Consideration of Photoactivity of TiO2 Pigments via the Photodegration of Methyl Orange under UV Irradiation
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. TiO2 Pigment Characterization
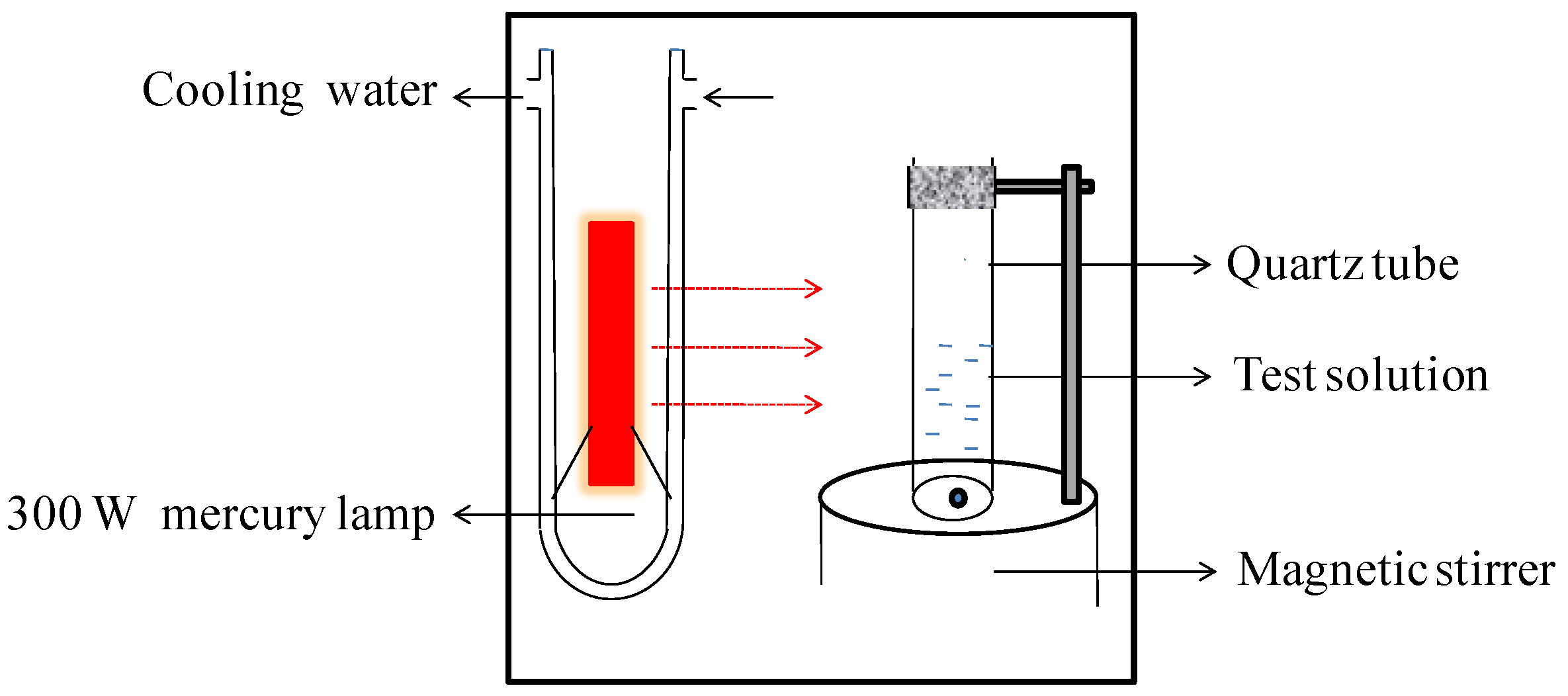
2.3. Photoactivity Tests
2.4. Theoretical Calculations
3. Results and Discussions
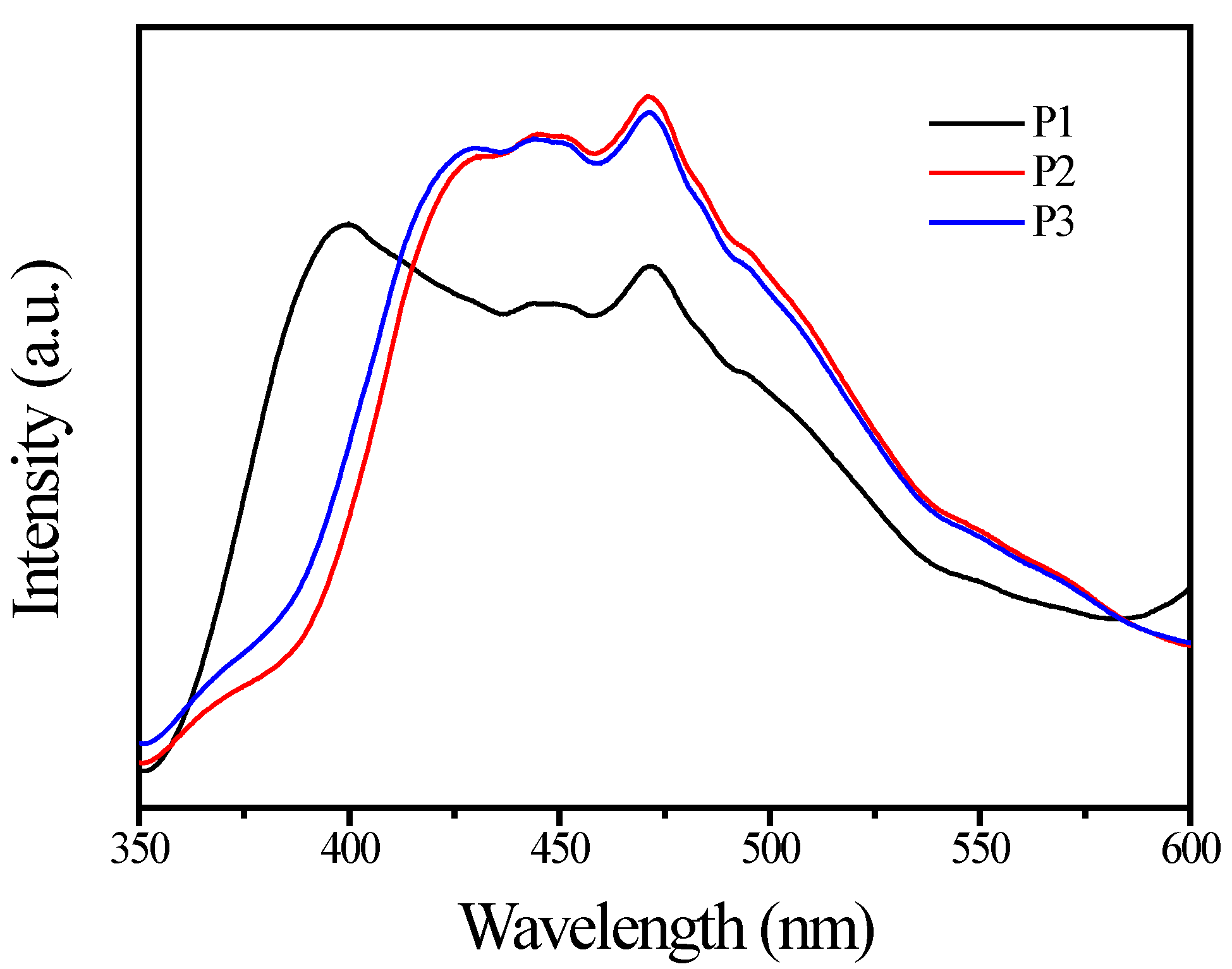
3.1. TiO2 Pigment Characterization
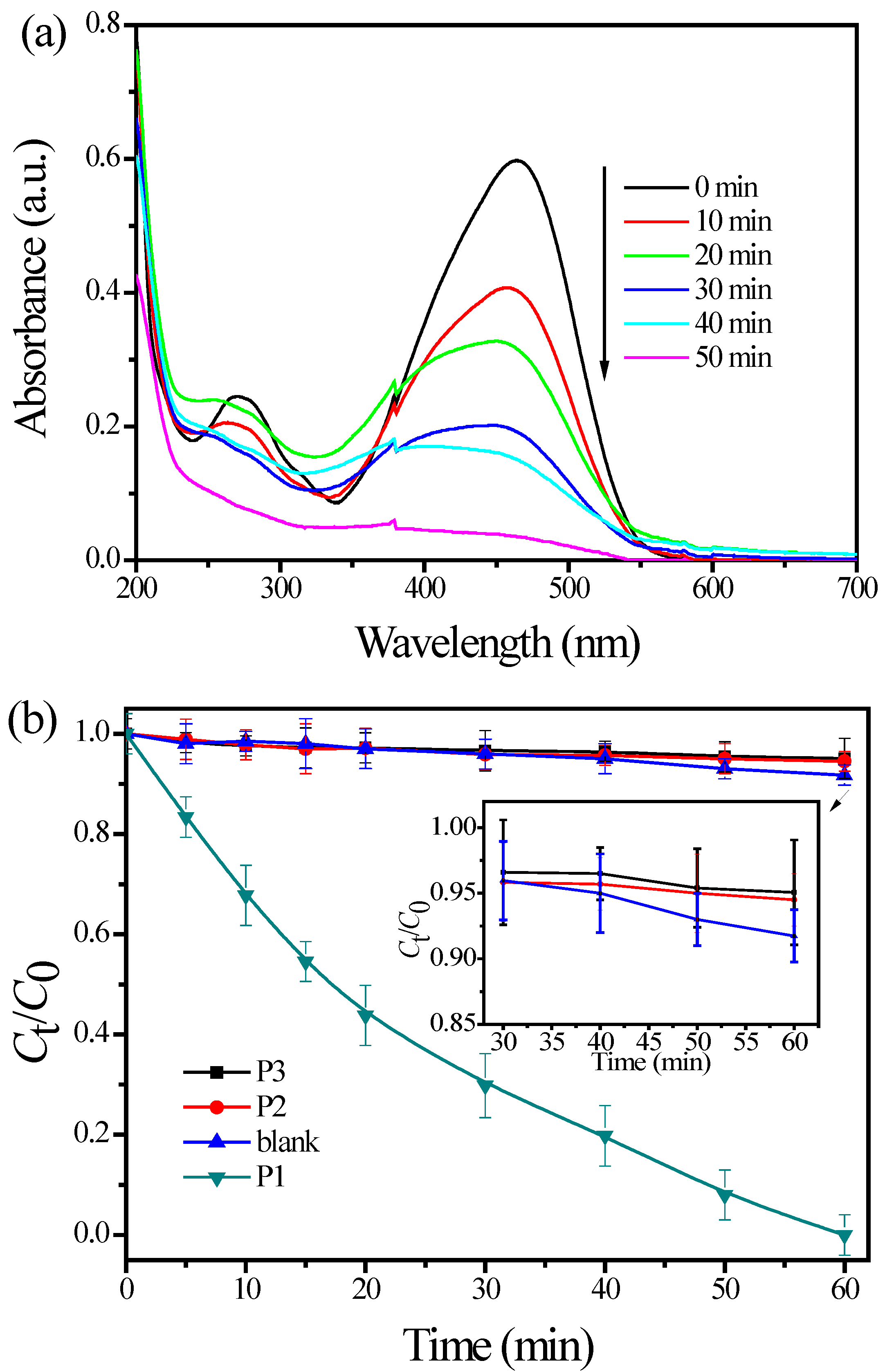
3.2. Photoactivity Tests
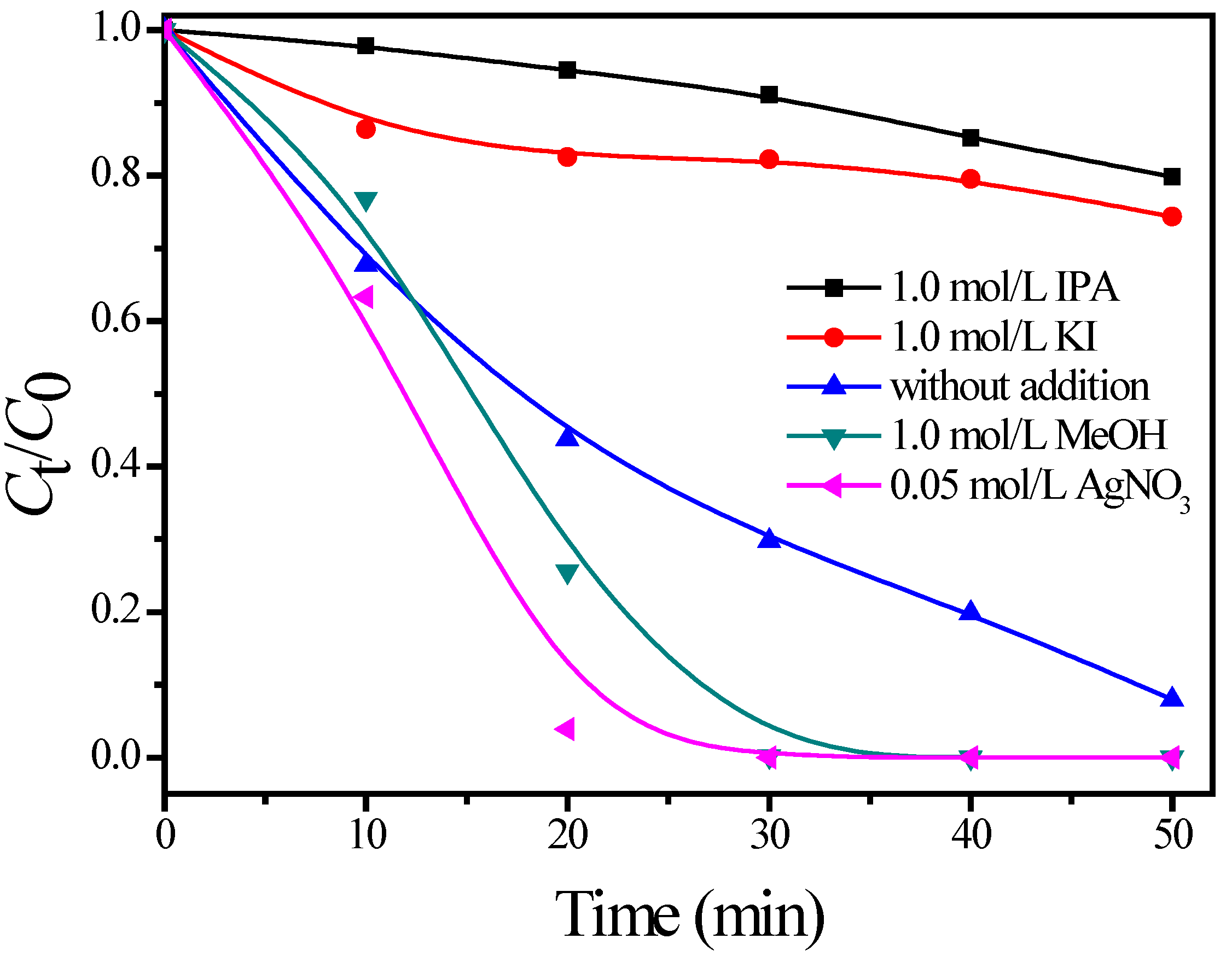
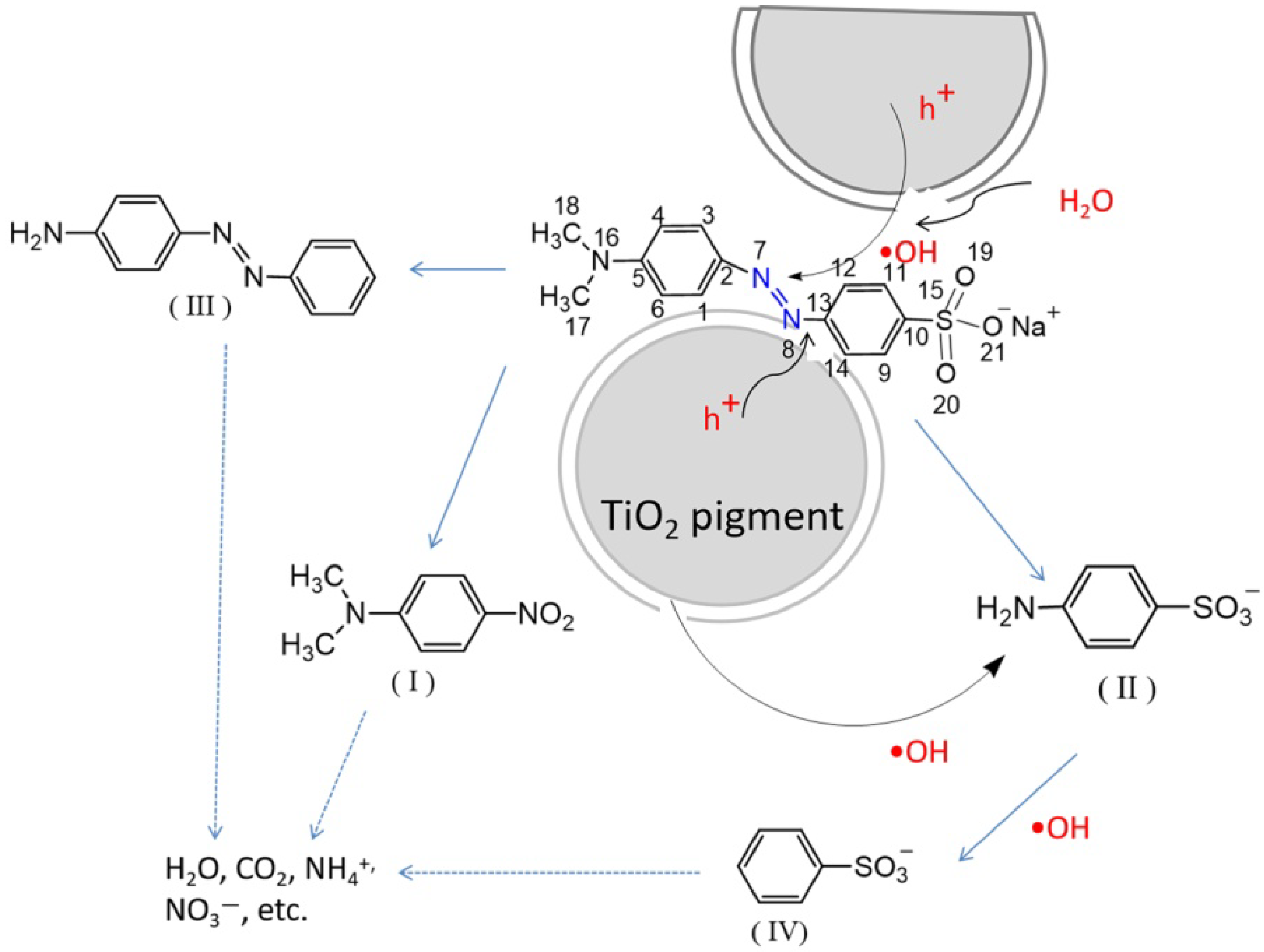
3.3. Photodegradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- George, J.; Gopalakrishnan, C.C.; Manikuttan, P.K.; Mukesh, K.; Sreenish, S. Preparation of multi-purpose TiO2 pigment with improved properties for coating applications. Powder Technol. 2021, 377, 269–273. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Chen, M.; Gu, M.; Wu, L. Scalable and waterborne titanium-dioxide-free thermochromic coatings for self-adaptive passive radiative cooling and heating. Cell Rep. Phys. Sci. 2022, 3, 100782. [Google Scholar] [CrossRef]
- Loosli, F.; Wang, J.; Rothenberg, S.; Bizimis, M.; Winkler, C.; Borovinskaya, O.; Flamigni, L.; Baalousha, M. Sewage spills are a major source of titanium dioxide engineered (nano)-particle release into the environment. Environ. Sci. Nano 2019, 6, 763–777. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Moreno, S.M.P.; Bolívar, J.P. Chapter 9: TiO2 as white pigment and valorization of the waste coming from its production. In Titanium Dioxide (TiO2) and Its Applications, 1st ed.; Parrino, F., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 311–335. [Google Scholar]
