Modernized Synthesis Technique of Pr2NiO4+δ-Based Complex Oxides Using Low-Temperature Salt Melts
Abstract
:1. Introduction
2. Materials and Methods
3. Results
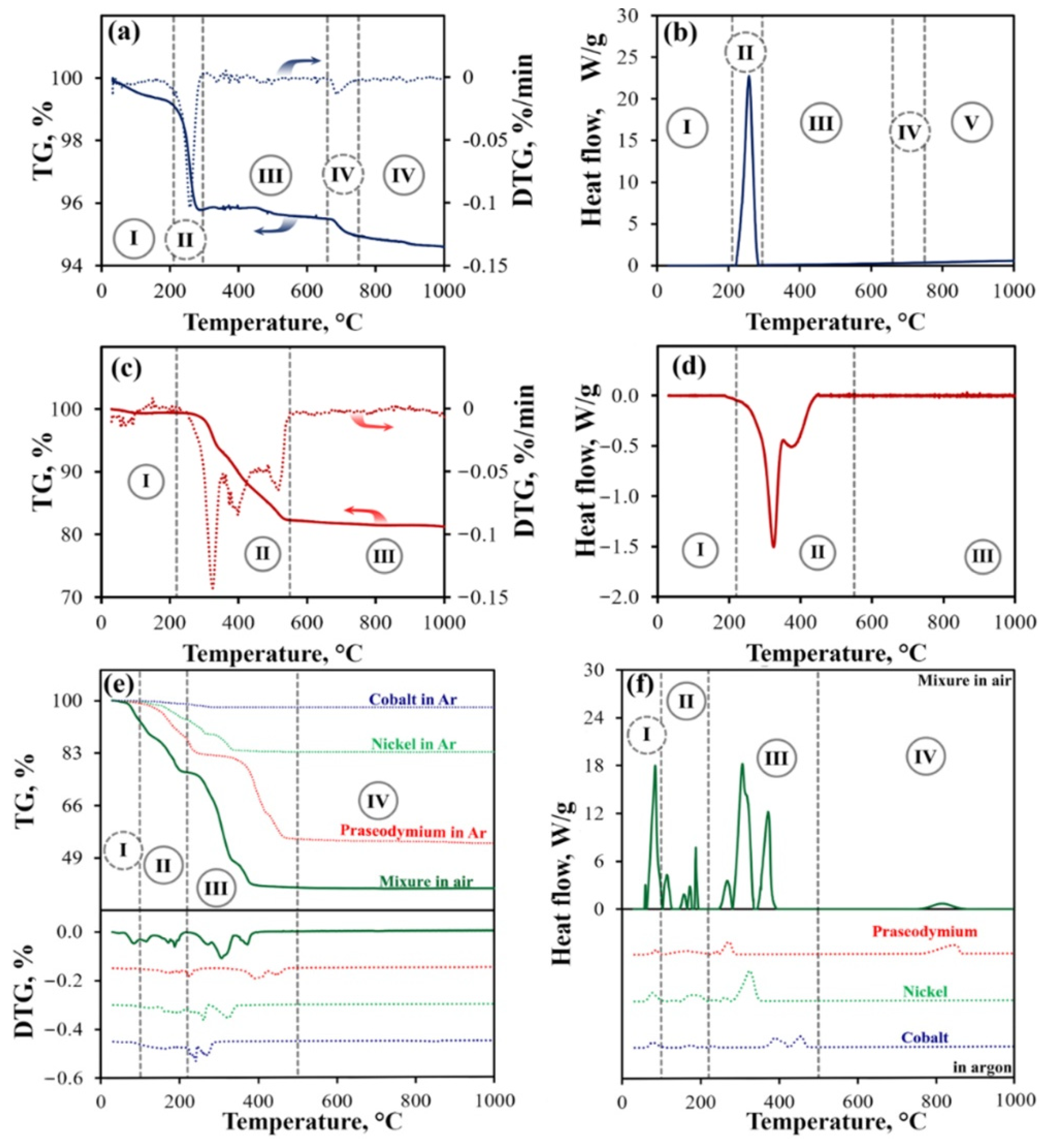
3.1. Thermal Behavior of Individual Nitrates
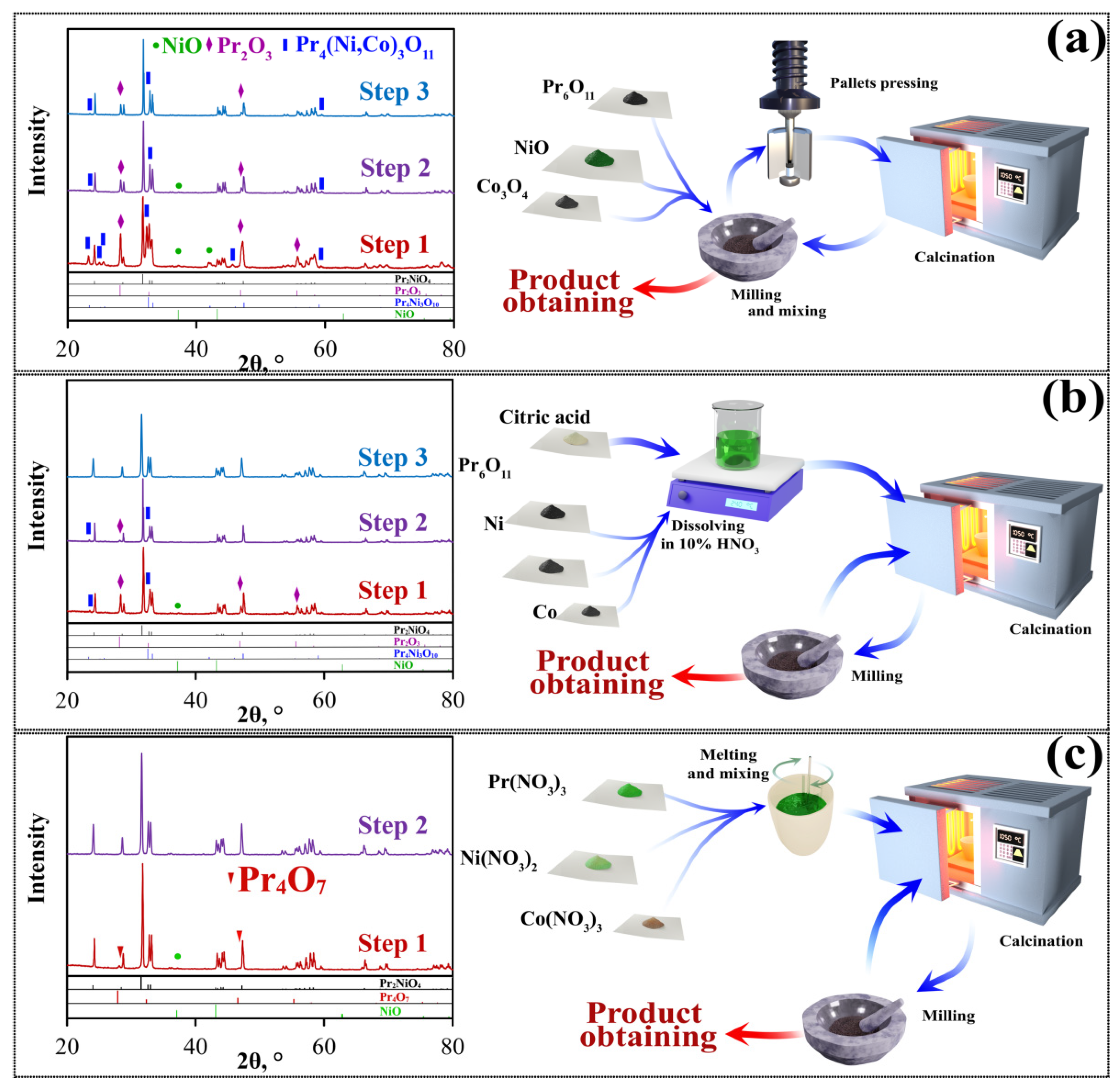
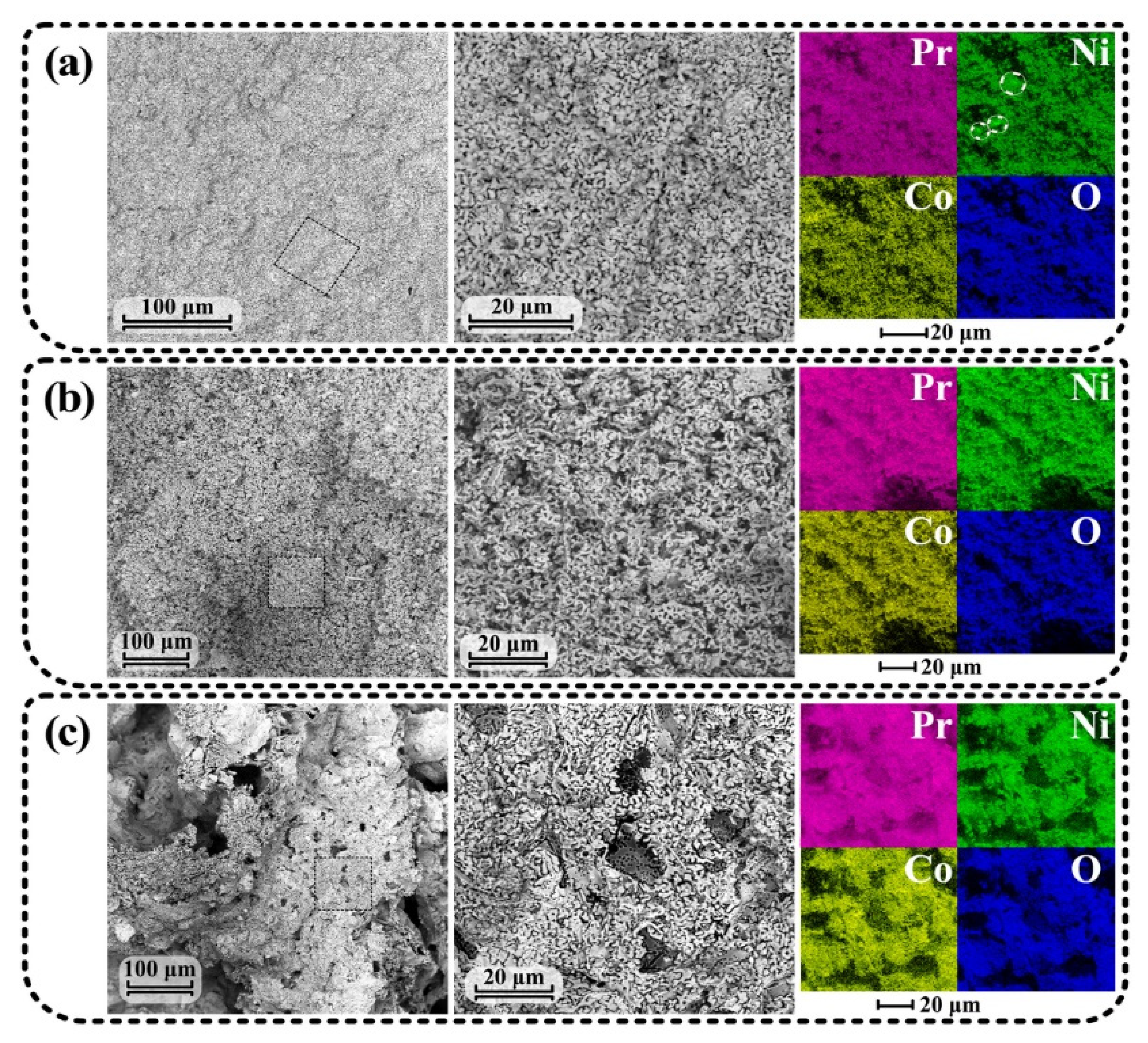
3.2. Comparison of Three Synthesis Routes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarutin, A.P.; Lyagaeva, J.G.; Medvedev, D.A.; Bi, L.; Yaremchenko, A.A. Recent advances in layered Ln2NiO4+δ nickelates: Fundamentals and prospects of their applications in protonic ceramic fuel and electrolysis cells. J. Mater. Chem. A 2021, 9, 154–195. [Google Scholar] [CrossRef]
- Ishihara, T. Oxide ion conductivity in defect perovskite, Pr2NiO4 and its application for solid oxide fuel cells. J. Ceram. Soc. Jpn. 2014, 122, 179–186. [Google Scholar] [CrossRef]
- Ge, H.-L.; Wang, Z.-Y.; Li, G.-R.; Liu, S.; Gao, X.-P. La2NiO4 nanoparticles as a core host of sulfur to enhance cathode volumetric capacity for lithium–sulfur battery. Electrochim. Acta 2022, 424, 140670. [Google Scholar] [CrossRef]
- Nowroozi, M.A.; Wissel, K.; Donzelli, M.; Hosseinpourkahvaz, N.; Plana-Ruiz, S.; Kolb, U.; Schoch, R.; Bauer, M.; Malik, A.M.; Rohrer, J.; et al. High cycle life all-solid-state fluoride ion battery with La2NiO4+δ high voltage cathode. Commun. Mater. 