Fabrication of Boron-Doped Diamond Film Electrode for Detecting Trace Lead Content in Drinking Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Instruments
2.2. Preparation of BDD Electrode
2.3. Electrochemical Measurement
3. Results and Discussion
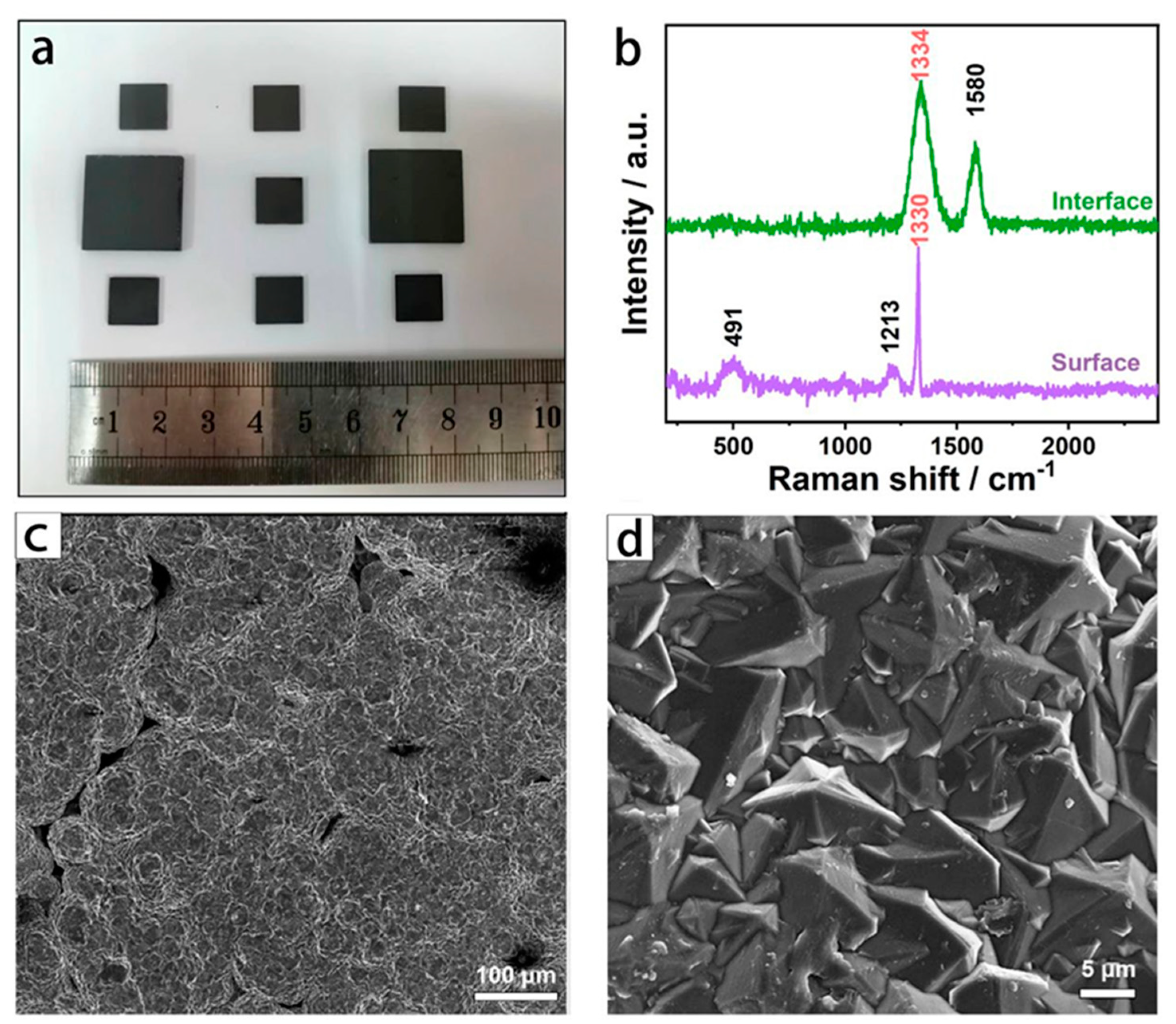
3.1. Structural Characterization of BDD Electrode
3.2. Electrochemical Characterization of BDD Electrode
3.3. Determination of Trace Pb2+ in Water
3.3.1. Dissolution Parameter Optimization
3.3.2. Sensitivity Detection
3.3.3. Anti-Interference Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Memon, A.G.; Zhou, X.H.; Xing, Y.P.; Wang, R.Y.; Liu, L.H.; Khan, M.; He, M. Label-free colorimetric nanosensor with improved sensitivity for Pb2 + in water by using a truncated 8–17 DNAzyme. Front. Env. Sci. Eng. 2019, 13, 125–130. [Google Scholar] [CrossRef]
- Hanna, A.M.; La, C.J.; Sadler, R.C.; Champney, S.A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am. J. Public. Health. 2016, 106, 283–290. [Google Scholar] [CrossRef]
- Fan, M.Y.; Li, T.J.; Hu, J.W.; Cao, R.S.; Wu, Q.; Wei, X.H.; Li, L.Y.; Shi, X.D.; Ruan, W.Q. Synthesis and characterization of reduced graphene oxide-supported nanoscale zero-valent Iron (nZVI/rGO) composites used for Pb(II) removal. Materials 2016, 9, 687. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total. Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Zhang, J.M.; Xiong, Z.H.; Li, C.; Wu, C.S. Exploring a thiol-functionalized MOF for elimination of lead and cadmium from aqueous solution. J. Mol. Liq. 2016, 221, 43–50. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Popoola, E.O.; Alayande, O.S.; Msagati, T.A.M. Synthesis and characterization of biopolymeric chitosan derived from land snail shells and its potential for Pb2+ removal from aqueous solution. Materials 2015, 8, 8630–8640. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Sun, Y.; Cai, L.; Gao, Y.P.; Cai, Y. Optical fiber sensors for measurement of heavy metal ion concentration: A review. Measurement 2020, 158, 107742. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Liang, X.Q.; Niyungeko, C.; Zhou, J.J.; Xu, J.M.; Tian, G. M A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Bansod, B.K.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Guo, C.Y.; Zheng, J.G.; Deng, H.W.; Shi, P.H.; Zhao, G.H. Photoelectrocatalytic interface of boron-doped diamond: Modification, functionalization and environmental applications. Carbon 2020, 175, 454–466. [Google Scholar] [CrossRef]
