Effect of Post-Heat Treatment Temperature on Interfacial Mechanical Properties of Cold-Rolled Ti/Al Clad Material
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
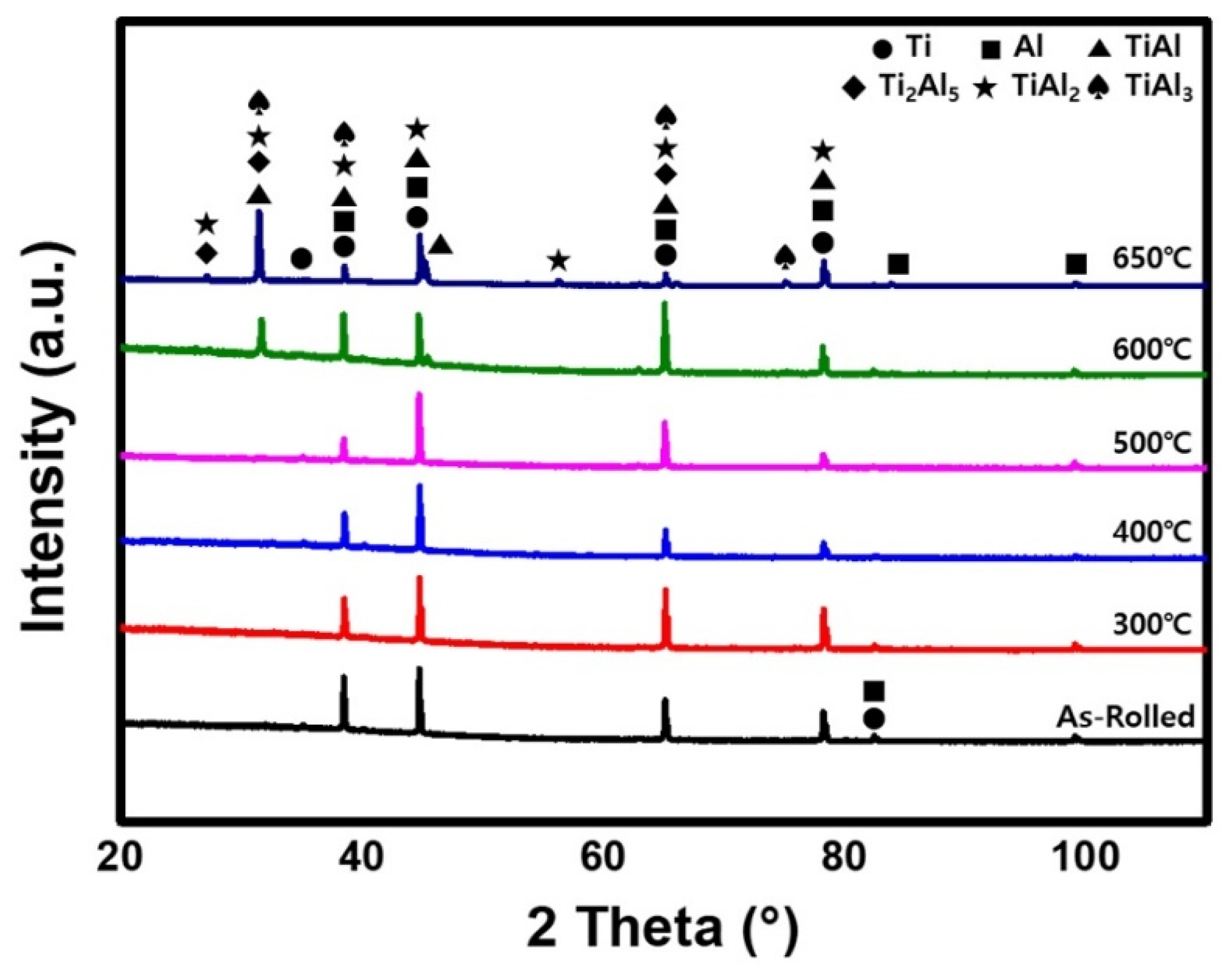
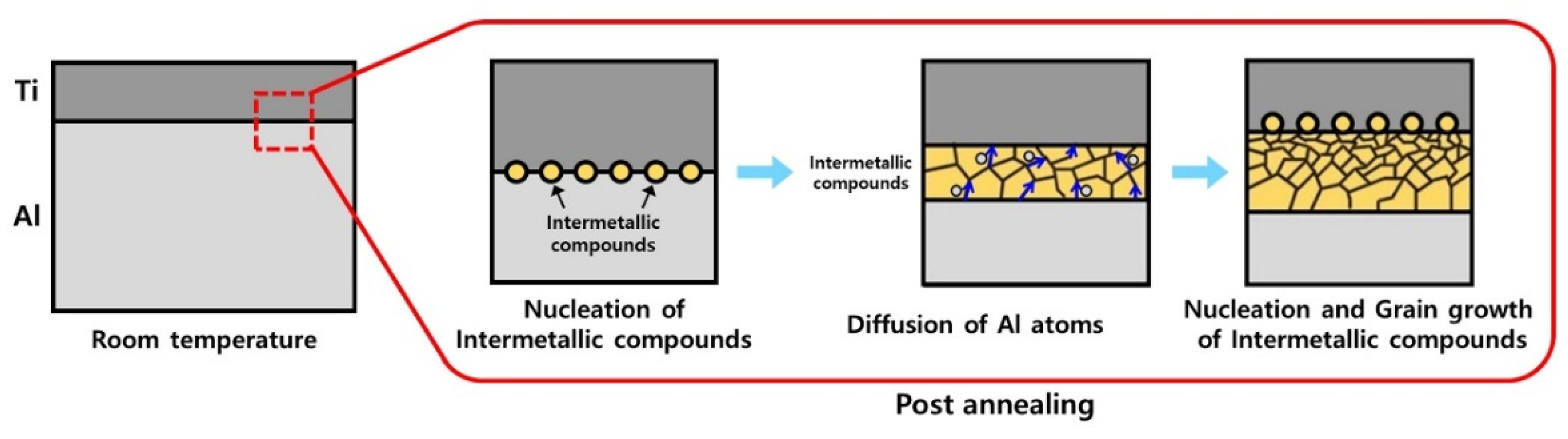
- Compared to the as-rolled specimen, no significant microstructural differences were observed after the post-heat treatment at 300, 400, or 500 °C. However, after the post-heat treatment at 600 °C and beyond, a new phase layer was formed between Ti and Al. The thickness of this layer increased with post-heat treatment temperature owing to the active diffusion of Al atoms. XRD analysis shows that this layer consisted of four different intermetallic compounds.