2020, 1, 27. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Zhang, Y.; Gong, Y.; Zhang, Y.; Lin, J.Y.S. Double-layer ceramic-carbonate hollow fiber membrane with superior mechanical strength for CO2 separation. J. Membr. Sci. 2022, 658, 120701. [Google Scholar] [CrossRef]
- Han, N.; Guo, X.; Cheng, J.; Liu, P.; Zhang, S.; Huang, S.; Rowles, M.R.; Fransaer, J.; Liu, S. Inhibiting in situ phase transition in Ruddlesden-Popper perovskite via tailoring bond hybridization and its application in oxygen permeation. Matter 2021, 4, 1720–1734. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, L.; Ma, Z.; Zhang, C.; Zhang, Y.; Zhang, D.; Pan, D.; Wu, J.; Li, G. Near-infrared-driven photoelectrocatalytic oxidation of urea on La-Ni-based perovskites. Chem. Eng. J. 2022, 446, 137240. [Google Scholar] [CrossRef]
- Amira, S.; Ferkhi, M.; Khaled, A.; Pireaux, J.-J. Electrochemical properties of La2BO4+δ/C electrocatalysts and study of the mechanism of the oxygen reduction reaction in alkaline medium. J. Iran. Chem. Soc. 2022, 19, 1787–1803. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Y.; Yang, B.; Lou, X.; Huang, J.; Ma, L.; Jing, D. Enhanced charge separation in La2NiO4 nanoplates by coupled piezocatalysis and photocatalysis for efficient H2 evolution. Nanoscale 2022, 14, 7083–7095. [Google Scholar] [CrossRef]
