Liquid-Phase-Assisted Catalytic Nitridation of Silicon and In Situ Growth of α-Si3N4
Abstract
:1. Introduction
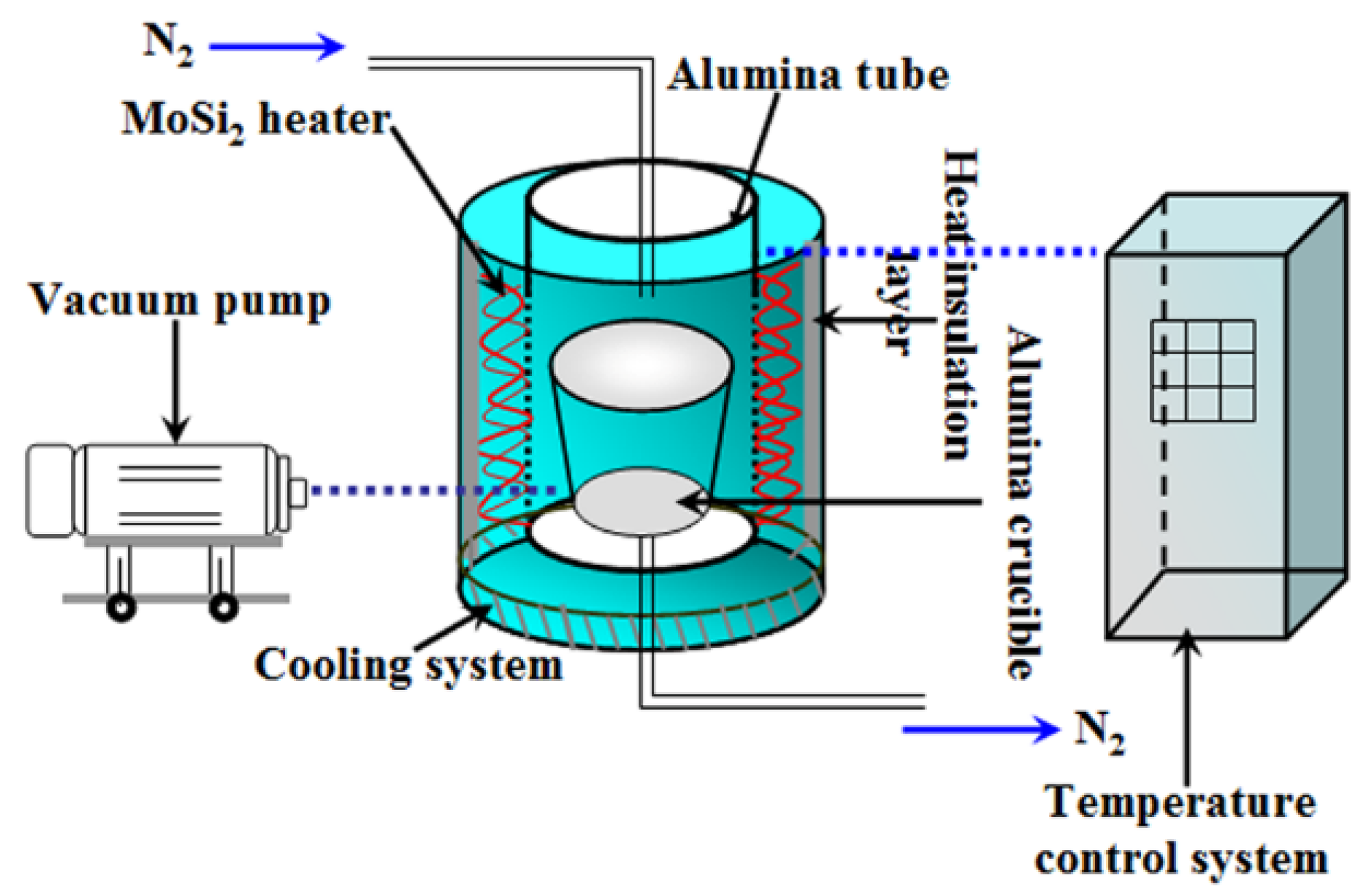
2. Materials and Methods
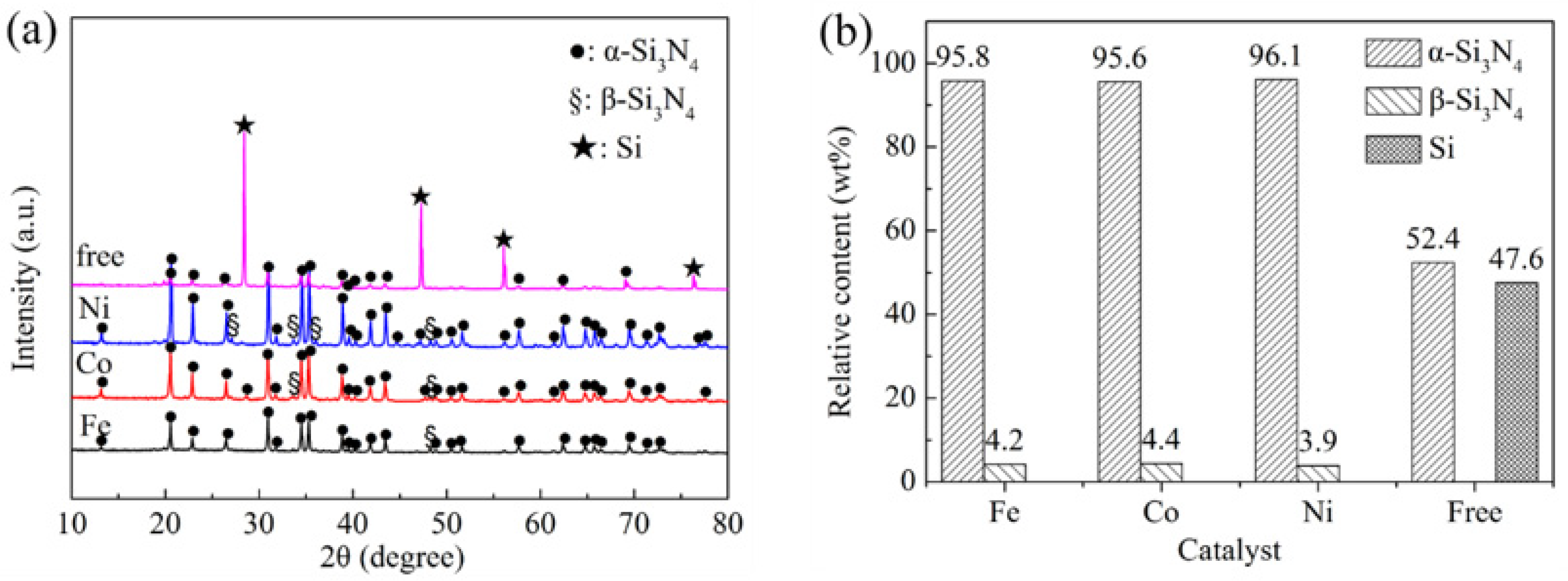
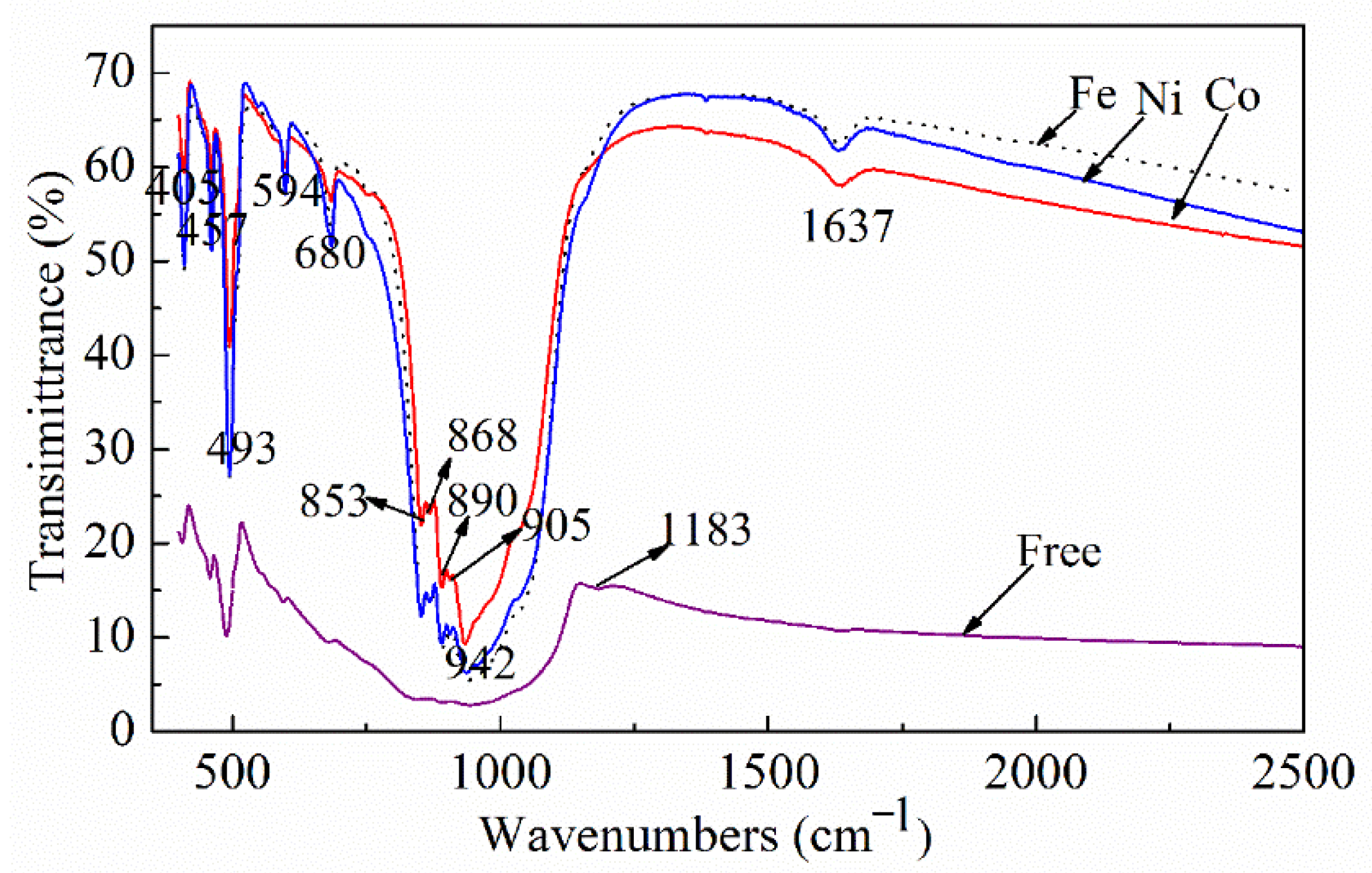
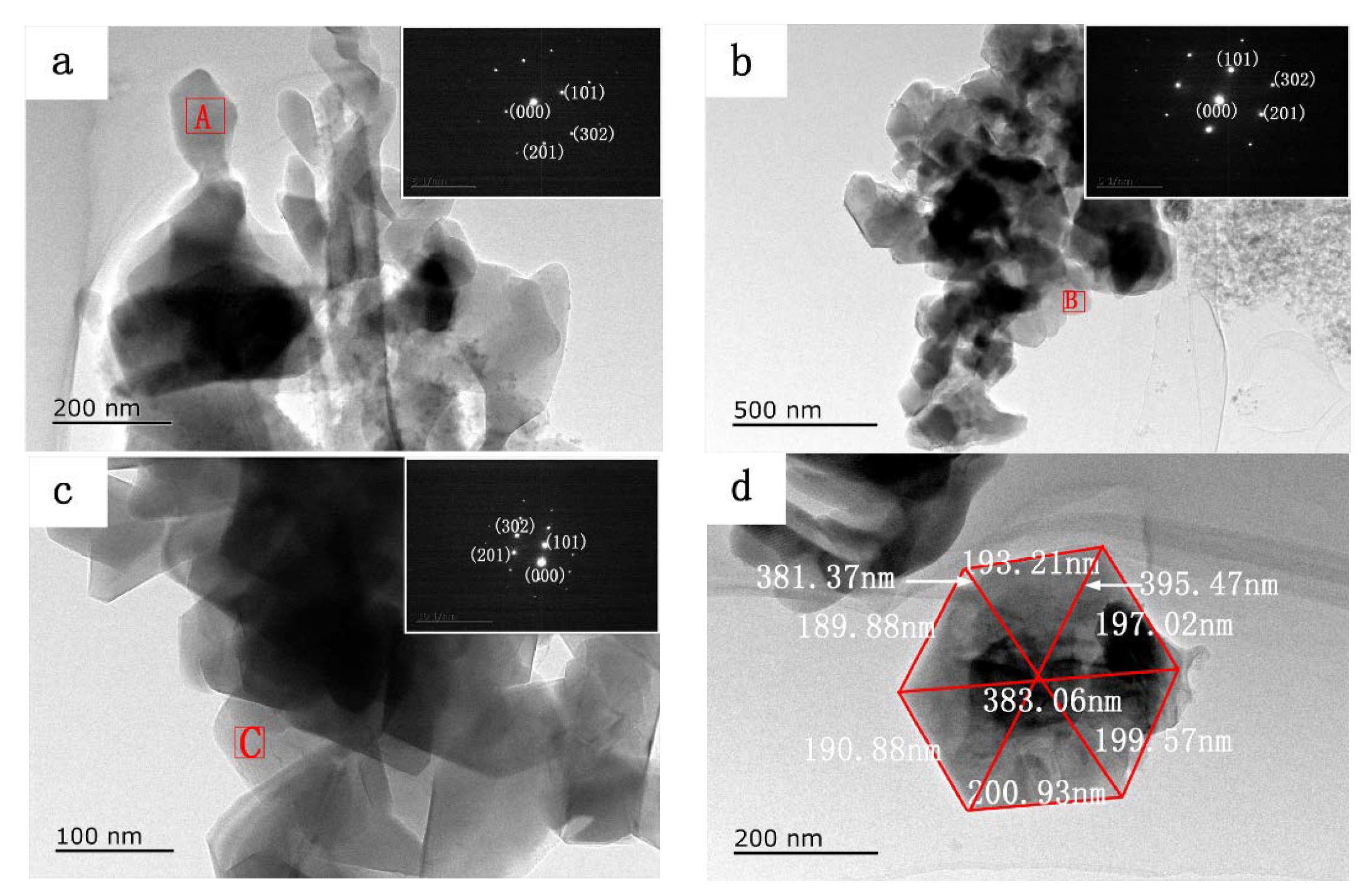
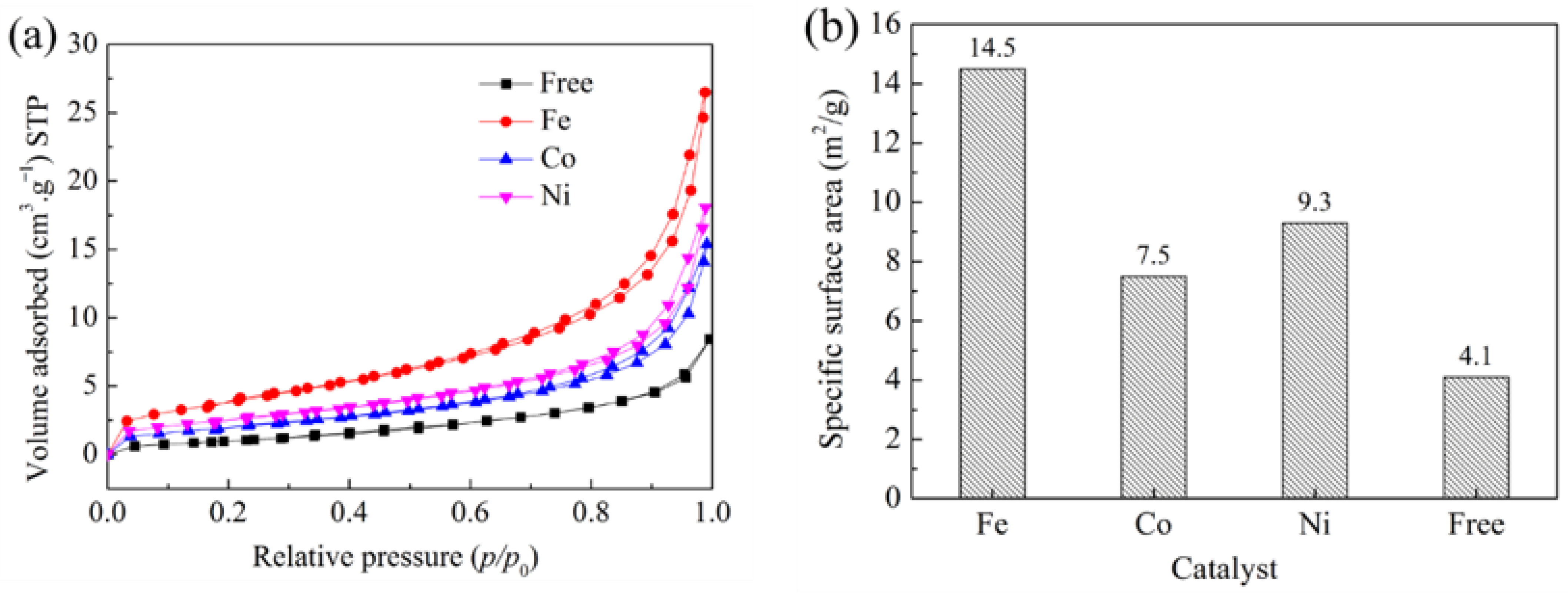
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riley, F.L. Silicon nitride and related materials. J. Am. Ceram. Soc. 2000, 83, 245–265. [Google Scholar] [CrossRef]
- Krstic, Z.; Krstic, V.D. Silicon nitride: The engineering material of the future. J. Mater. Sci. 2012, 47, 535–552. [Google Scholar] [CrossRef]
- Collins, J.F.; Gerby, R.W. New refractory uses for silicon nitride reported. J. Mater. Sci. 1955, 5, 612–615. [Google Scholar] [CrossRef]
- Zhu, X.W.; Zhou, Y.; Hirao, K.; Lenčéš, Z. Processing and thermal conductivity of sintered reaction-bonded silicon nitride. I: Effect of Si powder characteristics. J. Am. Ceram. Soc. 2006, 89, 3331–3339. [Google Scholar] [CrossRef]
- Xing, H.Y.; Liu, B.Q.; Sun, J.; Zou, B. Mechanical properties of Si3N4 ceramics from an in-situ synthesized α-Si3N4/β-Si3N4 composite powder. Ceram. Int. 2017, 43, 2150–2154. [Google Scholar] [CrossRef]
- Bocanegra-Bernal, M.H.; Matovic, B. Mechanical properties of silicon nitride-based ceramics and its use in structural applications at high temperatures. Mater. Sci. Eng. A 2010, 527, 1314–1338. [Google Scholar] [CrossRef]
