Isothermal Hydrogen Reduction of a Lime-Added Bauxite Residue Agglomerate at Elevated Temperatures for Iron and Alumina Recovery
Abstract
:1. Introduction
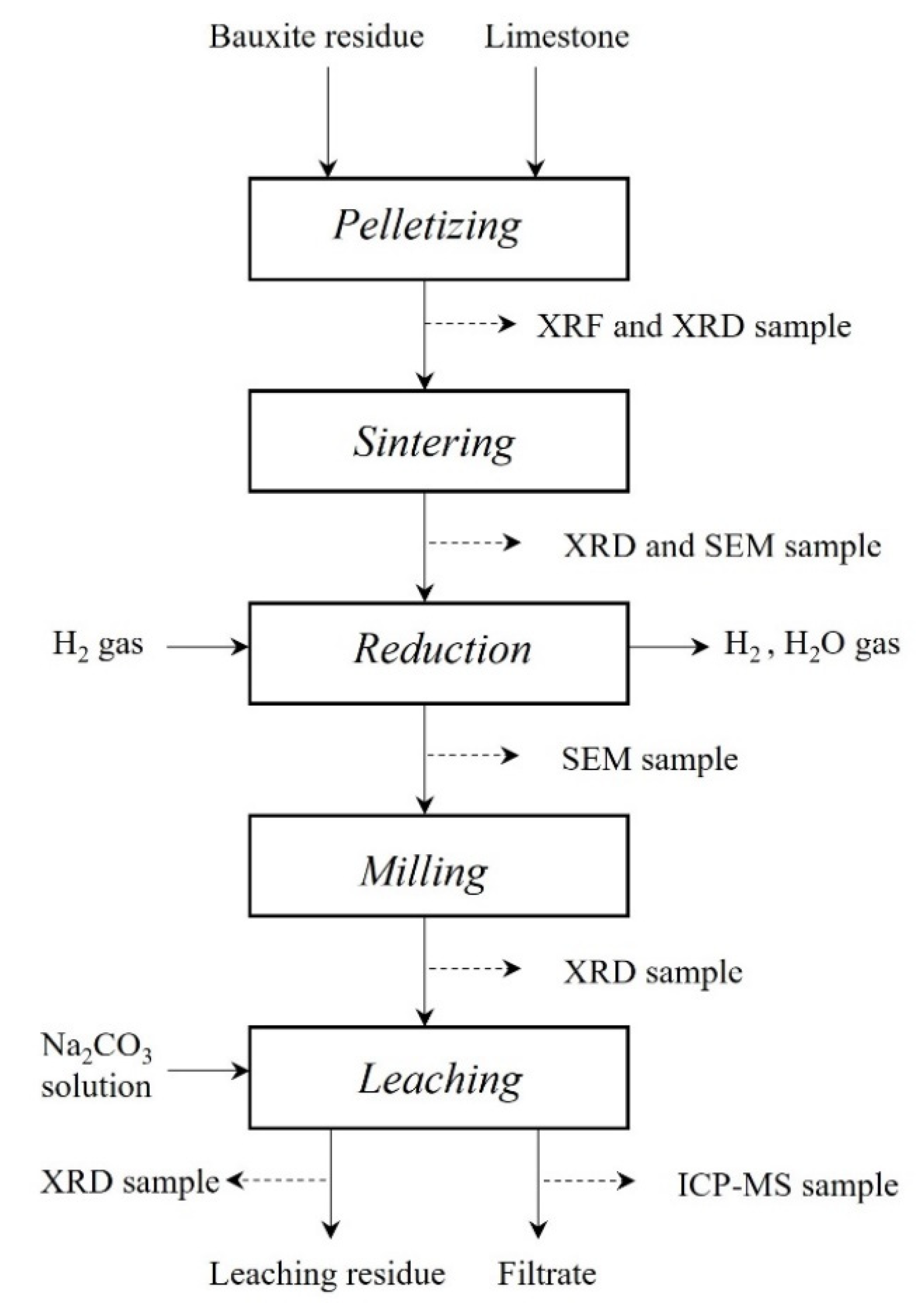
2. Materials and Methods
2.1. Materials and Pelletizing
2.2. Sample Preparation and Pelletizing
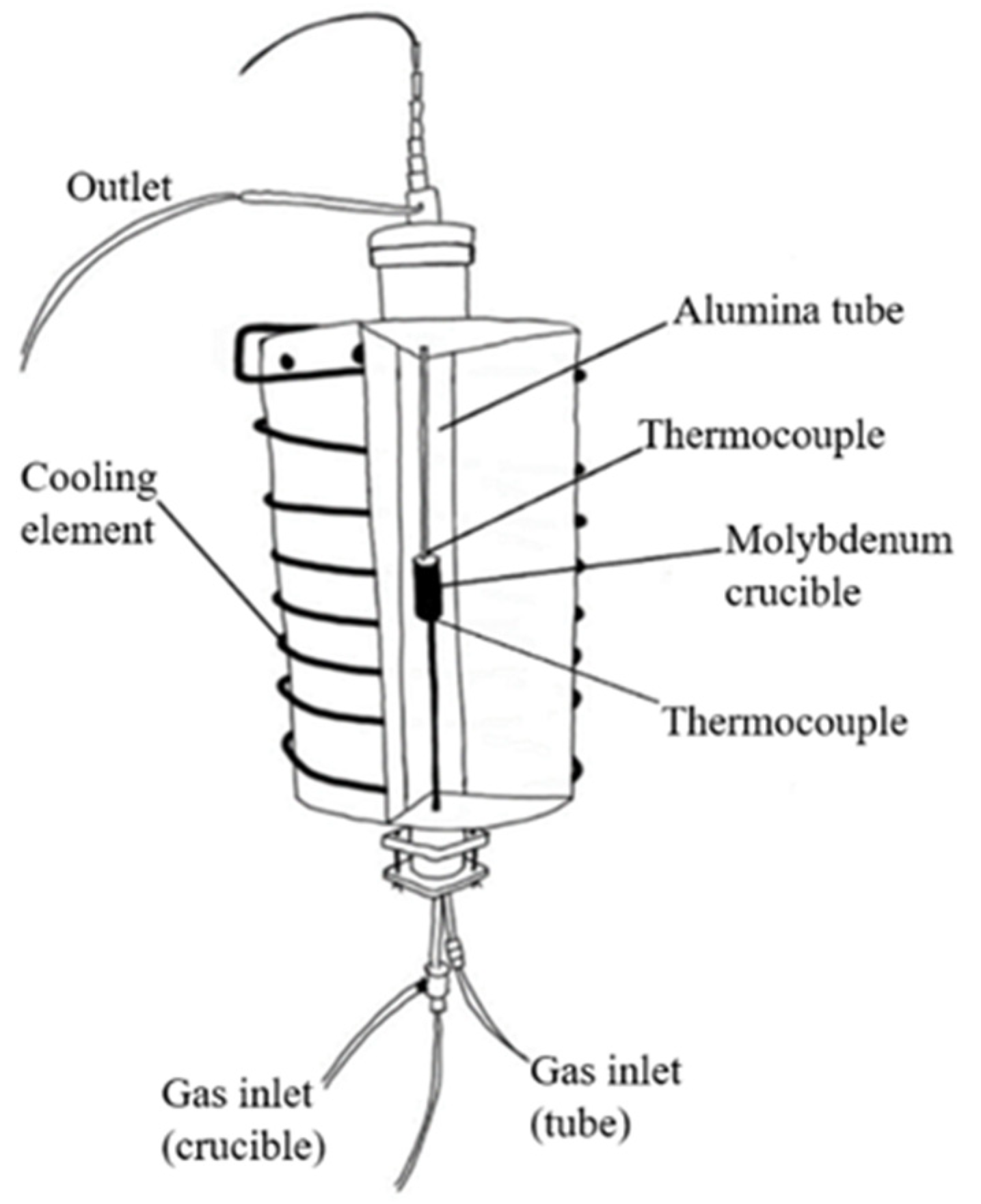
2.3. Hydrogen Reduction
2.4. Leaching of Reduced Samples
2.5. Characterization of Solid Materials
3. Results
3.1. Direct Observations
3.2. Phase Analysis