- Ma, X.; Luo, B.; Zeng, Z.; Hu, S.; Jing, D. Significantly Enhanced Photocatalytic Hydrogen Generation over a 2D/2D Z-Scheme La2NiO4/g-C3N4 Hybrid Free of Noble Metal Cocatalyst. ACS Appl. Energy Mater. 2021, 4, 10721–10730. [Google Scholar] [CrossRef]
- Zine, A.; Ferkhi, M.; Khaled, A.; Savan, E.K. A2BO4±δ as New Materials for Electrocatalytic Detection of Paracetamol and Diclofenac Drugs. Electrocatalysis 2022, 13, 524–538. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Koventhan, C.; Chen, S.-M.; Huang, Y.-F. A facile development of rare earth neodymium nickelate nanoparticles for selective electrochemical determination of antipsychotic drug prochlorperazine. J. Ind. Eng. Chem. 2022, 109, 253–266. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Meng, W.; Dai, L.; Wang, L. Layered perovskite oxides Lan+1NinO3n+1 (n = 1, 2, and 3) for detecting ammonia under high temperature. Sens. Actuators B Chem. 2021, 344, 130289. [Google Scholar] [CrossRef]
- Garali, M.; Kahlaoui, M.; Mohammed, B.; Mater, A.; Ben Azouz, C.; Chefi, C. Synthesis, characterization and electrochemical properties of La2–xEuxNiO4+δ Ruddlesden-Popper-type layered nickelates as cathode materials for SOFC applications. Int. J. Hydrog. Energy 2019, 44, 11020–11032. [Google Scholar] [CrossRef]
