Effect of Silicon Content on Microstructures and Properties of Directionally Solidified Fe-B Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
3.1. As-Cast Microstructure of DS Fe-B Alloy with Various Si Additions
3.2. XRD Analysis and Hardness Tests of As-Cast DS Fe-B Alloy
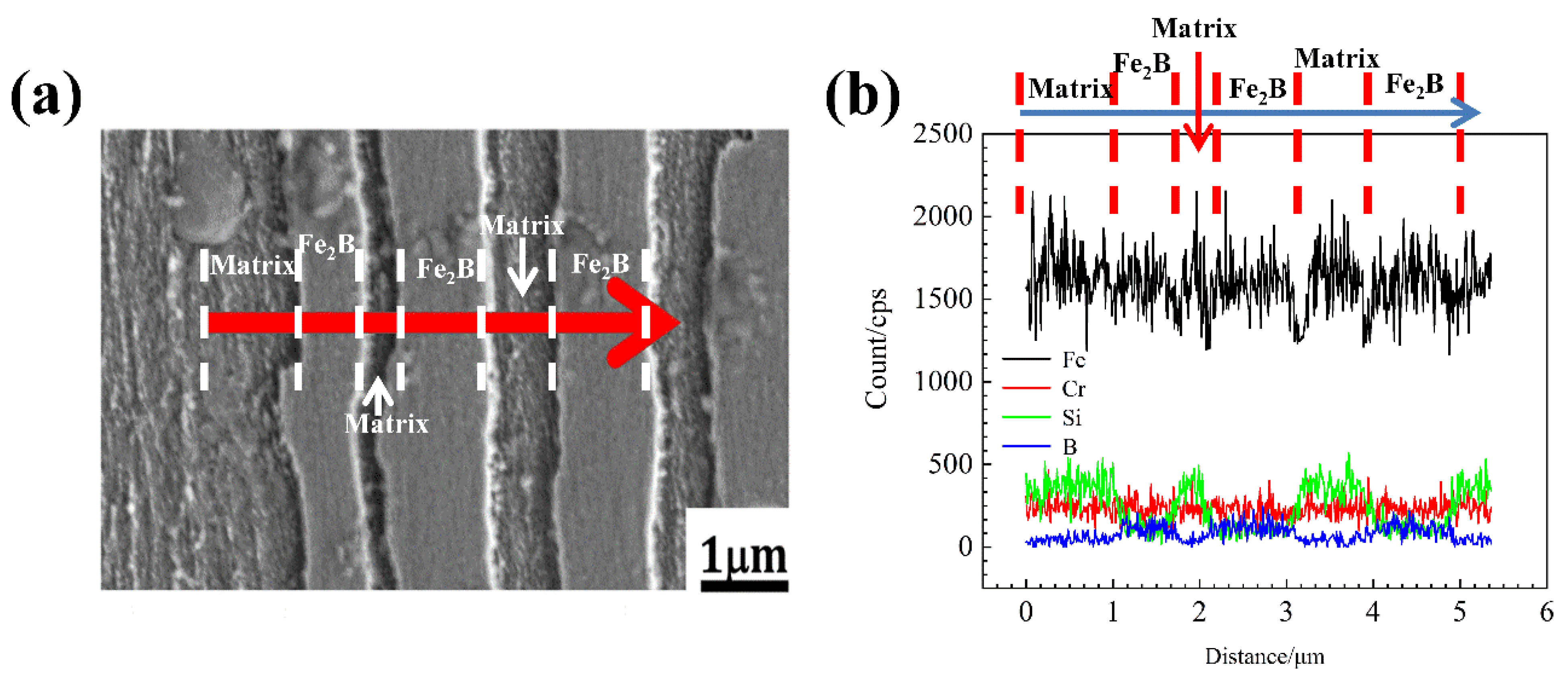
3.3. Element Distribution of As-Cast DS Fe-B Alloy
4. Conclusions
- (1).