- Pfeifer, V.; Erhart, P.; Li, S.; Rachut, K.; Morasch, J.; Brötz, J.; Reckers, P.; Mayer, T.; Rühle, S.; Zaban, A.; et al. Energy band alignment between anatase and rutile TiO2. J. Phys. Chem. Lett. 2013, 4, 4182–4187. [Google Scholar] [CrossRef]
- Mahmood, P.H.; Amiri, O.; Ahmed, S.S.; Hama, J.R. Simple microwave synthesis of TiO2/NiS2 nanocomposite and TiO2/NiS2/cu nanocomposite as an efficient visible driven photocatalyst. Ceram. Int. 2019, 45, 14167–14172. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; Martínez, M.R.; Martín, I.S. Effect of reduced graphene oxide load into TiO2 P25 on the generation of reactive oxygen species in a solar photocatalytic reactor. Application to antipyrine degradation. Chem. Eng. J. 2020, 380, 122410. [Google Scholar] [CrossRef]
- Tao, C.; Jia, Q.; Han, B.; Ma, Z. Tunable selectivity of radical generation over TiO2 for photocatalysis. Chem. Eng. Sci. 2020, 214, 115438. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar]
- Yu, L.; Xi, J.; Li, M.-D.; Chan, H.T.; Su, T.; Phillips, D.L.; Chan, W.K. The degradation mechanism of methyl orange under photo-catalysis of TiO2. Phys. Chem. Chem. Phys. 2012, 14, 3589–3595. [Google Scholar] [CrossRef]
- Man, C.; Zhang, C.; Liu, Y.; Wang, W.; Ren, W.; Jiang, L.; Reisdorffer, F.; Nguyen, T.P.; Dan, Y. Poly (lactic acid)/titanium dioxide composites: Preparation and performance under ultraviolet irradiation. Polym. Degrad. Stab. 2012, 97, 856–862. [Google Scholar] [CrossRef]
- Morsch, S.; van Driel, B.A.; van den Berg, K.J.; Dik, J. Investigating the photocatalytic degradation of oil paint using ATR-IR and AFM-IR. ACS Appl. Mat. Interfaces 2017, 9, 10169–10179. [Google Scholar] [CrossRef]
- van Driel, B.A.; van den Berg, K.J.; Smout, M.; Dekker, N.; Kooyman, P.J.; Dik, J. Investigating the effect of artists’ paint formulation on degradation rates of TiO2-based oil paints. Herit. Sci. 2018, 6, 21. [Google Scholar] [CrossRef]