- Haddadnezhad, M.; Babaei, A.; Molaei, M.J.; Ataie, A. Synthesis and characterization of lanthanum nickelate nanoparticles with Rudllesden-Popper crystal structure for cathode materials of solid oxide fuel cells. J. Ultrafine Grained Nanostruct. Mater. 2020, 53, 98–109. [Google Scholar] [CrossRef]
- Boumaza, S.; Brahimi, R.; Boudjellal, L.; Belhadi, A.; Trari, M. Photoelectrochemical study of La2NiO4 synthesized using citrate sol gel method—application for hydrogen photo-production. J. Solid State Electrochem. 2020, 24, 329–337. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Zakharchuk, K.; Boiba, D.; Tsvinkinberg, V.; Filonova, E.; Khrustov, A.; Yaremchenko, A. Mixed ionic-electronic conductivity, phase stability and electrochemical activity of Gd-substituted La2NiO4+δ as oxygen electrode material for solid oxide fuel/electrolysis cells. Int. J. Hydrogen Energy 2021, 46, 16932–16946. [Google Scholar] [CrossRef]
- Pikalova, E.; Sadykov, V.; Sadovskaya, E.; Yeremeev, N.; Kolchugin, A.; Shmakov, A.; Vinokurov, Z.; Mishchenko, D.; Filonova, E.; Belyaev, V. Correlation between Structural and Transport Properties of Ca-Doped La Nickelates and Their Electrochemical Performance. Crystals 2021, 11, 297. [Google Scholar] [CrossRef]
