Numerical and Experimental Investigation of the Effect of Current Density on the Anomalous Codeposition of Ternary Fe-Co-Ni Alloy Coatings
Abstract
:1. Introduction
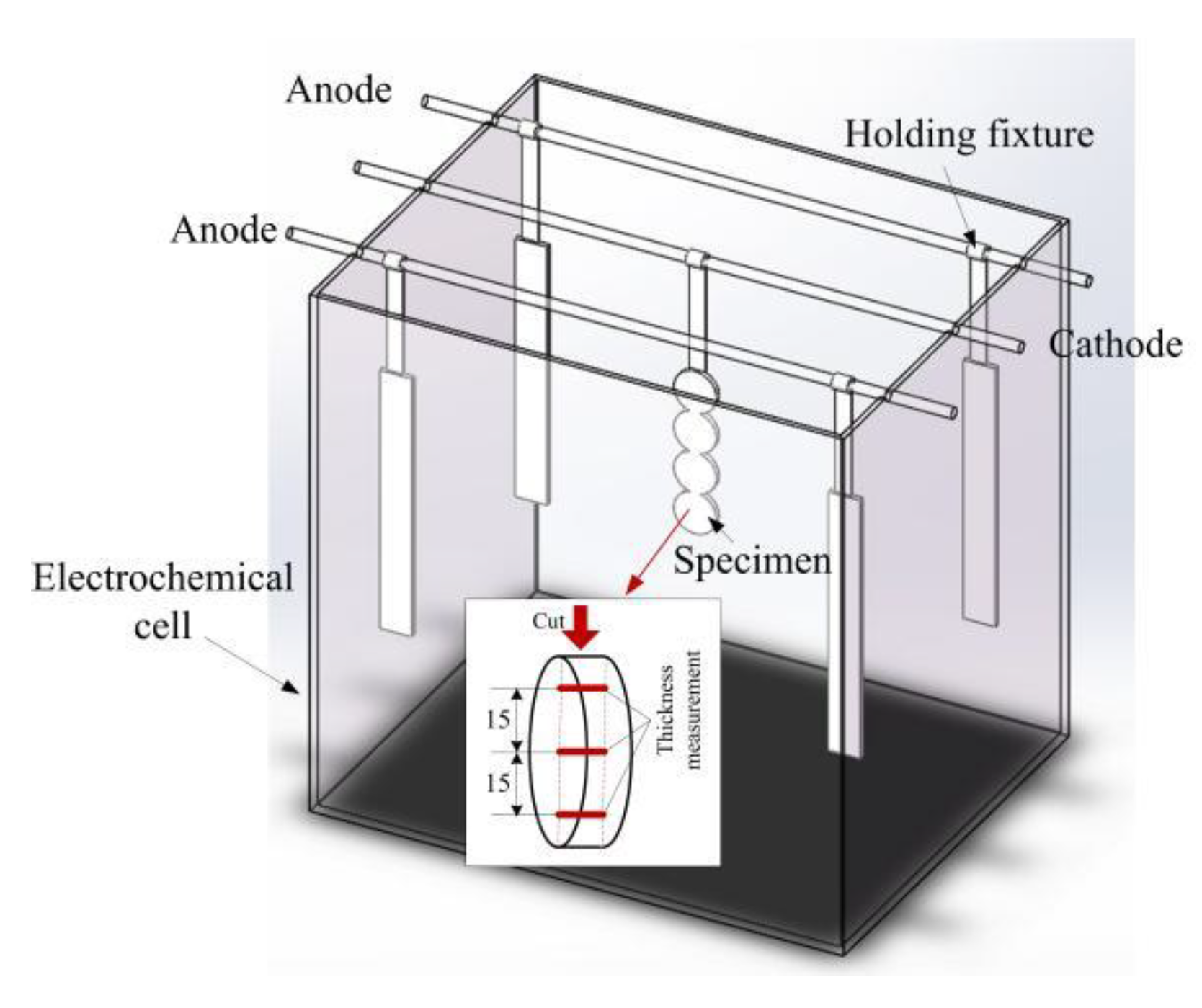
2. Experimental Procedure for Electrodeposition
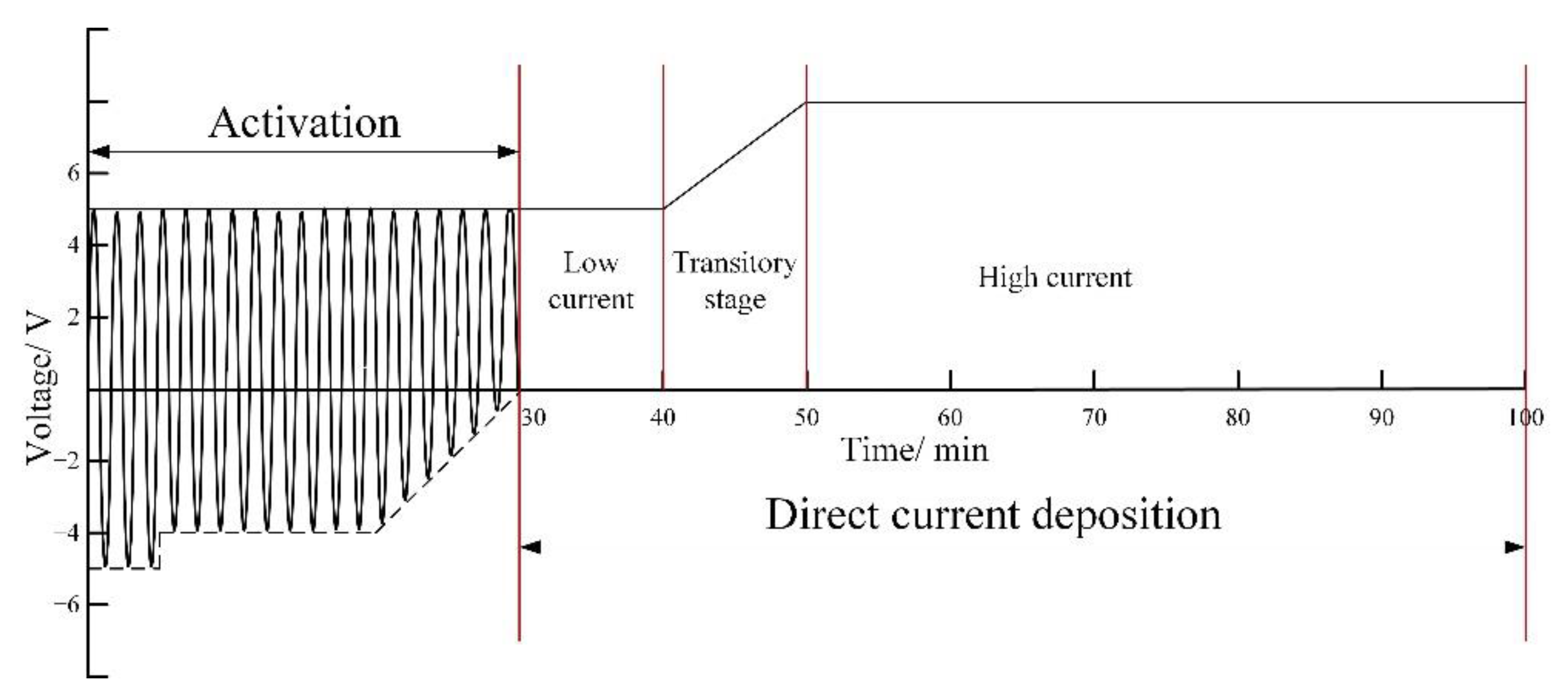
2.1. Materials and Electrodeposition
2.2. Electrodeposited Coating Analysis
3. Modelling of the Electrodeposition
3.1. Basic Assumptions
- 3.
