Evaluation of Green-Synthesized Cuprospinel Nanoparticles as a Nanosensor for Detection of Low-Concentration Cd(II) Ion in the Aqueous Solutions by the Quartz Crystal Microbalance Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Psidium Guajava L. Leaf Extract
2.3. Green Synthesis of Cuprospinel (CuFe2O4) Nanoparticles
2.4. Characterization
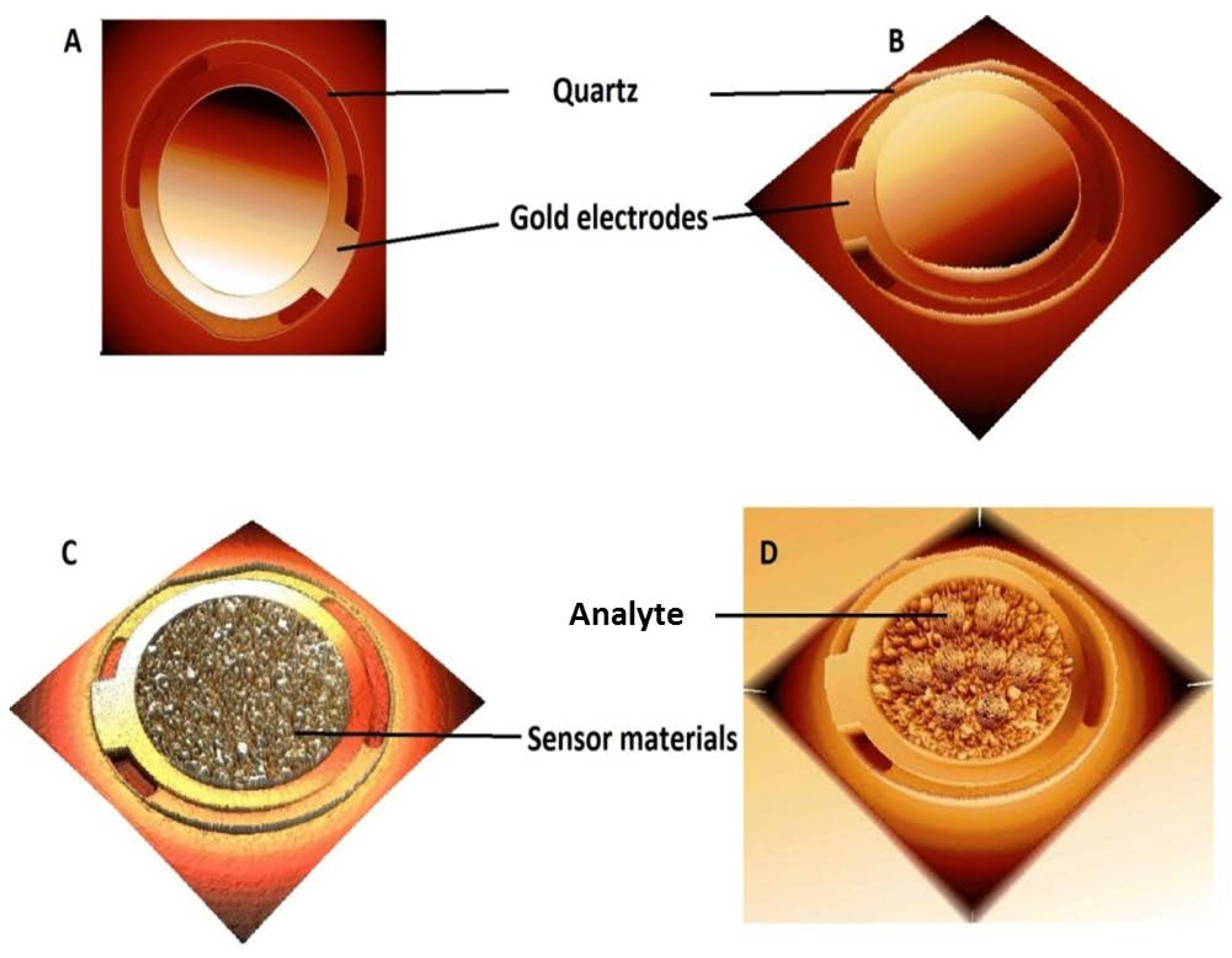
2.5. Quartz Crystal Micro-Balance (QCM) Technique
3. Results
3.1. Characterization of Cuprospinel Nanoparticles
3.1.1. Identification Analysis
3.1.2. Structural Analyses
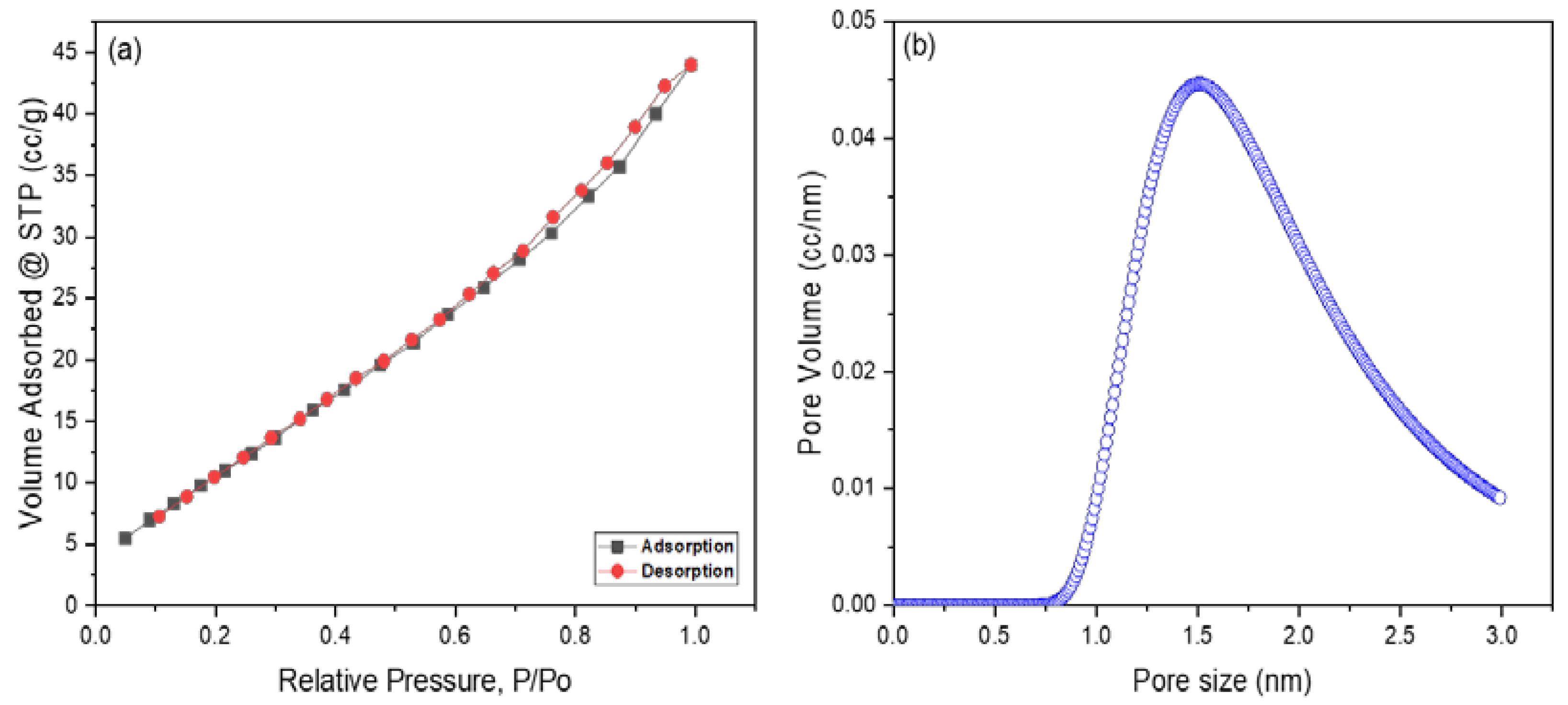