- The Si-containing structures of the four components all show good orientation effects, and that the DS Fe-B alloy consists of a columnar Fe2B boride and α-Fe ferrite. The columnar Fe2B borides display a preferential growth along the (002) orientation of Fe2B, which makes the DS Fe-B alloy a dual-phase oriented microstructures.
- (2).
- The columnar grain thickness of oriented Fe2B decreases sharply with the increase of Si content, which is attributed to the segregation and refinement Si in the DS Fe-B alloy.
- (3).
- The bulk hardness of the transverse and longitudinal section showed an upward trend with the increase of Si content, whereas the microhardness of the oriented Fe2B in the transverse section has little change, first increasing then decreasing in the longitudinal section.
- (4).
- The Si is mainly distributed in the ferrite matrix, and almost does not dissolve in boride. As the Si content increases, more Si is segregated in the center of the columnar ferrite distributed between the two columnar Fe2B borides, which results in the refinement of the Fe2B hard phase and solid solution strengthening of the ferrite matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, H.G.; Xiao, Q.; Fu, H.F. Heat treatment of multi-element low alloy wear-resistant steel. Mater. Sci. Eng. A 2005, 396, 206–212. [Google Scholar] [CrossRef]
- Uslu, I.; Comert, H.; Ipek, M.; Ozdemir, O.; Bindal, C. Evaluation of borides formed on AISI P20 steel. Mater. Des. 2007, 28, 55–61. [Google Scholar] [CrossRef]