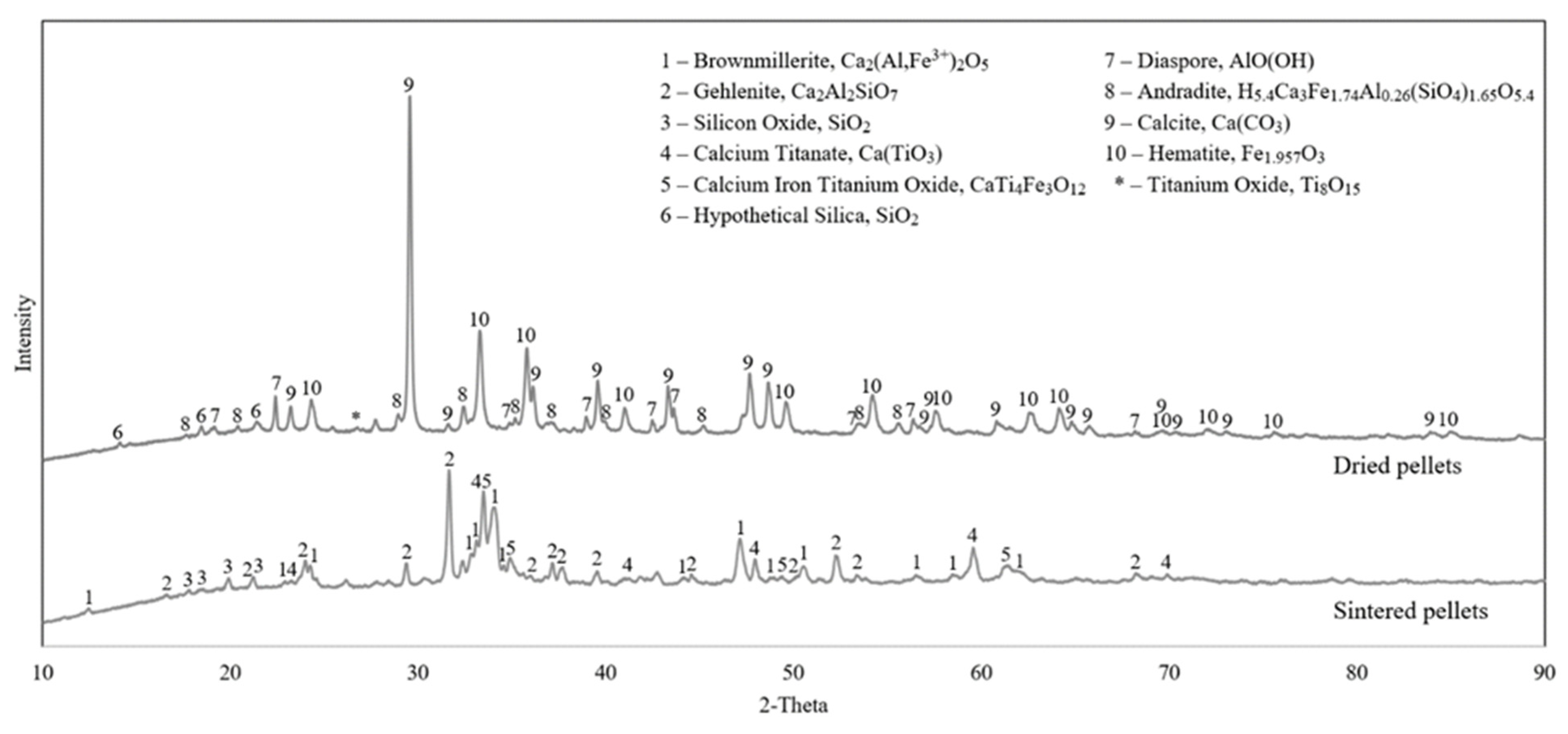
3.2.1. Dried and Sintered Pellets
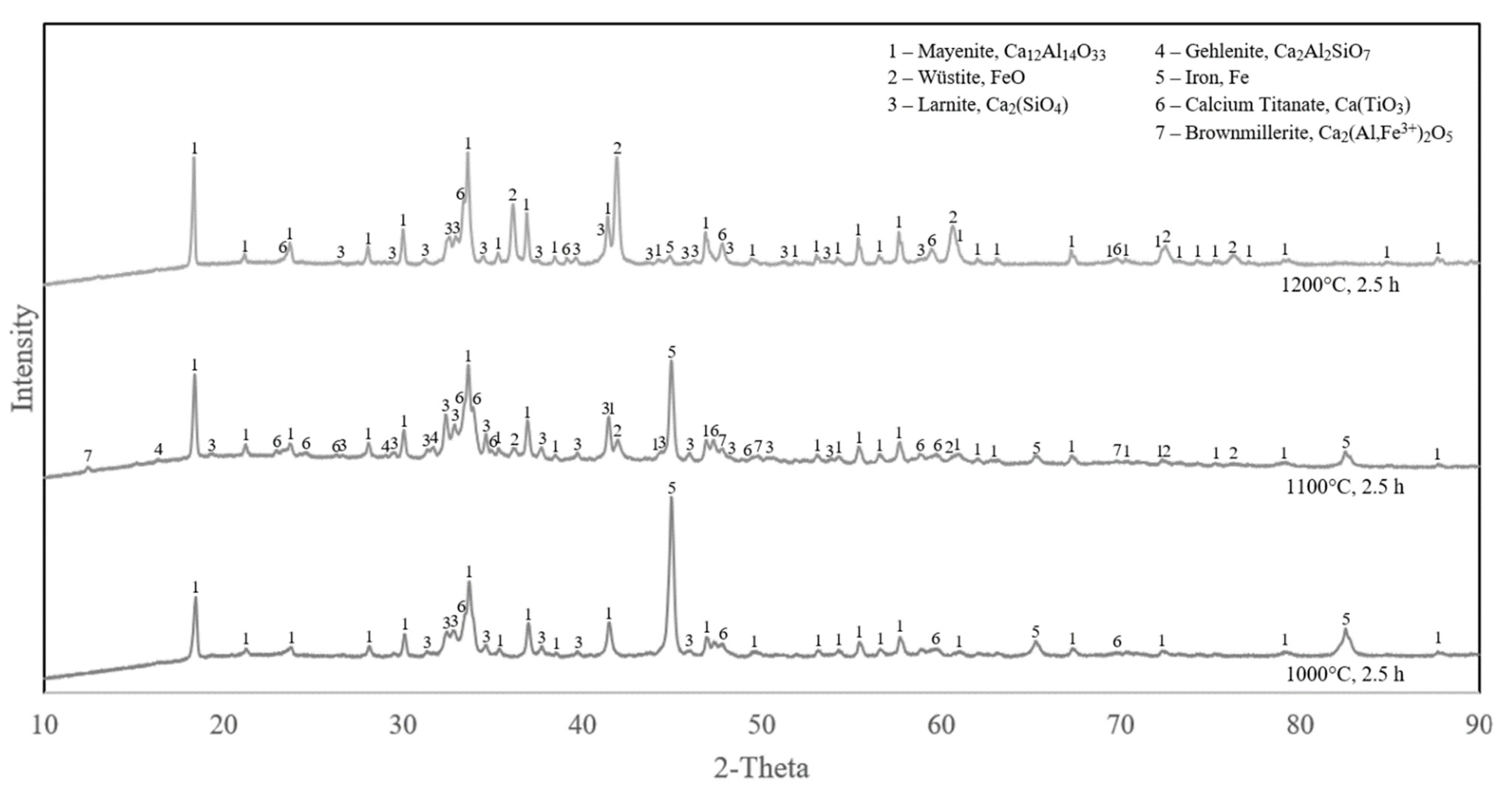
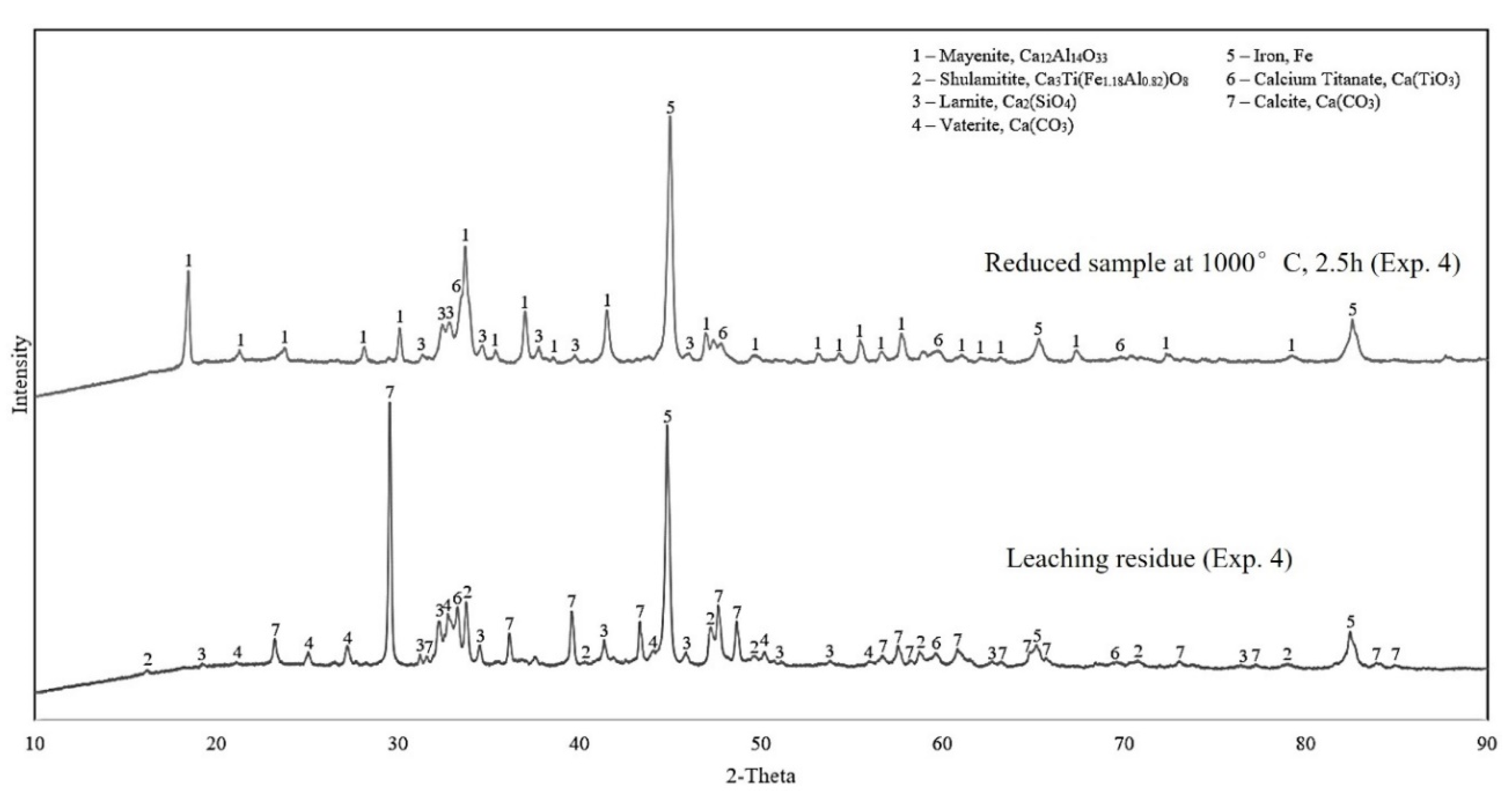
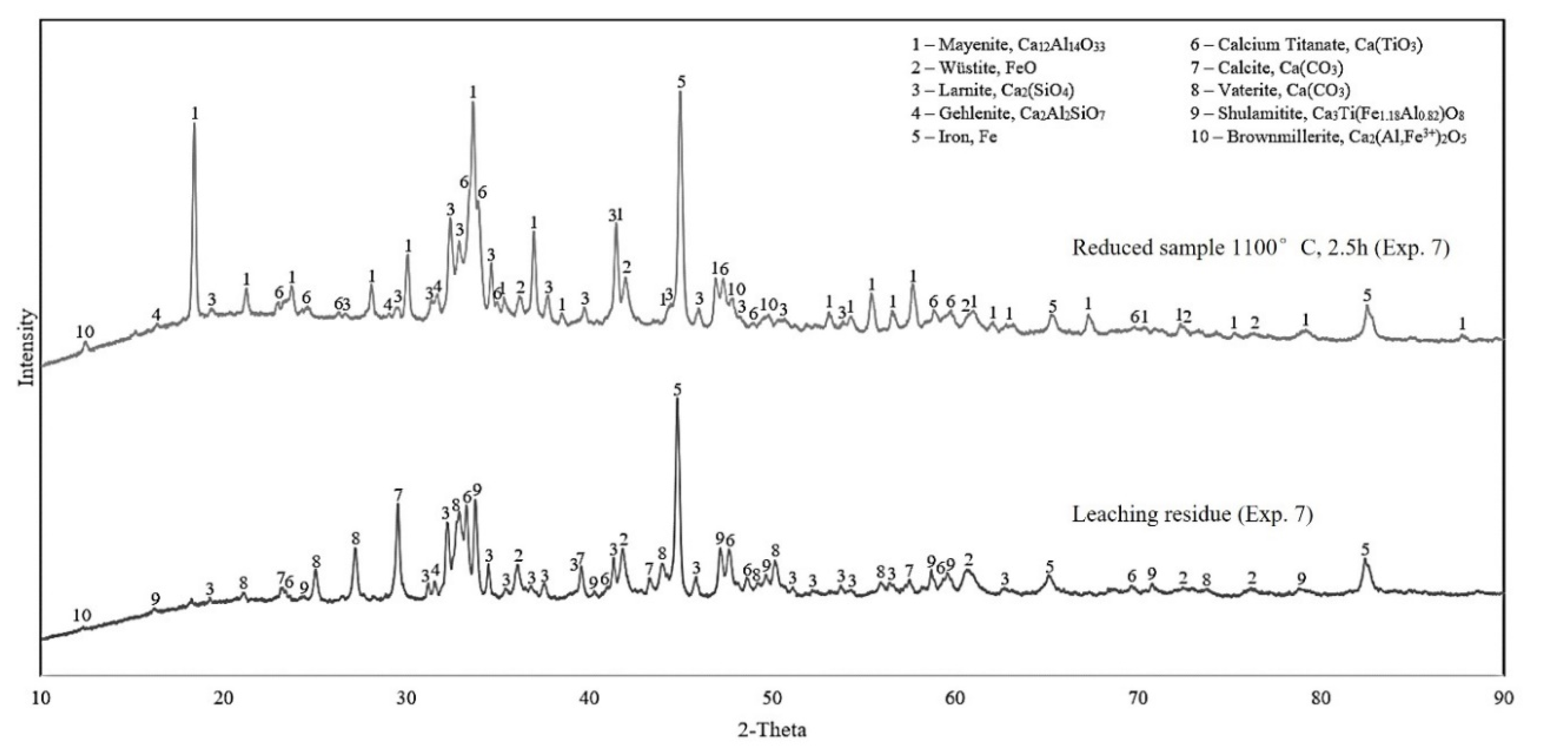
3.2.2. Reduced Samples
3.2.3. Leaching Residue Analysis
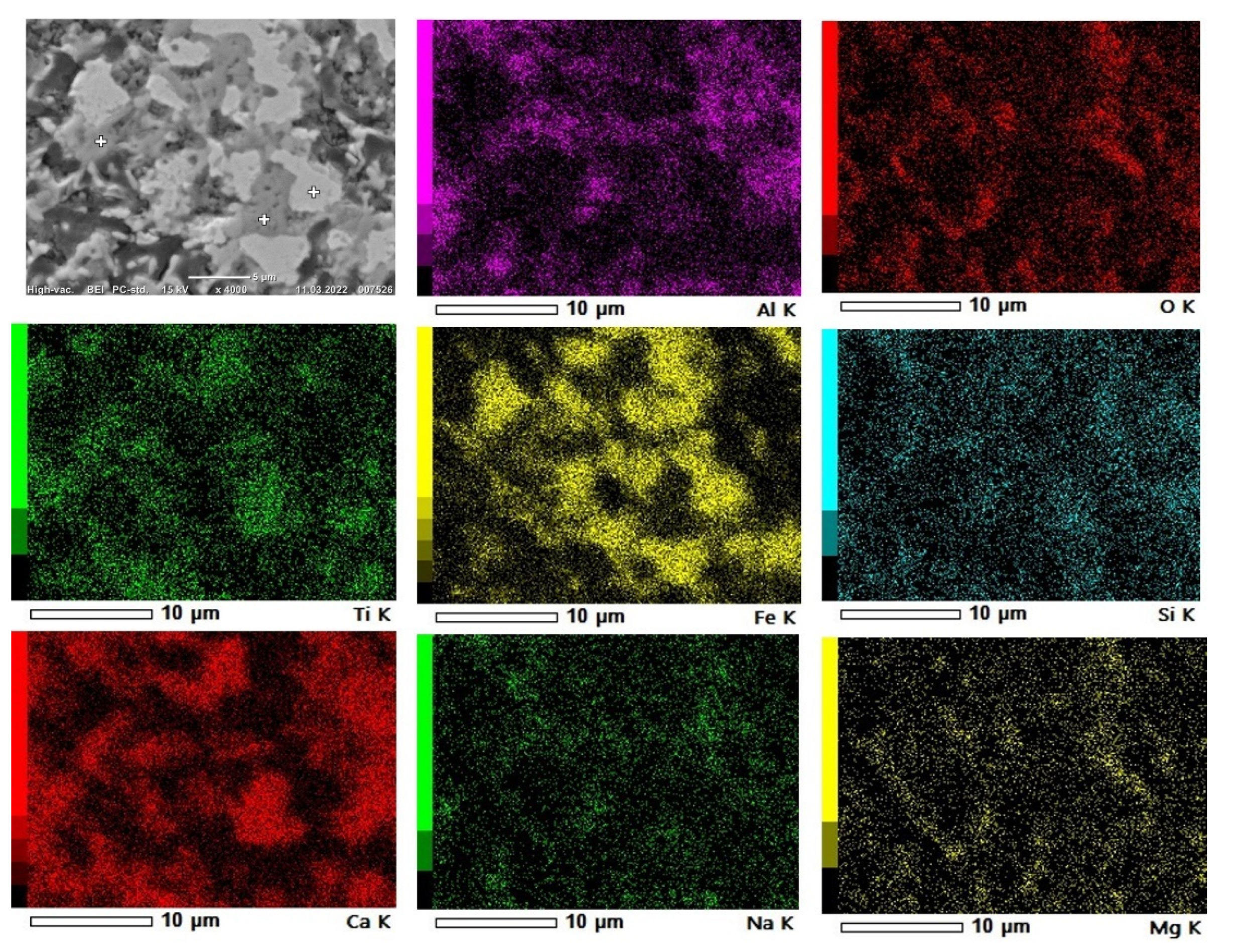
3.3. Microstructural Analysis
3.3.1. Sintered Pellets
3.3.2. Reduced Samples
3.4. Leachate Composition
4. Discussion
4.1. Evolution of Phases in Sintering
4.2. Reducibility of Pellets by Hydrogen
4.2.1. Effect of Temperature