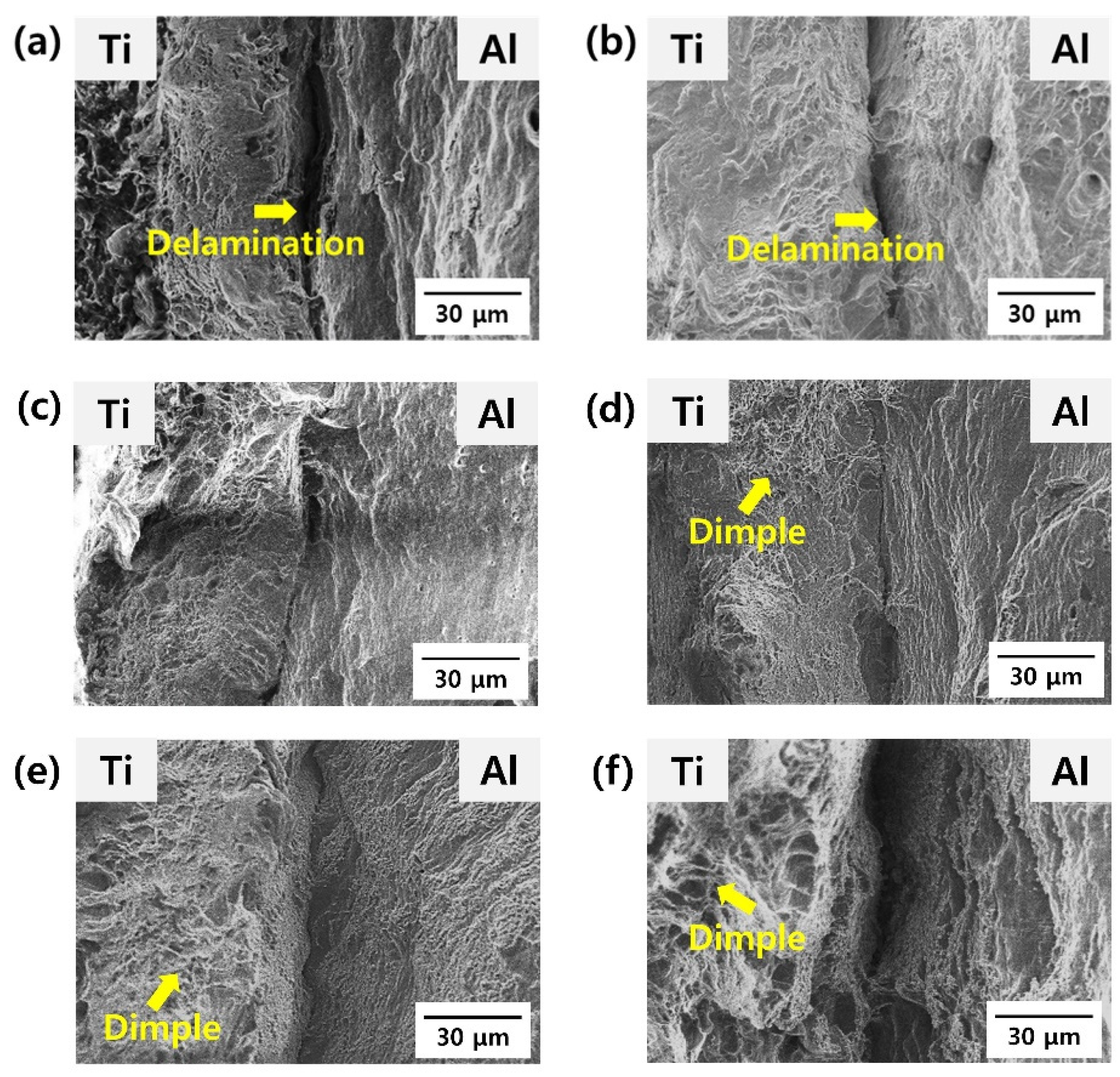
- With an increasing heat treatment temperature, the elongation at break increased, but the strength decreased because of the recovery of Al, which occupied a relatively large area of the cross-section. However, after the post-heat treatment at 600 and 650 °C, the strength remained constant, but the elongation at break decreased because of the growth of the intermetallic compound at the Ti/Al interface, which could act as a stress concentration area.