- Wiengmoon, A.; Chairuangsri, T.; Brown, A.; Brydson, R.; Edmonds, D.V.; Pearce, J.T.H. Microstructural and crystallographical study of carbides in 30 wt.% Cr cast irons. Acta Mater. 2005, 53, 4143–4154. [Google Scholar] [CrossRef]
- Ren, X.Y.; Fu, H.G.; Xing, J.D.; Yi, Y.L. Research on high-temperature dry sliding friction wear behavior of Ca Ti modified high boron high speed steel. Tribol. Int. 2019, 132, 165–176. [Google Scholar] [CrossRef]
- Yang, Y.W.; Fu, H.G.; Wang, K.M.; Zhu, L.L.; Jang, L. Thermodynamic calculation and analysis for aluminum-alloyed high boron high speed steel. Materialwiss. Werkstofftech. 2018, 49, 39–53. [Google Scholar] [CrossRef]
- Boccalini, M.; Goldenstein, H. Solidification of high speed steels. Int. Mater. Rev. 2001, 46, 92–115. [Google Scholar] [CrossRef]
- Fischmeister, H.F.; Riedl, R.; Karagöz, S. Solidification of high speed tool steels. Metall. Trans. A. 1989, 20, 2133–2148. [Google Scholar] [CrossRef]
- Cao, H.T.; Dong, X.P.; Pan, Z.; Wu, X.W.; Huang, Q.W.; Pei, Y.T. Surface alloying of high-vanadium high-speed steel on ductile iron using plasma transferred arc technique: Microstructure and wear properties. Mater. Des. 2016, 100, 223–234. [Google Scholar] [CrossRef]
- QiHong, C.; Zhan-Wen, W.; Yi, J.; Yehua, J.; Fei, L.; Hanguang, F. A study of centrifugal cast high boron high speed steel. Materialwiss. Werkstofftech. 2014, 45, 582–590. [Google Scholar] [CrossRef]