- Veronovski, N.; Lešnik, M.; Verhovšek, D. Surface treatment optimization of pigmentary TiO2 from an industrial aspect. J. Coat. Technol. Res. 2014, 11, 255–264. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, S.; Yu, Y.; van Ommen, J.R.; Van Bui, H.; Liang, B. Room-temperature pulsed cvd-grown SiO2 protective layer on TiO2 particles for photocatalytic activity suppression. RSC Adv. 2017, 7, 4547–4554. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Jiang, L.; Li, C.; Zheng, S. Investigation on the film-coating mechanism of alumina-coated rutile TiO2 and its dispersion stability. Adv. Powder Technol. 2017, 28, 1982–1988. [Google Scholar] [CrossRef]
- Guo, J.; Bui, H.V.; Gonzalez, D.V.; Yuan, S.; Liang, B.; van Ommen, J.R. Suppressing the photocatalytic activity of TiO2 nanoparticles by extremely thin Al2O3 films grown by gas-phase deposition at ambient conditions. Nanomaterials 2018, 8, 61. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Wang, A.; Liu, C.; Yu, L.; Jiang, T.; Hang, Y. Evolution of zirconia coating layer on rutile TiO2 surface and the pigmentary property. J. Phys. Chem. Solids 2010, 71, 1458–1466. [Google Scholar] [CrossRef]
- Gao, H.; Qiao, B.; Wang, T.-J.; Wang, D.; Jin, Y. Cerium oxide coating of titanium dioxide pigment to decrease its photocatalytic activity. Ind. Eng. Chem. Res. 2014, 53, 189–197. [Google Scholar] [CrossRef]
- Wang, D.L.; Watson, S.S.; Sung, L.-P.; Tseng, I.H.; Bouis, C.J.; Fernando, R. Effect of TiO2 pigment type on the uv degradation of filled coatings. J. Coat. Technol. Res. 2011, 8, 19–33. [Google Scholar] [CrossRef]