- Tsvinkinberg, V.A.; Tolkacheva, A.S.; Filonova, E.A.; Gyrdasova, O.I.; Pikalov, S.M.; Vorotnikov, V.A.; Vylkov, A.I.; Moskalenko, N.I.; Pikalova, E.Y. Structure, thermal expansion and electrical conductivity of La2–xGdxNiO4+δ (0.0 ≤x≤ 0.6) cathode materials for SOFC applications. J. Alloys Compd. 2021, 853, 156728. [Google Scholar] [CrossRef]
- Hyodo, J.; Tominaga, K.; Ju, Y.-W.; Ida, S.; Ishihara, T. Electrical conductivity and oxygen diffusivity in Cu- and Ga-doped Pr2NiO4. Solid State Ion. 2014, 256, 5–10. [Google Scholar] [CrossRef]
- Ogier, T.; Prestipino, C.; Figueroa, S.; Mauvy, F.; Mougin, J.; Grenier, J.C.; Demourgues, A.; Bassat, J.M. In-situ study of cationic oxidation states in Pr2NiO4+δ using X-ray absorption near-edge spectroscopy. Chem. Phys. Lett. 2019, 727, 116–120. [Google Scholar] [CrossRef]
- Melnikov, P.; Arkhangelsky, I.V.; Nascimento, V.A.; Oliveira, L.C.S.; Guimaraes, W.R.; Zanoni, L.Z. Thermal decomposition of praseodymium nitrate hexahydrate Pr(NO3)3·6H2O. J. Therm. Anal. Calorim. 2018, 133, 929–934. [Google Scholar] [CrossRef]
- Hussein, G.A.M.; Balboul, B.A.A.; A-Warith, M.A.; Othman, A.G.M. Thermal genesis course and characterization of praseodymium oxide from praseodymium nitrate hydrate. Thermochim. Acta 2001, 369, 59–66. [Google Scholar] [CrossRef]