- Fu, H.G.; Xiao, Q.; Kuang, J.C.; Jiang, Z.Q.; Xing, J.D. Effect of rare earth and titanium additions on the microstructures and properties of low carbon Fe–B cast steel. Mater. Sci. Eng. A 2007, 466, 160–165. [Google Scholar] [CrossRef]
- Fu, H.G.; Ma, S.Q.; Hou, J.Q.; Lei, Y.P.; Xing, J.D. Microstructure and Properties of Cast B-Bearing High Speed Steel. J. Mater. Eng. Perform. 2013, 22, 1194–1200. [Google Scholar] [CrossRef]
- Sorour, A.A.; Chromik, R.R.; Gauvin, R.; Jung, I.H.; Brochu, M. Understanding the solidification and microstructure evolution during CSC-MIG welding of Fe–Cr–B-based alloy. Mater. Charact. 2013, 86, 127–138. [Google Scholar] [CrossRef]
- Ma, S.Q.; Xing, J.D.; Fu, H.G.; Yi, D.W.; Li, Y.F.; Zhang, J.J.; Zhu, B.J.; Gao, Y. Microstructure and interface characteristics of Fe–B alloy in liquid 0.25wt.% Al–Zn at various bath temperatures. Mater. Chem. Phys. 2012, 132, 977–986. [Google Scholar]
- Lentz, J.; Röttger, A.; Theisen, W. Solidification and phase formation of alloys in the hypoeutectic region of the Fe–C–B system. Acta Mater. 2015, 99, 119–129. [Google Scholar] [CrossRef]
- Röttger, A.; Weber, S.; Theisen, W. Supersolidus liquid-phase sintering of ultrahigh-boron high-carbon steels for wear-protection applications. Mater. Sci. Eng. A 2012, 532, 511–521. [Google Scholar] [CrossRef]
- Yi, Y.L.; Li, Q.; Long, S.L.; Lv, Z.; Li, S.J.; Liu, Y.Z.; Zheng, B.C.; Li, W. Effect of matrix microstructure on the matrix/M2B wear interaction and its quantitative characterization in an Fe-2 wt.% B alloy. Wear 2021, 472–473, 203608. [Google Scholar] [CrossRef]