- van Driel, B.A.; Wezendonk, T.A.; van den Berg, K.J.; Kooyman, P.J.; Gascon, J.; Dik, J. Determination of early warning signs for photocatalytic degradation of titanium white oil paints by means of surface analysis. Spectrochim. Acta Part A 2017, 172, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Mirabedini, S.M.; Sabzi, M.; Zohuriaan-Mehr, J.; Atai, M.; Behzadnasab, M. Weathering performance of the polyurethane nanocomposite coatings containing silane treated TiO2 nanoparticles. Appl. Surf. Sci. 2011, 257, 4196–4203. [Google Scholar] [CrossRef]
- de Sá, M.H.; Eaton, P.; Ferreira, J.L.; Melo, M.J.; Ramos, A.M. Ageing of vinyl emulsion paints—An atomic force microscopy study. Surf. Interface Anal. 2011, 43, 1160–1164. [Google Scholar] [CrossRef]
- Farmakalidis, H.V.; Boyatzis, S.; Douvas, A.M.; Karatasios, I.; Sotiropoulou, S.; Argitis, P.; Chryssoulakis, Y.; Kilikoglou, V. The effect of TiO2 component on the properties of acrylic and urea-aldehyde resins under accelerated ageing conditions. Pure Appl. Chem. 2017, 89, 1659–1671. [Google Scholar] [CrossRef]
- Jin, C.; Christensen, P.A.; Egerton, T.A.; Lawson, E.J.; White, J.R. Rapid measurement of polymer photo-degradation by FTIR spectrometry of evolved carbon dioxide. Polym. Degrad. Stab. 2006, 91, 1086–1096. [Google Scholar] [CrossRef]
- van Driel, B.; Artesani, A.; van den Berg, K.J.; Dik, J.; Mosca, S.; Rossenaar, B.; Hoekstra, J.; Davies, A.; Nevin, A.; Valentini, G.; et al. New insights into the complex photoluminescence behaviour of titanium white pigments. Dyes Pigment. 2018, 155, 14–22. [Google Scholar] [CrossRef]
- Watson, S.; Tseng, I.H.; Marray, T.; Pellegrin, B.; Comte, J. Pigment and nanofiller photoreactivity database. J. Coat. Technol. Res. 2012, 9, 443–451. [Google Scholar] [CrossRef]
- Guan, R.; He, Z.; Liu, S.; Han, Y.; Wang, Q.; Cui, W.; He, T. A novel photoelectrochemical approach for efficient assessment of TiO2 pigments weatherability. Powder Technol. 2021, 380, 334–340. [Google Scholar] [CrossRef]
