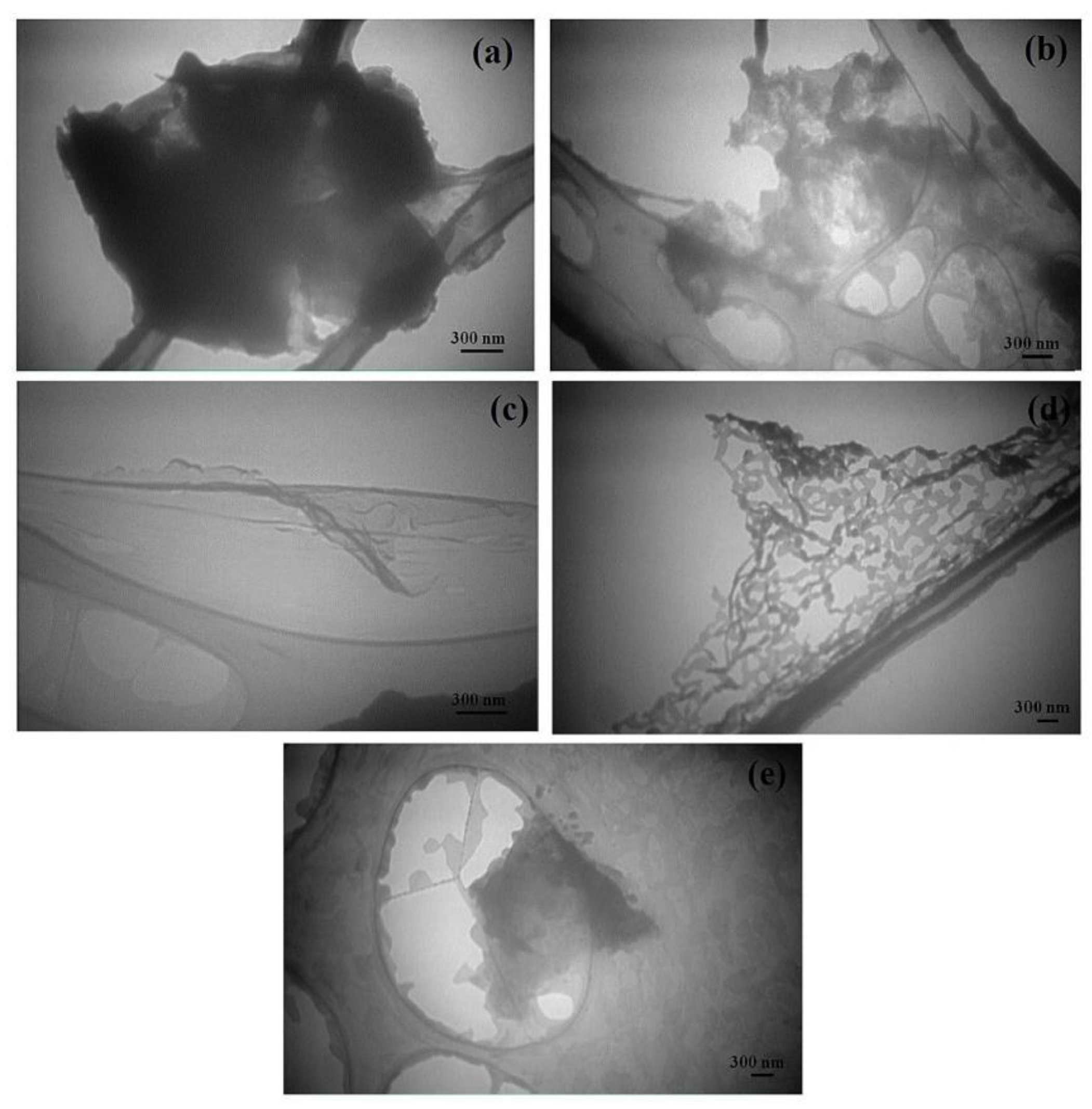
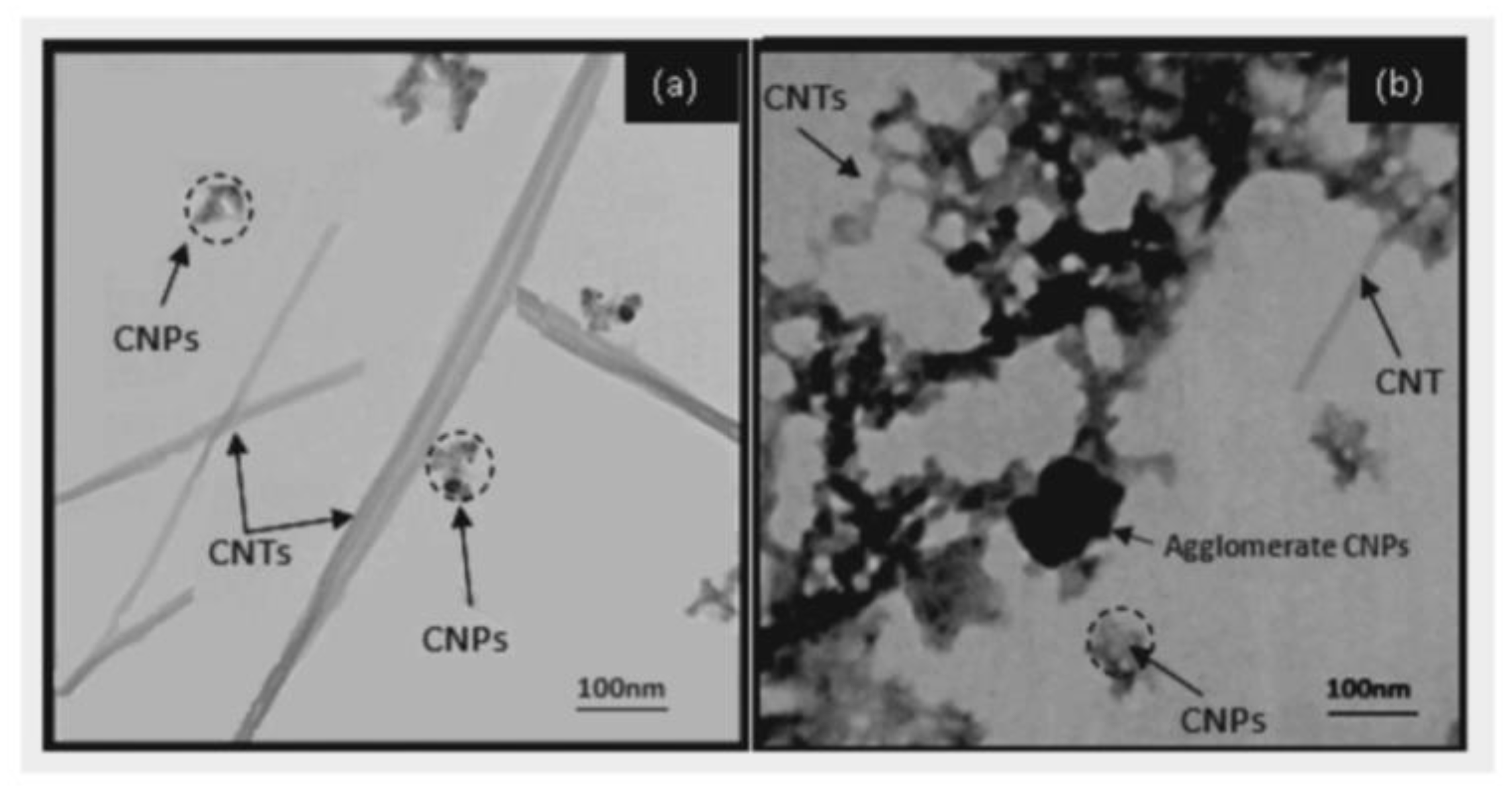




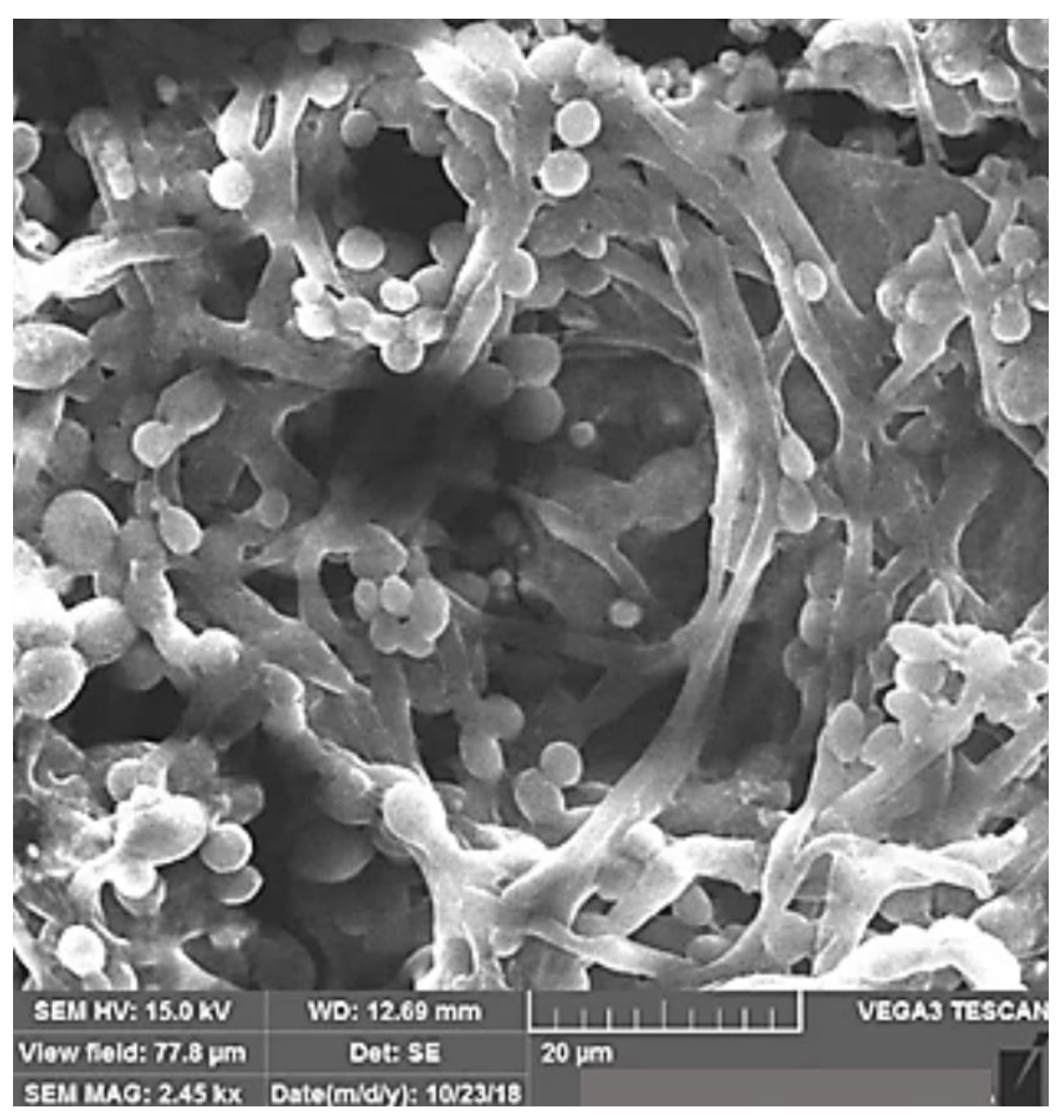


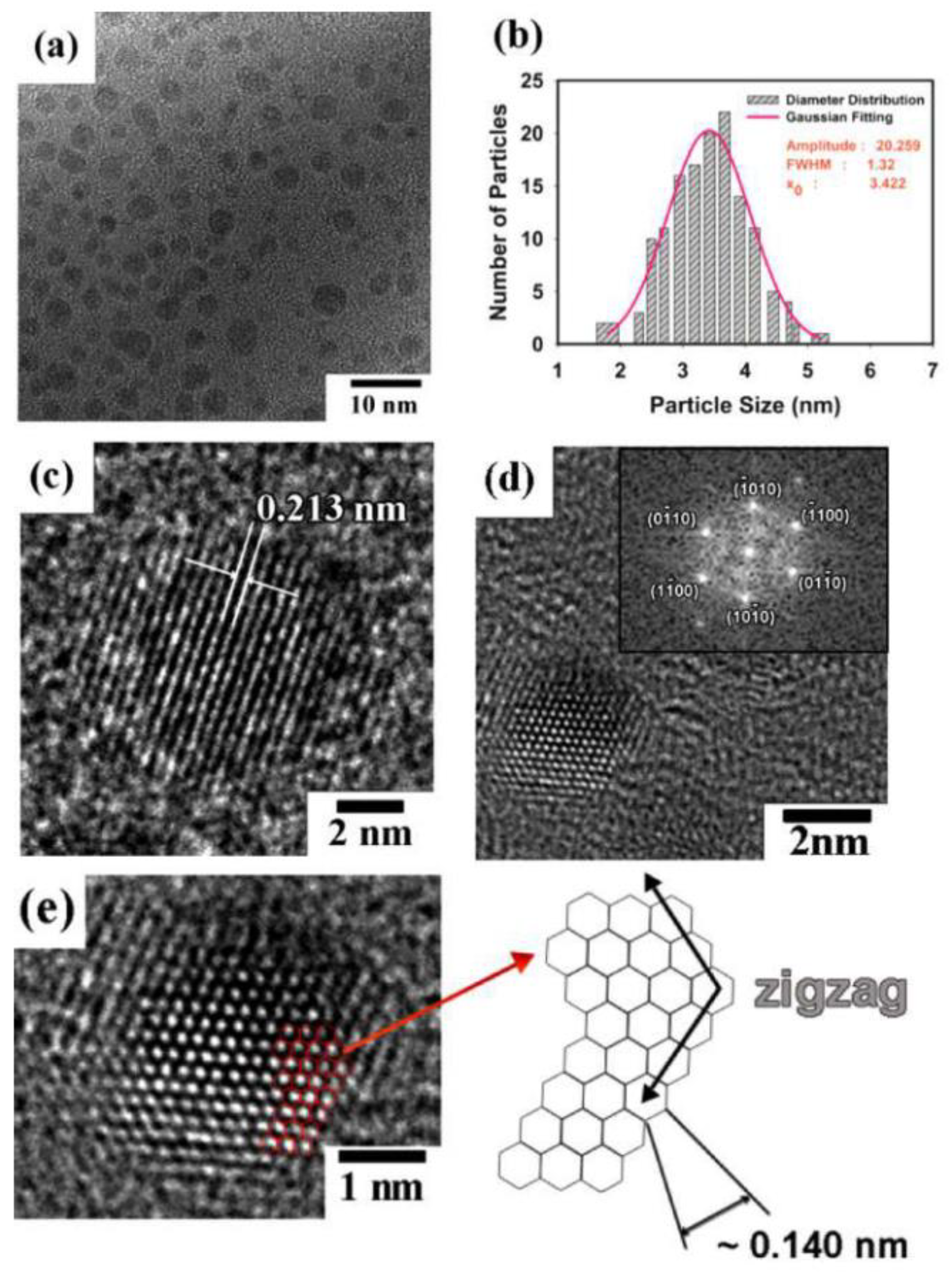
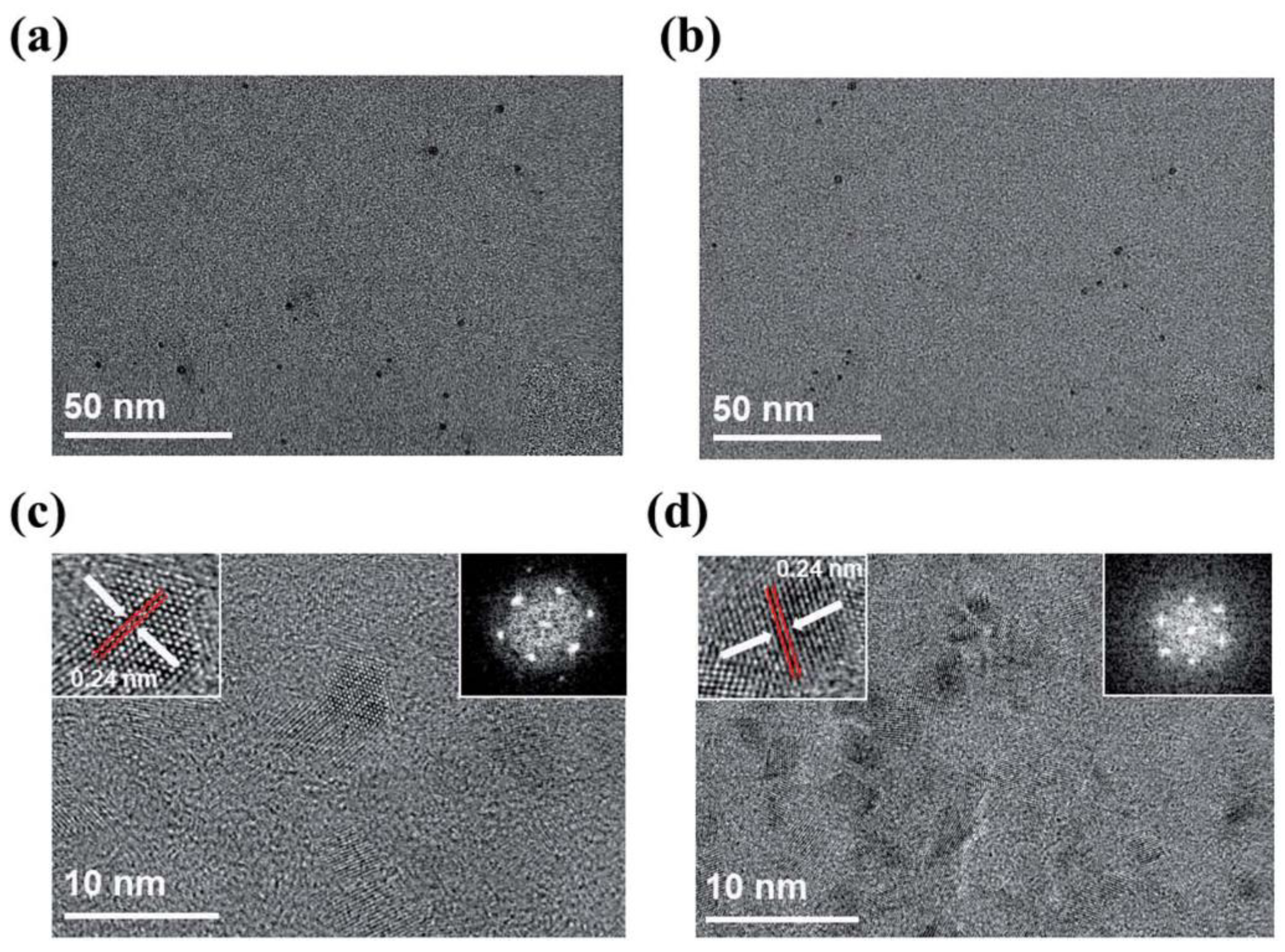
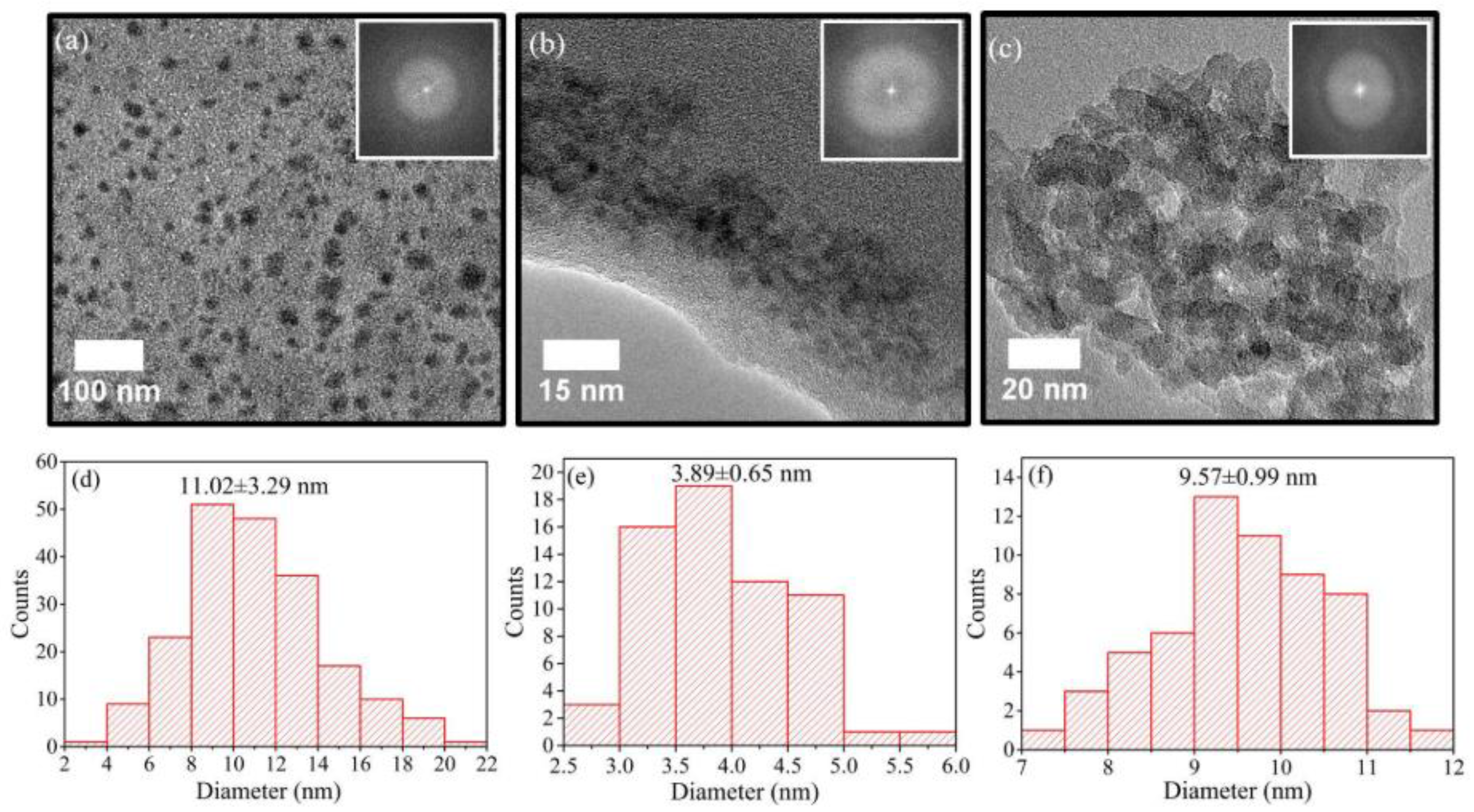
| Characterization Method | Description | Reference | |
|---|---|---|---|
| XRD | Graphene | 2θ = 26.5° (002), 42.3° (100), 44.5°(101), 54.6° (004), and 77.4° (110) | [63] |
| Graphene oxide | 2θ ≅ 9–11° (001), shifts depending on amount of trapped oxygen to 2θ ≅ 12–17° | [16,24] | |
| XPS | C 1s O 1s | Binding energy = 284 eV Binding energy = 532 eV | [64] |
| Raman spectroscopy | D-band G-band 2D-band | Raman shift = 1330–1360 cm−1 Raman shift = 1560–1600 cm−1 Raman shift = 2400–1700 cm−1 I2D > IG decreased layer thickness ID > IG increased disorder and poor quality | [65,66] |
| UV-Vis spectroscopy | Graphene Carbon material | No peak due to transparency Peak in the range 260–270 nm (π–π* transition of C=C bond). Peak in the range 270–350 nm (n-π* transition of the C=O bond). | [63,67] |
| PL emission spectroscopy | GQDs GOQDs | Blue emission Mixed blue and green emission | [68] |