- Strydom, C.A.; Van Vuuren, C.P.J. The thermal decomposition of lanthanum(III), praseodymium(III) and europium(III) nitrates. Thermochim. Acta 1988, 124, 277–283. [Google Scholar] [CrossRef]
- Mikuli, E.; Migdal-Mikuli, A.; Chyzy, R.; Grad, B.; Dziembaj, R. Melting and thermal decomposition of [Ni(H2O)6](NO3)2. Thermochim. Acta 2001, 370, 65–71. [Google Scholar] [CrossRef]
- Paulik, F.; Paulik, J.; Arnold, M. Investigation of the phase diagram for the system Ni(NO3)2-H2O and examination of the decomposition of Ni(NO3)2·6H2O. Thermochim. Acta 1987, 121, 137–149. [Google Scholar] [CrossRef]
- Malecki, A.; Malecka, A.; Gajerski, R.; Prochowska-Klisch, B.; Podgorecka, A. The mechanism of thermal decomposition of Co(NO3)2·2H2O. J. Therm. Anal. 1988, 34, 203–209. [Google Scholar] [CrossRef]
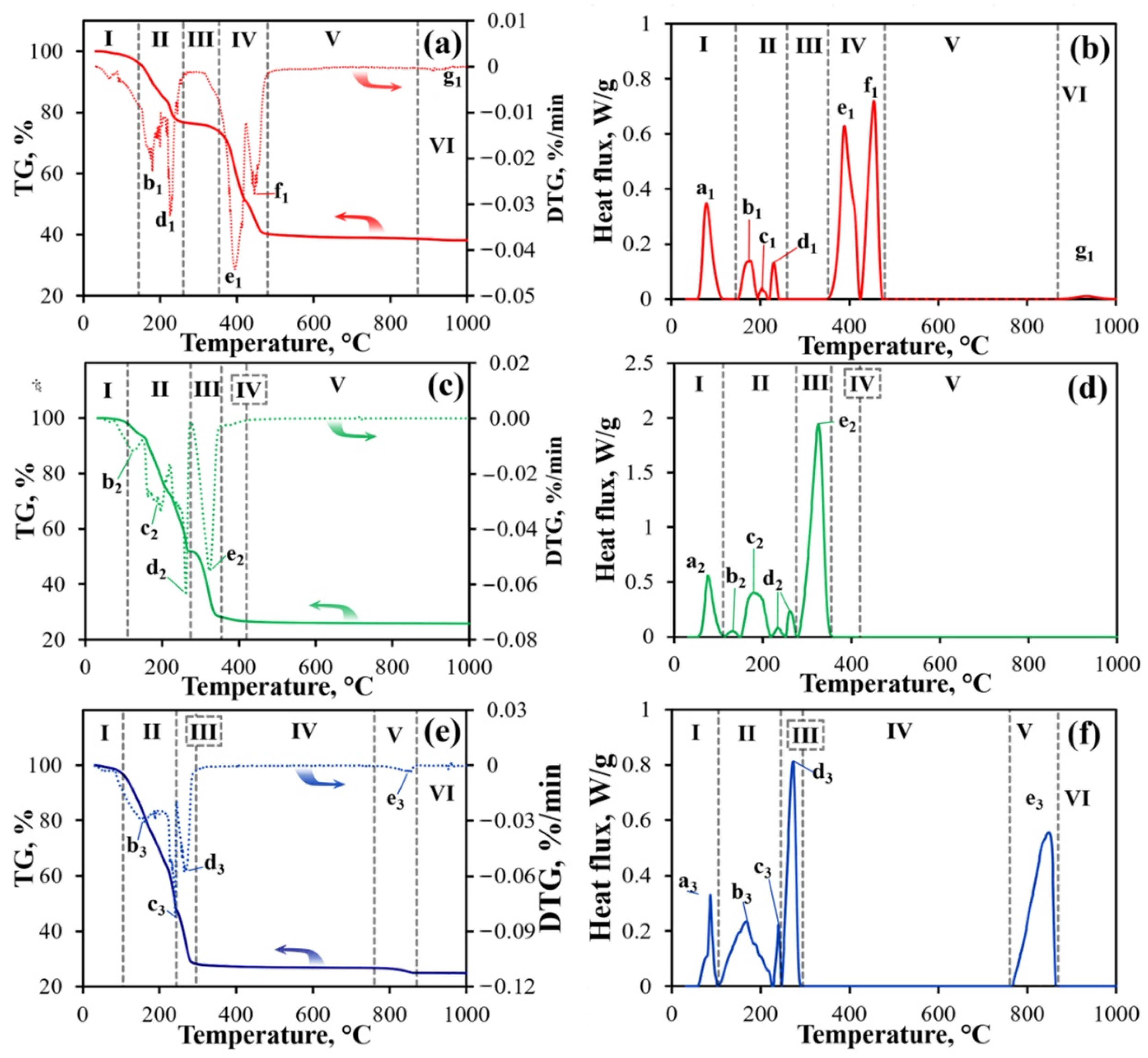
| Processes | Peak Index | Tstart, °C | Tpeak, °C | ΔH, kJ mol–1 |
|---|---|---|---|---|
| Pr(NO3)3·6H2O | ||||
| Melting of Pr(NO3)3·6H2O | a1 | 60 | 80 | 40.2 |
| Pr(NO3)3·6H2O → Pr(NO3)3·3H2O+ 3H2O↑ | b1 | 150 | 170 | 17.4 |
| Pr(NO3)3·3H2O = Pr(NO3)3·2H2O + H2O↑ | c1 | 195 | 200 | 2.2 |
| Pr(NO3)3·2H2O = Pr(NO3)3 + 2H2O↑ | d1 | 220 | 230 | 6.2 |
| Pr(NO3)3 = PrONO3 + 2NO2↑ + 0.5O2 | e1 | 350 | 390 | 66.3 |
| PrONO3 = PrO2 + NO2↑ | f1 | 425 | 455 | 40.3 |
| 2PrO2 = Pr2O3 + 0.5O2↑ | g1 | ~880 | - | - |
| Ni(NO3)2·6H2O | ||||
| Melting of Ni(NO3)2·6H2O | a2 | 60 | 80 | 40.3 |