- Santos, C.; Ribeiro, S.; Strecker, K.; Rodrigues, D., Jr.; Silva, C.R.M. Highly dense Si3N4 crucibles used for A1 casting: An investigation of the aluminum ceramic interface at high temperatures. J. Mater. Process. Tech. 2007, 184, 108–114. [Google Scholar] [CrossRef]
- Seyferth, D.; Wiseman, G.H.; Prud’homme, C. A liquid silazane precursor to silicon nitride. J. Am. Ceram. Soc. 1983, 66, C-13–C-14. [Google Scholar] [CrossRef]
- Burns, G.T.; Chandra, G. Pyrolysis of preceramic polymers in ammonia: Preparation of silicon nitride powders. J. Am. Ceram. Soc. 1989, 72, 333–337. [Google Scholar] [CrossRef]
- Isoda, T.; Kaya, H.; Nishii, H.; Funayama, O.; Suzuki, T.; Tashiro, Y. Perhydropolysilazane precursors to silicon nitride ceramics. J. Inorg. Organomet. Polym. 1992, 2, 151–160. [Google Scholar] [CrossRef]
- Yao, G.S.; Li, Y.; Jiang, P.; Jin, X.M.; Long, M.L.; Qin, H.X.; Vasant Kumar, R. Formation mechanisms of Si3N4 and Si2N2O in silicon powder nitridation. Solid State Sci. 2017, 66, 50–56. [Google Scholar] [CrossRef]
- Chen, K.; Huang, Z.H.; Liu, Y.G.; Fang, M.H.; Huang, J.T.; Xu, Y.G. Synthesis of β-Si3N4 powder from quartz via carbothermal reduction nitridation. Powder Technol. 2013, 235, 728–734. [Google Scholar] [CrossRef]