- Mortet, V.; Taylor, A.; Zivcova, Z.V.; Machon, D.; Frank, O.; Hubik, P.; Tremouilles, D.; Kavan, L. Analysis of heavily boron-doped diamond Raman spectrum. Diam. Relat. Mat. 2018, 88, 163–166. [Google Scholar] [CrossRef]
- Ashcheulov, P.; Šebera, J.; Kovalenko, A.; Petrák, V.; Fendrych, F.; Nesládek, M.; Taylor, A.; Živcová, Z.V.; Frank, O.; Kavan, L. Conductivity of boron-doped polycrystalline diamond films: Influence of specific boron defects. Eur. Phys. J. B 2013, 86, 443–455. [Google Scholar] [CrossRef]
- Volodin, V.A.; Mortet, V.; Taylor, A.; Remes, Z.; Stuchliková, T.H.; Stuchlik, J. Raman scattering in boron doped nanocrystalline diamond films: Manifestation of Fano interference and phonon confinement effect. Solid. State. Commun. 2018, 276, 33–36. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Zhang, Z.Q.; Zhao, Z.Y. Preparation of boron-doped diamond foam film for supercapacitor applications. Appl. Surf. Sci. 2020, 506, 1–7. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, E.S.; Lim, Y.K.; Park, K.H.; Park, H.D.; Lim, D.S. Enhanced electrochemical oxidation of phenol by boron-doped diamond nanowire electrode. RSC. Adv. 2017, 7, 6229–6235. [Google Scholar] [CrossRef]
- Li, X.J.; Li, H.J.; Li, M.J.; Li, C.P.; Sun, D.Z.; Lei, Y.J.; Yang, B.H. Preparation of a porous boron-doped diamond/Ta electrode for the electrocatalytic degradation of organic pollutants. Carbon 2018, 129, 543–551. [Google Scholar] [CrossRef]
- Shah, A.; Sultan, S.; Zahid, A.; Aftab, S.; Nisar, J.; Nayab, S.; Qureshi, R.; Khan, G.S.; Hussain, H.; Ozkan, S.A. Highly sensitive and selective electrochemical sensor for the trace level detection of mercury and cadmium. Electrochim. Acta 2017, 258, 1397–1403. [Google Scholar] [CrossRef]
- Sopha, H.; Baldrianová, L.; Tesařová, E.; Grincienė, G.; Weidlich, T.; Švancara, I.; Hočevar, S.B. A new type of bismuth electrode for electrochemical stripping analysis based on the ammonium tetrafluorobismuthate bulk-modified carbon paste. Electroanalysis 2010, 22, 1489–1493. [Google Scholar] [CrossRef]