- The walls of the electrochemical cell were assumed to be insulated, and the calculation domain was an isothermal system.
- 4.
- There was no extra stirring in the electrolyte, and the convection generated by hydrogen escaping along the electrodes was neglected [31].
- 5.
3.2. Governing Equations
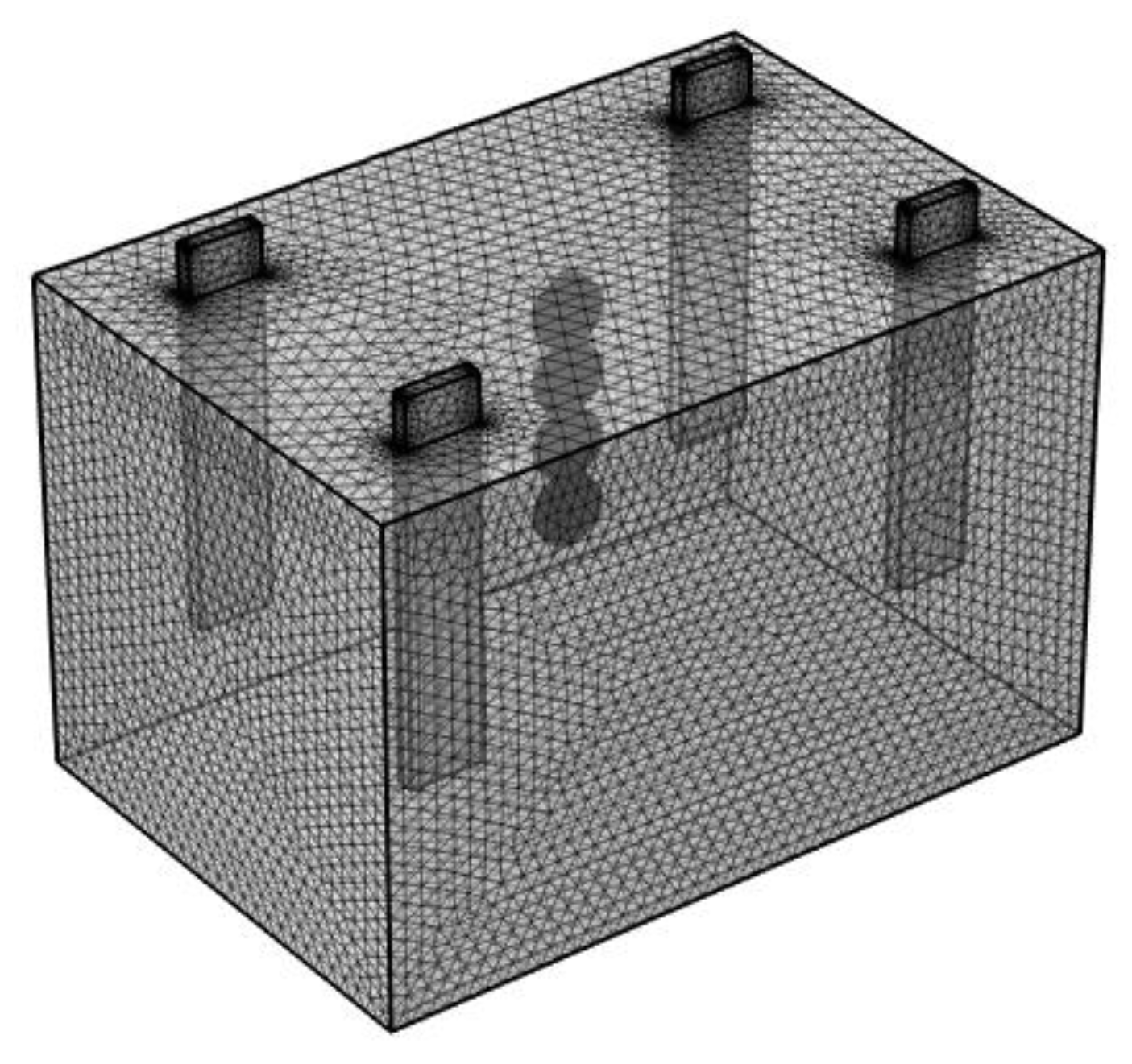