4.2.2. Effect of Reduction Duration
4.2.3. Pellet Physical Properties Effect
4.3. Leaching Behavior of Reduced Pellets
4.3.1. Characteristics of Leaching Residue
4.3.2. Alumina Recovery
4.3.3. Silica Dissolution in Leaching
5. Conclusions
- In the pelletizing and sintering process iron- and Al-containing phases in raw materials are converted mainly to brown millerite and gehlenite, respectively.
- In hydrogen reduction at elevated temperatures, metallic iron is formed from brownmillerite and calcium–iron–titanate phases via the intermediate wüstite phase formation.
- The hydrogen reduction at 1000 °C occurs as a gas–solid reaction, while at higher temperatures it is changed to a gas–solid–liquid system due to the partial melting of pellet components.
- The rate and extent of hydrogen reduction was highest for 1000 °C and the lowest for 1200 °C. The process is more chemical-reaction-controlled for the former, while it is diffusion-controlled for the latter.
- During hydrogen reduction, gehlenite is converted to a leachable mayenite phase.
- Al recovery via the alkaline leaching of reduced pellets with sodium carbonate solutions yields high Al recovery (up to 87% measured) with no effect on metallic iron, while calcite is evolved in the leaching residue.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Liu, X. Applications of red mud as an environmental remediation material: A review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Y.; He, F.; Gao, P.; Yuan, S. Characteristic, hazard and iron recovery technology of red mud—A critical review. J. Hazard. Mater. 2021, 420, 126542. [Google Scholar] [CrossRef] [PubMed]
- Production ESa. Background ensureal.com: ENSUREAL: Sustainable Alumina Production. 2021. Available online: https://ensureal.com/background/ (accessed on 1 July 2022).
- HARARE. Let’s Talk about Red Mud (Bauxite Residue). 2021. Available online: https://h2020harare.eu/lets-talk-about-red-mud-bauxite-residue (accessed on 1 July 2022).