- Dey, M.; Satpati, A.; Sahoo, S.; Kameswaran, R.; Reddy, A.; Mukherjee, T. Bi-Film on a carbon paste electrode modified with nafion film embedded with multiwall carbon nano tubes for the determination of heavy metals. Anal. Methods 2011, 3, 2540–2546. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Mercury films on commercial carbon screen-printed devices for the analysis of heavy metalions: A critical evaluation. Electroanalysis 2015, 27, 1345–1349. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.D.L.G.; Marín, M.R.P.; Blázquez, L.C.; Gil, E.P. Applicability of the bismuth bulk rotating disk electrode for heavy metal monitoring in undisturbed environmental and biological samples: Determination of Zn (II) in rainwater, tap water and urine. Anal. Methods 2014, 6, 8668–8674. [Google Scholar] [CrossRef]
- Ayres, Z.J.; Newton, M.E.; Macpherson, J.V. Quantitative analysis of trace palladium contamination in solution using electrochemical X-ray fluorescence (EC-XRF). Analyst 2016, 141, 3349–3357. [Google Scholar] [CrossRef]
- Bergmann, M.H.; Rollin, J. Product and by-product formation in laboratory studies on disinfection electrolysis of water using boron-doped diamond anodes. Catal. Today 2007, 124, 198–203. [Google Scholar] [CrossRef]
- Vanhove, E.; De Sanoit, J.; Arnault, J.; Saada, S.; Mer, C.; Mailley, P.; Bergonzo, P.; Nesladek, M. Stability of H-terminated BDD electrodes: An insight into the influence of the surface preparation. Phys Status. Solidi. A 2007, 204, 2931–2939. [Google Scholar] [CrossRef]
- Pujol, L.; Evrard, D.; Gros, P.; Freyssinier, M.; Ruffien-Cizsak, A.; Serrano, G.K. Boron-doped diamond as an all-in-one system for the mineralization and detection of lead in waters. Chemelectrochem 2019, 6, 1225–1228. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Liu, X.; Yu, X.; Xu, S.; Zhang, S.; Guo, S. Fabrication of Boron-Doped Diamond Film Electrode for Detecting Trace Lead Content in Drinking Water. Materials 2022, 15, 6013. https://doi.org/10.3390/ma15176013
Wu L, Liu X, Yu X, Xu S, Zhang S, Guo S. Fabrication of Boron-Doped Diamond Film Electrode for Detecting Trace Lead Content in Drinking Water. Materials. 2022; 15(17):6013. https://doi.org/10.3390/ma15176013
Chicago/Turabian StyleWu, Liang, Xinghong Liu, Xiang Yu, Shijue Xu, Shengxiang Zhang, and Shiman Guo. 2022. "Fabrication of Boron-Doped Diamond Film Electrode for Detecting Trace Lead Content in Drinking Water" Materials 15, no. 17: 6013. https://doi.org/10.3390/ma15176013





