Investigation of the Effect of Particle Surface Charge and Dispersion Stability on Latex Behavior in Cement Using Non-Ionic and Traditional Latexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of the Polyurethane-Polystyrene Latex
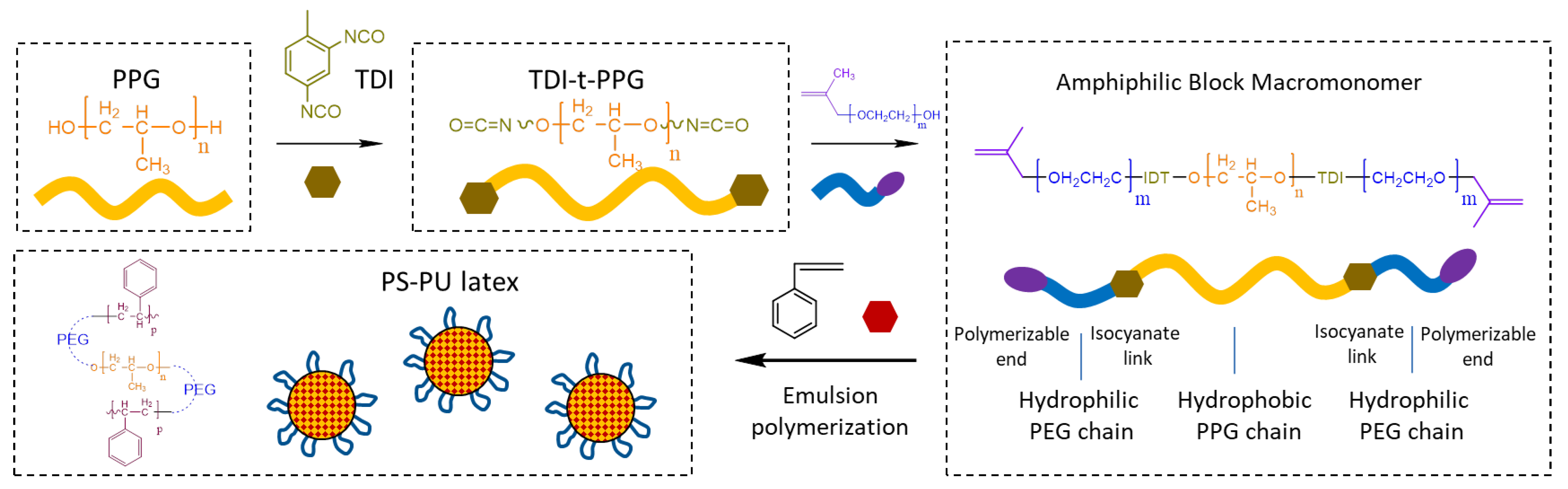
2.2.1. Preparation of the Latex
2.2.2. Characterization of the Latex
2.3. Mortar Testing
2.4. Paste Characterization
2.4.1. Paste Preparation
2.4.2. Characterization of Early Age Hydration
2.4.3. Characterization of Hydration at Later Ages
3. Results
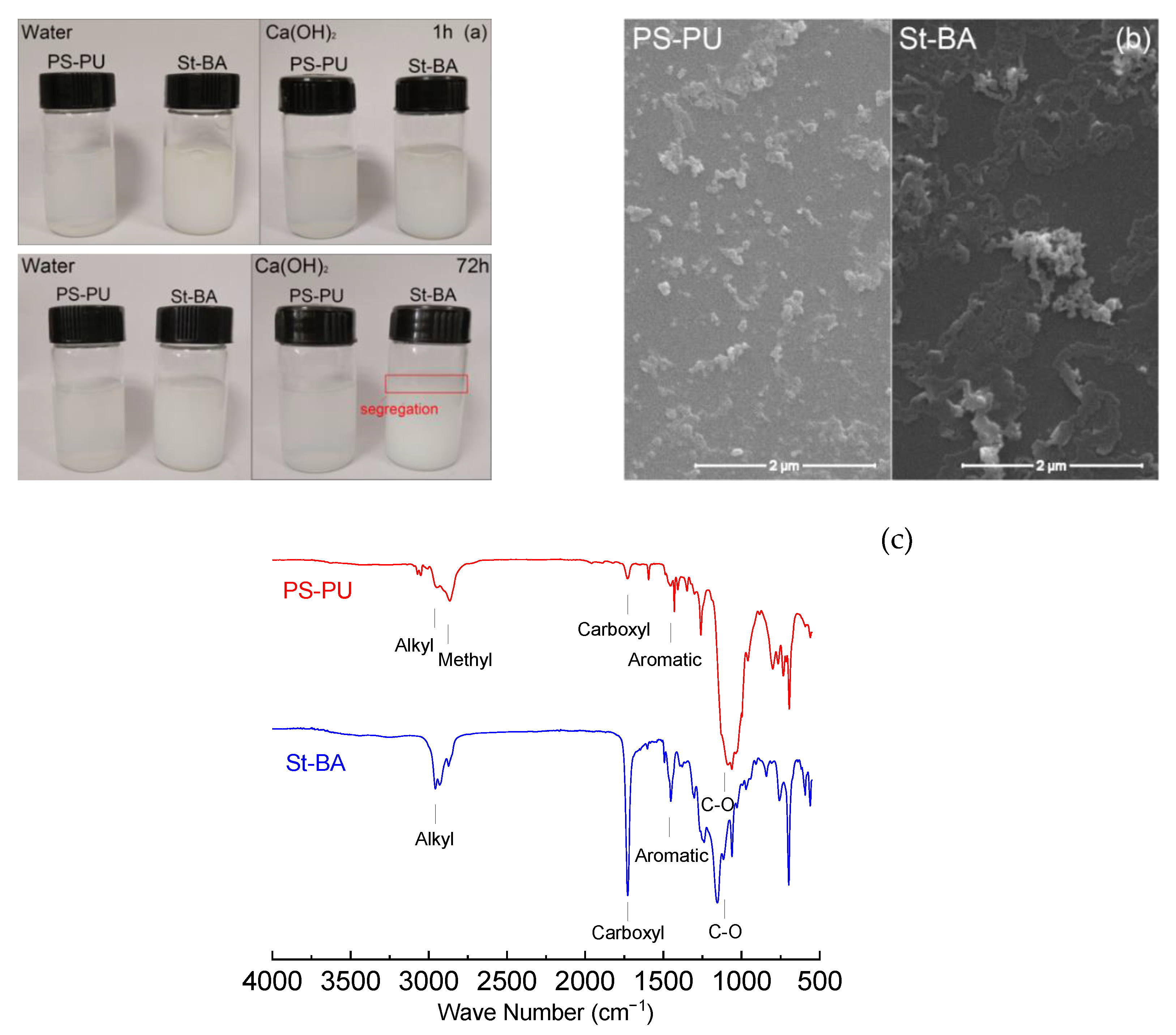
3.1. Preparation and Characteristics of Polymeric Nanoparticles
3.2. Effect of the Nano Latexes on Mortars
3.2.1. Mortar Fresh Properties
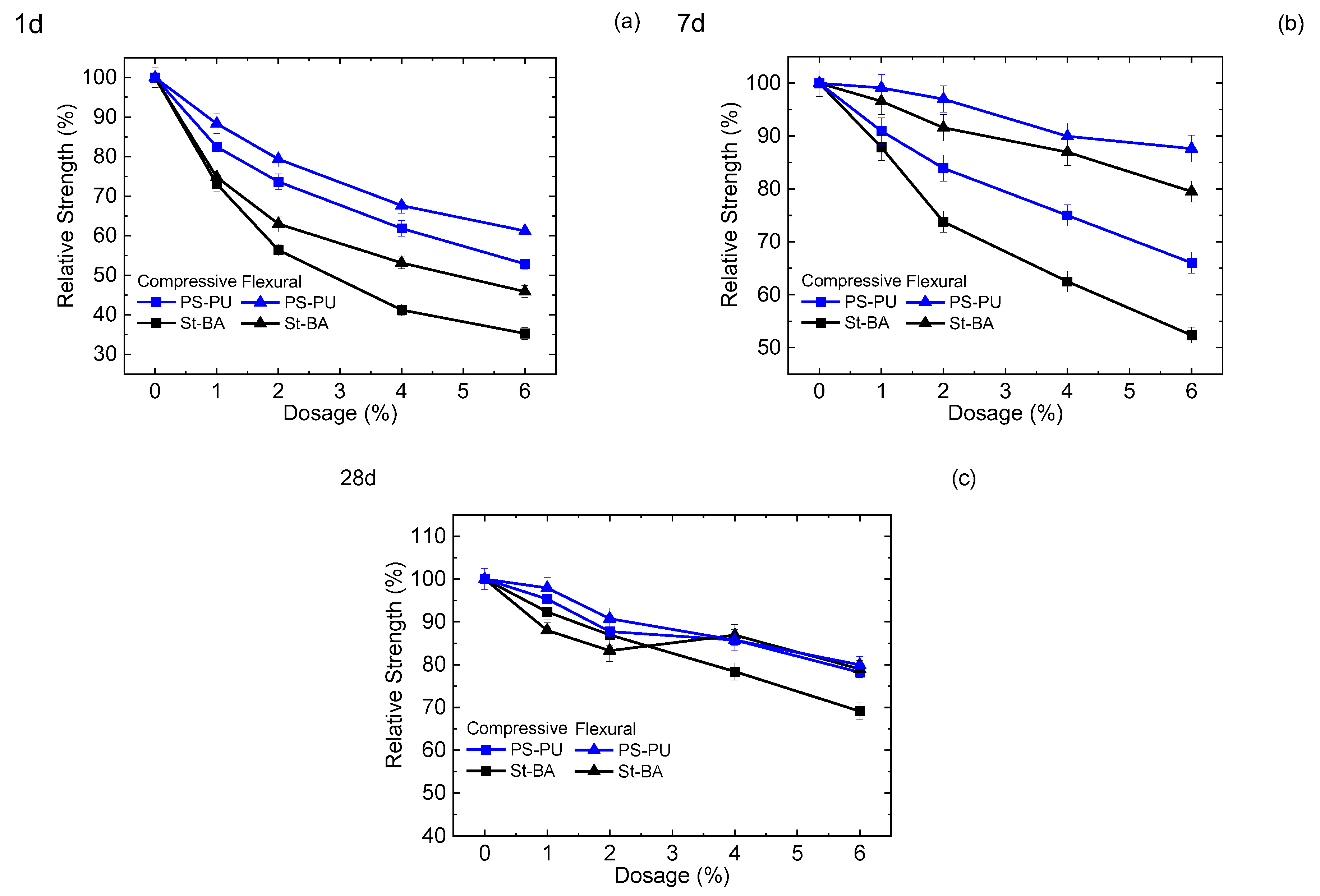
3.2.2. Mortar Strength
3.3. Interaction between the Latexes and Cement
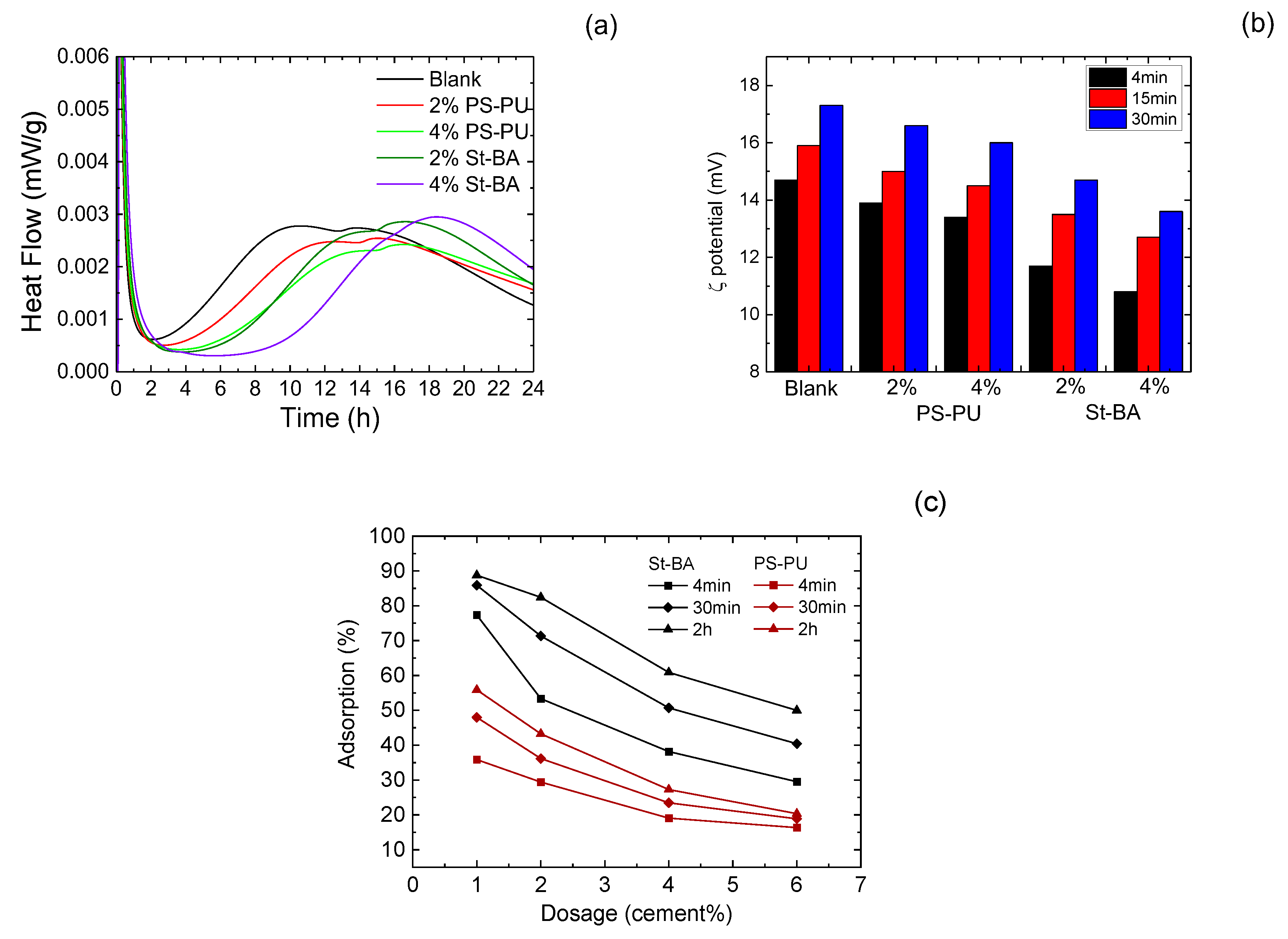
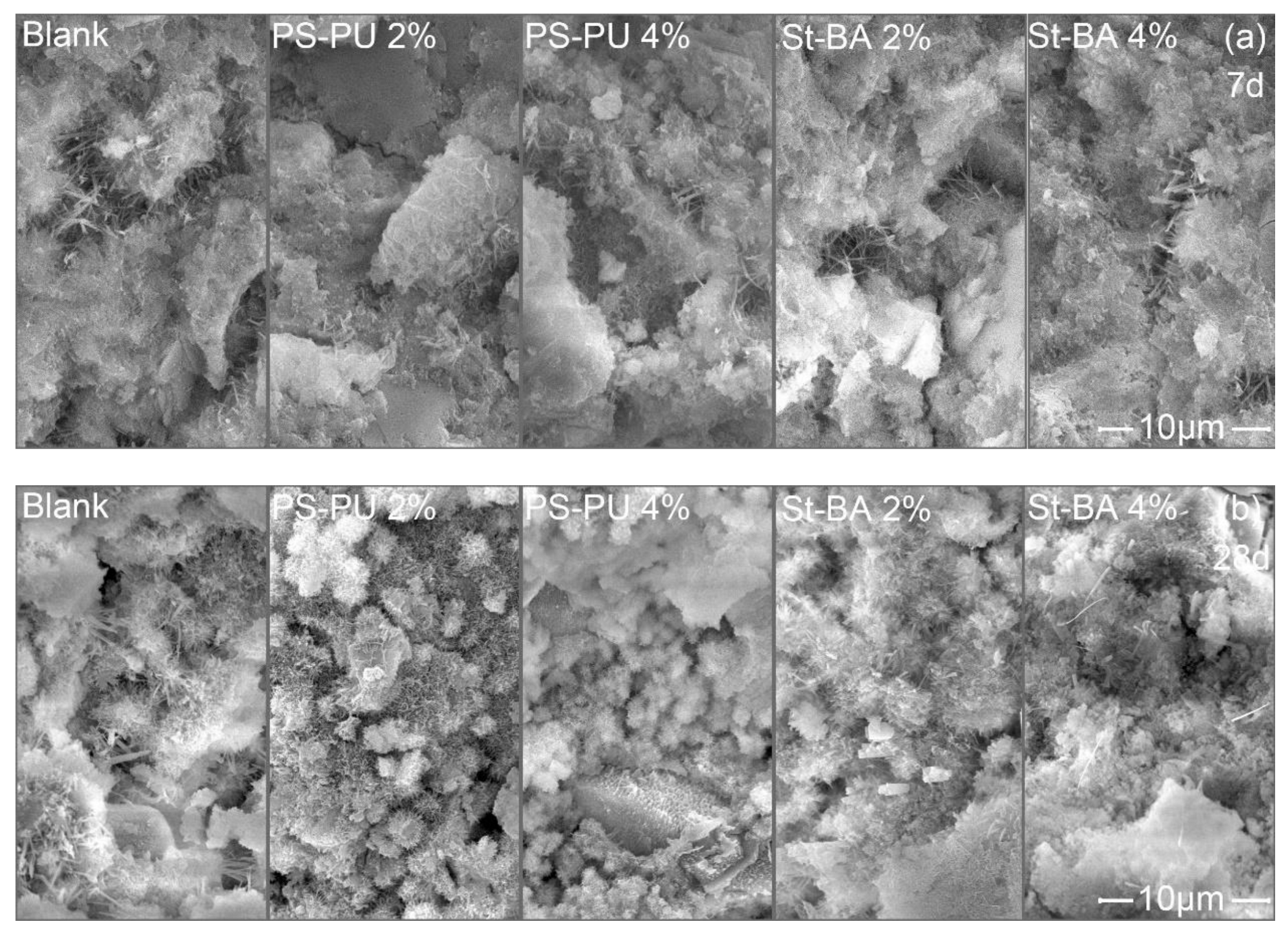
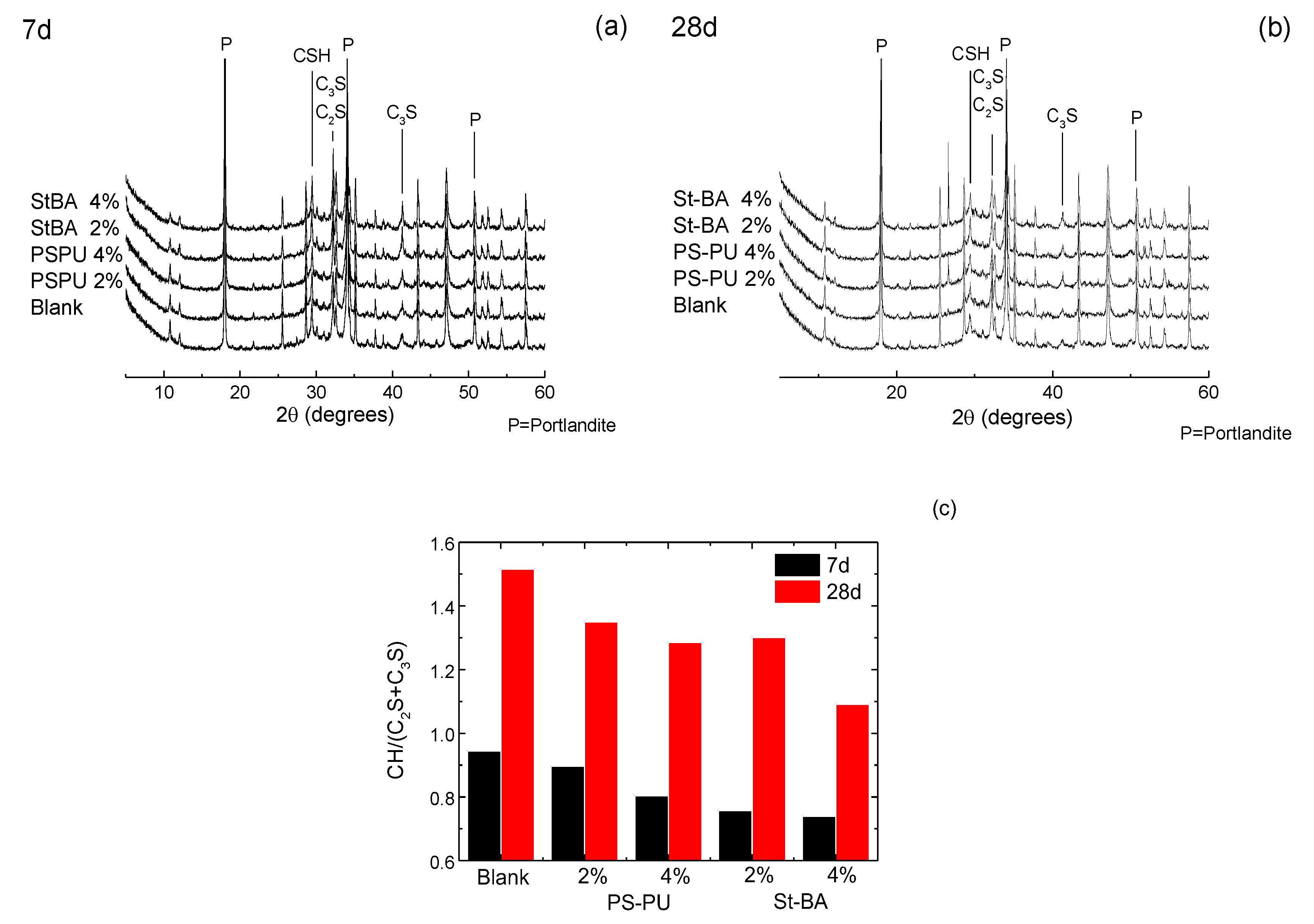
3.3.1. Interaction in Early Ages
3.3.2. Interaction in Later Ages
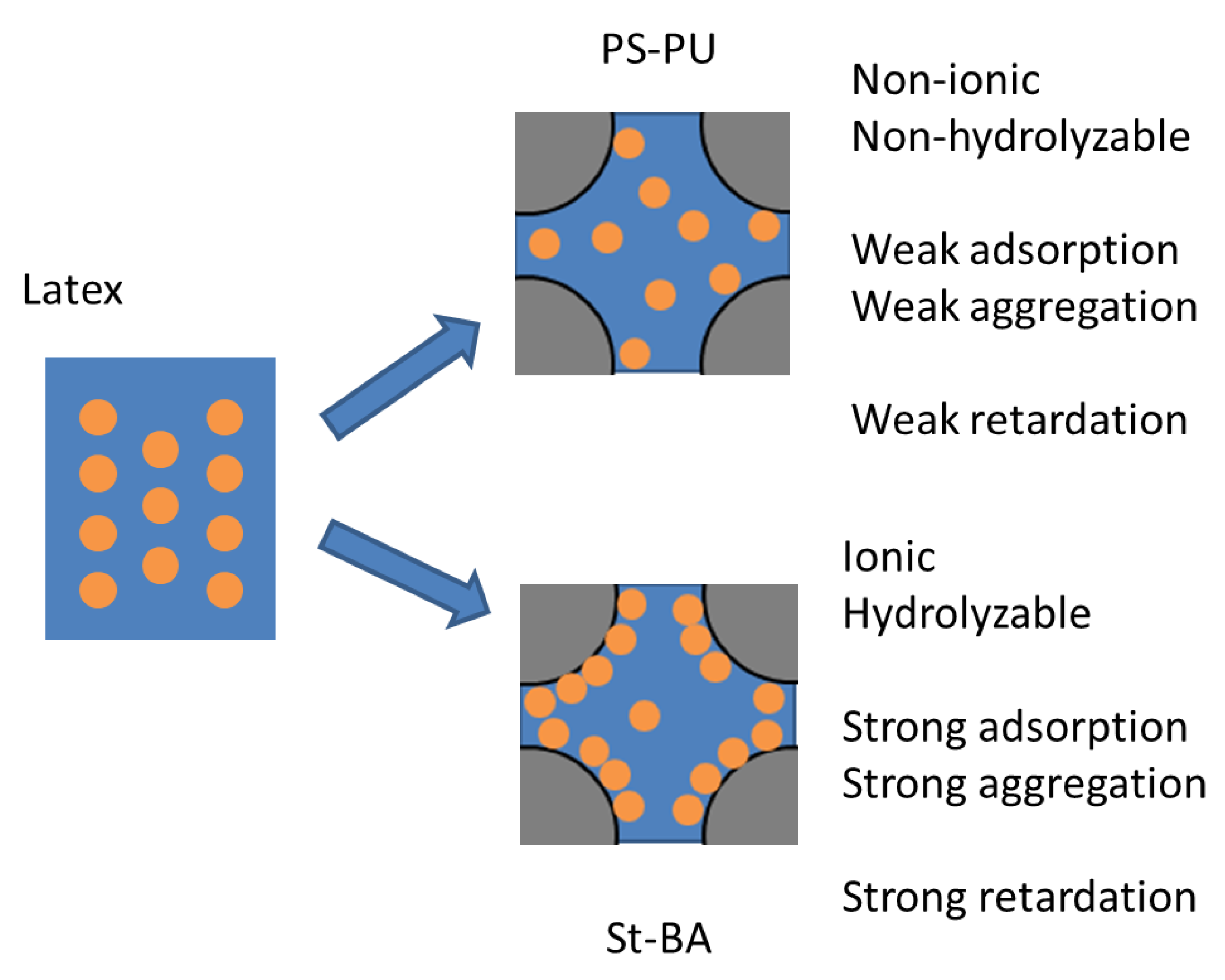
