Preparation, Characterization, and Photocatalytic Performance of Ag/BiOBr0.85I0.15 Nanocomposites
Abstract
:1. Introduction
2. Preparation and Experimental Details
2.1. Photocatalyst Preparation
2.2. Characterization and Analysis
2.3. Photocatalytic Experiments
3. Results and Discussion
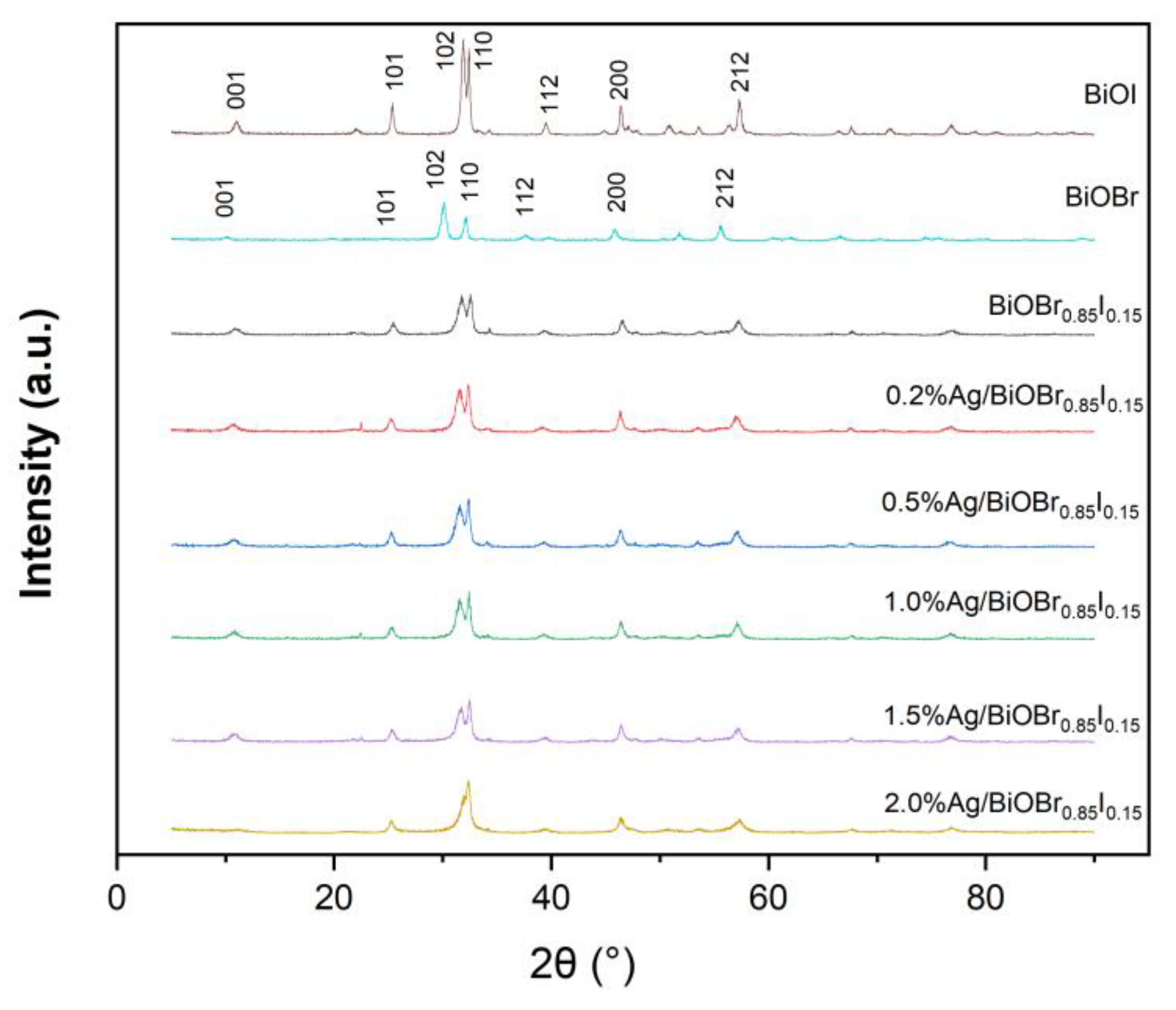
3.1. X-ray Diffraction (XRD)
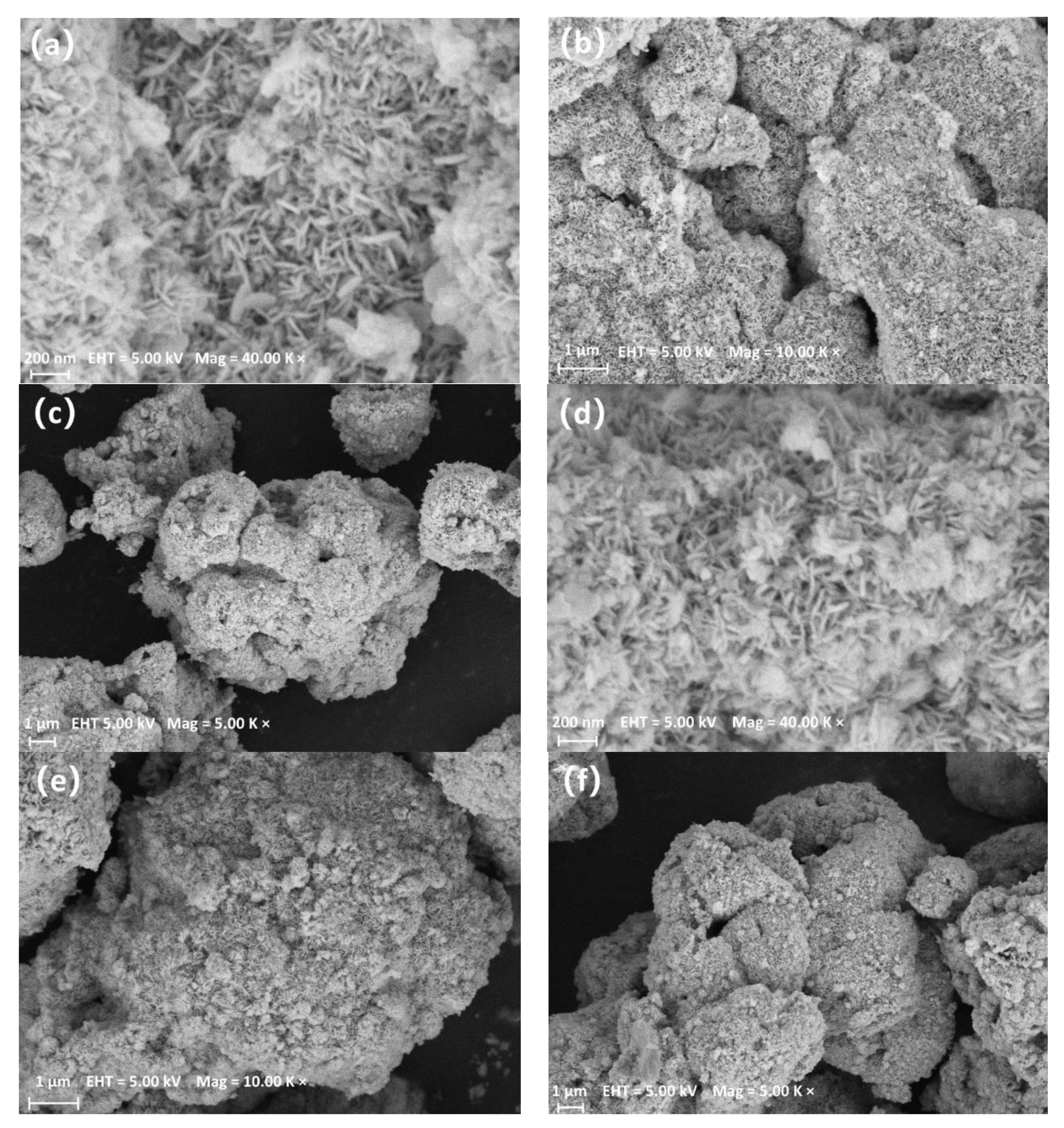
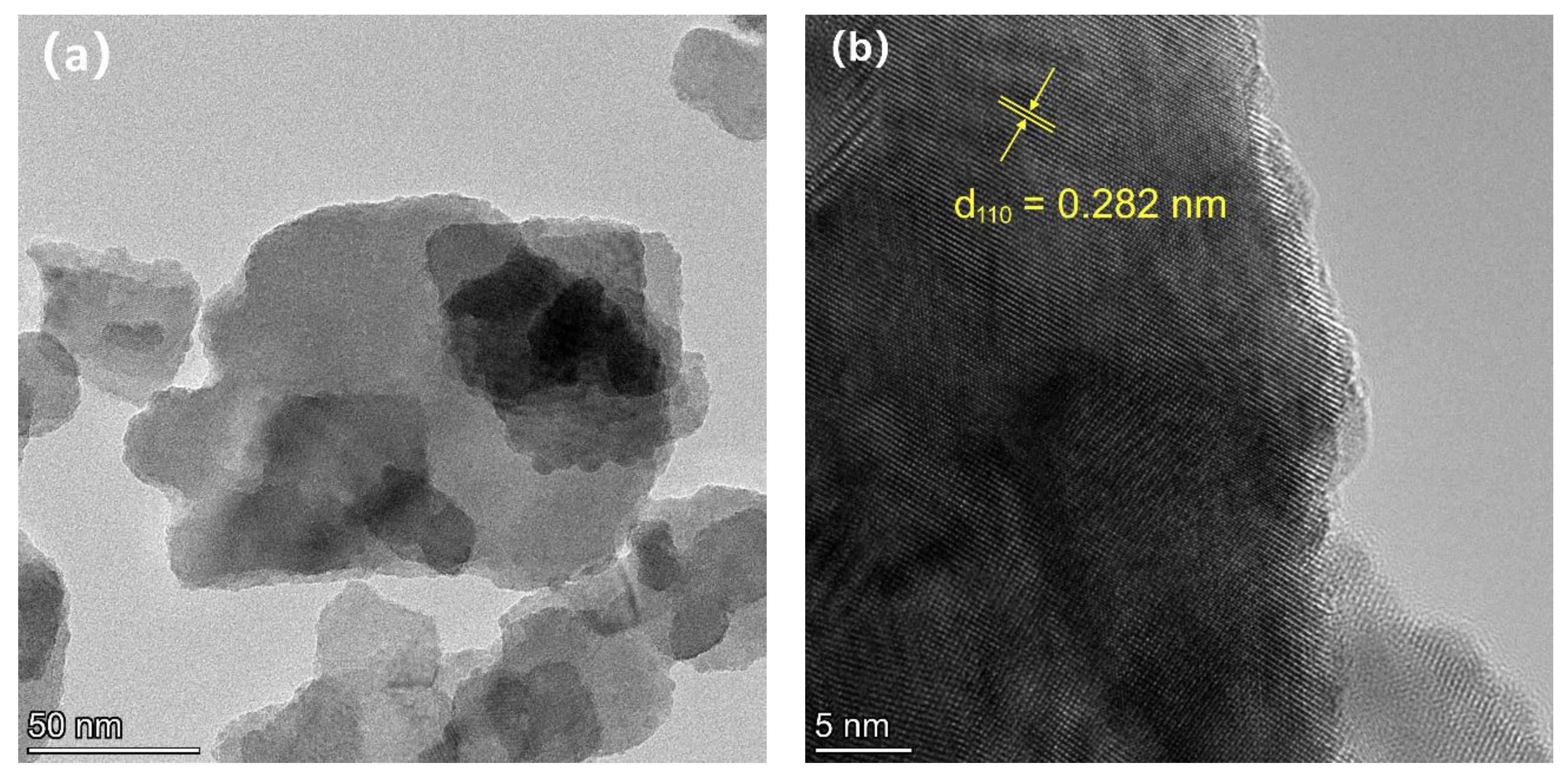
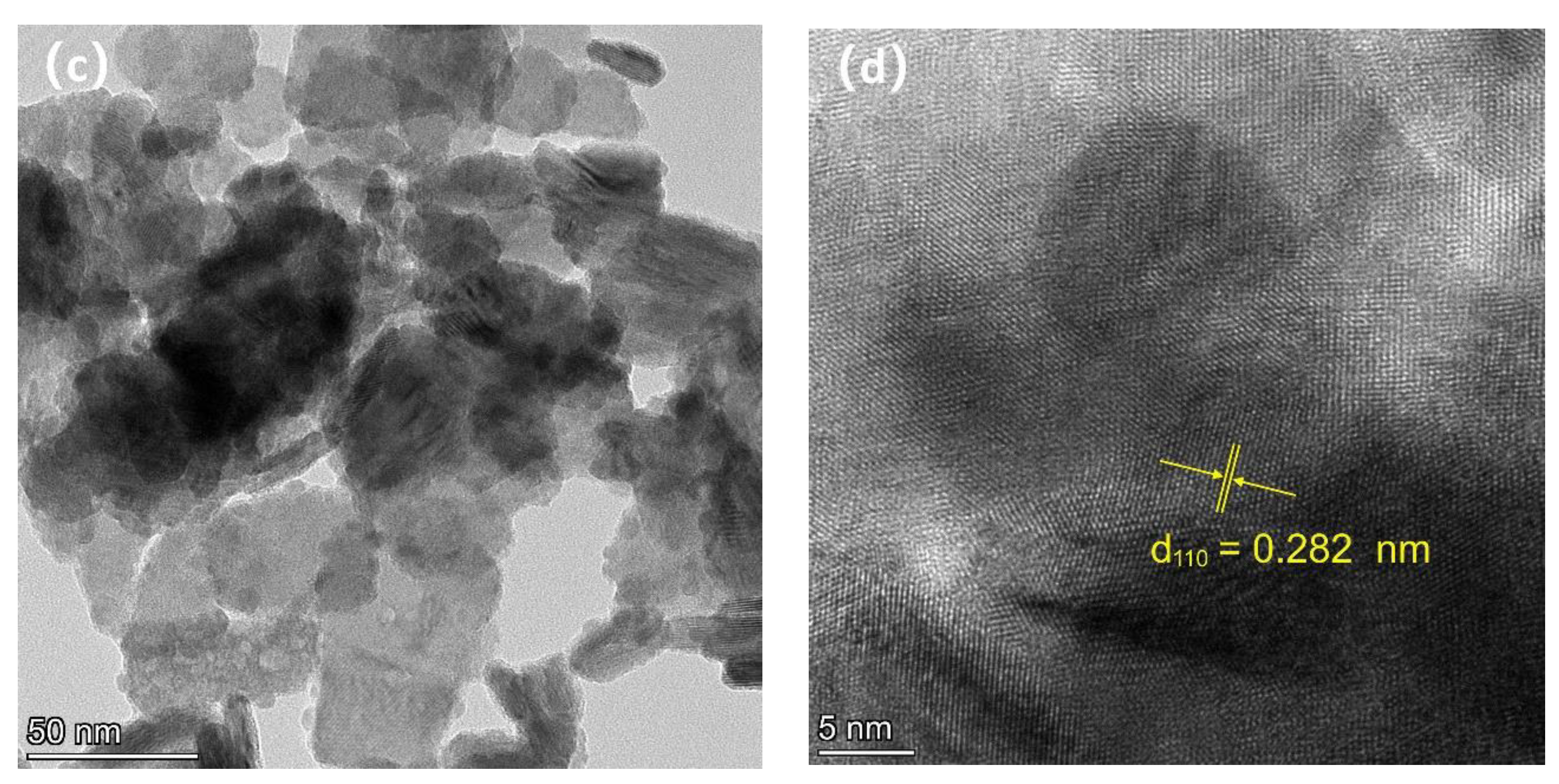
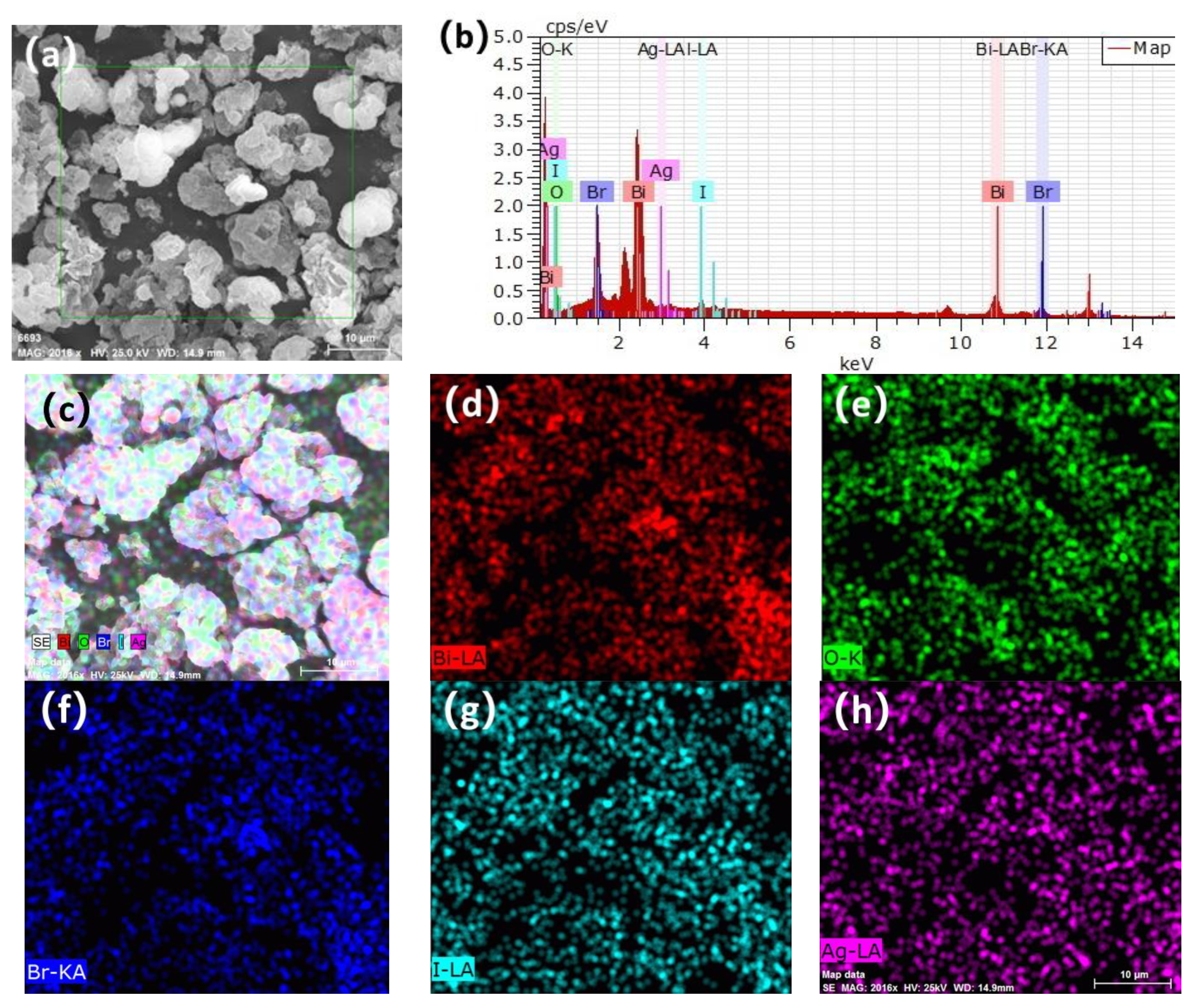
3.2. Scanning Electron Microscopy (SEM), Ttransmission Electron Microscopy (TEM), and Energy-Dispersive X-ray (EDX)
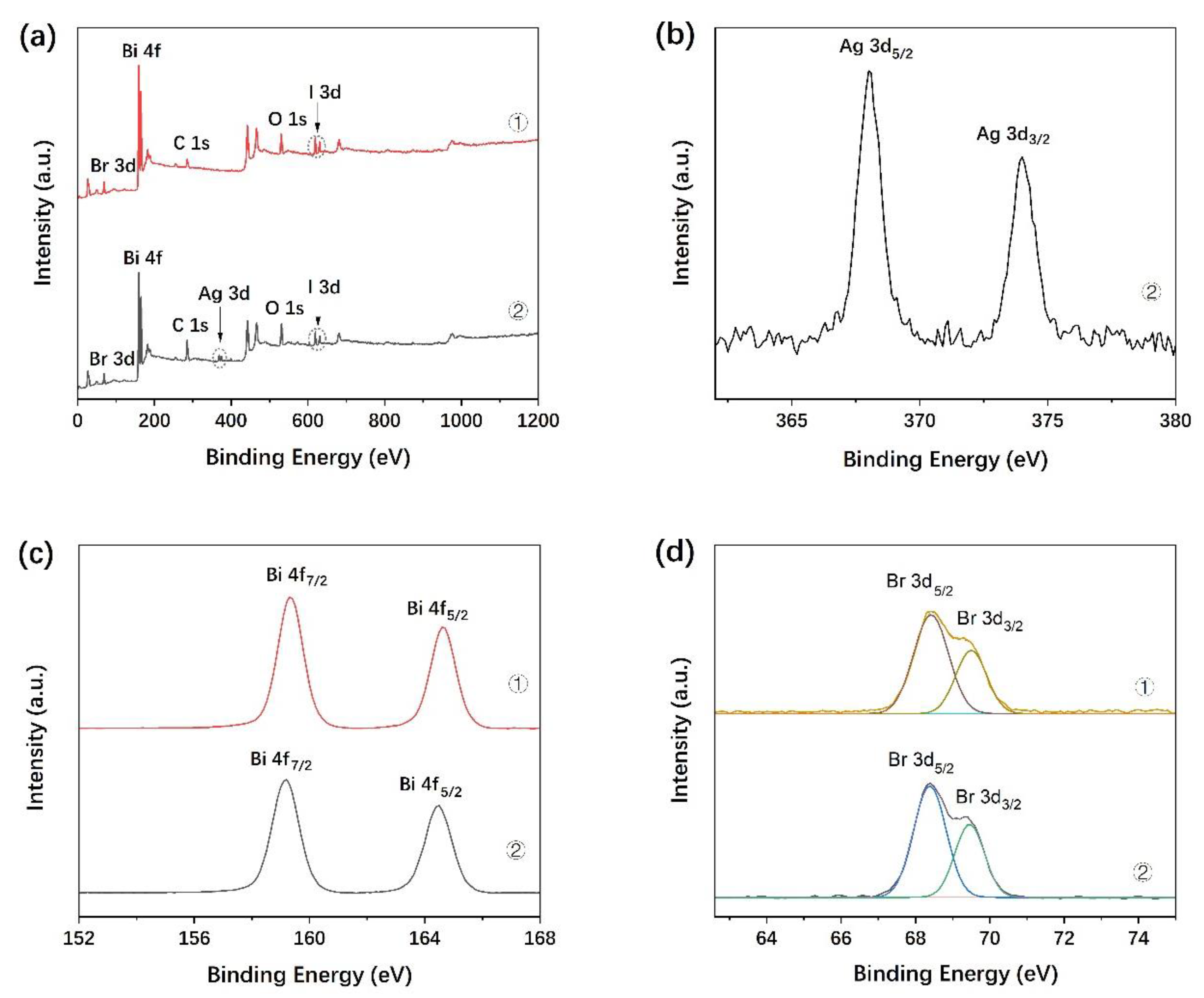
3.3. X-ray Photoelectron Spectroscopy (XPS)
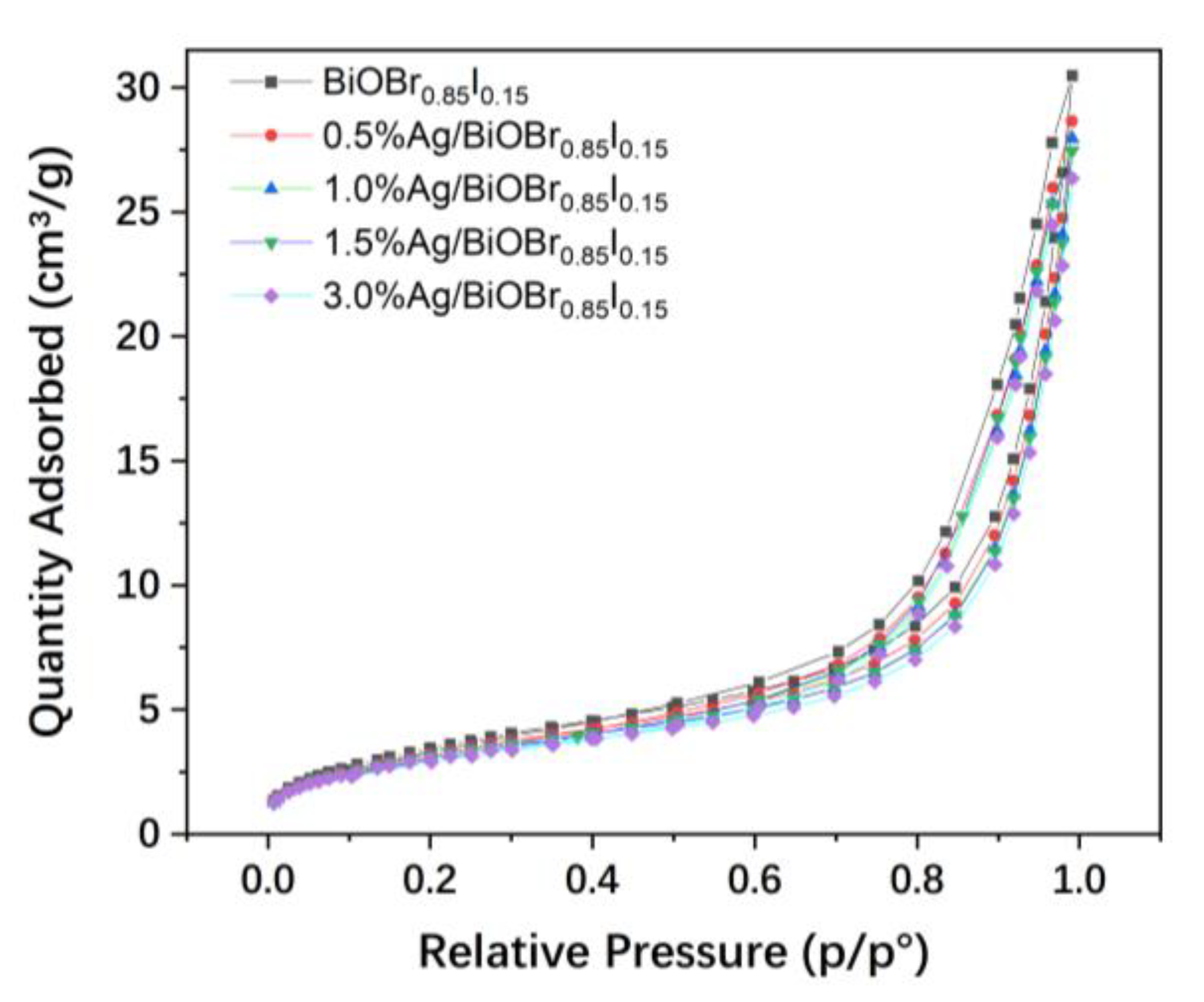
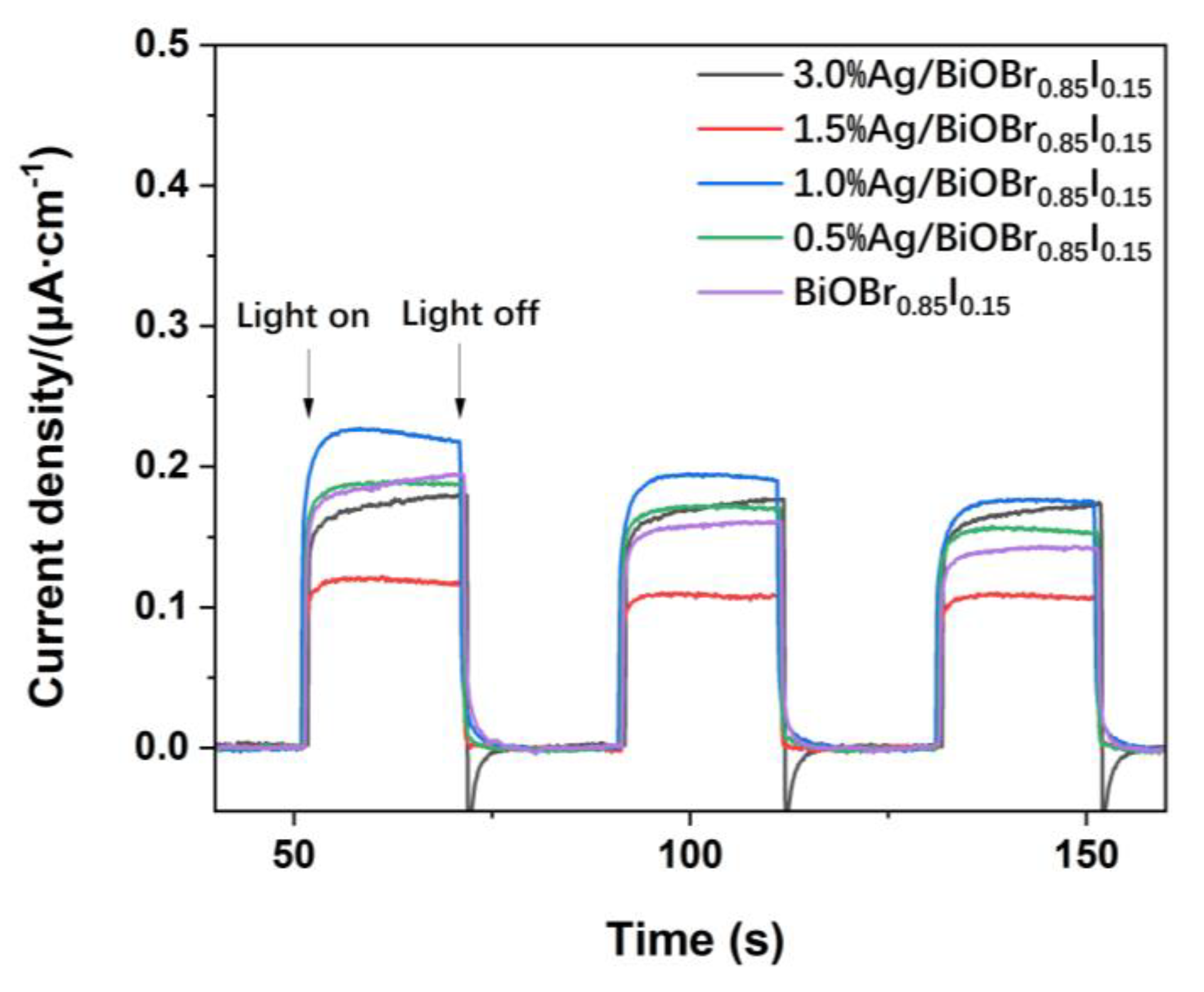
3.4. N2 Adsorption–Desorption Isotherms
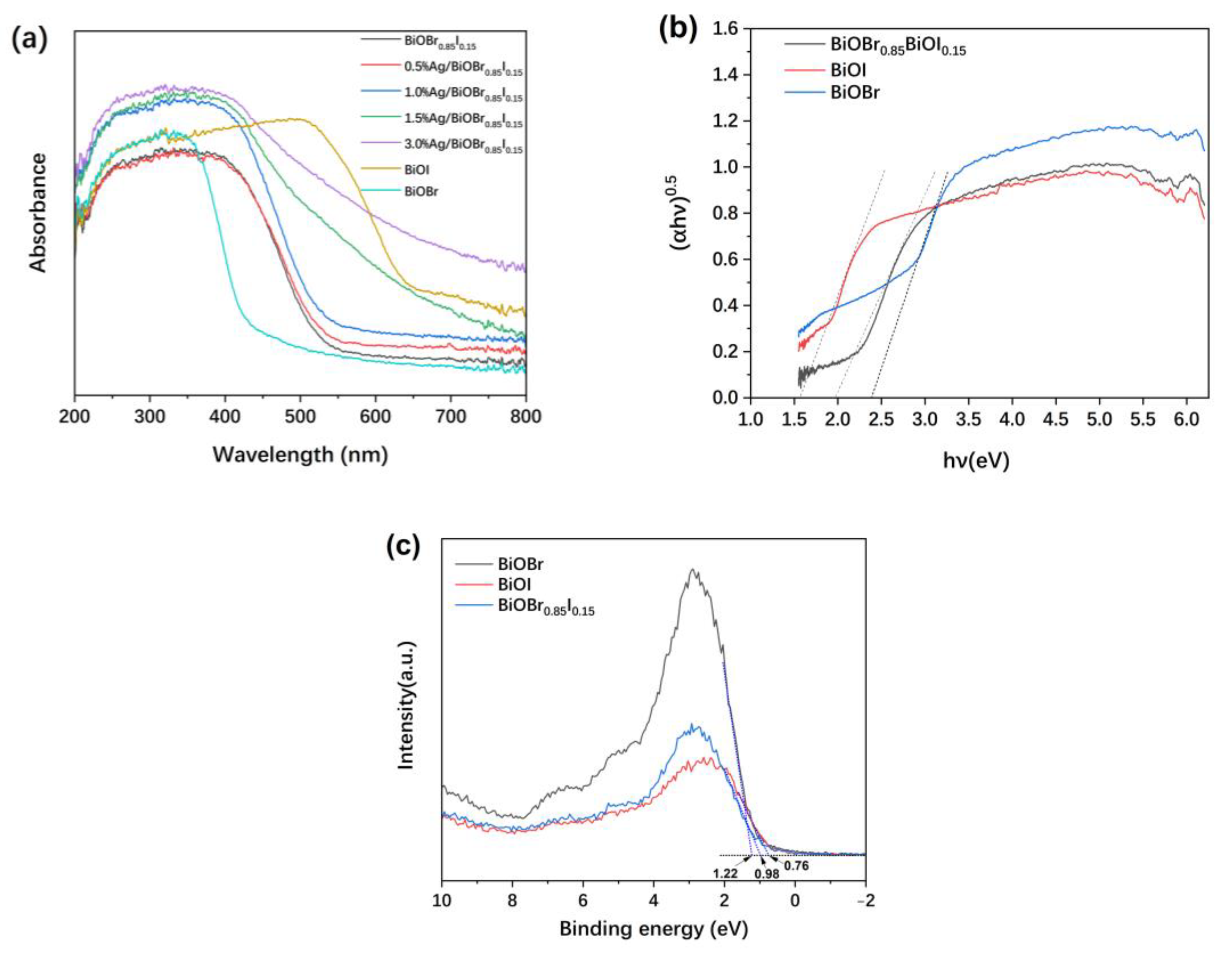
3.5. UV-Visible Diffuse Reflectance Spectra (UV-Vis DRS)
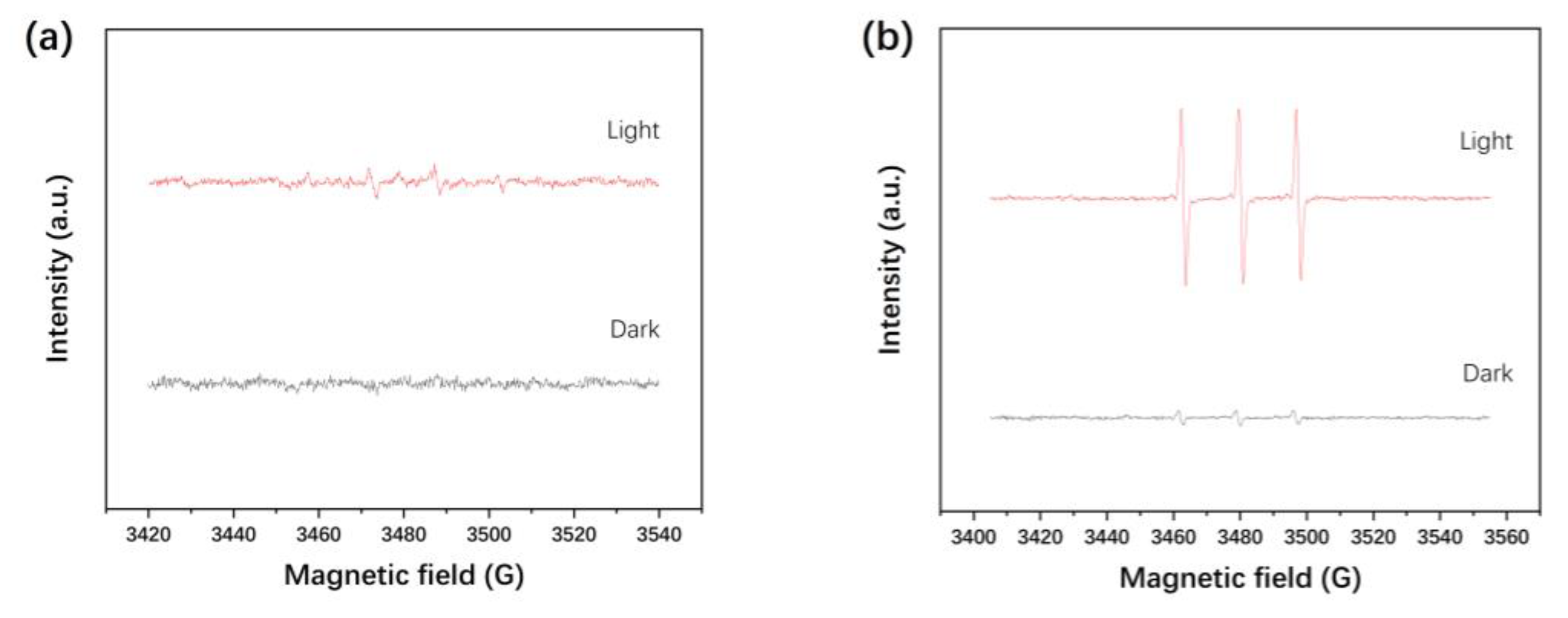
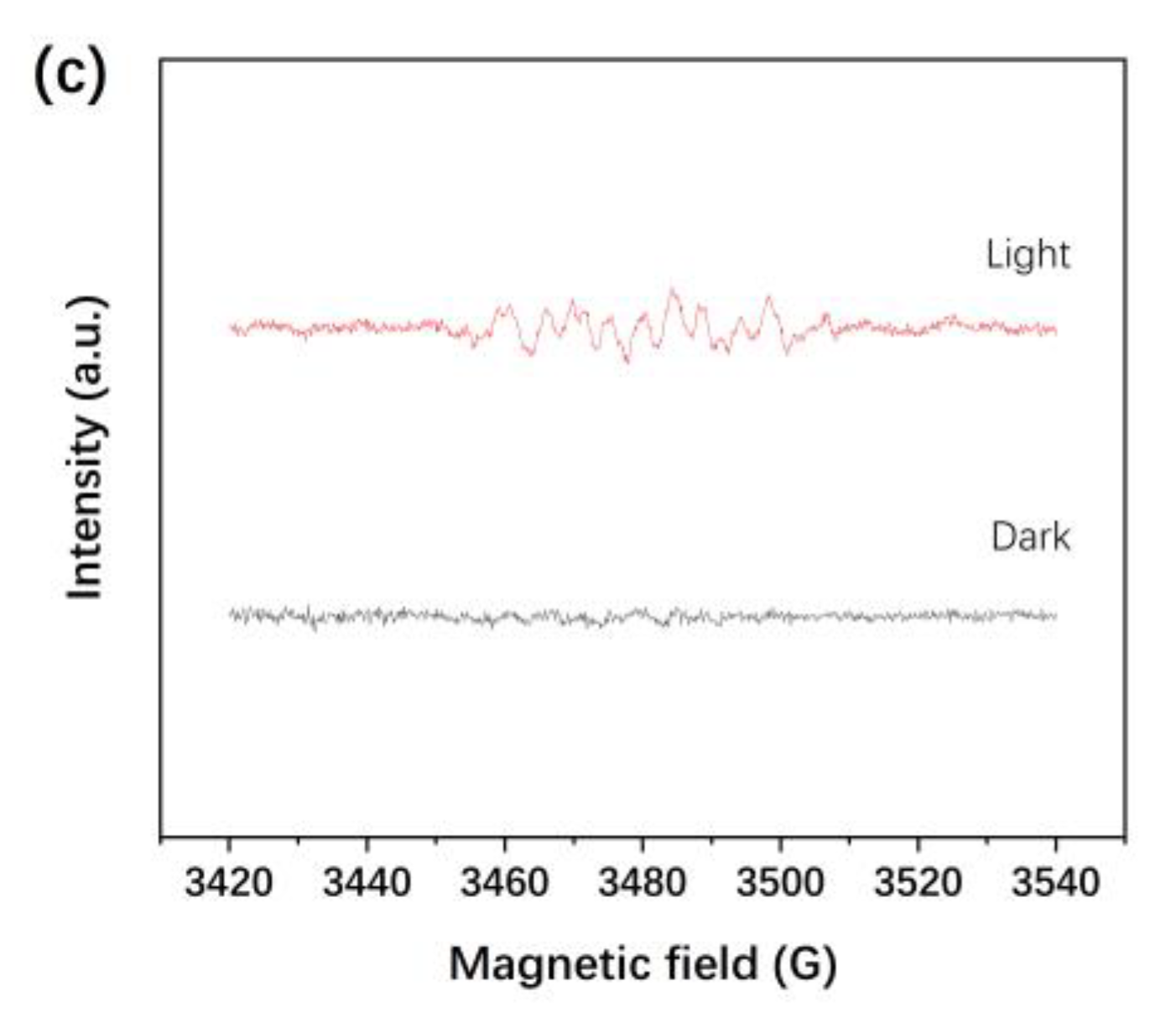
3.6. Electron Spin Resonance (ESR) Spectroscopy
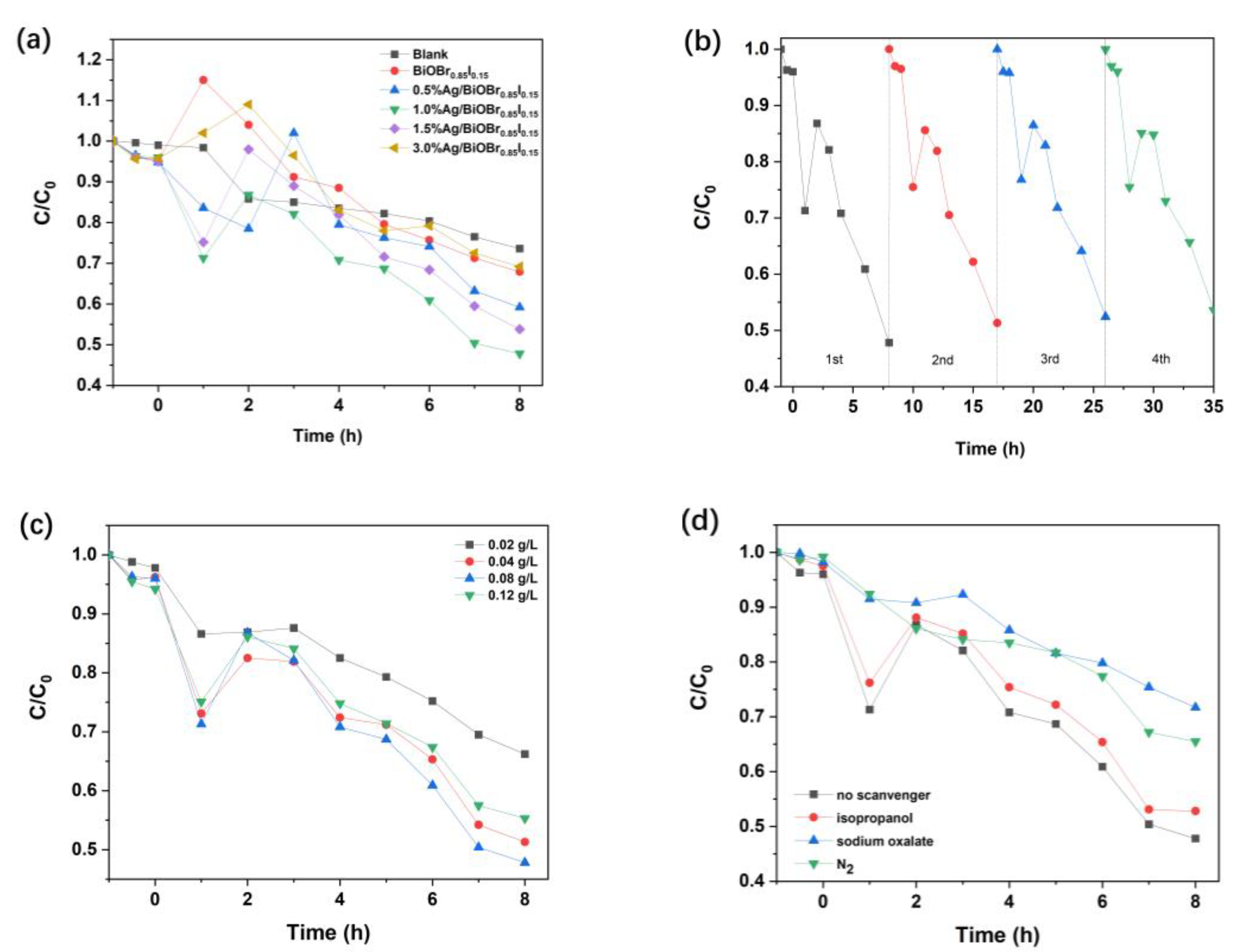
3.7. Photocatalytic Degradation of Ammonia Nitrogen
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Serna-Galvis, E.A.; Maria Botero-Coy, A.; Martinez-Pachon, D.; Moncayo-Lasso, A.; Ibanez, M.; Hernandez, F.; Torres-Palma, R.A. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019, 154, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Maria-Hormigos, R.; Pacheco, M.; Jurado-Sanchez, B.; Escarpa, A. Carbon nanotubes- ferrite- manganese dioxide micromotors for advanced oxidation processes in water treatment. Environ. Sci. Nano 2018, 5, 2993–3003. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sanchez-Perez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination-A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [PubMed]
- dos Santos, A.J.; Tossi de Araujo Costa, E.C.; da Silva, D.R.; Garcia-Segura, S.; Martinez-Huitle, C.A. Electrochemical advanced oxidation processes as decentralized water treatment technologies to remediate domestic washing machine effluents. Environ. Sci. Pollut. Res. 2018, 25, 7002–7011. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, Y.; Heo, Y.-J.; Park, S.-J. Advanced Design and Synthesis of Composite Photocatalysts for the Remediation of Wastewater: A Review. Catalysts 2019, 9, 122. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar]
- Guo, C.; Ge, M.; Liu, L.; Gao, G.; Feng, Y.; Wang, Y. Directed Synthesis of Mesoporous TiO2 Microspheres: Catalysts and Their Photocatalysis for Bisphenol A Degradation. Environ. Sci. Technol. 2010, 44, 419–425. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, X. Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Jing, D.; Guo, L. A novel method for the preparation of a highly stable and active CdS photocatalyst with a special surface nanostructure. J. Phys. Chem. B 2006, 110, 11139–11145. [Google Scholar] [CrossRef] [PubMed]
- Bantawal, H.; Shenoy, U.S.; Bhat, D.K. Tuning the Photocatalytic Activity of SrTiO3 by Varying the Sr/Ti Ratio: Unusual Effect of Viscosity of the Synthesis Medium. J. Phys. Chem. C 2018, 122, 20027–20033. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Luo, W.; Chen, X.; Wei, Z.; Liu, D.; Yao, W.; Zhu, Y. Three-dimensional network structure assembled by g-C3N4 nanorods for improving visible-light photocatalytic performance. Appl. Catal. B Environ. 2019, 255, 117761. [Google Scholar] [CrossRef]
- Kumar, S.; Karthikeyan, S.; Lee, A.F. g-C3N4-Based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 74. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Tungsten-based nanomaterials (WO3 & Bi2WO6): Modifications related to charge carrier transfer mechanisms and photocatalytic applications. Appl. Surf. Sci. 2015, 355, 939–958. [Google Scholar]
- Huang, G.-F.; Ma, Z.-L.; Huang, W.-Q.; Tian, Y.; Jiao, C.; Yang, Z.-M.; Wan, Z.; Pan, A. Ag3PO4 Semiconductor Photocatalyst: Possibilities and Challenges. J. Nanomater. 2013, 2013, 371356. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet Effect of Single-Crystalline Ag3PO4 Sub-microcrystals on Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, Z.; Liu, J. Facile synthesis of Ag3VO4/beta-AgVO3 nanowires with efficient visible-light photocatalytic activity. Rsc. Adv. 2017, 7, 27515–27521. [Google Scholar] [CrossRef]
- Wu, S.-Z.; Li, K.; Zhang, W.-D. On the heterostructured photocatalysts Ag3VO4/g-C3N4 with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2015, 324, 324–331. [Google Scholar] [CrossRef]
- Duy Trinh, N.; Hong, S.-S. Synthesis of Needle-Like BiVO4 with Improved Photocatalytic Activity Under Visible Light Irradiation. J. Nanosci. Nanotechnol. 2019, 19, 7696–7701. [Google Scholar]