- Pazokifard, S.; Mirabedini, S.M.; Esfandeh, M.; Mohseni, M.; Ranjbar, Z. Silane grafting of TiO2 nanoparticles: Dispersibility and photoactivity in aqueous solutions. Surf. Interface Anal. 2012, 44, 41–47. [Google Scholar] [CrossRef]
- van Driel, B.A.; Kooyman, P.J.; van den Berg, K.J.; Schmidt-Ott, A.; Dik, J. A quick assessment of the photocatalytic activity of TiO2 pigments—From lab to conservation studio! Microchem. J. 2016, 126, 162–171. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Zukowska, G.Z.; Korczagin, I.; Malanowski, P. Effect of TiO2 on uv stability of polymeric binder films used in waterborne facade paints. Prog. Org. Coat. 2015, 85, 123–130. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.M. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Luo, Q.; Bao, L.; Wang, D.; Li, X.; An, J. Preparation and strongly enhanced visible light photocatalytic activity of TiO2 nanoparticles modified by conjugated derivatives of polyisoprene. J. Phys. Chem. C 2012, 116, 25806–25815. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Maiti, S.; Malik, M.A.; Xu, S. Modification of geopolymer with size controlled TiO2 nanoparticle for enhanced durability and catalytic dye degradation under uv light. J. Clean. Prod. 2020, 255, 120183. [Google Scholar] [CrossRef]
- Godnjavec, J.; Zabret, J.; Znoj, B.; Skale, S.; Veronovski, N.; Venturini, P. Investigation of surface modification of rutile TiO2 nanoparticles with SiO2/Al2O3 on the properties of polyacrylic composite coating. Prog. Org. Coat. 2014, 77, 47–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Wang, A.; Ren, M.; Gu, Z.; Liu, Y.; Shen, Y.; Yu, L.; Jiang, T. Deposition and characterization of binary Al2O3/SiO2 coating layers on the surfaces of rutile TiO2 and the pigmentary properties. Appl. Surf. Sci. 2010, 257, 1351–1360. [Google Scholar] [CrossRef]