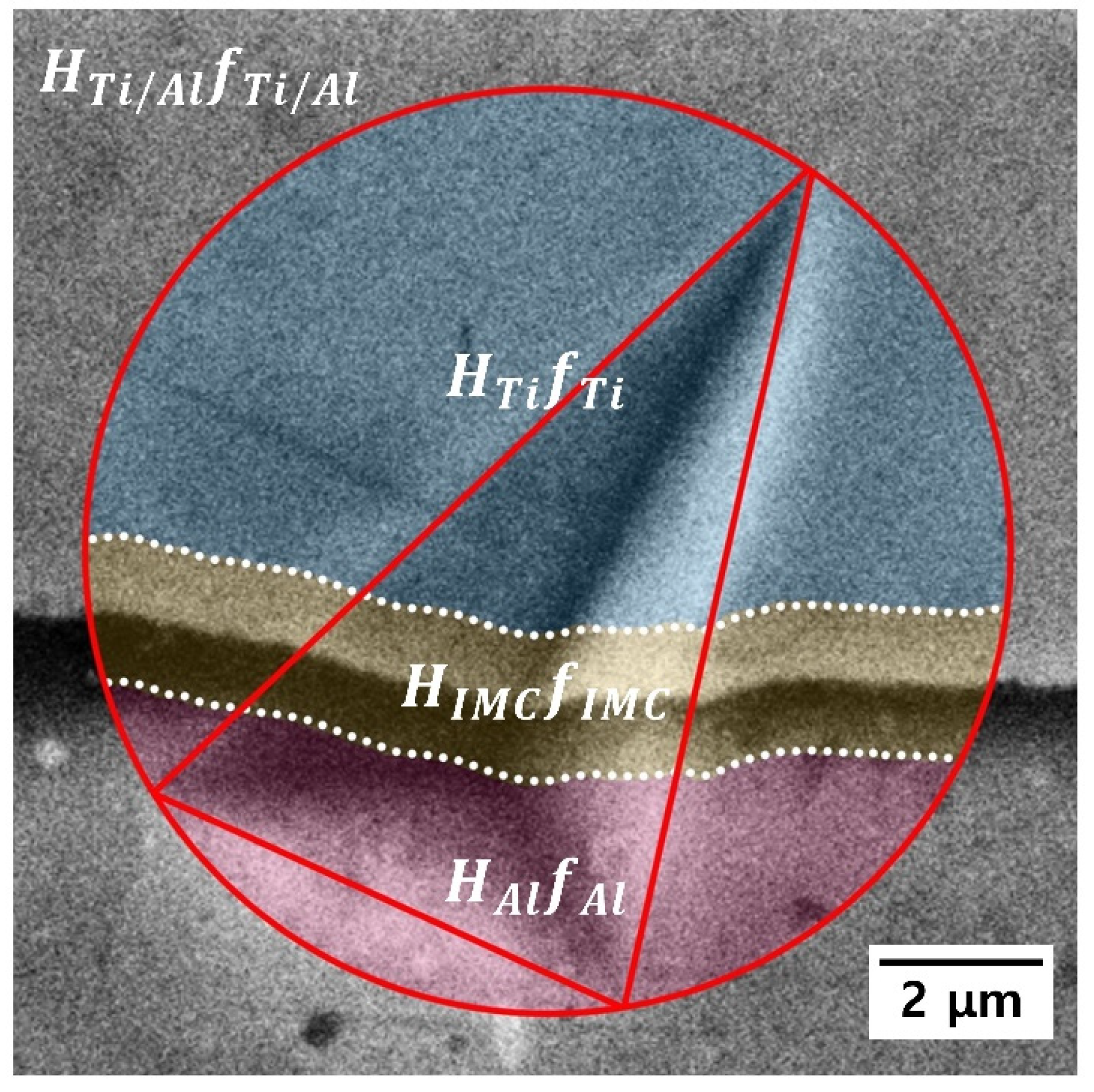
- Post-heat treatment decreased the nanohardness of Al owing to recovery, recrystallization, and grain growth; at the same time, the nanohardness of Ti was not affected. The nanohardness of the intermetallic compound at the Ti/Al interface was estimated to be 16.11 ± 4.85 GPa using a simple rule of mixture. Thus, a rapid increase in HTi/Al at the post-heat treatment temperature of 600 °C and higher was attributed to the formation of intermetallic compounds.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welsch, G.; Boyer, R.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Novelty, OH, USA, 1993; pp. 3–122. [Google Scholar]
- Lütjering, G.; Williams, J.C. Titanium Matrix Composites; Springer: New York, NY, USA, 2007; pp. 4–48. [Google Scholar]
- Munroe, N.; Tan, X.; Gu, H. Orientation dependence of slip and twinning in HCP metals. Scr. Mater. 1997, 36, 1383–1386. [Google Scholar] [CrossRef]
- Kim, Y.K.; Pouraliakbar, H.; Hong, S.I. Effect of interfacial intermetallic compounds evolution on the mechanical response and fracture of layered Ti/Cu/Ti clad materials. Mater. Sci. Eng. A 2020, 772, 138802. [Google Scholar] [CrossRef]
- Pengju, W.; Zejun, C.; Huang, H.; Jianshu, L.; Boxin, L.I.; Qing, L. Fabrication of Ti/Al/Mg laminated composites by hot roll bonding and their microstructures and mechanical properties. Chin. J. Aeronaut. 2021, 34, 192–201. [Google Scholar] [CrossRef]
- Matts, O.E.; Tarasov, S.Y.; Domenichini, B.; Lazurenko, D.V.; Filippov, A.V.; Bataev, V.A.; Rashkovets, M.V.; Chakin, I.K.; Emurlaev, K.I. Tribo-oxidation of Ti-Al-Fe and Ti-Al-Mn cladding layers obtained by non-vacuum electron beam treatment. Surf. Coat. Technol. 2021, 421, 127442. [Google Scholar] [CrossRef]
- Schubert, E.; Klassen, M.; Zerner, I.; Walz, C.; Sepold, G. Light-weight structures produced by laser beam joining for future applications in automobile and aerospace industry. J. Mater. Process. Technol. 2001, 115, 2–8. [Google Scholar] [CrossRef]