- El-Hakam, S.A.; Alshorifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeed, W.S.A.; Ibrahim, A.A.; Ahmed, A.I. Application of nanostructured mesoporous silica/bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, S.; Chen, D. Preparation of Ce3+ doped Bi2O3 hollow needle-shape with enhanced visible-light photocatalytic activity. J. Rare Earths 2019, 37, 726–731. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Tian, G.; Ren, Z.; Tian, C.; Fu, H. Visible-Light-Induced Self-Cleaning Property of Bi2Ti2O7-TiO2 Composite Nanowire Arrays. Langmuir 2015, 31, 5962–5969. [Google Scholar] [CrossRef]
- Gao, H.; Liu, F.; Li, X.; Li, F.; Sui, X. Hydrangeas-Like Bi2WO6: Facile Synthesis, Visible-Light Driven Photocatalysis and Theoretical Analysis. J. Nanosci. Nanotechnol. 2012, 12, 6321–6326. [Google Scholar] [CrossRef]
- Yu, Z.; Bahnemann, D.; Dillert, R.; Lin, S.; Lu, L. Photocatalytic degradation of azo dyes by BiOX (X = Cl, Br). J. Mol. Catal. A Chem. 2012, 365, 1–7. [Google Scholar]
- Dong, F.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Room temperature synthesis and highly enhanced visible light photocatalytic activity of porous BiOI/BiOCl composites nanoplates microflowers. J. Hazard. Mater. 2012, 219, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Boruah, B.; Gupta, R.; Modak, J.M.; Madras, G. Novel insights into the properties of AgBiO3 photocatalyst and its application in immobilized state for 4-nitrophenol degradation and bacteria inactivation. J. Photochem. Photobiol. A Chem. 2019, 373, 105–115. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Xu, X.; Lu, R.; Zhao, X.; Xu, S.; Lei, X.; Zhang, F.; Evans, D.G. Fabrication and photocatalytic performance of a ZnxCd1-xS solid solution prepared by sulfuration of a single layered double hydroxide precursor. Appl. Catal. B Environ. 2011, 102, 147–156. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.-W.; Wang, C.-Y.; Wang, W.-K.; Chen, Y.-L.; Huang, Y.-X.; Li, W.-W.; Feng, Y.-J.; Yu, H.-Q. Synthesis of BiOClxBr1-x Nanoplate Solid Solutions as a Robust Photocatalyst with Tunable Band Structure. Chem. A Eur. J. 2015, 21, 11872–11877. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.; Liu, D.; Liu, G.; Yang, M.; Cui, F.; Wang, W. Ultrathin two-dimensional BiOBrxI1−x solid solution with rich oxygen vacancies for enhanced visible-light-driven photoactivity in environmental remediation. Appl. Catal. B Environ. 2018, 236, 222–232. [Google Scholar] [CrossRef]
- Bera, S.; Won, D.-I.; Rawal, S.B.; Kang, H.J.; Lee, W.I. Design of visible-light photocatalysts by coupling of inorganic semiconductors. Catal. Today 2019, 335, 3–19. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Wang, D.-J.; Zhang, J.; Guo, L.; Shen, H.-D.; Fu, F.; Xue, G.-L.; Fang, Y.-F. Modification Strategies for Semiconductor Photocatalyst Based on Energy Band Structure Theory. J. Inorg. Mater. 2015, 30, 683–693. [Google Scholar]