| FTIR spectroscopy | O-H stretching C-H stretching  C-O-C bonds | 3420 cm−1 2930 cm−1 1633 cm−1 1068 cm−1 | [69] |
| Wavelength | Fluence/Energy/Power | Repetition Rate | Pulse Width | Target/Solvent | Irradiation Time | Size | Ref. |
|---|---|---|---|---|---|---|---|
| 248 nm | 2.5, 3.12 to 3.75 J/cm2 | 10 Hz | Graphite target/ de-ionized water | 30 min | Bilayer graphene | [66] | |
| 532 nm | 5J/cm2 | 10 Hz | 5 ns | Graphite target/ distilled water with assisted electric field | Few min–2 h | 2–6 layers flower-like | [54] |
| 532 nm | 0.5, 2.2 and 3.6 J/cm2 | 10 Hz | 5 ns | Graphite disk/ distilled water | 15 min | 1–4 layers | [55] |
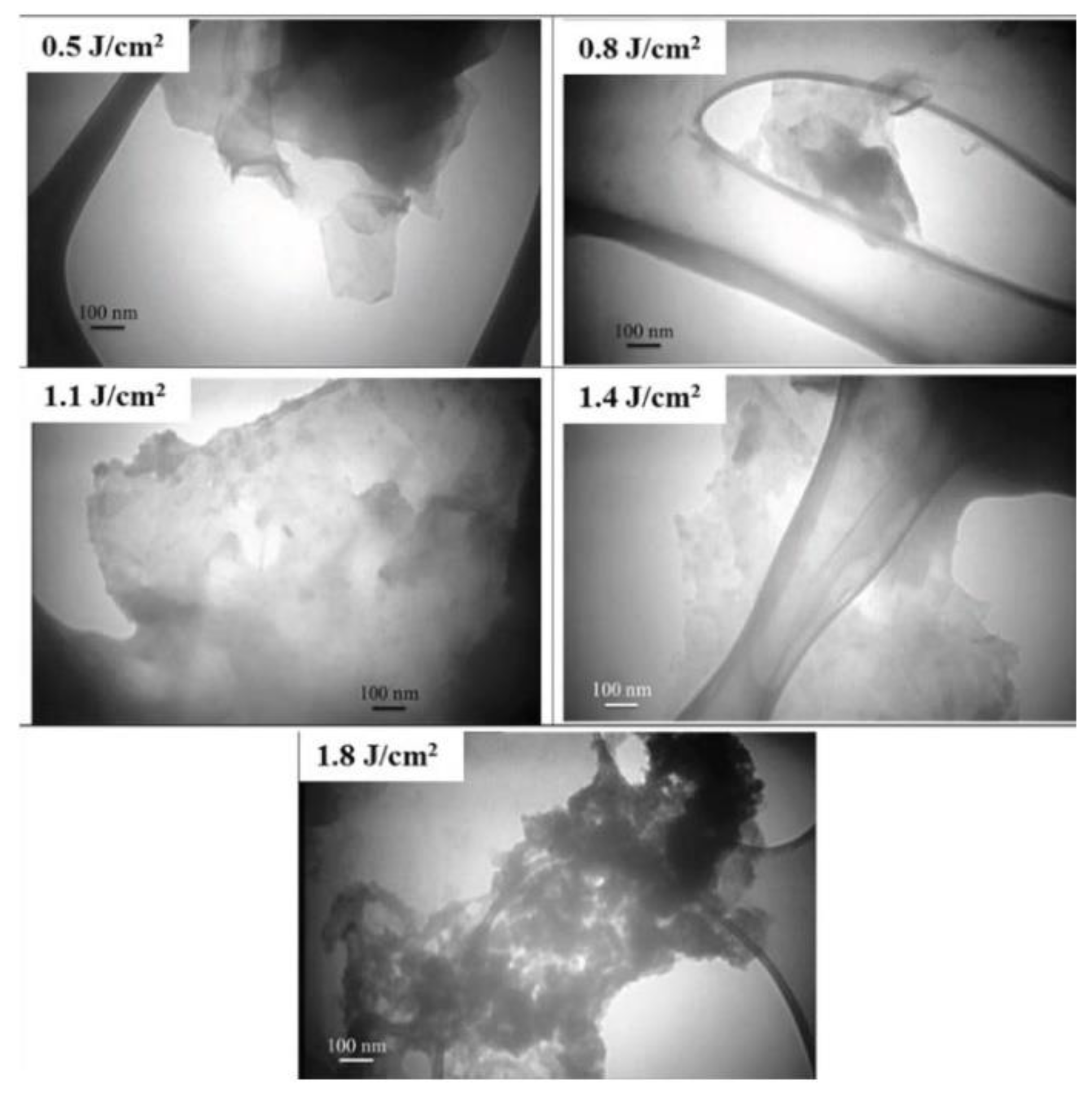
| 532 nm | 0.5, 0.8, 1.1, 1.4, and 1.8 J/cm2 | 5 Hz | 7 ns | Graphite plate/ liquid nitrogen | -- | Bi- to multi-layer | [56] |
| 532 nm | 0.5 J/mm2 | -- | 7 ns | Graphite plate/ acetone | 10, 20, 30, 40, 60 s | Bi- and multi-layer graphene | [58] |
| 532 nm | 0.5 J/cm2 | 5 Hz | 7 ns | Graphite target/ Liquid nitrogen, distilled water | -- | few-layer graphene | [65] |
| 532 nm | 0.4 J/cm2 | 10 Hz | 6 ns | Graphite rod/ distilled water | 10 min | rGO nanosheets | [76] |