- Christodoulou, P.; Calos, N. A step towards designing Fe-Cr-B-C cast alloys, Mater. Sci. Eng. A 2001, 301, 103–117. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Li, J.; Li, B.H. Effects of hot rolling and titanium content on the microstructure and mechanical properties of high boron Fe-B alloys. Mater. Des. 2012, 36, 88–93. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, X.; Li, Y.X.; Hu, K.H. Microstructure and mechanical properties of high boron white cast iron. Mater. Sci. Eng. A 2008, 486, 112–116. [Google Scholar] [CrossRef]
- Lentz, J.; Röttger, A.; Theisen, W. Mechanism of the Fe3(B,C) and Fe23(C,B)6 solid-state transformation in the hypoeutectic region of the Fe-C-B system. Acta Mater. 2016, 119, 80–91. [Google Scholar] [CrossRef]
- Yang, C.; Mu, D.; Shen, B.; Li, L.; Yue, C.; Gao, Z.; Yang, Q. Effect of boronizing on wear resistance of ductile iron. Mod. Cast. Iron. 2008, 1, 66–69. [Google Scholar]
- Kim, J.H.; Ko, K.H.; Noh, S.D.; Kim, G.G.; Kim, S.J. The effect of boron on the abrasive wear behavior of austenitic Fe-based hardfacing alloys. Wear 2009, 267, 1415–1419. [Google Scholar] [CrossRef]
- Hartono, W.; Goto, S.; Aso, S.; Komatsu, Y. Wear characteristics of Fe-25Cr-C-B eutectic cast alloys. Int. J. Cast. Mater. Res. 2004, 17, 206–212. [Google Scholar] [CrossRef]