- Ji, H.P.; Huang, Z.H.; Chen, K.; Li, W.J.; Gao, Y.F.; Fang, M.H.; Liu, Y.G.; Wu, X.W. Synthesis of Si3N4 powder with tunable α/β-Si3N4 content from waste silica fume using carbothermal reduction nitridation. Powder Technol. 2014, 252, 51–55. [Google Scholar] [CrossRef]
- Wang, Q.S.; Liu, G.H.; Yang, J.H.; Chen, Y.X.; Li, J.T. Preheating-assisted combustion synthesis of β-Si3N4 powders at low N2 pressure. Mater. Res. Bull. 2013, 48, 1321–1323. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, L.E.; Wang, P.L.; Huang, Z.K. Pretreatment and sintering of Si3N4 powder synthesized by the high-temperature self-propagation method. Mater. Res. Bull. 2009, 44, 21–24. [Google Scholar] [CrossRef]
- Xue, P.J.; Hu, Y.; Xia, W.R.; Wu, H.; Zhu, X.H. Molten-salt synthesis of BaTiO3 powders and their atomic-scale structural characterization. J. Alloys Comp. 2017, 695, 2870–2877. [Google Scholar] [CrossRef]
- Kan, X.Q.; Ding, J.; Zhu, H.X.; Deng, C.J.; Yu, C. Low temperature synthesis of nanoscale titanium nitride via molten-salt mediated magnesiothermic reduction. Powder Technol. 2017, 315, 81–86. [Google Scholar] [CrossRef]
- Ding, J.; Guo, D.; Deng, C.J.; Zhu, H.X.; Yu, C. Low-temperature synthesis of nanocrystalline ZrC coatings on flake graphite by molten salts. Appl. Surf. Sci. 2017, 407, 315–321. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, C.J.; Wang, X.; Yu, C.; Ding, J.; Zhu, H.X. Evolution of c-ZrN nanopowders in low-carbon MgO–C refractories and their properties. J. Eur. Ceram. Soc. 2021, 41, 963–977. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, H.X.; Li, G.Q.; Deng, C.J.; Li, J. Growth of SiC nanowires on wooden template surface using molten salt media. Appl. Surf. Sci. 2014, 320, 620–626. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, J.F.; Cao, L.Y.; Ouyang, H.B.; Wu, J.P.; Liu, J.T.; Wang, X.; Hao, W. Synthesis of β-Y2Si2O7 nanowires via a facile molten salt method and their thermal properties. J. Alloys Comp. 2017, 699, 210–215. [Google Scholar] [CrossRef]
- Ding, J.; Deng, C.J.; Yuan, W.J.; Zhu, H.X.; Zhang, X.J. Novel synthesis and characterization of silicon carbide nanowires on graphite flakes. Ceram. Int. 2014, 40, 4001–4007. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Deng, C.J.; Yu, C.; Ding, J.; Zhu, H.X. Molten salt synthesis of Cr3C2-coated flake graphite and its effect on the physical properties of low-carbon MgO–C refractories. Adv. Powder Technol. 2021, 32, 2566–2576. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, H.X.; Deng, C.J.; Li, G.Q.; Wang, K.L.; Liu, J.P. Preparation and characterisation of porous biomorphic SiC/C ceramic from molten salt. Ceram. Int. 2015, 41, 11539–11545. [Google Scholar] [CrossRef]