| 532 nm | 1 J/cm2 | 5 Hz | 7 ns | HOPG/ NMP, SDBS | 2 h | thickness 1 nm and 10 μm in size. | [77] |
| 532 nm | 0.7 J/cm2 | 5 Hz | 7 ns | Graphite target/ liquid nitrogen, de-ionized water, 0.01 M CTAB | 5000 pulses | Few-layer GO | [78] |
| 532 nm | 0.5 J/cm2 | 5 Hz | 7 ns | Graphite targets/ 0.02, 0.04, 0.06, 0.08, and 0.1 M CTAB | -- | multilayer GO nanosheets | [79] |
| 532 nm | 0.5 J/ cm2 | 5 Hz | 7 ns | Graphite targets/ liquid nitrogen and 0.04 M CTAB | 5000 pulses | Multilayer GO nanosheets | [80] |
| 532 nm | 0.813 mW | 1, 10, 20, 50, and 100 Hz | 1.5 ns | Graphite sheet/ de-ionized water | 10 min | 110–287 nm | [81] |
| 800 nm | 20–30 J/cm2 | 1 kHz | 35 fs | HOPG/ water | 20 min | PG 6 layers thick Pores 15–20 nm | [82] |
| 1064 nm | 0.92 J/cm2 | 10 Hz | 10 ns | Graphene nanoplatelets/ ethanol | 30 min | 26 nm | [16] |
| 1064 nm | 0.1, 0.2, 0.3, and 0.4 J. | 10 Hz | 10 ns | Graphite flakes/ de-ionized water | -- | 3–10 layers | [32] |
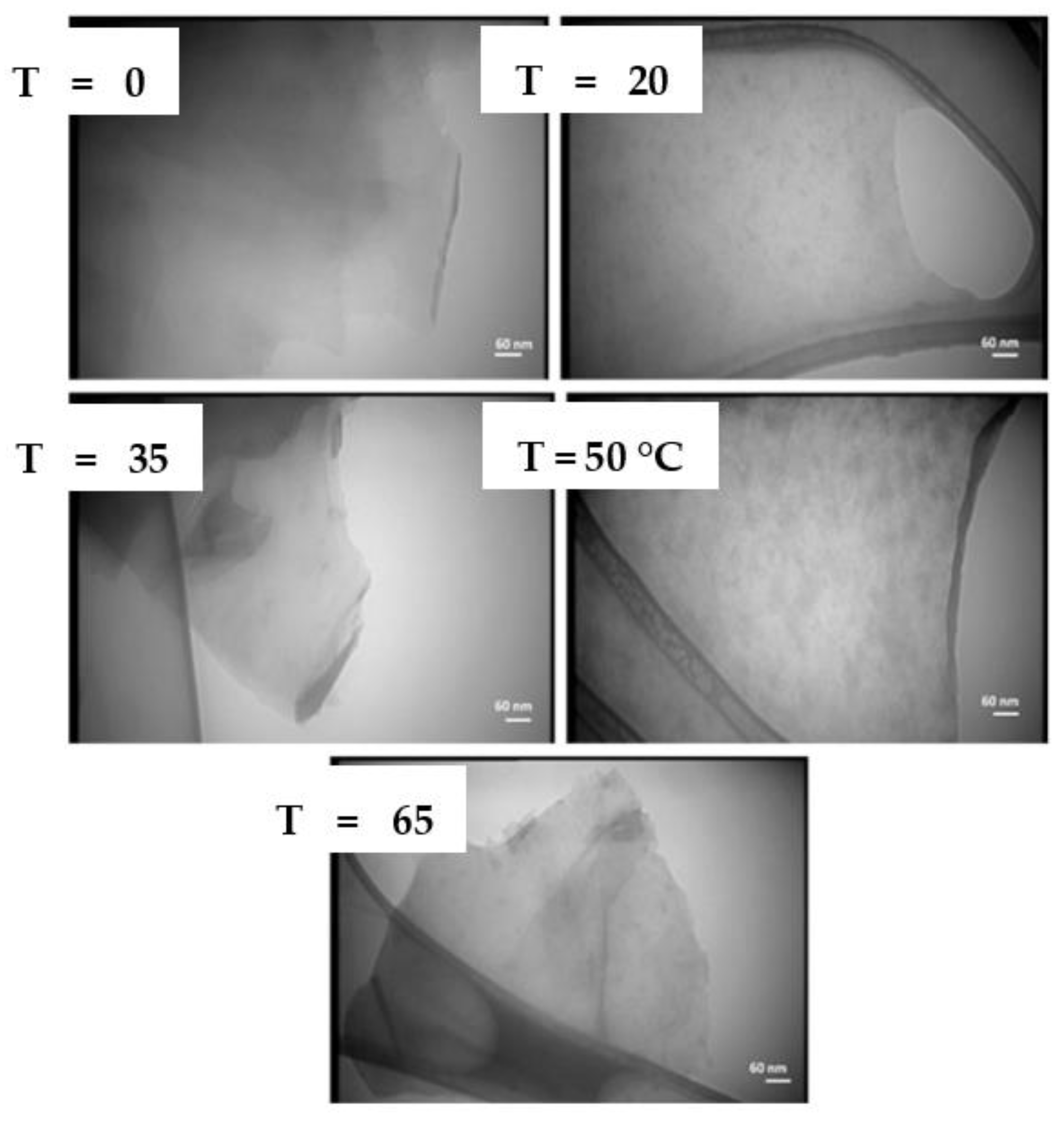
| 1064 nm | 0.6 J/cm2 | 5 Hz | 7 ns | Graphite target/ water @ Temp: 0, 20, 35, 50 and 65 °C | 1000 s | Few-layer graphene | [57] |