- Liu, D.S.; Liu, R.P.; Wei, Y.H.; Ma, Y.; Zhu, K. Microstructure and wear properties of Fe-15Cr-2.5Ti-2C-xB wt.% hardfacing alloys. Appl. Surf. Sci. 2013, 271, 253–259. [Google Scholar] [CrossRef]
- Kassfeldt, E.; Lundmark, J. Tribological properties of hardened high strength Boron steel at combined rolling and sliding condition. Wear 2009, 267, 2287–2293. [Google Scholar] [CrossRef]
- Pan, F.S.; Hirohashi, M.; Lu, Y.; Ding, P.D.; Tang, A.T.; Edmonds, D.V. Carbides in high-speed steels containing silicon. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2004, 35A, 2757–2766. [Google Scholar] [CrossRef]
- Setia, P.; Venkateswaran, T.; Tharian, K.T.; Jain, J.; Singh, S.S.; Shekhar, S. Influence of Si content on the microstructure and mechanical properties of silicon stainless steel. Mater. Sci. Eng. A 2022, 829, 142141. [Google Scholar] [CrossRef]
- Cai, M.H.; Ding, H.; Zhang, J.S.; Li, L.; Li, X.B.; Du, L.X. Transformation Behavior of Low Carbon Steels Containing Two Different Si Contents. J. Iron Steel Res. Int. 2009, 16, 55–60. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. Analysis of mechanical properties and microstructure of high-silicon dual-phase steel. Met. Sci. 1980, 14, 41–49. [Google Scholar] [CrossRef]
- Coronado, J.J. Effect of (Fe,Cr)7C3 carbide orientation on abrasion wear resistance and fracture toughness. Wear 2011, 270, 287–293. [Google Scholar] [CrossRef]
- Dogan, O.N.; Hawk, J.A. Effect of carbide orientation on abrasion of high Cr white cast iron. Wear 1995, 189, 136–142. [Google Scholar] [CrossRef]