- Kan, X.Q.; Ding, J.; Yu, C.; Zhu, H.X.; Deng, C.J.; Li, G.Y. Low-temperature fabrication of porous ZrC/C composite material from molten salts. Ceram. Int. 2017, 43, 6377–6384. [Google Scholar] [CrossRef]
- Ding, J.; He, W.Z.; Yu, C.; Liu, L.Z.; Deng, C.J.; Zhu, H.X. Waste biomass derived carbon supported Mo2C/C composite materials for heavy metal adsorption and hydrogen evolution reaction. Mater. Today Commun. 2022, 31, 103646. [Google Scholar] [CrossRef]
- Boyer, S.M.; Moulson, A.J. A mechanism for the nitridation of Fe-contaminated silicon. J. Mater. Sci. 1978, 13, 1637–1646. [Google Scholar] [CrossRef]
- Pavarajarn, V.; Kimura, S. Catalytic effects of metals on direct nitridation of silicon. J. Am. Ceram. Soc. 2001, 84, 1669–1674. [Google Scholar] [CrossRef]
- Gu, Y.J.; Lu, L.L.; Zhang, H.J.; Cao, Y.N.; Li, F.L.; Zhang, S.W. Nitridation of silicon powders catalyzed by cobalt nanoparticles. J. Am. Ceram. Soc. 2015, 98, 1762–1768. [Google Scholar] [CrossRef]
- Hyug, H.; Yoshid, K.; Kondo, N.; Kita, H.; Sugai, J.; Okano, H.; Tsuchida, J. Nitridation enhancing effect of ZrO2 on silicon powder. Mater. Lett. 2008, 62, 3475–3477. [Google Scholar] [CrossRef]
- Guo, W.M.; Wu, L.X.; Xie, H.; You, Y.; Lin, H.T. Effect of TiO2 additives on nitridation of Si powders. Mater. Lett. 2016, 177, 61–63. [Google Scholar] [CrossRef]