| 1064 nm | 1.5 J/cm2 | 5 Hz | 7 ns | Graphite plate/ 0.02, 0.04, 0.06, 0.08, and 0.1 M CTAB | 8000 pulses | few layers graphene | [62] |
| 1064 nm | 600 mJ | 10 Hz | 10 ns | Graphene nanoplatelets/ ethanol | 60 min | 100–300 nm | [63] |
| 1064 nm | 6 J/cm2 | 5 Hz | 10 ns | Graphite target/ Liquid nitrogen | 20 min | few-layer graphene | [83] |
| 1064 nm | 1.5 J/cm2 | 5 Hz | 7 ns | Graphite plate/ distilled water, acetone, alcohol, CTAB | 1000 s | Few-layer graphene | [84] |
| 1064 nm | 1.5 J/cm2 | 5 Hz | 7 ns | Graphite plate/ distilled water, liquid nitrogen, acetone, alcohol, 0.01M and 0.1M CTAB | 1000 s | Few-layer graphene | [85] |
| 1064 nm | 0.5, 0.8, 1.1, 1.4 and 1.8 J/cm2 | 5 Hz | 7 ns | Graphite plate/ Liquid nitrogen | 5000 pulses | few-layer graphene | [86] |
| 1064 nm | 80 mJ 160 mJ | -- | 7 ns | Graphite target/ water | 100 pulses | MWCNTs diameter 25–75 nm PG sheet pours 7–16 nm | [87] |
| 1064 nm | 60 J/cm2 | 10 Hz | 10 ns | dry-cell graphite electrode (DGE) | 20–50 min | Bilayer graphene | [88] |
| 532 nm 1064 nm | 15.4 J/cm2 | 2 Hz | 9 ns | Graphite pellet/ double-distilled water | 30 min | MWCNT diameter 20–75 nm | [12] |