- Yazdani, Z.; Toroghinejad, M.R.; Edris, H.; Ngan, A. A novel method for the fabrication of Al-matrix nanocomposites reinforced by mono-dispersed TiAl3 intermetallic via a three-step process of cold-roll bonding, heat-treatment and accumulative roll bonding. J. Alloys Compd. 2018, 747, 217–226. [Google Scholar] [CrossRef]
- Xu, J.; Fu, J.; Li, S.; Xu, G.; Li, Y.; Wang, Z. Effect of annealing and cold rolling on interface microstructure and properties of Ti/Al/Cu clad sheet fabricated by horizontal twin-roll casting. J. Mater. Res. Technol. 2022, 16, 530–543. [Google Scholar] [CrossRef]
- Zhu, Z.; Lee, K.Y.; Wang, X. Ultrasonic welding of dissimilar metals, AA6061 and Ti6Al4V. Int. J. Adv. Manuf. Technol. 2012, 59, 569–574. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Lee, G.; Lee, S. An Overview and Prospects for Hybrid Materials. Trends Met. Mater. Eng. 2011, 24, 24–30. [Google Scholar]
- Jamaati, R.; Toroghinejad, M.R. Investigation of the parameters of the cold roll bonding (CRB) process. Mater. Sci. Eng. A 2010, 527, 2320–2326. [Google Scholar] [CrossRef]
- Khan, H.A.; Asim, K.; Akram, F.; Hameed, A.; Khan, A.; Mansoor, B. Roll bonding processes: State-of-the-art and future perspectives. Metals 2021, 11, 1344. [Google Scholar] [CrossRef]
- Li, L.; Nagai, K.; Yin, F. Progress in cold roll bonding of metals. Sci. Technol. Adv. Mater. 2008, 9, 023001. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.G.; Chen, P.; Ji, C. Solid-liquid cast-rolling bonding (SLCRB) and annealing of Ti/Al cladding strip. Mater. Des. 2017, 118, 233–244. [Google Scholar] [CrossRef]
- Youn, C.; Lee, D. Effects of post heat treatment on the mechanical properties of cold-rolled Ti/Cu clad sheet. Metals 2020, 10, 1672. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Liu, B.; Zhao, Y.; Ren, J.; Yan, Y.; Cao, R.; Chen, J. Microstructure characteristics and tensile properties of multilayer Al-6061/Ti-TA1 sheets fabricated by accumulative roll bonding. J. Mater. Process. Technol. 2020, 275, 116378. [Google Scholar] [CrossRef]