- Zhang, W.; Jia, B.; Wang, Q.; Dionysiou, D. Visible-light sensitization of TiO2 photocatalysts via wet chemical N-doping for the degradation of dissolved organic compounds in wastewater treatment: A review. J. Nanoparticle Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; Feng, J.; Jie, W. A review of noble metal (Pd, Ag, Pt, Au)-zinc oxide nanocomposites: Synthesis, structures and applications. J. Mater. Sci. -Mater. Electron. 2017, 28, 16585–16597. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Salama, R.S. Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 2022, 8, e09652. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.F.; Fu, W.Z.; Ge, X.H.; Wang, L.S.; Wang, J.H.; Zhong, W.Z. Preparation and characterization of a copper phosphotungstate/titanium dioxide (Cu-H3PW12O40/TiO2) composite and the photocatalytic oxidation of high-concentration ammonia nitrogen. Sci. Total Environ. 2020, 727, 138425. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Gao, Y.; Zhu, H.; Yang, G.; Liu, W.; Huo, Y.; Xie, Z.; Li, H. Coupling system of Ag/BiOBr photocatalysis and direct contact membrane distillation for complete purification of N-containing dye wastewater. Chem. Eng. J. 2017, 317, 386–393. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.Y.; Wang, L.W.; Huang, G.X.; Wang, W.K.; Yu, H.Q. Fabrication of BiOBrxI1−x photocatalysts with tunable visible light catalytic activity by modulating band structures. Sci. Rep. 2016, 6, 22800. [Google Scholar] [CrossRef]
- Park, M.-S.; Kang, M. The preparation of the anatase and rutile forms of Ag–TiO2 and hydrogen production from methanol/water decomposition. Mater. Lett. 2008, 62, 183–187. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Yin, S.; Li, H.; Xu, H.; Zhang, Q.; Li, H. Controllable synthesis of Bi4O5Br2 ultrathin nanosheets for photocatalytic removal of ciprofloxacin and mechanism insight. J. Mater. Chem. A 2015, 3, 15108–15118. [Google Scholar] [CrossRef]