| 532 nm 1064 nm | 50 mJ/pulse | 5 Hz | 10 ns | Flexible graphite targets and nuclear graphite/ acetone, DMF, de-ionized water | 5 min | <10 layers | [89] |
| 532 nm 1064 nm | 0.5 J/cm2 0.8 J/cm2 | 5 Hz | 7 ns | Graphite plate/ liquid nitrogen | 5000 pulses | Bilayer graphene | [90] |
| 266 nm 532 nm 1064 nm | 0.38 J/cm2 (@266 nm) 1.33 J/cm2 (@532 nm) 3.33 & 6.61 J/cm2 (@1064 nm) | 10 Hz | 18 ns | Graphite target/ double-distilled water. | -- | multilayer rGO nanosheets | [91] |
| Wavelength | Fluence/Energy/Power | Repetition Rate | Pulse Width | Target/Solvent | Irradiation Time | Size | Ref. |
|---|---|---|---|---|---|---|---|
| 355 nm | 0.50, 0.75, 1.00, 1.50, and 2.00 J | 10 Hz | -- | MWCNTs/ ethanol | 10 min | 1–5 nm 3–4 layers | [38] |
| 355 nm | 0.1 J | 20 Hz | 10 ns | Coal/ ethanol | 5 min | 5–30 nm | [95] |
| 355 nm | 1.5 W | 10 Hz | 10 ns | graphite flakes/ ethanol | 30 min | on-GQDs ~3.8 nm off-GOQDs ~4.1 nm | [96] |
| 355 nm | 1 W | 10 Hz | 10 ns | Graphite flakes/ ethanol with DETA | 30 min | <6 nm | [97] |
| 355 nm | 280 mJ/pulse | -- | -- | WO3 NPS/ de-ionized water | 30 min | -- | [98] |
| 355 nm | 2 J/cm2 | 10 Hz | 5 ns | Aqueous graphene dispersion | 30 min | Thickness 1 nm | [99] |