- Shablonin, E.; Popov, A.I.; Prieditis, G.; Vasil’chenko, E.; Lushchik, A. Thermal annealing and transformation of dimer F centers in neutron-irradiated Al2O3 single crystals. J. Nucl. Mater. 2021, 543, 152600. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y.; Luo, B. Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions. Mater. Sci. Semicond. Process. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Martin, P.; Jimenez-Rey, D.; Vila, R.; Sánchez, F.; Saavedra, R. Optical absorption defects created in SiO2 by Si, o and He ion irradiation. Fusion Eng. Des. 2014, 89, 1679–1683. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X.; Yu, X.; Jiang, Z.; Liu, X.; Li, C.; Sun, Z.; Zheng, S.; Dionysiou, D.D. A novel rutile TiO2/AlPO4 core-shell pigment with substantially suppressed photoactivity and enhanced dispersion stability. Powder Technol. 2020, 366, 537–545. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, Q.; Xiao, J.; Zhong, W.; Yu, N.; Kirk, S.R.; Shu, T.; Yin, D. Consideration of roles of commercial TiO2 pigments in aromatic polyurethane coating via the photodegradation of dimethyl toluene-2,4-dicarbamate in non-aqueous solution. Res. Chem. Intermed. 2015, 41, 7785–7797. [Google Scholar] [CrossRef]
- Shen, J.-H.; Chuang, H.-Y.; Jiang, Z.-W.; Liu, X.-Z.; Horng, J.-J. Novel quantification of formation trend and reaction efficiency of hydroxyl radicals for investigating photocatalytic mechanism of Fe-doped TiO2 during UV and visible light-induced degradation of acid orange 7. Chemosphere 2020, 251, 126380. [Google Scholar] [CrossRef]