3.3. Boundary Conditions and Mesh
4. Results and Discussion
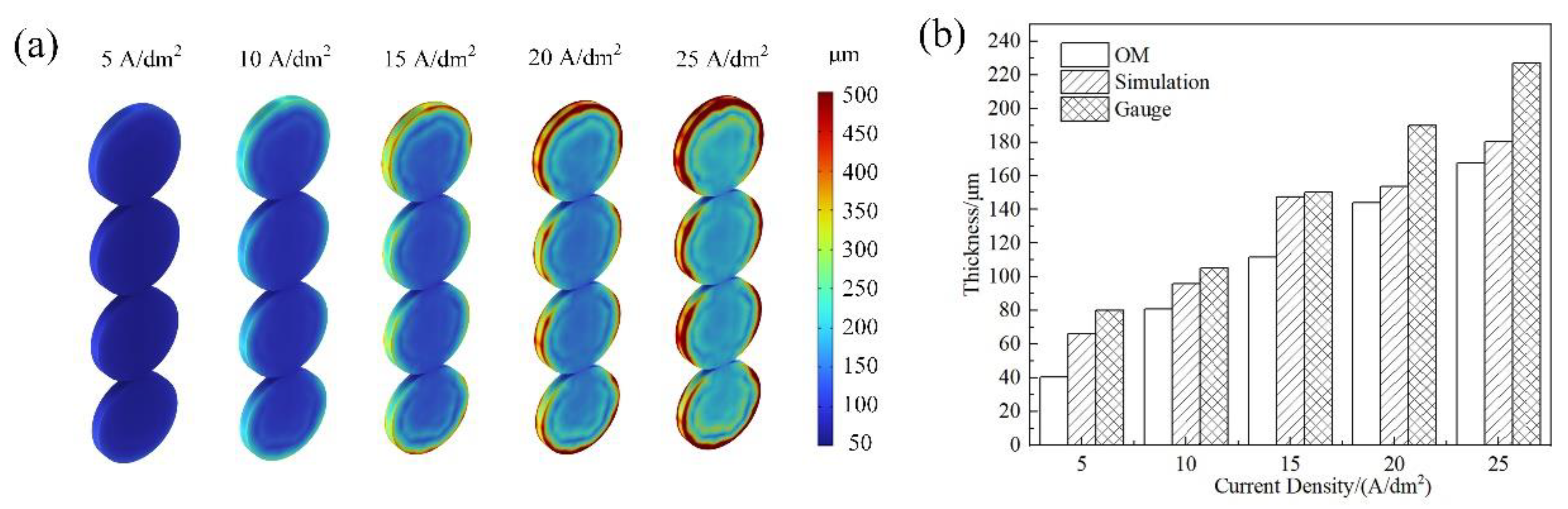
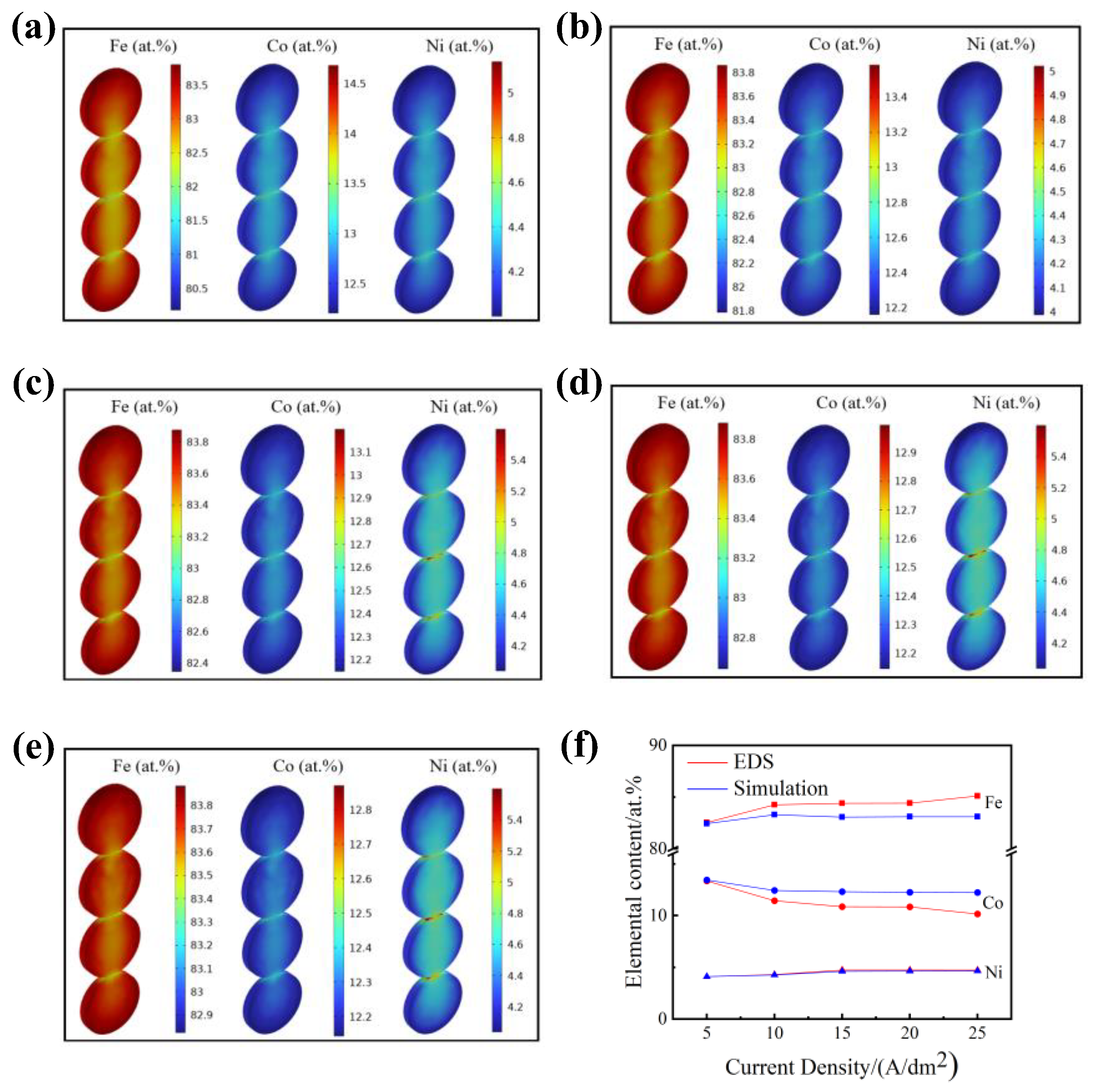
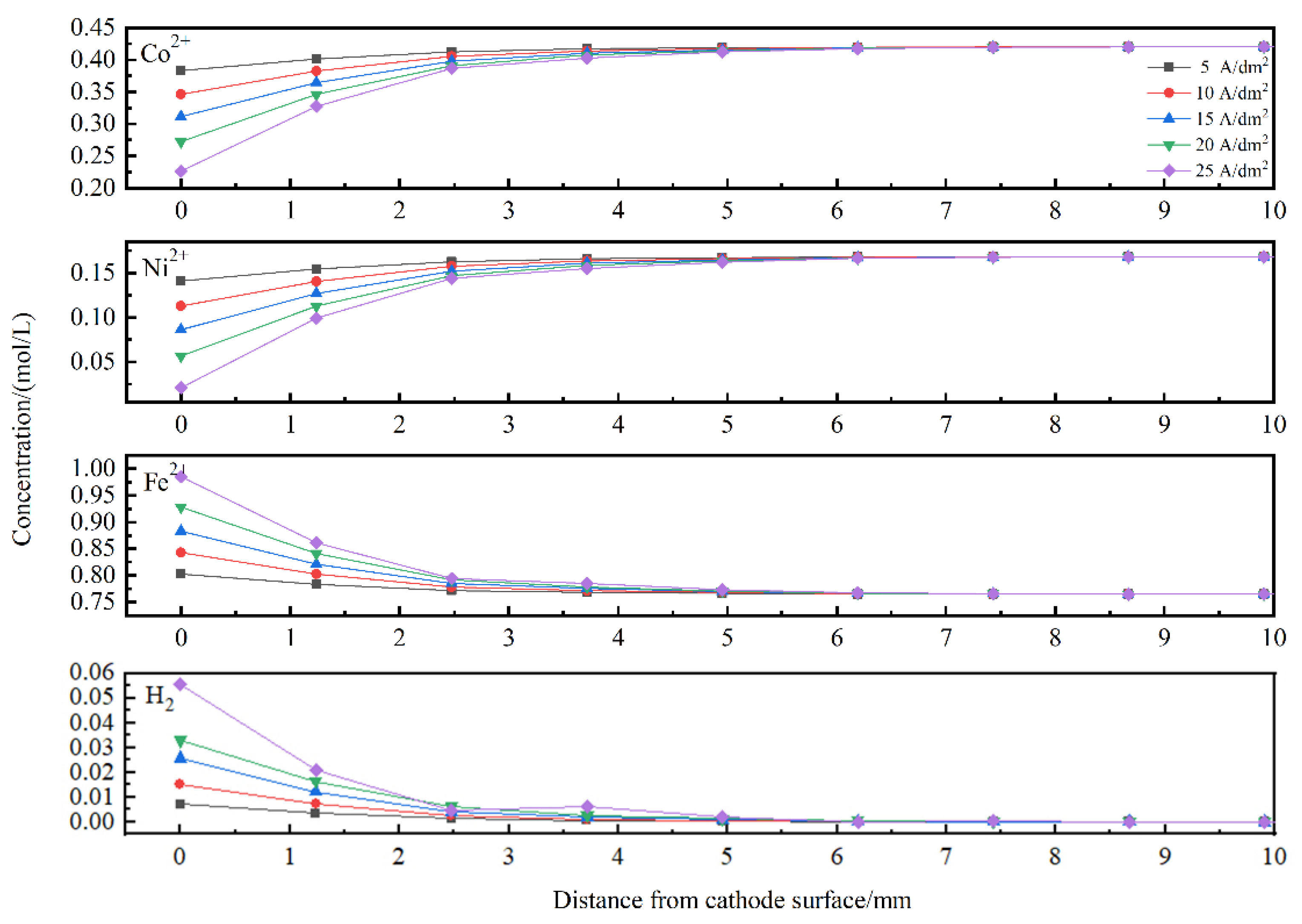
4.1. Simulating Results
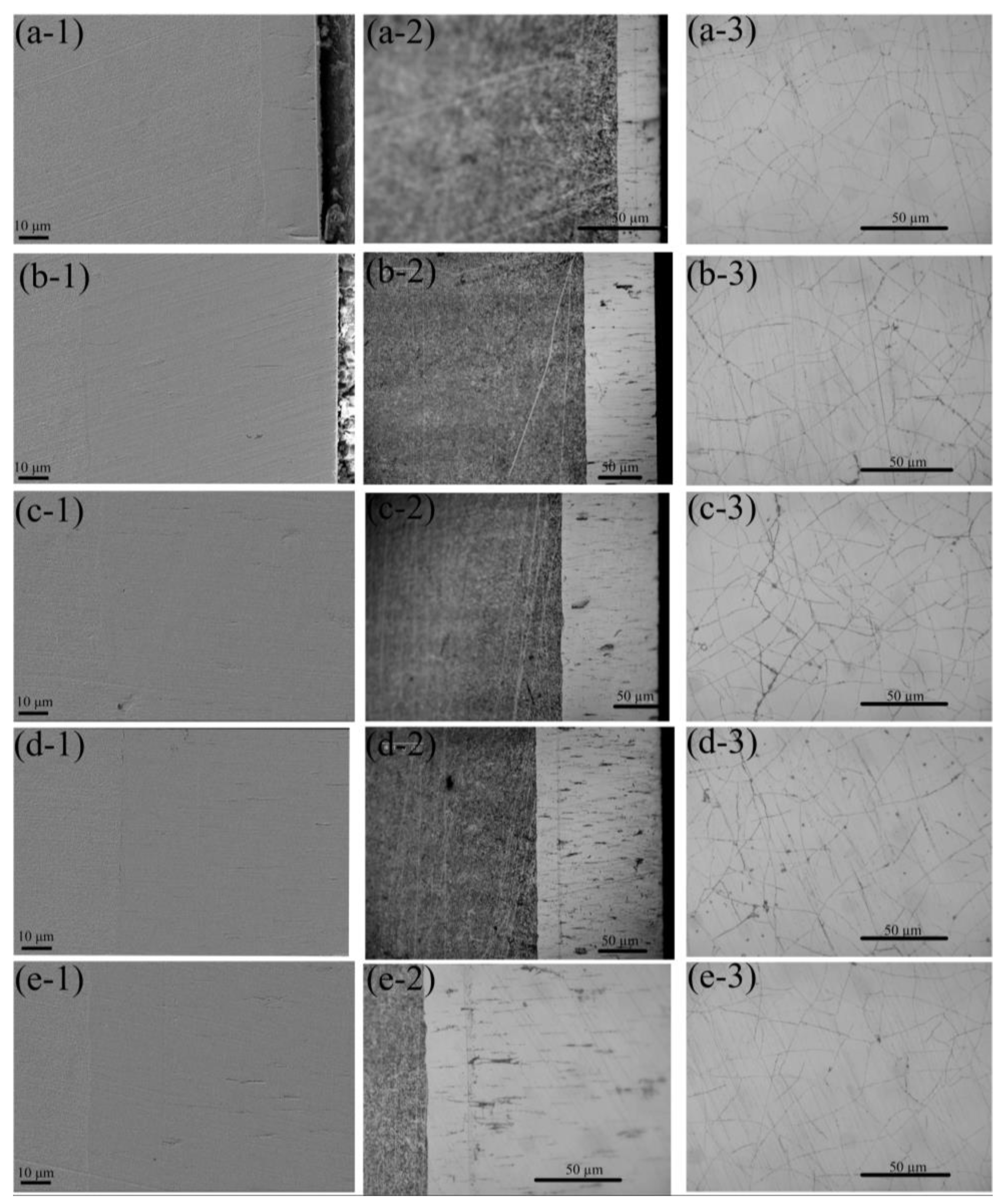
4.2. Experimental Results
4.3. Anomalous Behaviour Analysis
5. Conclusions
- The electrodeposition process was simulated based on the tertiary current distribution. Although there was a deviation between the simulated and experimental results in terms of the coating thickness and elemental contents, the numerical model was still able to predict the variation trend for thickness and element composition and to facilitate the design of the electrodeposition parameters.