| 532 nm | 7.5 J/cm2 | 100 Hz | 10 ns | Graphite powder/ DMF | -- | 1.5–7.5 nm | [18] |
| 532 nm | 1.30, 0.11, 0.57, 0.95 W | 10 Hz | 5–7 ns | nCNOs pellet/ de-ionized water | 7 h | 1.8 nm diameter Single-layer | [92] |
| 532 nm | 0.8 J/cm2 | 10 Hz | 7 ns | Glassy carbon plate/ de-ionized water | 5 min | 10–20 nm | [100] |
| 532 nm | 1 J/cm2 | 10 Hz | 7 ns | Glassy carbon plate/ de-ionized water and THF | 30 min | 6–15 nm | [101] |
| 532 nm | 0.131 J/cm2 | 10 Hz | 10 ns | activated carbon (4% ash)/ ethanol + double-distilled water ± NaOH | 30 min | 4–14 nm | [102] |
| 532 nm | 4.5 J/cm2 | 50 Hz | 6–8 ns | nCNOs pellet/ ammonia, ethylenediamine, pyridine | 1 h | 14 nm | [103] |
| 532 nm | 0.21, 0.025, 0.014, 0.006, 0.008 J/cm2 | 1, 10, 20, 50, 100 Hz | 1.5 ns | Graphite target/ de-ionized water | 10 min | 110–287 nm | [81] |
| 800 nm | -- | 76 MHz | 150 fs | Zn3N2 target/ ethanol | -- | ~ 4 nm | [17] |
| 800 nm | 20–30 J/cm2 | 1 kHz | 35 fs | HOPG/ water | 20 min | S 2–5 nm | [82] |
| 800 nm | 150–1000 J/cm2 | 1 kHz | 150 fs | carbon powder/ PEG200N | 3 h | 1–4 nm | [104] |
| 800 nm | 100–400 mW | 1 kHz | 150 fs | graphite powder/ aminotoluene liquid | 2 h | 2.87 nm | [105] |