- Liu, G.Z.; Liu, J.F.; Feng, L.L.; Kang, Y.; Yue, D. Effect of Silicon on Corrosion of Directional Fe-B-Si Alloy in Liquid Zinc. Metall. Mater. Trans. A 2021, 52, 691–699. [Google Scholar] [CrossRef]
- Ma, S.Q.; Xing, J.D.; He, Y.L.; Fu, H.G.; Li, Y.F.; Liu, G.Z. Effect of orientation and lamellar spacing of Fe2B on interfaces and corrosion behavior of Fe-B alloy in hot-dip galvanization. Acta Mater. 2016, 115, 392–402. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, J.D.; Ma, S.Q.; Zheng, B.C.; Liu, G.Z.; Yang, D.X.; Bai, Y.P. Interface characterization and erosion–corrosion behavior of directional Fe-3.5 wt.% B steel in flowing liquid zinc at various temperatures. Corros. Sci. 2016, 104, 260–268. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, J.D.; Ma, S.Q.; Liu, G.Z.; He, Y.L.; Yang, D.X.; Bai, Y.P. Effect of Fe2B orientation on erosion–corrosion behavior of Fe–3.5 wt.% B steel in flowing zinc. Corros. Sci. 2015, 98, 240–248. [Google Scholar] [CrossRef]
- Lv, Z.; Fu, H.G.; Xing, J.D.; Huang, Z.F.; Ma, S.Q.; Hu, Y. Influence of boron contents on oxidation behavior and the diffusion mechanism of Fe–B based alloys at 1073K in air. Corros. Sci. 2016, 108, 185–193. [Google Scholar] [CrossRef]
- Guo, P.J.; Ma, S.Q.; Jiao, M.; Lv, P.; Xing, J.D.; Xu, L.J.; Huang, Z.F. Effect of chromiumon microstructure and oxidation wear behavior of high-boron high-speed steel at elevated temperatures. Materials 2022, 15, 557. [Google Scholar] [CrossRef]