- Jafari, R.; Eghbali, B. Intermetallic growth behavior during post deformation annealing in multilayer Ti/Al/Nb composite interfaces. Int. J. Miner. Metall. Mater. 2022, 29, 1608–1617. [Google Scholar] [CrossRef]
- Choi, I.; Yoo, B.; Kim, Y.; Jang, J. Indentation creep revisited. J. Mater. Res. 2012, 27, 3–11. [Google Scholar] [CrossRef]
- Choi, I.; Jang, J. A Survey of Nanoindentation Studies on HPT-Processed Materials. Adv. Eng. Mater. 2020, 22, 1900648. [Google Scholar] [CrossRef]
- Choi, I.; Brandl, C.; Schwaiger, R. Thermally activated dislocation plasticity in body-centered cubic chromium studied by high-temperature nanoindentation. Acta Mater. 2017, 140, 107–115. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys (Revised Reprint); CRC Press: New York, NY, USA, 2009; pp. 57–100. [Google Scholar]
- Liu, Z.W.; Rakita, M.; Han, Q.; Li, J.G. A Developed Method for Fabricating In-Situ TiC p/Mg Composites by Using Quick Preheating Treatment and Ultrasonic Vibration. Metall. Mater. Trans. A 2012, 43, 2116–2124. [Google Scholar] [CrossRef]
- Du, Y.; Chang, Y.A.; Huang, B.; Gong, W.; Jin, Z.; Xu, H.; Yuan, Z.; Liu, Y.; He, Y.; Xie, F. Diffusion coefficients of some solutes in fcc and liquid Al: Critical evaluation and correlation. Mater. Sci. Eng. A 2003, 363, 140–151. [Google Scholar] [CrossRef]
- Krafcsik, I.; Gyulai, J.; Palmström, C.J.; Mayer, J.W. Influence of Cu as an impurity in Al/Ti and Al/W thin-film reactions. Appl. Phys. Lett. 1983, 43, 1015–1017. [Google Scholar] [CrossRef]
- Kráľ, J.; Ferdinandy, M.; Liška, D.; Diko, P. Formation of TiAl3 layer on titanium alloys. Mater. Sci. Eng. A 1991, 140, 479–485. [Google Scholar] [CrossRef]
- Xu, L.; Cui, Y.Y.; Hao, Y.L.; Yang, R. Growth of intermetallic layer in multi-laminated Ti/Al diffusion couples. Mater. Sci. Eng. A 2006, 435, 638–647. [Google Scholar] [CrossRef]