| 1030 nm | 3.5 W | 100 kHz | 365 fs | LIG/ water and ammonia | -- | 3 nm 1–3 layers | [106] |
| 1064 nm | 3.6 W | 10 Hz | 7 ns | Silver/copper disk/ GO + de-ionized water | -- | 3.5–27.3 nm | [14] |
| 1064 nm | 750 mJ/pulse | 10 Hz | 10 ns | Charcoal powder/ ethanol | 20 min | ~21 nm | [40] |
| 1064 nm | 3.0 J/pulse | 2 Hz | 5 ms | Graphite target/ ethanol | Stage 1: 5000 pulse Stage 2: 25,000 | 200–500 nm | [107] |
| 1064 nm | 3.0 J/pulse | 2 Hz | 5 ms | Graphite target/ DMF | Stage 1: 5000 pulse Stage 2: 25,000 | 80–130 nm | [108] |
| 1064 nm | 40 mJ | 10 Hz | 6 ns | Graphite plate/ PEG–water solution | 30 min | <10 nm | [109] |
| 1064 nm | 6.0 × 106 W/cm2 | -- | -- | Graphite powder/ water PEG200N | 2 h | ~3 nm | [110] |
| 1064 nm | 0.18–7.52W | 25 kHz | 200 ns | Graphite target/ de-ionized water, isopropanol | -- | <100 nm | [111] |
| 1064 nm | 0.17, 0.42, 0.70, 0.90, 1.0 J/cm2 | 15 Hz | 7 ns | Graphite disk/ acetone | 180 s | 4–20 nm | [112] |
| 1064 nm | 0.3, 0.6, 0.9 J/cm2 | 10, 12, 14 kHz | 700 ps | Graphite rods/ de-ionized water | 1200, 1500, and 1800 pulses | 42–75 nm | [113] |