- Lazou, A.; van der Eijk, C.; Tang, K.; Balomenos, E.; Kolbeinsen, L.; Safarian, J. The Utilization of Bauxite Residue with a Calcite-Rich Bauxite Ore in the Pedersen Process for Iron and Alumina Extraction. Metall. Mater. Trans. B 2021, 52, 11. [Google Scholar] [CrossRef]
- Azof, F.I.; Kolbeinsen, L.; Safarian, J. Kinetics of the Leaching of Alumina-containing Slag for Alumina Recovery. In Proceedings of the European Metallurgical Conference 2019, Düsseldorf, Germany, 23–26 June 2019; p. 15. [Google Scholar]
- Azof, F.I.; Yang, Y.; Panias, D.; Kolbeinsen, L.; Safarian, J. Leaching characteristics and mechanism of the synthetic calcium-aluminate slags for alumina recovery. Hydrometallurgy 2019, 185, 17. [Google Scholar] [CrossRef]
- Azof, F.I.; Safarian, J. Leaching kinetics and mechanism of slag produced from smelting-reduction of bauxite for alumina recovery. Hydrometallurgy 2020, 195, 13. [Google Scholar] [CrossRef]
- Azof, F.I.; Kolbeinsen, L.; Safarian, J. The Leachability of Calcium Aluminate Phases in Slags for the Extraction of Alumina. In Proceedings of the 35th International ICSOBA Conference, Hamburg, Germany, 2–5 October 2017; Volume 46, p. 12. [Google Scholar]
- Azof, F.I.; Vafeias, M.; Panias, D.; Safarian, J. The leachability of a ternary CaO-Al2O3-SiO2 slag produced from smelting reduction of low-grade bauxite for alumina recovery. Hydrometallurgy 2020, 191, 12. [Google Scholar] [CrossRef]
- Safarian, J.; Kolbeinsen, L. Sustainability in Alumina production from Bauxite. Miner. Process. 2016, 5, 7. [Google Scholar]
- Lazou, A.; van der Eijk, C.; Balomenos, E.; Kolbeinsen, L.; Safarian, J. On the Direct Reduction Phenomena of Bauxite Ore Using H2 Gas in a Fixed Bed Reactor. J. Sustain. Metall. 2020, 6, 11. [Google Scholar] [CrossRef]
- Mwase, D.J.M.; Safarian, J. Production of Aluminum Tri-hydroxides from Secondary Bauxite Materials. In Proceedings of the European Metallurgical Conference 2021, Salzburg, Austria, 27–30 June 2021; pp. 547–557. [Google Scholar]
- Safarian, J. Extraction of Iron and Ferrosilicon Alloys from Low-Grade Bauxite Ores. In Extraction 2018; Springer: Cham, Switzerland, 2018; Volume 12. [Google Scholar]
- Safarian, J.; Kolbeinsen, L. Smelting-reduction of bauxite for sustainable alumina production. Miner. Process. 2016, 5, 158. [Google Scholar]
- Azof, F.I.; Kolbeinsen, L.; Safarian, J. Characteristics of Calcium-Aluminate Slags and Pig Iron Produced from Smelting-Reduction of Low-Grade Bauxites. Metall. Mater. Trans. B 2018, 49, 20. [Google Scholar] [CrossRef]
- Production ESa. Results ensureal.com: ENSUREAL: Sustainable Alumina Production. 2021. Available online: https://ensureal.com/results/#WPs (accessed on 12 May 2022).
- Spreitzer, D.; Schenk, J. Iron Ore Reduction by Hydrogen Using a Laboratory Scale Fluidized Bed Reactor: Kinetic Investigation—Experimental Setup and Method for Determination. Metall. Mater. Trans. B 2019, 50, 13. [Google Scholar] [CrossRef]
- Patisson, F.; Mirgaux, O. Hydrogen Ironmaking: How It Works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- Heidari, A.; Niknahad, N.; Iljana, M.; Fabritius, T. A Review on the Kinetics of Iron Ore Reduction by Hydrogen. Materials 2021, 14, 7540. [Google Scholar] [CrossRef] [PubMed]
- HARARE. HARARE h2020harare.eu2021. Available online: https://h2020harare.eu/ (accessed on 1 July 2022).
- CORDIS err. Horizon 2020: Hydrogen as the Reducing Agent in the Recovery of Metals and Minerals from Metallurgical Waste cordis.europa.eu. European Commision. 2022. Available online: https://cordis.europa.eu/project/id/958307 (accessed on 15 July 2022).
- Production ESa. Ensuring Zero Waste Production of Alumina in Europe. 2021. Available online: https://ensureal.com/ (accessed on 10 July 2022).