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aitcin, P.C.; Flatt, R.J. Science and Techlology of Concrete Admixtures; WoodHead Publishing: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Peng, J.; Ellingham, T.; Sabo, R.; Turng, L.-S.; Clemons, C.M. Short cellulose nanofibrils as reinforcement in polyvinyl alcohol fiber. Cellulose 2014, 21, 4287–4298. [Google Scholar] [CrossRef]
- Richard, P.; Cheyrezy, M. Composition of reactive powder concretes. Cem. Concr. Res. 1995, 25, 1501–1511. [Google Scholar] [CrossRef]
- Aggarwal, L.K.; Thapliyal, P.C.; Karade, S.R. Properties of polymer-modified mortars using epoxy and acrylic emulsions. Constr. Build. Mater. 2007, 21, 379–383. [Google Scholar] [CrossRef]
- Li, P.; Lu, W.; An, X.; Zhou, L.; Du, S. Effect of Epoxy Latexes on the Mechanical Behavior and Porosity Property of Cement Mortar with Different Degrees of Hydration and Polymerization. Materials 2021, 14, 517. [Google Scholar] [CrossRef]
- Kujawa, W.; Olewnik-Kruszkowska, E.; Nowaczyk, J. Concrete Strengthening by Introducing Polymer-Based Additives into the Cement Matrix-A Mini Review. Materials 2021, 14, 6071. [Google Scholar] [CrossRef]
- Bian, Y.; Huang, Y.; Li, F.; Dong, D.; Zhao, H.; Zhao, P.; Lu, L. Polyvinyl-Alcohol-Modified Calcium Sulphoaluminate Cement Repair Mortar: Hydration and Properties. Materials 2021, 14, 7834. [Google Scholar] [CrossRef]
- Han, J.-W.; Jeon, J.-H.; Park, C.-G. Mechanical and Permeability Characteristics of Latex-Modified Pre-Packed Pavement Repair Concrete as a Function of the Rapid-Set Binder Content. Materials 2015, 8, 6728–6737. [Google Scholar] [CrossRef]
- Cao, C.; Mei, M.; Li, Q.-l.; Lo, E.; Chu, C. Methods for Biomimetic Mineralisation of Human Enamel: A Systematic Review. Materials 2015, 8, 2873–2886. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.G.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Ohama, Y. Recent progress in concrete-polymer composites. Adv. Cem. Based Mater. 1997, 5, 31–40. [Google Scholar] [CrossRef]
- Ohama, Y. Polymer-based admixtures. Cem. Concr. Compos. 1998, 20, 189–212. [Google Scholar] [CrossRef]