| 1064 nm | 30 mJ/pulse | 10 Hz | 10 ns | A mixture of nickel (II) oxide powder/benzene | 30 min | 2–6 nm | [114] |
| 1064 nm | Stage 1: 2 J/cm2 Stage 2: 15 J/cm2 | 10 Hz | 10 ns | Graphite target/ urea ± DIW | Stage 1: 15 min Stage 2: 60 min | <100 nm | [115] |
| 1064 nm | 20 mJ/pulse | 10 Hz | 3–6 ns | waste Platanus biomass/ formamide | 30 min | 8 nm | [116] |
| 1064 nm | 100 mJ | 20 Hz | 8 ns | carbon powder/ PEG200N | -- | 3 nm | [117] |
| 355 nm 532 nm | 50 mJ | 10 Hz | MWCNTs/ ethanol | 10 min | 1–5 nm | [68] | |
| 532 nm 1064 nm | 80 mJ | 10 Hz 20 Hz | 9 ns | graphite plate/ de-ionized water | 45 min | nanoballs 2.68 µm nanowires 1.89 µm | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altuwirqi, R.M. Graphene Nanostructures by Pulsed Laser Ablation in Liquids: A Review. Materials 2022, 15, 5925. https://doi.org/10.3390/ma15175925
Altuwirqi RM. Graphene Nanostructures by Pulsed Laser Ablation in Liquids: A Review. Materials. 2022; 15(17):5925. https://doi.org/10.3390/ma15175925
Chicago/Turabian StyleAltuwirqi, Reem M. 2022. "Graphene Nanostructures by Pulsed Laser Ablation in Liquids: A Review" Materials 15, no. 17: 5925. https://doi.org/10.3390/ma15175925