| Ni(NO3)2·6H2O = Ni(NO3)2·5H2O + H2O↑ | b2 | 110 | 140 | 3.2 |
| Ni(NO3)2·5H2O = Ni(NO3)2·1.5H2O + 3.5H2O↑ | c2 | 150 | 180 | 51.1 |
| Ni(NO3)2·1.5H2O = Ni(NO2)2 + O2↑ + 1.5H2O↑ | d2 | 220 | 230 | 9.2 |
| 2Ni(NO2)2 = Ni2O3 + 4NO + 0.5O2 | e2 | 280 | 330 | 85.3 |
| Ni2O3 = 2NiO + 0.5O2 | 350 | - | - | |
| Co(NO3)2·6H2O | ||||
| Melting of Co(NO3)2·6H2O | a3 | 60 | 90 | 5.6 |
| Co(NO3)2·6H2O = Co(NO3)2 + 6H2O↑ | b3 | 100 | 170 | 14.8 |
| Co(NO3)2 = Co(NO2)2 + O2↑ | c3 | 230 | 240 | 1.4 |
| 3Co(NO2)2 = Co3O4 + 2NO2↑ + 4NO↑ | d3 | 250 | 270 | 7.8 |
| Co3O4 = 3CoO + 0.5O2 | e3 | 765 | 850 | 9.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarutin, A.P.; Baratov, S.A.; Medvedev, D.A. Modernized Synthesis Technique of Pr2NiO4+δ-Based Complex Oxides Using Low-Temperature Salt Melts. Materials 2022, 15, 6148. https://doi.org/10.3390/ma15176148
Tarutin AP, Baratov SA, Medvedev DA. Modernized Synthesis Technique of Pr2NiO4+δ-Based Complex Oxides Using Low-Temperature Salt Melts. Materials. 2022; 15(17):6148. https://doi.org/10.3390/ma15176148
Chicago/Turabian StyleTarutin, Artem P., Stanislav A. Baratov, and Dmitry A. Medvedev. 2022. "Modernized Synthesis Technique of Pr2NiO4+δ-Based Complex Oxides Using Low-Temperature Salt Melts" Materials 15, no. 17: 6148. https://doi.org/10.3390/ma15176148