- Kattner, U.R.; Lin, J.; Chang, Y.A. Thermodynamic assessment and calculation of the Ti-Al system. Metall. Trans. A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, Z.; Hu, L.; Ren, J. Characterization of hot processing parameters of powder metallurgy TiAl-based alloy based on the activation energy map and processing map. Mater. Des. 2015, 86, 922–932. [Google Scholar] [CrossRef]
- Peng, L.M.; Wang, J.H.; Li, H.; Zhao, J.H.; He, L.H. Synthesis and microstructural characterization of Ti–Al3Ti metal–intermetallic laminate (MIL) composites. Scr. Mater. 2005, 52, 243–248. [Google Scholar] [CrossRef]
- Mishin, Y.; Herzig, C. Diffusion in the Ti–Al system. Acta Mater. 2000, 48, 589–623. [Google Scholar] [CrossRef]
- Mirjalili, M.; Soltanieh, M.; Matsuura, K.; Ohno, M. On the kinetics of TiAl3 intermetallic layer formation in the titanium and aluminum diffusion couple. Intermetallics 2013, 32, 297–302. [Google Scholar] [CrossRef]
- Assari, A.H.; Eghbali, B. Solid state diffusion bonding characteristics at the interfaces of Ti and Al layers. J. Alloys Compd. 2019, 773, 50–58. [Google Scholar] [CrossRef]
- Mori, T.; Kurimoto, S. Press-formability of stainless steel and aluminum clad sheet. J. Mater. Process. Technol. 1996, 56, 242–253. [Google Scholar] [CrossRef]
- Ahn, K.; Huh, H.; Yoon, J. Strain hardening model of pure titanium considering effects of deformation twinning. Met. Mater. Int. 2013, 19, 749–758. [Google Scholar] [CrossRef]
- ASM International Metals Handbook Vol 2: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM: Novelty, OH, USA, 1990; pp. 118–177.
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties; Elsevier: Amsterdam, The Netherlands, 2013; pp. 56–95. [Google Scholar]
- Kim, D.W.; Lee, D.H.; Kim, J.; Sohn, S.S.; Kim, H.S.; Lee, S. Novel twin-roll-cast Ti/Al clad sheets with excellent tensile properties. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Hong, S.I. Interface Cracking and Fracture in As-Roll-Bonded and Heat-Treated 3-Ply Cu/Al/Cu Hybrid Plate. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2013; pp. 284–287. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Salman, M.; Liang, L.; Xiong, J.; Zhang, F. Effect of friction time on mechanical and metallurgical properties of continuous drive friction welded Ti6Al4V/SUS321 joints. Mater. Des. 2014, 56, 649–656. [Google Scholar] [CrossRef]