- Lu, Z.; Merkl, J.-P.; Pulkin, M.; Firdous, R.; Wache, S.; Stephan, D. A Systematic Study on Polymer-Modified Alkali-Activated Slag-Part II: From Hydration to Mechanical Properties. Materials 2020, 13, 3418. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, G.; Deng, S.; Deng, C.; Wang, Z.; Zhang, Z. Effect of Carbon Nanotube and Styrene-Acrylic Emulsion Additives on Microstructure and Mechanical Characteristics of Cement Paste. Materials 2020, 13, 2807. [Google Scholar] [CrossRef]
- Kim, H.-H.; Kim, C.-S.; Jeon, J.-H.; Park, C.-G. Effects on the Physical and Mechanical Properties of Porous Concrete for Plant Growth of Blast Furnace Slag, Natural Jute Fiber, and Styrene Butadiene Latex Using a Dry Mixing Manufacturing Process. Materials 2016, 9, 84. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.-M. Action of redispersible vinyl acetate and versatate copolymer powder in cement mortar. Constr. Build. Mater. 2011, 25, 4210–4214. [Google Scholar] [CrossRef]
- Schulze, J. Influence of water-cement ratio and cement content on the properties of polymer-modified mortars. Cem. Concr. Res. 1999, 29, 909–915. [Google Scholar] [CrossRef]
- Lee, S.-J.; Shin, H.-J.; Park, C.-G. Mechanical and Durability Properties of Latex-Modified Hybrid Fiber-Reinforced Roller-Compacted Rapid-Set Cement Concrete for Pavement Repair. Materials 2021, 14, 3981. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Emmerling, S.; Pakusch, J.; Rueckel, M.; Nieberle, J. Retardation effect of styrene-acrylate copolymer latexes on cement hydration. Cem. Concr. Res. 2015, 75, 23–41. [Google Scholar] [CrossRef]
- Lazniewska-Piekarczyk, B. The type of air-entraining and viscosity modifying admixtures and porosity and frost durability of high performance self-compacting concrete. Constr. Build. Mater. 2013, 40, 659–671. [Google Scholar] [CrossRef]
- Du, L.X.; Folliard, K.J. Mechanisms of air entrainment in concrete. Cem. Concr. Res. 2005, 35, 1463–1471. [Google Scholar] [CrossRef]
- Nehdi, M.; Sumner, J. Recycling waste latex paint in concrete. Cem. Concr. Res. 2003, 33, 857–863. [Google Scholar] [CrossRef]