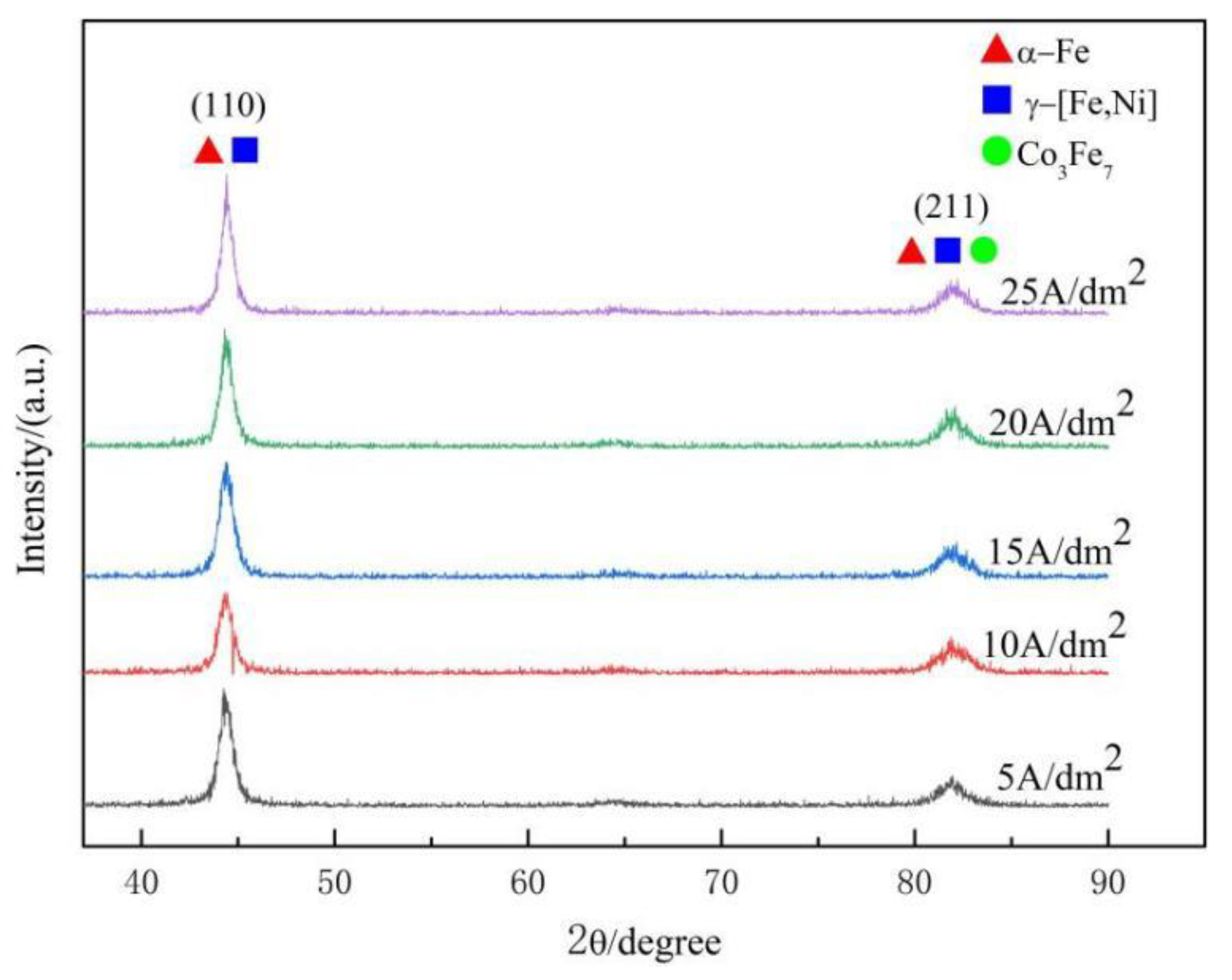
- Gradient-structured coatings were electrochemically fabricated using alternating current and direct current in sequence. Due to hydrogen evolution, microcracks perpendicular to the substrate occurred at the cross-section of the coating, and the network crack was distributed on the coating surface. The XRD patterns show that the deposited coatings consisted of the solid-solution phases α-Fe and γ(Fe, Ni) and the metallic compound Co3Fe7; the current density in the present studied range had a small influence on the phase composition. The grain sizes on the coating surface varied from 15 nm to 20 nm, and the current density showed no noticeable effect on the grain size. The microhardness of the deposited coatings ranged from 625 HV to 655 HV. Meanwhile, the average microhardness increased slightly as the current density increased from 5 A/dm2 to 10 A/dm2 and then decreased when the current density further increased.
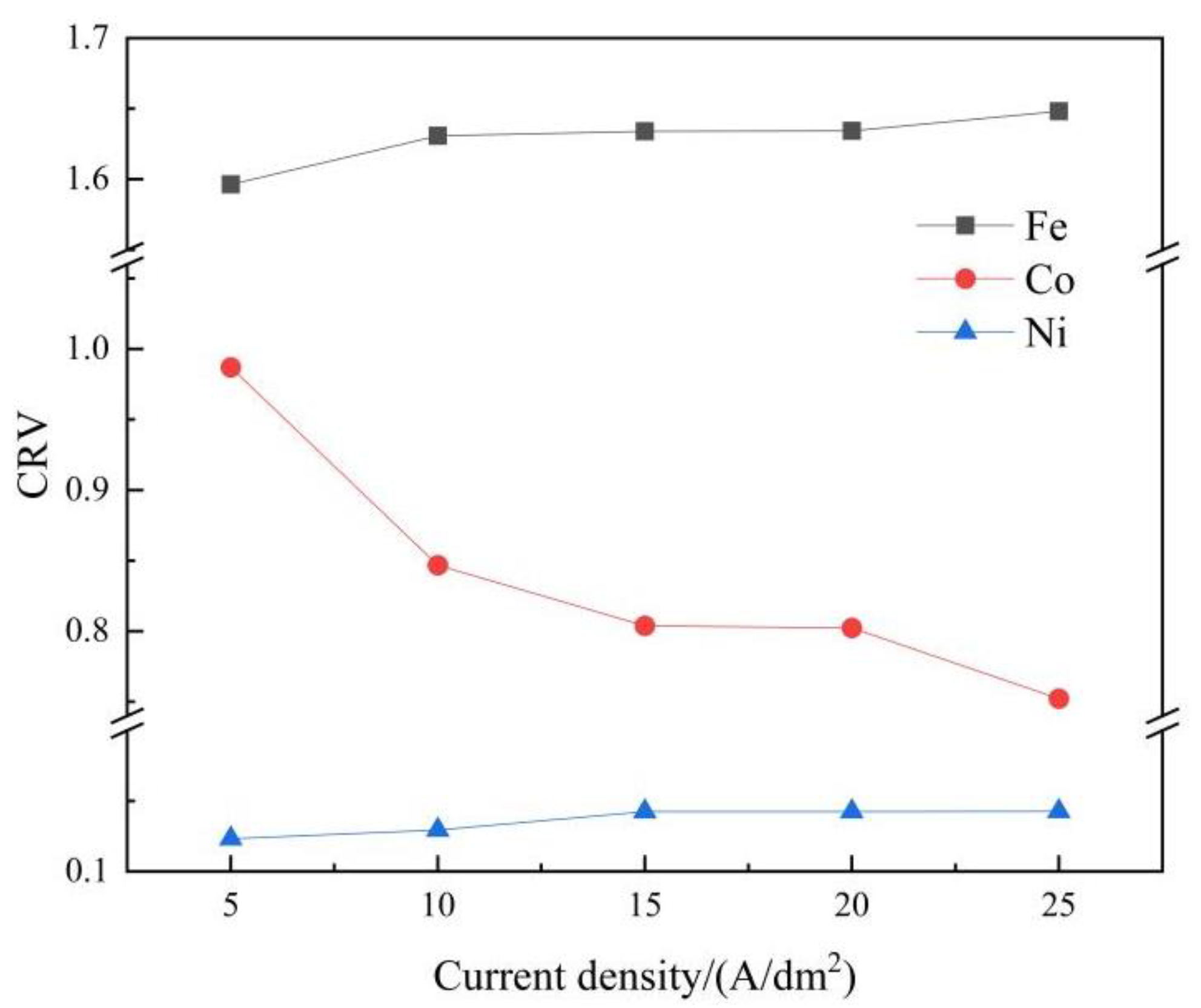
- The CRV was calculated to analyse the degree of anomaly. With increasing applied current density, the CRV of Fe increased slightly, while the CRV of Co decreased sharply, and the CRV of Ni remained steady at a relatively low current density and then increased gradually. Finally, the metal ions and hydrogen atom concentrations in the vicinity of the cathodic surface were numerically calculated. With increasing applied current density, the concentrations of Co2+ and Ni2+ decreased, while the Fe2+ and hydrogen atom concentration profiles showed the reverse trend.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karimzadeh, A.; Sabour, A.; Aliofkhazraei, M.; Miresmaeili, R. Sliding wear behavior of Ni–Co–P multilayer coatings electrodeposited by pulse reverse method. Tribol. Int. 2020, 141, 105914. [Google Scholar]
- Hyie, K.M.; Zabri, M.Z.; Roseley, N.R.N.; Masdek, N.R.N.M. Effect of deposition time on wear and corrosion performance of Co–Ni–Fe alloy coated mild steel. J. Mater. Res. 2016, 31, 1848–1856. [Google Scholar]
- Pellicer, E.; Varea, A.; Pané, S.; Sivaraman, K.M.; Nelson, B.J.; Suriñach, S.; Baró, M.D.; Sort, J. A comparison between fine-grained and nanocrystalline electrodeposited Cu–Ni films. Insights on mechanical and corrosion performance. Surf. Coat. Technol. 2011, 205, 5285–5293. [Google Scholar] [CrossRef]
- Harimkar, S.P.; Baksui, S.R.; Agarwal, A. Recent developments in surface engineering of materials. JOM 2013, 65, 739–740. [Google Scholar] [CrossRef]
- Larson, C.; Smith, J.; Armstrong, G. Current research on surface finishing and coatings for aerospace bodies and structures–a review. Trans. IMF 2013, 91, 120–132. [Google Scholar] [CrossRef]
- Phua, L.; Phuoc, N.; Ong, C. Effect of Ni concentration on microstructure, magnetic and microwave properties of electrodeposited NiCoFe films. J. Alloy. Compd. 2012, 543, 1–6. [Google Scholar]
- Koay, M.H.; Tukiran, M.A.A.; Halidi, S.N.A.M.; Murad, M.C.; Salleh, Z.; Yusoff, H.; Hyie, K. M Corrosion investigation of Co–Ni–Fe-coated mild steel electrodeposited at different current densities and deposition times. Ind. Lubr. Tribol. 2017, 69, 393–398. [Google Scholar]
- Torabinejad, V.; Aliofkhazraei, M.; Sabour Rouhaghdam, A.; Allahyarzadeh, M.H. Tribological properties of Ni-Fe-Co multilayer coatings fabricated by pulse electrodeposition. Tribol. Int. 2017, 106, 34–40. [Google Scholar]
- Ledwig, P.; Kac, M.; Kopia, A.; Falkus, J.; Dubiel, B. Microstructure and Properties of Electrodeposited Nanocrystalline Ni-Co-Fe Coatings. Materials 2021, 14, 3886. [Google Scholar] [CrossRef]
- Czerwiński, F.; Kedzierski, Z. On the mechanism of microcrack formation in nanocrystalline Fe–Ni electrodeposits. J. Mater. Sci. 1997, 32, 2957–2961. [Google Scholar]
- Xintan, H. Crankshaft restoration by iron plating. Met. Finish. 1997, 6, 98–100. [Google Scholar]
- Huang, R.X.; Ma, Z.; Dong, M.Z.; Shen, Y.; Du, F.M.; Xu, J.; Jin, M. On the Adhesive Strength Quantification and Tribological Performance of the Multilayered Fe–Ni Coating Fabricated by Electroplating. Strength Mater. 2019, 51, 280–290. [Google Scholar]
- Zech, N.; Podlaha, E.J.; Landolt, D. Anomalous codeposition of iron group metals: I. Experimental results. J. Electrochem. Soc. 1999, 146, 2886–2891. [Google Scholar]