- Hollomon, J.H. Tensile Deformation; Aime Trans: Englewood, CO, USA, 1945; Volume 12, pp. 268–290. [Google Scholar]
- Fizanne-Michel, C.; Cornen, M.; Castany, P.; Péron, I.; Gloriant, T. Determination of hardness and elastic modulus inverse pole figures of a polycrystalline commercially pure titanium by coupling nanoindentation and EBSD techniques. Mater. Sci. Eng. A 2014, 613, 159–162. [Google Scholar] [CrossRef]
- Stern, A.; Aizenshtein, M.; Moshe, G.; Cohen, S.R.; Frage, N. The nature of interfaces in Al-1050/Al-1050 and Al-1050/Mg-AZ31 couples joined by magnetic pulse welding (MPW). J. Mater. Eng. Perform. 2013, 22, 2098–2103. [Google Scholar] [CrossRef]
- Gashti, S.O.; Fattah-Alhosseini, A.; Mazaheri, Y.; Keshavarz, M.K. Effects of grain size and dislocation density on strain hardening behavior of ultrafine grained AA1050 processed by accumulative roll bonding. J. Alloys Compd. 2016, 658, 854–861. [Google Scholar] [CrossRef]
- Davis, J.R.; Davis, J.R. ASM International Handbook Committee, Aluminum and Aluminum Alloys, Volume 4: Heat Treating; ASM International: Novelty, OH, USA, 1991; pp. 1861–1960. [Google Scholar]
- Seok, M.; Choi, I.; Moon, J.; Kim, S.; Ramamurty, U.; Jang, J. Estimation of the Hall–Petch strengthening coefficient of steels through nanoindentation. Scr. Mater. 2014, 87, 49–52. [Google Scholar] [CrossRef]
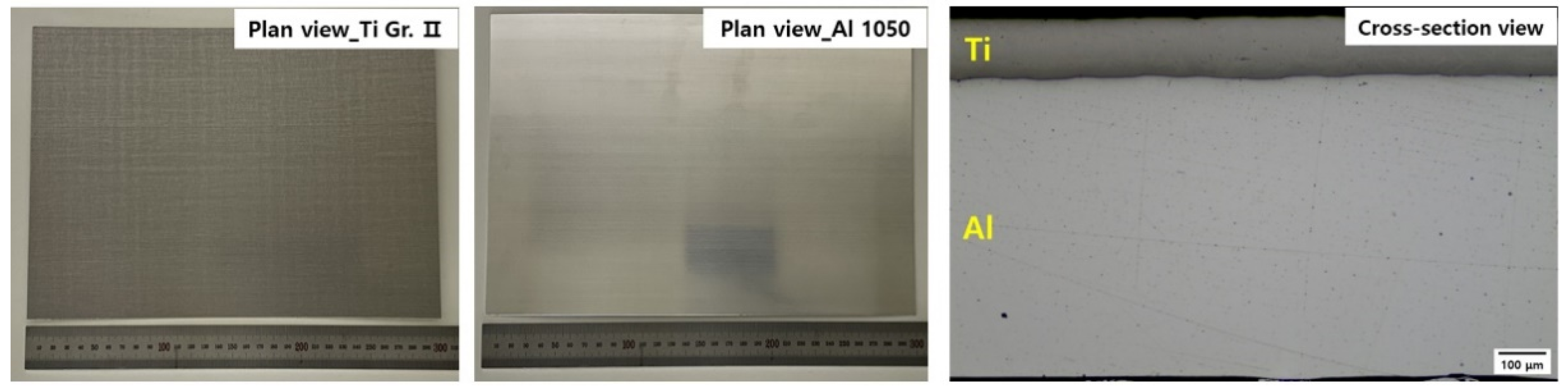
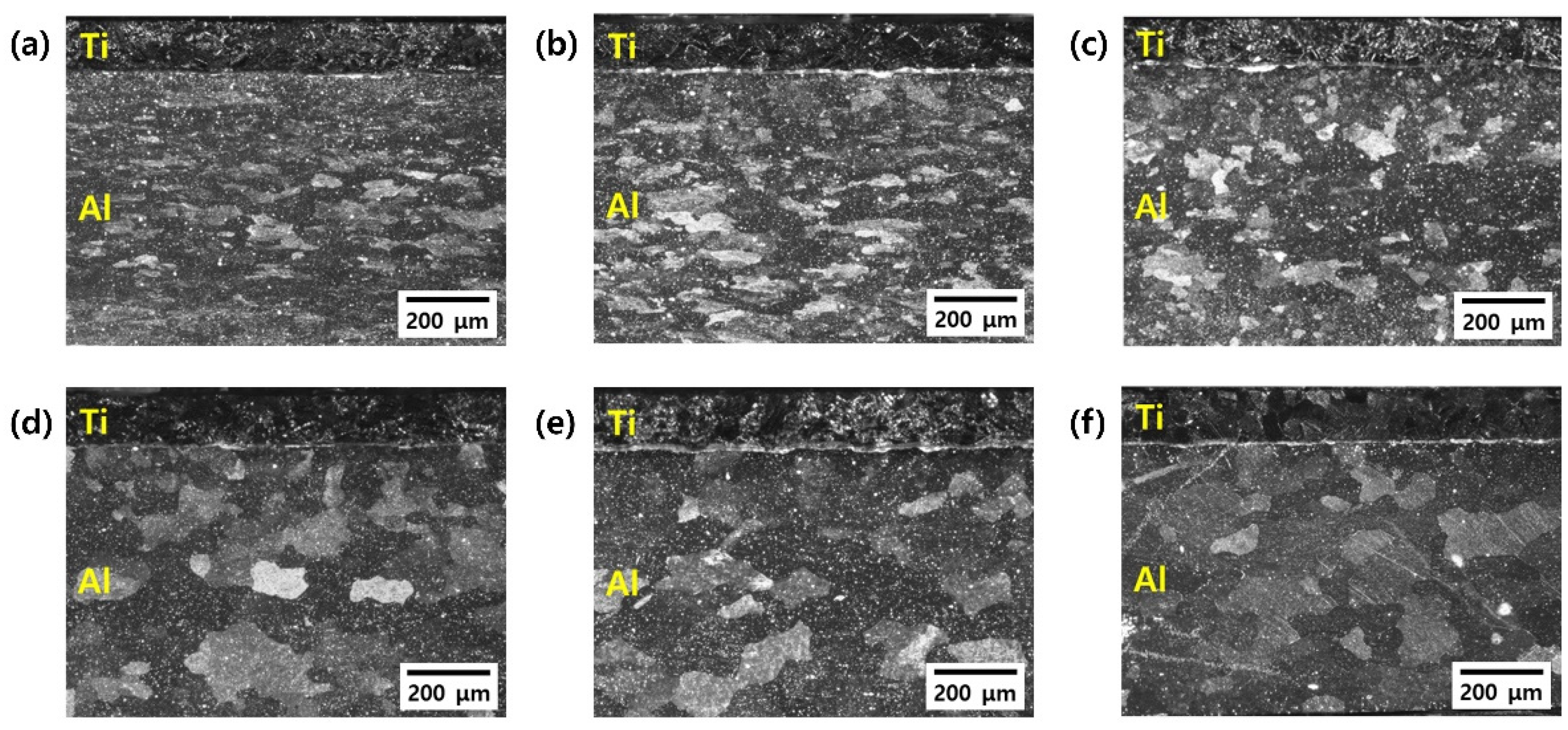

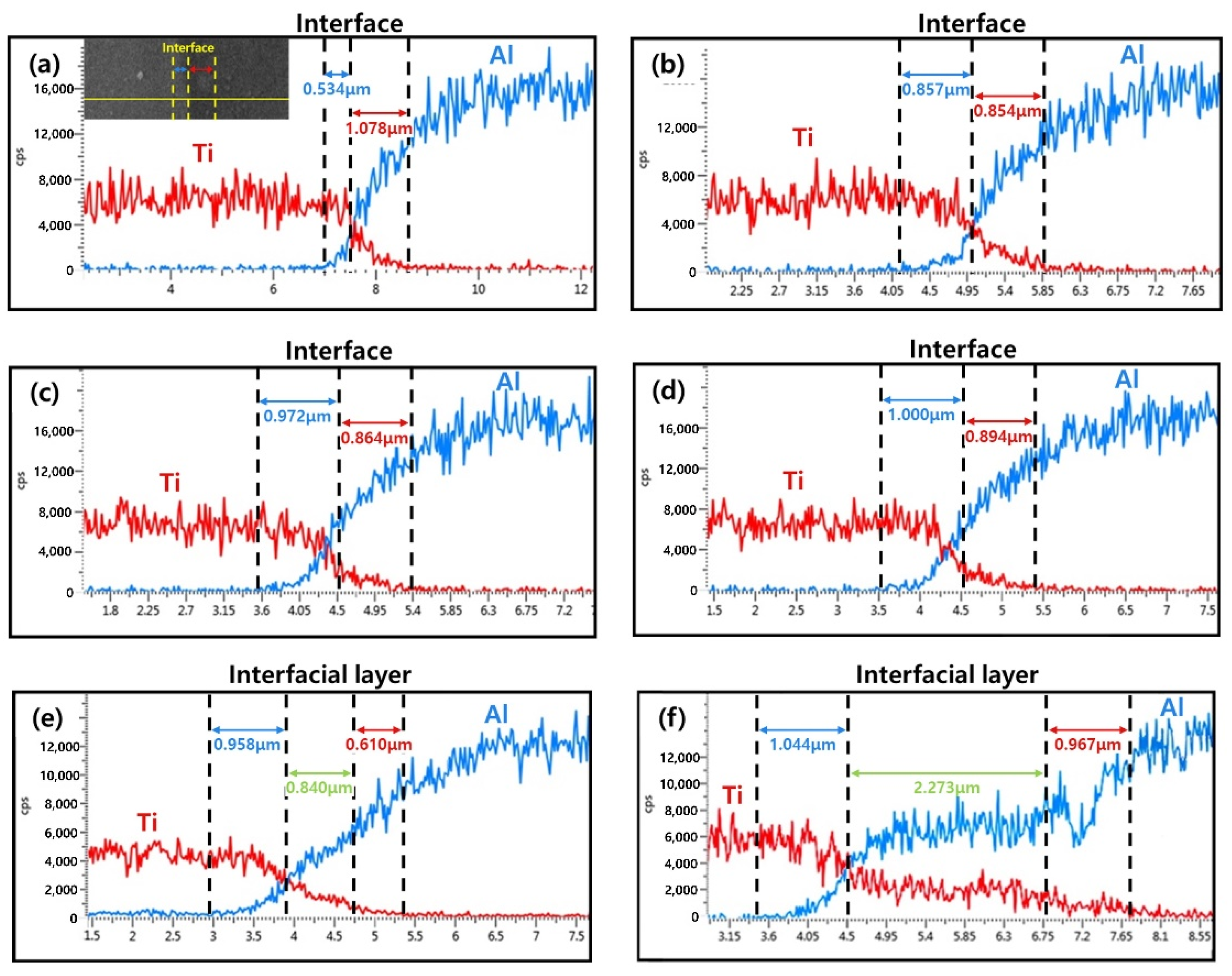
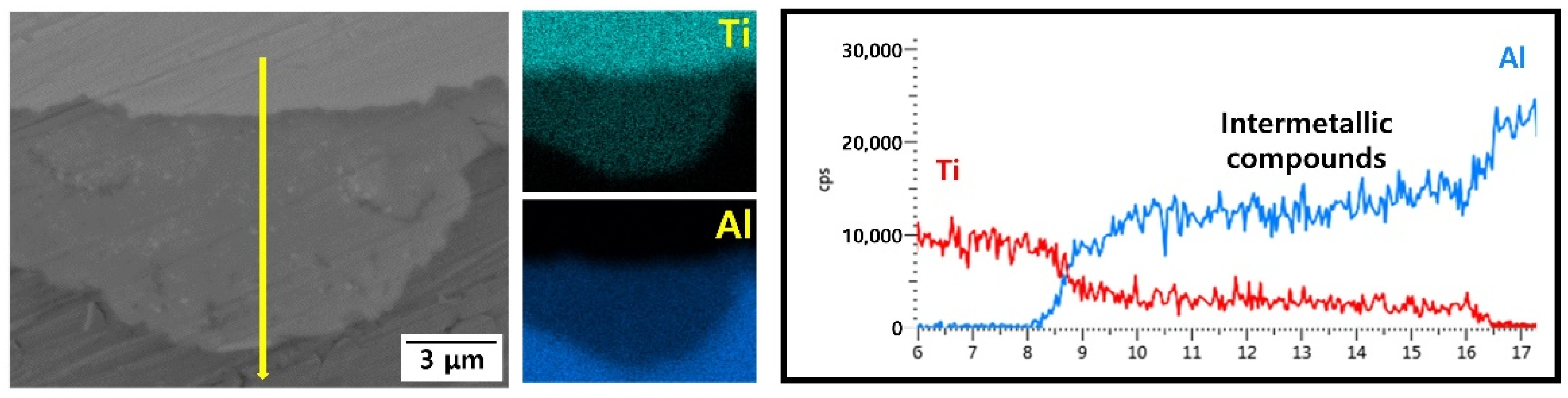

| Sample | Elements (wt. %) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | O | N | Al | Ti | Mg | Mn | Fe | Cu | Si | Zn | |
| Ti Gr. Ⅱ | 0.015 | 0.08 | 0.25 | 0.03 | - | Bal. | - | 0.30 | - | - | ||
| Al 1050 | - | - | - | - | Bal. | ≤0.03 | ≤0.05 | ≤0.05 | ≤0.40 | ≤0.05 | ≤0.25 | ≤0.05 |