- Smith, W.V.; Ewart, R.H. Kinetics of Emulsion Polymerization. J. Chem. Phys. 1948, 16, 592–599. [Google Scholar] [CrossRef]
- Ma, H.; Li, Z. Microstructures and mechanical properties of polymer modified mortars under distinct mechanisms. Constr. Build. Mater. 2013, 47, 579–587. [Google Scholar] [CrossRef]
- Kardon, J.B. Polymer-modified concrete: Review. J. Mater. Civ. Eng. 1997, 9, 85–92. [Google Scholar] [CrossRef]
- Silvestre, J.; Silvestre, N.; de Brito, J. Review on concrete nanotechnology. Eur. J. Environ. Civ. Eng. 2016, 20, 455–485. [Google Scholar] [CrossRef]
- Yousefi, N.; Gudarzi, M.M.; Zheng, Q.B.; Lin, X.Y.; Shen, X.; Jia, J.J.; Sharif, F.; Kim, J.K. Highly aligned, ultralarge-size reduced graphene oxide/polyurethane nanocomposites: Mechanical properties and moisture permeability. Compos. Part A-Appl. Sci. Manuf. 2013, 49, 42–50. [Google Scholar] [CrossRef]
- Van, V.-T.-A.; Rößler, C.; Bui, D.-D.; Ludwig, H.-M. Mesoporous structure and pozzolanic reactivity of rice husk ash in cementitious system. Constr. Build. Mater. 2013, 43, 208–216. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Ge, C.; Li, Q.; Guo, L.; Shu, X.; Liu, J. Investigation of the effectiveness of PC@GO on the reinforcement for cement composites. Constr. Build. Mater. 2016, 113, 470–478. [Google Scholar] [CrossRef]
- Kantarci, F.; Turkmen, I.; Ekinci, E. Improving elevated temperature performance of geopolymer concrete utilizing nano-silica, micro-silica and styrene-butadiene latex. Constr. Build. Mater. 2021, 286, 122980. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Raki, L.; Beaudoin, J.; Alizadeh, R.; Makar, J.; Sato, T. Cement and Concrete Nanoscience and Nanotechnology. Materials 2010, 3, 918–942. [Google Scholar] [CrossRef]
- GB/T 8076-2008; Regulations for Concrete Admixtures. Administration of Quality Supervision. Standardization Administration of China: Beijing, China, 2008.
- GB/T 17671-1999; Methods of Testing Cements—Determination of Strength. Administration of Quality Supervision. Standardization Administration of China: Beijing, China, 1999.