- Brenner, A. Electrodeposition of Alloys: Principles and Practice; Academic Press: New York, NY, USA, 1963; Volume II. [Google Scholar]
- Khazi, I.; Mescheder, U. Micromechanical properties of anomalously electrodeposited nanocrystalline Nickel-Cobalt alloys: A review. Mater. Res. Express 2019, 6, 082001. [Google Scholar]
- Rozik, R.; Oriňàková, K.; Markušová, L. Trnková. The study of Ni–Co alloy deposition on iron powder particles in a fluidized bed from sulphate bath. J. Solid State Electrochem. 2005, 10, 423–429. [Google Scholar]
- Sarac, U.; Kaya, M.; Baykul, M.C. A Comparative Study on Microstructures, Magnetic Features and Morphologies of Ternary Fe-Co-Ni Alloy Thin Films Electrochemically Fabricated at Different Deposition Potentials. J. Supercond. Nov. Magn. 2018, 32, 917–923. [Google Scholar]
- Zech, N.; Podlaha, E.J.; Landolt, D. Anomalous codeposition of iron group metals: II. Mathematical model. J. Electrochem. Soc. 1999, 146, 2892. [Google Scholar]
- Zhuang, Y.; Podlaha, E.J. NiCoFe ternary alloy deposition: III. A mathematical model. J. Electrochem. Soc. 2003, 150, 225–233. [Google Scholar]
- Dahms, H.; Croll, I.M. The anomalous codeposition of iron-nickel alloys. J. Electrochem. Soc. 1965, 112, 771–775. [Google Scholar]
- Ping, L.; Jiayuan, X.; ShaoMin, Z. Studies on Ni-Fe Alloy Codeposition. Acta Phys. Chim. Sin. 1989, 5, 693–698. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, P. Uncertainty Quantification Analysis on Silicon Electrodeposition Process Via Numerical Simulation Methods. ASME J. Risk Uncertain. B 2022, 8, 011105. [Google Scholar]
- Fu, X.; Li, J.; Zhang, H.; Xian, J. Simulation and experimental research on nickel-based coating prepared by jet electrodeposition at different scanning speeds. Int. J. Adv. Manuf. Technol. 2022, 121, 1851–1866. [Google Scholar]
- Zhang, M.; Wang, J.; Cai, Y.; Jiao, C.; Ochieng Adede, S.; Zhu, C. Electroanalytical and Electrodeposited Simulation of Ce3+ in Molten LiCl-KCl. J. Electrochem. Soc. 2019, 166, 868–874. [Google Scholar]
- Khazi, I.; Mescheder, U.; Wilde, J. Influence of Bath Hydrodynamics on the Micromechanical Properties of Electrodeposited Nickel-Cobalt Alloys. Materials 2021, 14, 3898. [Google Scholar]
- Manzano, C.V.; Schürch, P.; Pethö, L.; Bürki, G.; Michler, J.; Philippe, L. High Aspect-Ratio Nanocrystalline CuNi T-Structures and Micro-Gears: Synthesis, Numerical Modeling and Characterization. J. Electrochem. Soc. 2019, 166, 310–316. [Google Scholar]
- Maraschky, A.; Akolkar, R. Pulsed Current Plating Does Not Improve Microscale Current Distribution Uniformity Compared to Direct Current Plating at Equivalent Time-Averaged Plating Rates. J. Electrochem. Soc. 2021, 168, 062507. [Google Scholar]
- Yang, G.; Deng, D.; Zhang, Y.; Zhu, Q.; Cai, J. Numerical Optimization of Electrodeposition Thickness Uniformity with Respect to the Layout of Anode and Cathode. Electrocatalysis 2021, 12, 478–488. [Google Scholar]
- Yue, B.; Zhu, G.; Wang, Y.; Song, J.; Chang, Z.; Guo, N.; Xu, M. Uncertainty analysis of factors affecting coating thickness distribution during nickel electrodeposition. J. Electroanal. Chem. 2021, 891, 115274. [Google Scholar]
- Belov, I.; Zanella, C.; Edström, C.; Leisner, P. Finite element modeling of silver electrodeposition for evaluation of thickness distribution on complex geometries. Mater. Des. 2016, 90, 693–703. [Google Scholar]
- Palli, S.; Dey, S.R. Theoretical and Experimental Study of Copper Electrodeposition in a Modified Hull Cell. Int. J. Electrochem. 2016, 2016, 3482406. [Google Scholar]
- Zhang, Z.; Zhang, J.Q.; Leng, W.H.; Cao, C.N. Investigation of the secondary current distribution in a hull cell. J. Electrochem. Soc. 2001, 148, 488–491. [Google Scholar]
- Solovjev, D.S.; Solovjeva, I.A.; Konkina, V.V.; Litovka, Y.V. Improving the uniformity of the coating thickness distribution during electroplating treatment of products using multi anode baths. Mater. Today Proc. 2019, 19, 1895–1898. [Google Scholar]
- ISO 2064:1996; Metallic and Other Inorganic Coatings-Definitions And Conventions Concerning The Measurement Of Thickness. ISO: Geneva, Switzerland, 1996.