- Devi, L.G.; Kumar, S.G.; Reddy, K.M.; Munikrishnappa, C. Photo degradation of methyl orange an azo dye by advanced fenton process using zero valent metallic iron: Influence of various reaction parameters and its degradation mechanism. J. Hazard. Mater. 2009, 164, 459–467. [Google Scholar] [CrossRef]
- Suárez-Escobar, A.; Rodríguez-González, V.; Gallardo-Vega, C.; Sarria, E.; Clavijo, L. Ketoprofen degradation by surface reactive species on TiO2, AgxOy/MoxOy/catalysts. Mater. Lett. 2021, 294, 129687. [Google Scholar] [CrossRef]
- Guivarch, E.; Trevin, S.; Lahitte, C.; Oturan, M.A. Degradation of azo dyes in water by electro-fenton process. Environ. Chem. Lett. 2003, 1, 38–44. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A. Mechanistic of photocatalytic decolorization and mineralization of methyl orange dye by immobilized TiO2/chitosan-montmorillonite. J. Water Process Eng. 2019, 31, 100843. [Google Scholar] [CrossRef]
- Karkmaz, M.; Puzenat, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of the alimentary azo dye amaranth: Mineralization of the azo group to nitrogen. Appl. Catal. B Environ. 2004, 51, 183–194. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Lin, Y.; Wang, P.; Chen, W.; Fu, X.; Shao, Y. Evidence for the active species involved in the photodegradation process of methyl orange on TiO2. J. Phys. Chem. C 2012, 116, 3552–3560. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Sandoval, G.; Verran, J.; Catalina, F.; Bygott, C.; Kerrod, J. Research perspectives on the photocatalytic activity of titanium dioxide: Catalytic assessment methods in solution and solid-state in relation to particle surface activity. Polym. Degrad. Stab. 2021, 190, 109624. [Google Scholar] [CrossRef]



| TiO2 Pigment Sample | Composition | Surface Treatment | Applications |