- GB/T 8077-2012; Methods for Testing Uniformity of Concrete Admixture. Administration of Quality Supervision. Standardization Administration of China: Beijing, China, 2012.
- GB/T 1346-2011; Measurment for Water Use, Setting Time and Stability of Standard-Viscoucity Cement. Administration of Quality Supervision. Standardization Administration of China: Beijing, China, 2011.
- Kong, X.; Shi, Z.; Lu, Z. Synthesis of novel polymer nano-particles and their interaction with cement. Constr. Build. Mater. 2014, 68, 434–443. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Shah, S.P.; Mishra, G.; Sharma, U. Studies on early stage hydration of tricalcium silicate incorporating silica nanoparticles: Part II. Constr. Build. Mater. 2016, 102, 943–949. [Google Scholar] [CrossRef]
- Grangeon, S.; Claret, F.; Linard, Y.; Chiaberge, C. X-ray diffraction: A powerful tool to probe and understand the structure of nanocrystalline calcium silicate hydrates. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2013, 69, 465–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Compound | Content (%) |
|---|---|
| SiO2 | 21.23 |
| Al2O3 | 4.83 |
| CaO | 64.34 |
| MgO | 1.81 |
| Fe2O3 | 3.12 |
| SO3 | 3.32 |
| K2O | 0.68 |
| Na2O | 0.19 |
| Free lime | 1.15 |
| Total | 99.26 |
| Content | Blank | Latex-Modified Samples | |||
|---|---|---|---|---|---|
| 1.0% | 2.0% | 4.0% | 6.0% | ||
| Cement(kg/m3) | 560 ± 0.5 | ||||
| Water(kg/m3) | 224 ± 0.2 | ||||
| Sand(kg/m3) | 1512 ± 5 | ||||
| Latex((kg/m3) in solid weight) | None | 5.60 ± 0.02 | 11.20 ± 0.05 | 22.40 ± 0.10 | 33.60 ± 0.10 |
| Samples | Solid Content (%) | Conversion (%) | Zeta Potential (Intrinsic pH) | Rh (water) | Rh (Ca(OH)2) |
|---|---|---|---|---|---|
| PS-PU | 16.96 | 92.54 | −5.2 (5.3) | 40.3 | 75.3 |
| St-BA | 27.08 | 92.96 | −27.3 (5.1) | 163.7 | 256.2 |
| Samples | Flow (mm) | Standard Deviation | Superplasticizer (% Cement) | Density before Defoaming (kg/m3) | Density after Defoaming (kg/m3) | Defoamer (g) | |
|---|---|---|---|---|---|---|---|
| Blank | 157 | 2.4 | 1.85 | 2.29 | 2.29 | 0.02 | |
| PS-PU | 1.0% | 162 | 2.6 | 1.53 | 2.27 | 2.27 | 0.02 |
| 2.0% | 163 | 1.7 | 1.13 | 2.24 | 2.27 | 0.04 | |
| 4.0% | 159 | 3.6 | 0.67 | 2.19 | 2.24 | 0.04 | |
| 6.0% | 160 | 2.6 | 0.03 | 2.12 | 2.21 | 0.04 | |
| St-BA | 1.0% | 158 | 2.0 | 1.60 | 2.17 | 2.28 | 0.04 |
| 2.0% | 162 | 2.6 | 1.36 | 1.96 | 2.26 | 0.10 | |
| 4.0% | 157 | 1.0 | 0.97 | 1.88 | 2.24 | 0.22 | |
| 6.0% | 161 | 1.7 | 0.53 | 1.83 | 2.20 | 0.30 | |
| Sample | Initial Set (min) | Final Set (min) | |
|---|---|---|---|
| Blank | 190 | 255 | |
| PS-PU | 2% | 260 | 355 |
| 4% | 350 | 475 | |
| St-BA | 2% | 360 | 470 |
| 4% | 520 | 680 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Yan, H.; Yang, Y.; Shu, X.; Chen, L.; Li, C.; Ran, Q. Investigation of the Effect of Particle Surface Charge and Dispersion Stability on Latex Behavior in Cement Using Non-Ionic and Traditional Latexes. Materials 2022, 15, 6145. https://doi.org/10.3390/ma15176145
Zhou D, Yan H, Yang Y, Shu X, Chen L, Li C, Ran Q. Investigation of the Effect of Particle Surface Charge and Dispersion Stability on Latex Behavior in Cement Using Non-Ionic and Traditional Latexes. Materials. 2022; 15(17):6145. https://doi.org/10.3390/ma15176145
Chicago/Turabian StyleZhou, Dongliang, Han Yan, Yong Yang, Xin Shu, Lei Chen, Changcheng Li, and Qianping Ran. 2022. "Investigation of the Effect of Particle Surface Charge and Dispersion Stability on Latex Behavior in Cement Using Non-Ionic and Traditional Latexes" Materials 15, no. 17: 6145. https://doi.org/10.3390/ma15176145