- Wang, F.; Qin, X.F.; Yang, L.X.; Meng, Y.F.; Sun, L.X. Synthesis and photoluminescence of Si3N4 nanowires from La/SiO2 composites and Si powders. Ceram. Int. 2015, 41, 1505–1510. [Google Scholar] [CrossRef]
- Huang, J.T.; Huang, Z.H.; Yi, S.; Liu, Y.G.; Fang, M.H.; Zhang, S.W. Fe-catalyzed growth of one-dimensional α-Si3N4 nanostructures and their cathodoluminescence properties. Sci. Rep. 2013, 3, 3504. [Google Scholar] [CrossRef] [PubMed]
- Pavarajarn, V.; Vongthavorn, T.; Praserthdam, P. Enhancement of direct nitridation of silicon by common metals in silicon nitride processing. Ceram. Int. 2007, 33, 675–680. [Google Scholar] [CrossRef]
- Zheng, C.S.; Yan, Q.Z.; Xia, M. Combustion synthesis of SiC/Si3N4-NW composite powders: The influence of catalysts and gases. Ceram. Int. 2012, 38, 4549–4554. [Google Scholar] [CrossRef]
- Chen, Y.X.; Yang, J.H.; Jiang, Z.J.; Lin, Z.M.; Li, J.T. Combustion synthesis of α-Si3N4 powder using AC as additive. J. Inorg. Mater. 2012, 27, 169–173. [Google Scholar] [CrossRef]
- Cao, Y.G.; Chen, H.; Li, J.T.; Ge, C.C.; Tang, S.Y.; Tang, J.X.; Chen, X. Formation of α-Si3N4 whiskers with addition of NaN3 as catalyst. J. Cryst. Growth 2002, 234, 9–11. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, H.X.; Li, G.Q.; Deng, C.J.; Chai, Z.N. Catalyst-assisted synthesis of α-Si3N4 in molten salt. Ceram. Int. 2016, 42, 2892–2898. [Google Scholar] [CrossRef]