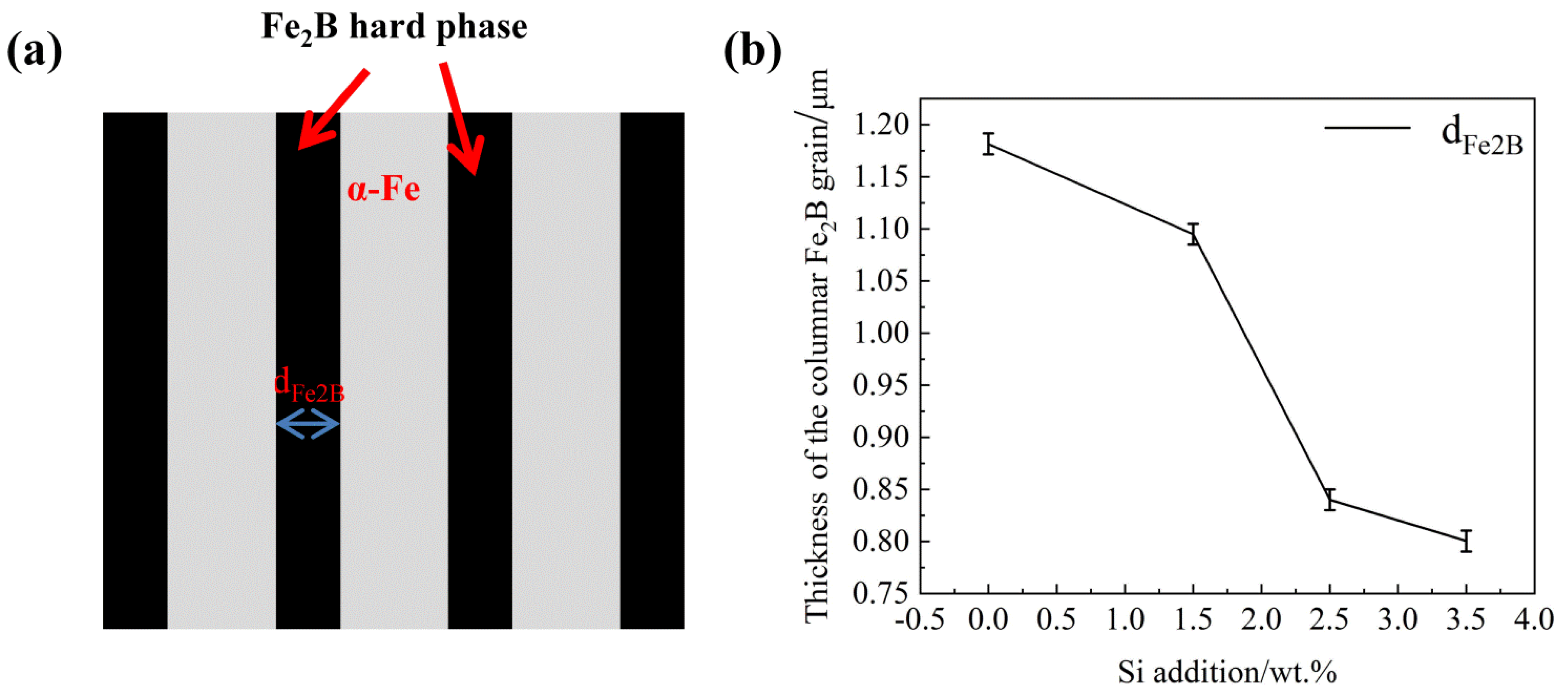

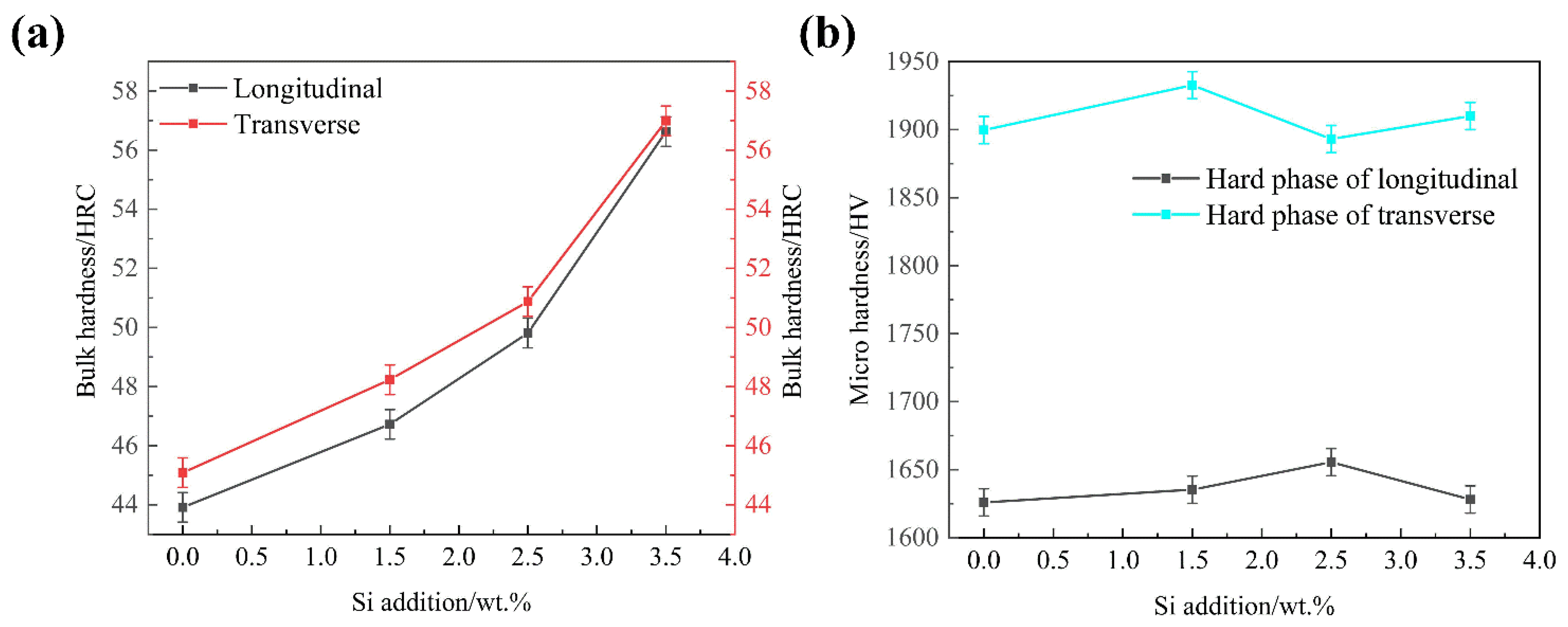
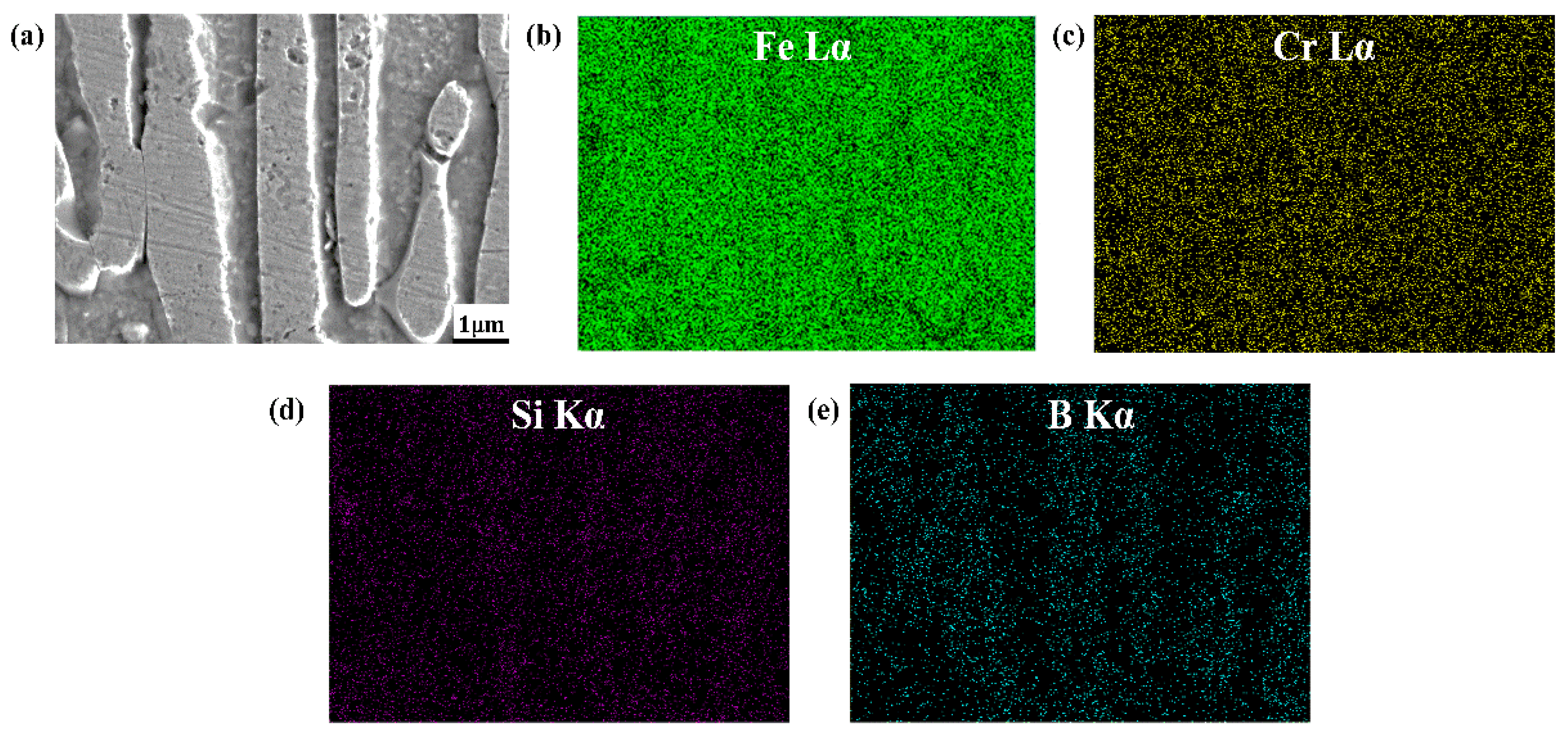
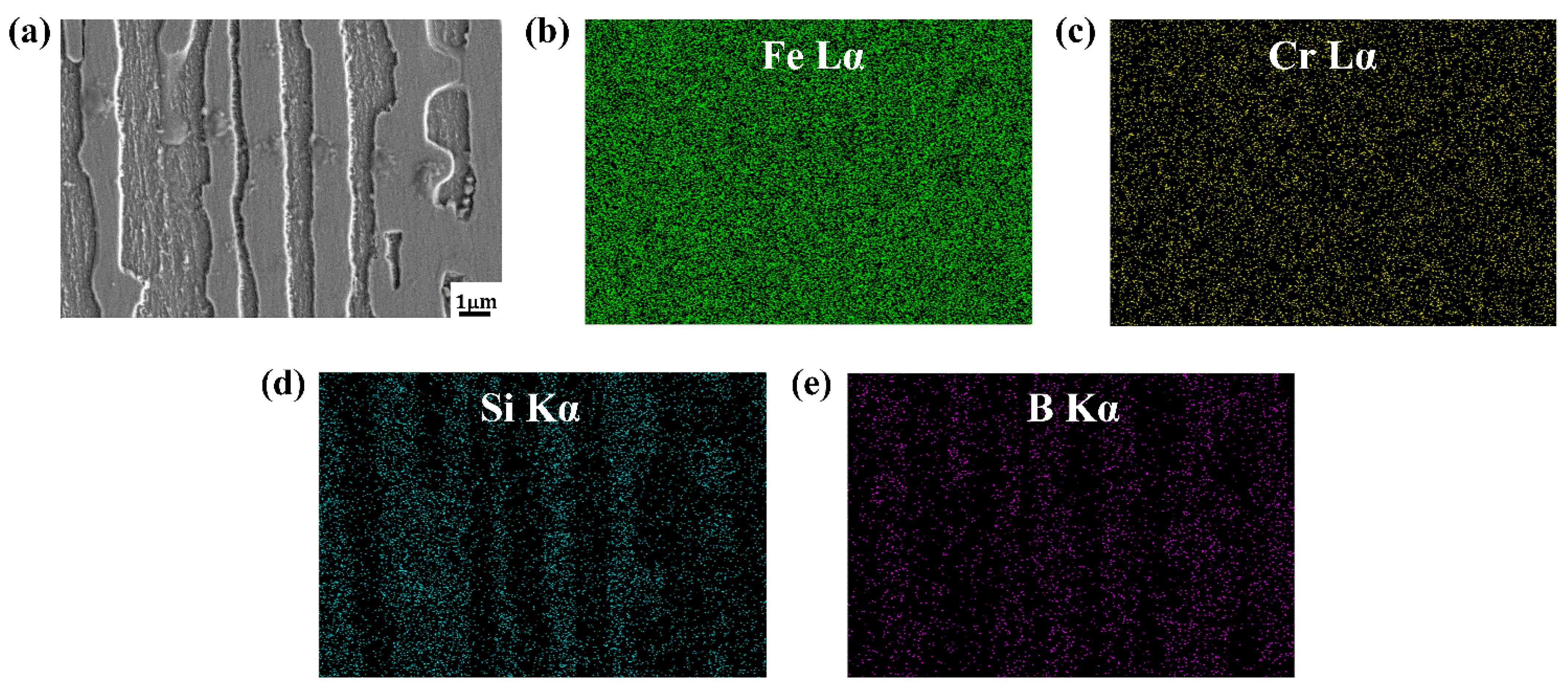

| Samples | B | Cr | Si | C | Fe |
|---|---|---|---|---|---|
| A1 | 3.51 | 0.52 | 0.00 | 0.11 | Bal |
| A2 | 3.51 | 0.50 | 1.50 | 0.10 | Bal |
| A3 | 3.49 | 0.50 | 2.50 | 0.12 | Bal |
| A4 | 3.50 | 0.51 | 3.50 | 0.11 | Bal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Ma, S.; He, X.; Shah, I.A.; Lv, P.; Chen, H.; Xing, J.; Xu, L.; Zhang, J. Effect of Silicon Content on Microstructures and Properties of Directionally Solidified Fe-B Alloy. Materials 2022, 15, 5937. https://doi.org/10.3390/ma15175937
Guo P, Ma S, He X, Shah IA, Lv P, Chen H, Xing J, Xu L, Zhang J. Effect of Silicon Content on Microstructures and Properties of Directionally Solidified Fe-B Alloy. Materials. 2022; 15(17):5937. https://doi.org/10.3390/ma15175937
Chicago/Turabian StyleGuo, Pengjia, Shengqiang Ma, Xuebin He, Intizar Ali Shah, Ping Lv, Hantao Chen, Jiandong Xing, Liujie Xu, and Jiankang Zhang. 2022. "Effect of Silicon Content on Microstructures and Properties of Directionally Solidified Fe-B Alloy" Materials 15, no. 17: 5937. https://doi.org/10.3390/ma15175937
APA StyleGuo, P., Ma, S., He, X., Shah, I. A., Lv, P., Chen, H., Xing, J., Xu, L., & Zhang, J. (2022). Effect of Silicon Content on Microstructures and Properties of Directionally Solidified Fe-B Alloy. Materials, 15(17), 5937. https://doi.org/10.3390/ma15175937