| Diffusion of Al in Ti | Diffusion of Ti in Al | ||||
|---|---|---|---|---|---|
| Temp. Range (℃) | (m2/s) | Q (J/mol) | (m2/s) | Q (J/mol) | Refs. |
| 300–450 | 8.32 × 10−6 | 159,275 | 1.12 × 10−1 | 260,000 | [24,25,26] |
| 530–600 | 2.28 × 10−7 | 105,100 | 1.12 × 10−1 | 260,000 | [24,25,27] |
| UTS (MPa) | YS (MPa) | EL (%) | |
|---|---|---|---|
| As-rolled | 166.0 ± 2.01 | 139.6 ± 4.23 | 11.4 ± 0.87 |
| 300 °C | 152.6 ± 6.12 | 131.6 ± 5.32 | 17.8 ± 4.91 |
| 400 °C | 128.4 ± 1.33 | 97.0 ± 3.69 | 36.8 ± 0.88 |
| 500 °C | 122.1 ± 1.72 | 86.6 ± 2.42 | 38.0 ± 0.56 |
| 600 °C | 115.5 ± 1.42 | 74.7 ± 2.04 | 43.6 ± 1.82 |
| 650 °C | 120.3 ± 2.41 | 75.1 ± 0.29 | 43.1 ± 1.13 |
| Specimen | HTi (GPa) | ETi (GPa) | HAl (GPa) | EAl (GPa) | HTi/Al (GPa) |
|---|---|---|---|---|---|
| As-Rolled | 2.41 ± 0.15 | 118.79 ± 2.88 | 0.71 ± 0.03 | 83.99 ± 2.10 | 3.92 ± 0.52 |
| 300 °C | 2.71 ± 0.18 | 122.72 ± 3.16 | 0.65 ± 0.05 | 82.08 ± 2.18 | 3.29 ± 0.40 |
| 400 °C | 2.54 ± 0.21 | 119.60 ± 2.92 | 0.61 ± 0.03 | 79.74 ± 1.83 | 3.88 ± 0.61 |
| 500 °C | 2.78 ± 0.20 | 122.45 ± 3.24 | 0.59 ± 0.06 | 78.61 ± 3.33 | 3.00 ± 0.39 |
| 600 °C | 2.36 ± 0.16 | 122.61 ± 3.84 | 0.58 ± 0.03 | 79.86 ± 1.45 | 4.74 ± 0.71 |
| 650 °C | 2.66 ± 0.34 | 122.21 ± 4.50 | 0.58 ± 0.02 | 80.92 ± 1.25 | 5.08 ± 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.-K.; Kim, J.-W.; Oh, M.-H.; Choi, I.-C. Effect of Post-Heat Treatment Temperature on Interfacial Mechanical Properties of Cold-Rolled Ti/Al Clad Material. Materials 2022, 15, 6103. https://doi.org/10.3390/ma15176103
Yoo S-K, Kim J-W, Oh M-H, Choi I-C. Effect of Post-Heat Treatment Temperature on Interfacial Mechanical Properties of Cold-Rolled Ti/Al Clad Material. Materials. 2022; 15(17):6103. https://doi.org/10.3390/ma15176103
Chicago/Turabian StyleYoo, Sang-Kyu, Ji-Won Kim, Myung-Hoon Oh, and In-Chul Choi. 2022. "Effect of Post-Heat Treatment Temperature on Interfacial Mechanical Properties of Cold-Rolled Ti/Al Clad Material" Materials 15, no. 17: 6103. https://doi.org/10.3390/ma15176103