- Ji, L.; Wang, H.; Yu, R. Preparation, Characterization and Visible-light Photocatalytic Activities of p-n Heterojunction BiOBr/NaBiO3 Composites. Chem. J. Chin. Univ. Chin. 2014, 35, 2170–2176. [Google Scholar]
- Wang, Q.; Wang, W.; Zhong, L.; Liu, D.; Cao, X.; Cui, F. Oxygen vacancy-rich 2D/2D BiOCl-g-C3N4 ultrathin heterostructure nanosheets for enhanced visible-light-driven photocatalytic activity in environmental remediation. Appl. Catal. B: Environ. 2018, 220, 290–302. [Google Scholar] [CrossRef]
- Fan, J.; Qin, H.; Jiang, S. Mn-doped g-C3N4 composite to activate peroxymonosulfate for acetaminophen degradation: The role of superoxide anion and singlet oxygen. Chem. Eng. J. 2019, 359, 723–732. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, Y.; Li, C.; Fan, W.; Yan, X.; Yan, M.; Shi, W. In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B Environ. 2016, 180, 663–673. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Chen, S.; Li, D.; Zhang, X.; Shao, W.; Sun, X.; Xie, J.; Zhao, Z.; Zhang, Q.; et al. Enhanced Singlet Oxygen Generation in Oxidized Graphitic Carbon Nitride for Organic Synthesis. Adv. Mater. 2016, 28, 6940–6945. [Google Scholar] [CrossRef] [PubMed]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.P.; Ortega-Méndez, J.A.; Doña-Rodríguez, J.M. Effect of TiO2–Pd and TiO2–Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, Y.Y.; Miao, S.; Liu, T.F.; Mu, L.C.; Li, R.G.; Fan, F.T.; Li, C. Positioning the Water Oxidation Reaction Sites in Plasmonic Photocatalysts. J. Am. Chem. Soc. 2017, 139, 11771–11778. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Kataria, J.; Bhardwaj, V.K.; Sharma, S. Green biomimetic preparation of efficient Ag-ZnO heterojunctions with excellent photocatalytic performance under solar light irradiation: A novel biogenic-deposition-precipitation approach. Nanoscale Adv. 2019, 1, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Shen, Z.; Wu, Y.; Wang, J.; Yang, L. Photocatalytic degradation of organics and germicidal treatment of shrimp breeding wastewater. Acta Sci. Nat. Univ. Sunyatseni 2009, 48, 60–64. [Google Scholar]
- Lu, K.; Zhang, X.; Wang, Y.; Wang, Y.; Fan, C. Fabrication, Characterization and Photocatalytic Performance of Au/BiOBr Nanosheets. J. Synth. Cryst. 2015, 44, 2095–2100. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Zhao, M.; Zheng, W.; Zhu, J. Preparation, Characterization, and Photocatalytic Performance of Ag/BiOBr0.85I0.15 Nanocomposites. Materials 2022, 15, 6022. https://doi.org/10.3390/ma15176022
Hu X, Zhao M, Zheng W, Zhu J. Preparation, Characterization, and Photocatalytic Performance of Ag/BiOBr0.85I0.15 Nanocomposites. Materials. 2022; 15(17):6022. https://doi.org/10.3390/ma15176022
Chicago/Turabian StyleHu, Xiaobin, Mingxing Zhao, Weihong Zheng, and Junjie Zhu. 2022. "Preparation, Characterization, and Photocatalytic Performance of Ag/BiOBr0.85I0.15 Nanocomposites" Materials 15, no. 17: 6022. https://doi.org/10.3390/ma15176022