- Frank, M.; Kaußen, B.F. Methods for Alkaline Recovery of Aluminum from Bauxite Residue. Miner. Met. Mater. Soc. 2016, 2, 353–364. [Google Scholar]
- Blackman, L.G.A. SI Chemical Data, 7th ed.; John Wiley & Sons Ltd.: Milton, Australia, 2014; p. 185. [Google Scholar]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Azof, F.I.; Tang, K.; You, J.; Safarian, J. Synthesis and Characterization of 12CaOÆ7Al2O3 Slags: The Effects of Impurities and Atmospheres on the Phase Relations. Metall. Mater. Trans. B 2020, 51, 2689–2710. [Google Scholar] [CrossRef]





| Materials | Al2O3 | CaO | Fe | Fe2O3 | K2O | MgO | MnO | Na2O | P | P2O5 | S | SO3 | SiO2 | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LS [wt%] | 0.90 | 52.70 | - | 0.15 | 0.12 | 0.95 | - | - | - | 0.01 | - | 0.06 | 2.07 | 0.03 | 42.60 |
| BR [wt%] | 22.00 | 8.80 | 28.50 | - | 0.09 | 0.23 | 0.08 | 3.10 | 0.05 | - | 0.38 | - | 7.10 | 5.00 | 24.67 |
| Materials | Al2O3 | CaO | Cr2O3 | Fe2O3 | K2O | MgO | MnO | Na2O | P | S | SiO2 | SrO | TiO2 | V2O5 | ZrO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DP [wt%] | 14.60 | 27.70 | 0.11 | 27.07 | 0.09 | 0.48 | 0.09 | 2.09 | 0.03 | 0.23 | 5.42 | 0.09 | 3.25 | 0.09 | 0.11 | 18.56 |
| SP [wt%] | 18.15 | 34.34 | - | 33.12 | 0.05 | 0.62 | 0.10 | 2.05 | 0.04 | 0.31 | 6.77 | 0.10 | 4.10 | 0.10 | 0.10 | 0.07 |
| Temperature [°C] | 1000 | 1100 | 1200 | ||||||
| Hydrogen gas flow | 0.2 NL/min | ||||||||
| Time [h] | 0.5 | 1 | 2 | 2.5 | 0.5 | 1 | 2.5 | 0.5 | 2.5 |
| Reduction sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Sample weight [g] | 25.0 | 25.2 | 25.1 | 25.1 | 25.0 | 25.1 | 25.7 | 24.9 | 24.1 |
| Initial Weight [g] | Mass Loss [%] | Theoretical Mass Loss [g] | Actual: Theoretical Mass Loss [%] |
|---|---|---|---|
| 82.6 | 22.7 | 17.9 | 104.8 |
| 84.2 | 22.5 | 18.3 | 103.9 |
| 85.6 | 22.3 | 18.6 | 102.7 |
| 76.0 | 22.5 | 16.5 | 103.0 |
| 88.7 | 22.5 | 19.3 | 103.8 |
| 78.5 | 22.7 | 17.1 | 104.4 |
| Average: | 22.5 | 17.9 | 103.8 |
| Reduction No. | Mass Loss [g] | Mass Loss [%] | Theoretical Mass Loss [g] | Fe Yield [%] |
|---|---|---|---|---|
| 1 | 1.44 | 5.75 | 2.49 | 57.8 |
| 2 | 1.78 | 7.07 | 2.50 | 71.1 |
| 3 | 1.92 | 7.65 | 2.50 | 76.8 |
| 4 | 2.07 | 8.27 | 2.50 | 82.9 |
| 5 | 1.09 | 4.34 | 2.49 | 43.8 |
| 6 | 1.46 | 5.84 | 2.50 | 58.5 |
| 7 | 1.8 | 7.02 | 2.55 | 70.5 |
| 8 | 0.73 | 3.04 | 2.40 | 30.5 |
| Sample | Concentration [mg/L] | ||
|---|---|---|---|
| Al | Si | Fe | |
| Blank | 0.12 | 3.78 | 0.00 |
| Reduction 2 | 4163 | 70 | 0.78 |
| Reduction 4 | 3943 | 66 | 0.74 |
| Reduction 6 | 3915 | 64 | 0.68 |
| Reduction 7 | 3647 | 66 | 0.55 |
| Sample | Yield [%] | |
|---|---|---|
| Al | Si | |
| Reduction 2 | 86.6 | 4.40 |
| Reduction 4 | 82.0 | 4.14 |
| Reduction 6 | 81.5 | 4.07 |
| Reduction 7 | 75.9 | 4.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skibelid, O.B.; Velle, S.O.; Vollan, F.; Van der Eijk, C.; Hoseinpur-Kermani, A.; Safarian, J. Isothermal Hydrogen Reduction of a Lime-Added Bauxite Residue Agglomerate at Elevated Temperatures for Iron and Alumina Recovery. Materials 2022, 15, 6012. https://doi.org/10.3390/ma15176012
Skibelid OB, Velle SO, Vollan F, Van der Eijk C, Hoseinpur-Kermani A, Safarian J. Isothermal Hydrogen Reduction of a Lime-Added Bauxite Residue Agglomerate at Elevated Temperatures for Iron and Alumina Recovery. Materials. 2022; 15(17):6012. https://doi.org/10.3390/ma15176012
Chicago/Turabian StyleSkibelid, Olivia Bogen, Sander Ose Velle, Frida Vollan, Casper Van der Eijk, Arman Hoseinpur-Kermani, and Jafar Safarian. 2022. "Isothermal Hydrogen Reduction of a Lime-Added Bauxite Residue Agglomerate at Elevated Temperatures for Iron and Alumina Recovery" Materials 15, no. 17: 6012. https://doi.org/10.3390/ma15176012
APA StyleSkibelid, O. B., Velle, S. O., Vollan, F., Van der Eijk, C., Hoseinpur-Kermani, A., & Safarian, J. (2022). Isothermal Hydrogen Reduction of a Lime-Added Bauxite Residue Agglomerate at Elevated Temperatures for Iron and Alumina Recovery. Materials, 15(17), 6012. https://doi.org/10.3390/ma15176012