|---|---|---|---|
| P1 | TiO2 (anatase, >98%) | SiO2/Al2O3 | Paints, fiber, rubber, metallurgy, etc. |
| P2 | TiO2 (rutile, >98%) | None | - |
| P3 | TiO2 (rutile, >92%) | SiO2/Al2O3 | Coatings, rubber, plastic, etc. |
| Atom Label | Point Charges | Bond | Bond Length (Å) |
|---|---|---|---|
| C1 | 0.239 | C1–C2 | 1.4419 |
| C2 | −0.886 | C2–C3 | 1.4369 |
| C3 | 0.070 | C3–C4 | 1.3639 |
| C4 | −0.070 | C4–C5 | 1.4419 |
| C5 | 0.402 | C5–C6 | 1.4471 |
| C6 | −0.138 | C6–C1 | 1.3618 |
| N7 | 0.055 | N7–C2 | 1.3356 |
| N8 | −0.088 | N7–N8 | 1.3267 |
| C9 | −0.214 | C9–C10 | 1.4593 |
| C10 | −0.637 | C10–C11 | 1.4613 |
| C11 | 0.066 | O11–C12 | 1.3543 |
| C12 | 0.550 | O12–C13 | 1.4505 |
| C13 | −0.776 | C13–C14 | 1.4455 |
| C14 | 0.060 | C14–C9 | 1.3552 |
| S15 | 1.341 | S15–C10 | 1.8431 |
| N16 | −0.079 | N16–C5 | 1.3401 |
| C17 | −0.285 | N16–C17 | 1.4735 |
| C18 | −0.276 | N16–C18 | 1.4737 |
| O19 | −0.467 | S15–O19 | 1.4557 |
| O20 | −0.465 | S15–O20 | 1.4558 |
| O21 | −0.320 | S15–O21 | 1.6345 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Bai, J.; Huang, K.; Ye, X.; Peng, Y.; Lei, M. Consideration of Photoactivity of TiO2 Pigments via the Photodegration of Methyl Orange under UV Irradiation. Materials 2022, 15, 6044. https://doi.org/10.3390/ma15176044
Zhou S, Bai J, Huang K, Ye X, Peng Y, Lei M. Consideration of Photoactivity of TiO2 Pigments via the Photodegration of Methyl Orange under UV Irradiation. Materials. 2022; 15(17):6044. https://doi.org/10.3390/ma15176044
Chicago/Turabian StyleZhou, Shuolin, Junzhuo Bai, Keying Huang, Xinlu Ye, Yingqing Peng, and Min Lei. 2022. "Consideration of Photoactivity of TiO2 Pigments via the Photodegration of Methyl Orange under UV Irradiation" Materials 15, no. 17: 6044. https://doi.org/10.3390/ma15176044