- Pérez, T.; Arenas, L.; Villalobos-Lara, D.; Zhou, N.; Wang, S.; Walsh, F.; Nava, J.; de León, C. Simulations of fluid flow, mass transport and current distribution in a parallel plate flow cell during nickel electrodeposition. J. Electroanal. Chem. 2020, 873, 114359. [Google Scholar]
- Mahapatro, A.; Suggu, S.K. Modeling and simulation of electrodeposition: Effect of electrolyte current density and conductivity on electroplating thickness. Adv. Mater. Sci. 2018, 3, 1–9. [Google Scholar]
- Chung, C.K.; Zhou, R.X.; Chang, W.T. The anomalous behavior and properties of Ni–Co films codeposited in the sulfamate-chloride electrolyte. Microsyst. Technol. 2008, 14, 1279–1284. [Google Scholar]
- Podlaha, E.J.; Landolt, D. Induced Codeposition: II. A Mathematical Model Describing the Electrodeposition of Ni-Mo Alloys. J. Electrochem. Soc. 1996, 143, 893. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Y.-S.; Liu, Z.-M. Pyrolysis of iron phthalocyanine on activated carbon as highly efficient non-noble metal oxygen reduction catalyst in microbial fuel cells. Chem. Eng. J. 2019, 361, 416–427. [Google Scholar]
- Zheng, J.; Zhao, Y.; Xi, H.; Li, C. Seawater splitting for hydrogen evolution by robust electrocatalysts from secondary M (M = Cr, Fe, Co, Ni, Mo) incorporated Pt. RSC Adv. 2018, 8, 9423–9429. [Google Scholar]
- Seri, O. Differentiating approach to the Tafel slope of hydrogen evolution reaction on nickel electrode. Electrochem. Commun. 2017, 81, 150–153. [Google Scholar]
- Kumar, S.; Pande, S.; Verma, P. Factor effecting electro-deposition process. Int. J. Curr. Eng. Technol. 2015, 5, 700–703. [Google Scholar]
- Apelt, S.; Zhang, Y.; Zhu, J.; Leyens, C. Electrodeposition of Co-Mn3O4 composite coatings. Surf. Coat. Technol. 2015, 280, 208–215. [Google Scholar]
- Al Radadi, R.M.; Ibrahim, M.A.M. Nickel-cobalt alloy coatings prepared by electrodeposition Part I: Cathodic current efficiency, alloy composition, polarization behavior and throwing power. Korean J. Chem. Eng. 2020, 37, 1599–1608. [Google Scholar]
- Zhang, C. Influence of the Ratio of Total Acidity to pH Value of Conversion Bath and Surface Condition of Magnesium Alloy on the Corrosion Resistance of Phosphate Conversion Coating on Magnesium Alloy. Ph.D. Thesis, Harbin Engineering University, Harbin, China, 2019. (in Chinese). [Google Scholar]
- Su, Y.Z.; Fu, Y.C.; Wei, Y.M.; Yan, J.W.; Mao, B.W. The electrode/ionic liquid interface: Electric double layer and metal electrodeposition. Chemphyschem 2010, 11, 2764–2778. [Google Scholar]
- Marichev, V. A new possibility of application of electron tunneling effects in electrochemical double layer structure investigations. Surf. Sci. 1991, 250, 220–228. [Google Scholar]
- Hanafi, I.; Daud, D.A.R.; Radiman, S. Potentiostatic Electrodeposition of Co-Ni-Fe Alloy Particles Thin Film in a Sulfate Medium. Port. Electrochim. Acta 2017, 35, 1–12. [Google Scholar] [CrossRef]
- Yang, Y. Preparation of Fe-Co-Ni Ternary Alloys with Electrodeposition. Int. J. Electrochem. Sci. 2015, 10, 5164–5175. [Google Scholar]
- Zhang, Y.; Ivey, D.G. Characterization of Co-Fe and Co-Fe-Ni soft magnetic films electrodeposited from citrate-stabilized sulfate baths. Mater. Sci. Eng. B 2007, 140, 15–22. [Google Scholar]
- Grill, C.; Fries, M.; Jung, A.; Diebels, S. Numerical and experimental investigations of the electrodeposition process on open porous foams, determination of the parameter influence on the coating homogeneity. Int. J. Heat Mass Transf. 2021, 180, 121791. [Google Scholar]
- Bastos, A.; Zaefferer, S.; Raabe, D.; Schuh, C. Characterization of the microstructure and texture of nanostructured electrodeposited NiCo using electron backscatter diffraction (EBSD). Acta Mater. 2006, 54, 2451–2462. [Google Scholar]
- Brittain, C.P.; Smith, G.C. A Preliminary Investigation of the formation of cracks in hard chromium electrodeposits and the evolution of hydrogen during deposition. Trans. IMF 1955, 33, 289–305. [Google Scholar]
- Lin, Y.; Duan, F.; Pan, J.; Zhang, C.; Chen, Q.; Lu, J.; Liu, L. On the adhesion performance of gradient-structured Ni-P metallic coatings. Mater. Sci. Eng. A 2022, 844, 143170. [Google Scholar] [CrossRef]
- Wu, T.; Pan, B.; Tian, Y. Effect of Low Temperature Iron Plating Current Density on Microstructure, Microstructure and Hardness of Coatings. Mater. Prot. 2012, 45, 27–29. (In Chinese) [Google Scholar]
- Guo, T.; Huang, P.; Xu, K.; Wang, F.; Lu, T. Solid solution effects on hardness and strain rate sensitivity of nanocrystalline NiFe alloy. Mater. Sci. Eng. A 2016, 676, 501–505. [Google Scholar] [CrossRef]
- Liu, X.; Zangari, G.; Shamsuzzoha, M. Structural and magnetic characterization of electrodeposited, high moment FeCoNi films. J. Electrochem. Soc. 2003, 150, 159–168. [Google Scholar] [CrossRef]