- Chai, Z.N.; Ding, J.; Deng, C.J.; Zhu, H.X.; Li, G.Q. Ni-catalyzed synthesis of hexagonal plate-like alpha silicon nitride from nitridation of Si powder in molten salt media. Adv. Powder Technol. 2016, 27, 1637–1644. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Deng, C.J.; Yu, C.; Ding, J.; Zhu, H.X. Controllable synthesis of Si@SiC plate@Si3N4 whisker with core-shell structure and their electrochemical performances. Powder Technol. 2021, 377, 324–335. [Google Scholar] [CrossRef]
- Alem, A.; Pugh, M.D.; Drew, R.A.L. Reaction bonded silicon nitride foams: The influence of iron disilicide on microstructure and mechanical strength. Ceram. Int. 2015, 41, 4966–4974. [Google Scholar] [CrossRef]
- Zhu, H.L.; Han, F.D.; Bi, J.Q.; Bai, Y.J.; Qi, Y.X.; Pang, L.L.; Wang, C.G.; Li, S.J.; Lu, C.W. Facile synthesis of Si3N4 nanocrystals via an organic-inorganic reaction route. J. Am. Ceram. Soc. 2009, 92, 535–538. [Google Scholar] [CrossRef]
- Lu, J.G.; Rozgonyi, G.; Rand, J.; Jonczyk, R. Secondary phase inclusions in polycrystalline sheet silicon. J. Cryst. Growth 2004, 269, 599–605. [Google Scholar] [CrossRef]
- Batan, A.; Franquet, A.; Vereecken, J.; Reniers, F. Characterization of the silicon nitride thin films deposited by plasma magnetron. Surf. Interface Anal. 2008, 40, 754–757. [Google Scholar] [CrossRef]
- Schmit, F.; Bois, L.; Chiriac, R.; Toche, F.; Chassagneux, F.; Descorme, C.; Besson, M.; Khrouz, L. Porous microspheres of manganese-cerium mixed oxides by a polyvinylpyrrolidone assisted solvothermal method. J. Phys. Chem. Solids 2017, 103, 22–32. [Google Scholar] [CrossRef]
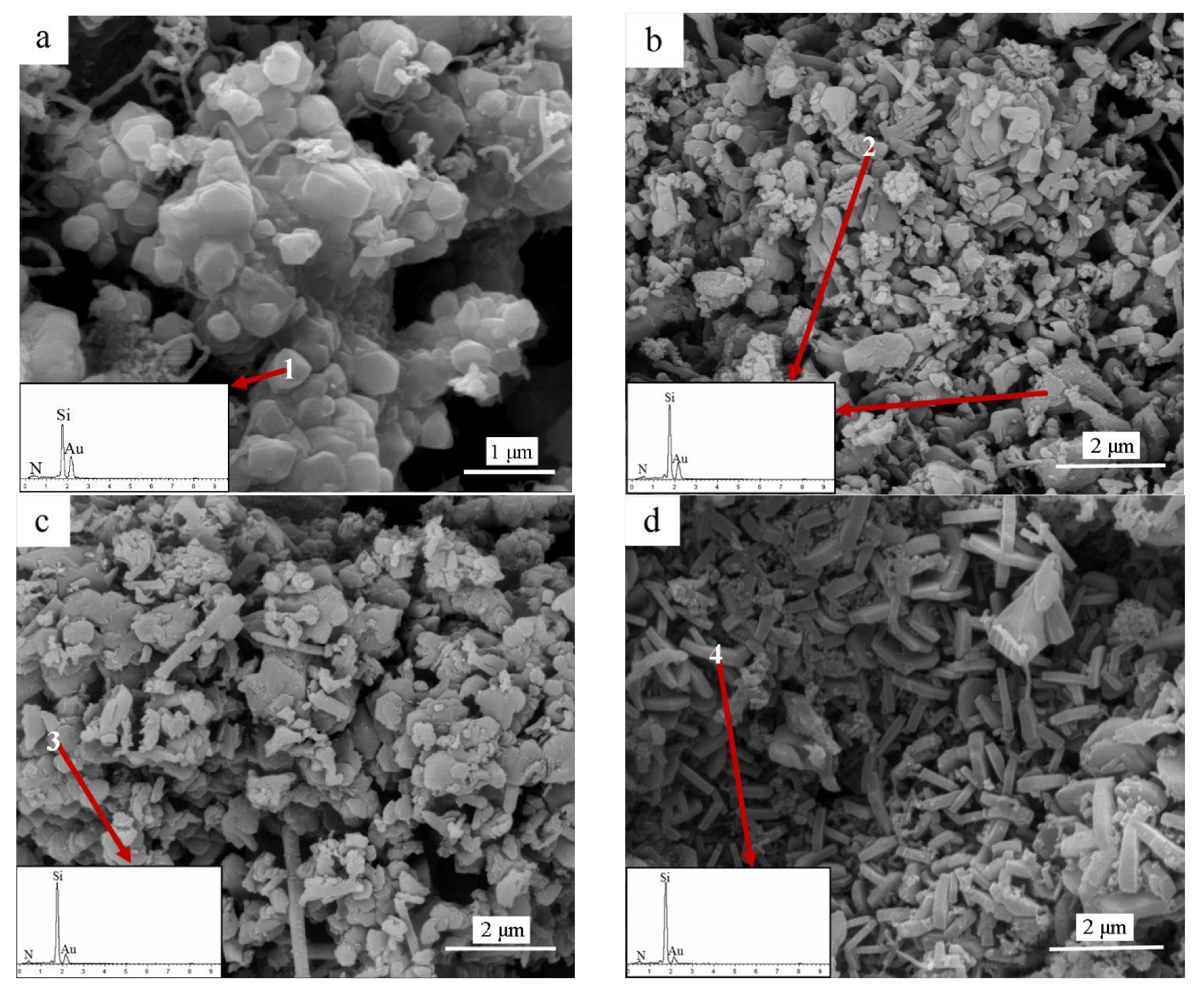

| Element | N | Si | Au |
|---|---|---|---|
| 1 | 40.25 | 50.45 | 9.30 |
| 2 | 48.40 | 44.65 | 6.96 |
| 3 | 37.63 | 55.56 | 6.81 |
| 4 | 51.55 | 41.62 | 6.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Chai, Z.; Yu, C.; Ding, J.; Deng, C.; Li, X.; Zhu, H. Liquid-Phase-Assisted Catalytic Nitridation of Silicon and In Situ Growth of α-Si3N4. Materials 2022, 15, 6074. https://doi.org/10.3390/ma15176074
Liu Z, Chai Z, Yu C, Ding J, Deng C, Li X, Zhu H. Liquid-Phase-Assisted Catalytic Nitridation of Silicon and In Situ Growth of α-Si3N4. Materials. 2022; 15(17):6074. https://doi.org/10.3390/ma15176074
Chicago/Turabian StyleLiu, Zhenglong, Zhinan Chai, Chao Yu, Jun Ding, Chengji Deng, Xiangcheng Li, and Hongxi Zhu. 2022. "Liquid-Phase-Assisted Catalytic Nitridation of Silicon and In Situ Growth of α-Si3N4" Materials 15, no. 17: 6074. https://doi.org/10.3390/ma15176074
APA StyleLiu, Z., Chai, Z., Yu, C., Ding, J., Deng, C., Li, X., & Zhu, H. (2022). Liquid-Phase-Assisted Catalytic Nitridation of Silicon and In Situ Growth of α-Si3N4. Materials, 15(17), 6074. https://doi.org/10.3390/ma15176074