- Thiemig, D.; Bund, A.; Talbot, J.B. Influence of hydrodynamics and pulse plating parameters on the electrocodeposition of nickel–alumina nanocomposite films. Electrochim. Acta 2009, 54, 2491–2498. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Wu, G.-X.; Lin, J.-C.; Hwang, Y.-R.; Wei, D.-H.; Chang, S.-Y.; Peng, K.-C. Preparation of Co-Fe-Ni alloy micropillar by microanode-guided electroplating. J. Alloy. Compd. 2021, 885, 160873. [Google Scholar] [CrossRef]
- Ghaferi, Z.; Sharafi, S.; Bahrololoom, M. Effect of current density and bath composition on crystalline structure and magnetic properties of electrodeposited FeCoW alloy. Appl. Surf. Sci. 2015, 355, 766–773. [Google Scholar] [CrossRef]
- Cheung, C.; Djuanda, F.; Erb, U.; Palumbo, G. Electrodeposition of nanocrystalline Ni-Fe alloys. Nanostructured materials 1995, 5, 513–523. [Google Scholar] [CrossRef]
- Wu, B.Y.C.; Schuh, C.A.; Ferreira, P.J. Nanostructured Ni-Co alloys with tailorable grain size and twin density. Metall. Mater. Trans. A 2005, 36, 1927–1936. [Google Scholar] [CrossRef]
- Rasmussen, F.; Ravnkilde, J.; Tang, P.; Hansen, O.; Bouwstra, S. Electroplating and characterization of cobalt-nickel-iron and nickel-iron for magnetic microsystems applications. Sens. Actuators A 2001, 92, 242–248. [Google Scholar] [CrossRef]
- Zamani, M.; Amadeh, A.; Baghal, S.L. Effect of Co content on electrodeposition mechanism and mechanical properties of electrodeposited Ni-Co alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 484–491. [Google Scholar] [CrossRef]
- Sasaki, K.Y.; Talbot, J.B. Electrodeposition of Iron-Group Metals and Binary Alloys from Sulfate Baths. II. Modeling. J. Electrochem. Soc. 2000, 147, 189–197. [Google Scholar] [CrossRef]
- Matlosz, M. Competitive adsorption effects in the electrodeposition of iron-nickel alloys. J. Electrochem. Soc. 1993, 140, 2272–2279. [Google Scholar] [CrossRef]
- Hessami, S.; Tobias, C.W. A Mathematical Model for Anomalous Codeposition of Nickel-Iron on a Rotating Disk Electrode. J. Electrochem. Soc. 1989, 136, 3611–3616. [Google Scholar] [CrossRef]

| Research Objects | Governing Equations | Parameter Explanations and Notes |
|---|---|---|
| Electrodes | ||
| Electrolyte | is electric flux. | |
| Electrode interface | ||
| Local current density | are the species . |
| Symbol | Terminology | Value | Unit |
|---|---|---|---|
| Eeq_Fe | Iron equilibrium potential | −0.444 | V |
| Eeq_Co | Cobalt equilibrium potential | −0.290 | V |
| Eeq_Ni | Nickel equilibrium potential | −0.276 | V |
| j0 | Current density | 5~25 | A/dm2 |
| jFe | Exchange current density of iron [39] | 2.09 | A/m2 |
| jCo | Exchange current density of cobalt [40] | 0.23 | A/m2 |
| jNi | Exchange current density of nickel [29] | 0.1 | A/m2 |
| jH | Exchange current density of hydrogen [26] | 2 × 10−5 | A/m2 |
| ρFe | Density of iron | 7900 | kg/m3 |
| ρCo | Density of cobalt | 8900 | kg/m3 |
| ρNi | Density of nickel | 8910 | kg/m3 |
| MFe | Molar mass iron | 56 | g/mol |
| MCo | Molar mass of cobalt | 58.93 | g/mol |
| MNi | Molar mass of nickel | 58.693 | g/mol |
| cFe | Fe2+ concentration | 0.765 | mol/L |
| cCo | Co2+ concentration | 0.42 | mol/L |
| cNi | Ni2+ concentration | 0.168 | mol/L |
| zFe | Charge of iron | 2 | - |
| zCo | Charge of cobalt | 2 | - |
| zNi | Charge of nickel | 2 | - |
| DFe | Diffusion coefficient of iron | 1.59 × 10−9 | m2/s |
| DCo | Diffusion coefficient of cobalt | 1.31 × 10−9 | m2/s |
| DNi | Diffusion coefficient of nickel | 1.27 × 10−9 | m2/s |
| A | Cathode surface area | 1 | dm2 |
| σ | Electrolyte conductivity [29] | 10 | S/m |
| T0 | Temperature | 348.15 | K |
| Ac | Tafel slope for hydrogen evolution [41] | −188 | mV |
| αa | Anode transfer coefficient [41] | 0.5 | - |
| αc | Cathode transfer coefficient [41] | 0.5 | - |
| Boundary (Surface) | V | A | c |
|---|---|---|---|
| Electrochemical cell | - | ||
| Bath | - | ||
| Anode | |||
| Cathode |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Yu, J.; Liu, Z.; Yin, Y.; Qiao, C. Numerical and Experimental Investigation of the Effect of Current Density on the Anomalous Codeposition of Ternary Fe-Co-Ni Alloy Coatings. Materials 2022, 15, 6141. https://doi.org/10.3390/ma15176141
Zhang S, Yu J, Liu Z, Yin Y, Qiao C. Numerical and Experimental Investigation of the Effect of Current Density on the Anomalous Codeposition of Ternary Fe-Co-Ni Alloy Coatings. Materials. 2022; 15(17):6141. https://doi.org/10.3390/ma15176141
Chicago/Turabian StyleZhang, Shuai, Jing Yu, Zhengda Liu, Yanjun Yin, and Chenfeng Qiao. 2022. "Numerical and Experimental Investigation of the Effect of Current Density on the Anomalous Codeposition of Ternary Fe-Co-Ni Alloy Coatings" Materials 15, no. 17: 6141. https://doi.org/10.3390/ma15176141