Surface Area and Pore Size
Zeta Potential and Particle Size Distribution
3.1.3. Microscopic Analyses
Atomic Force Microscopy (AFM)
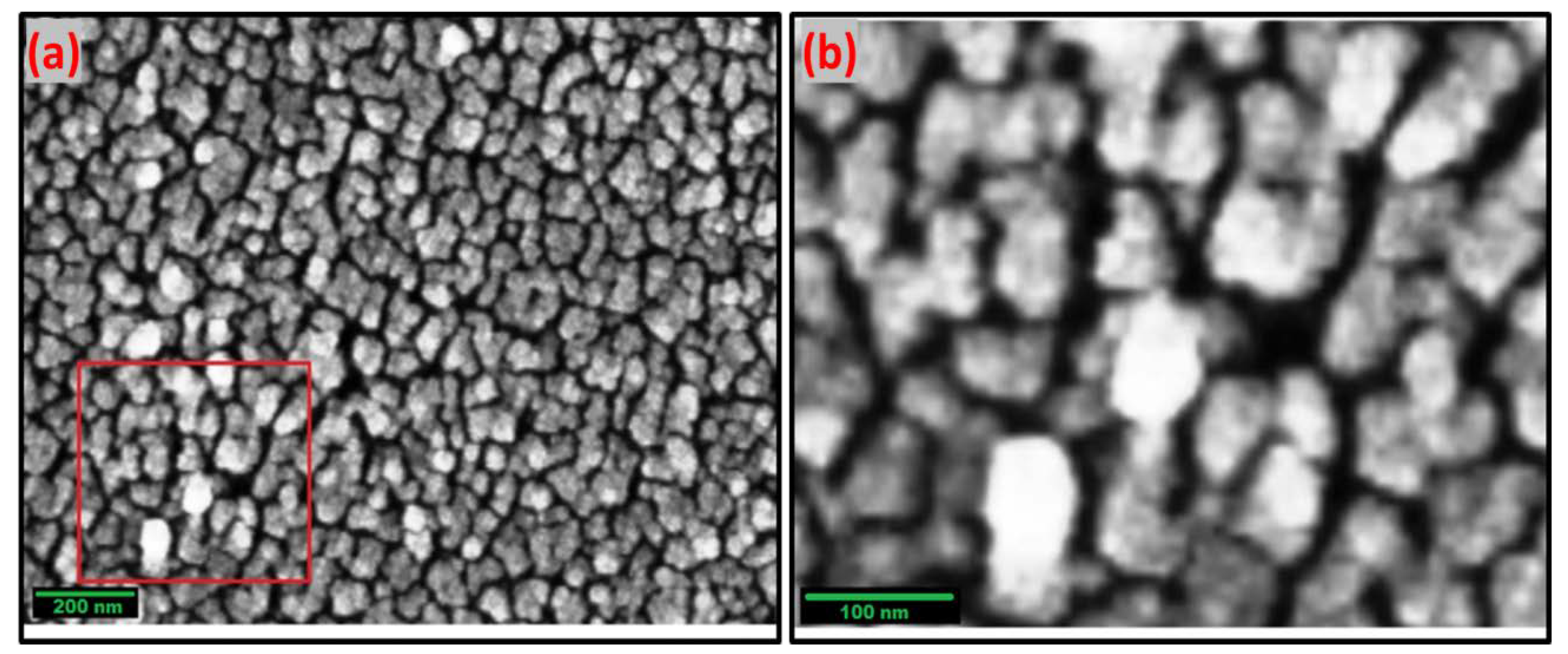
Scanning Electron Microscopy (SEM)
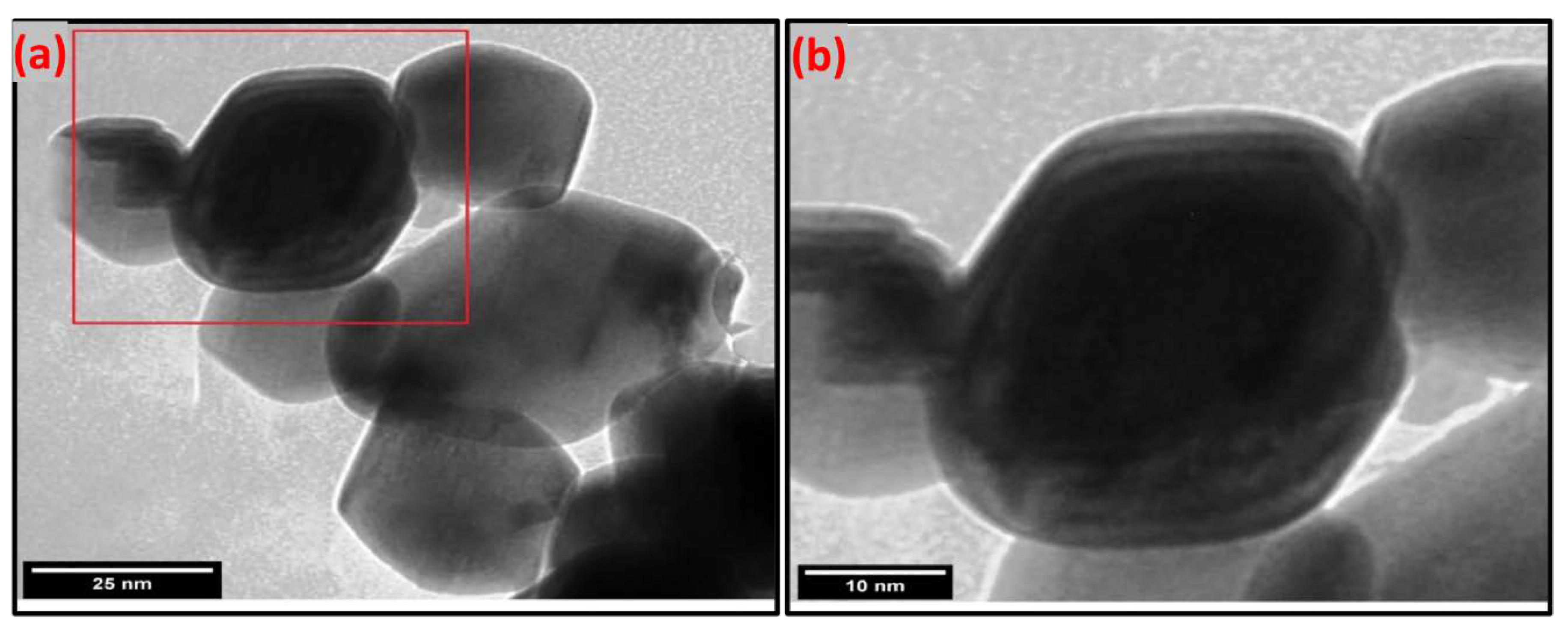
Transmission Electron Microscopy (TEM)
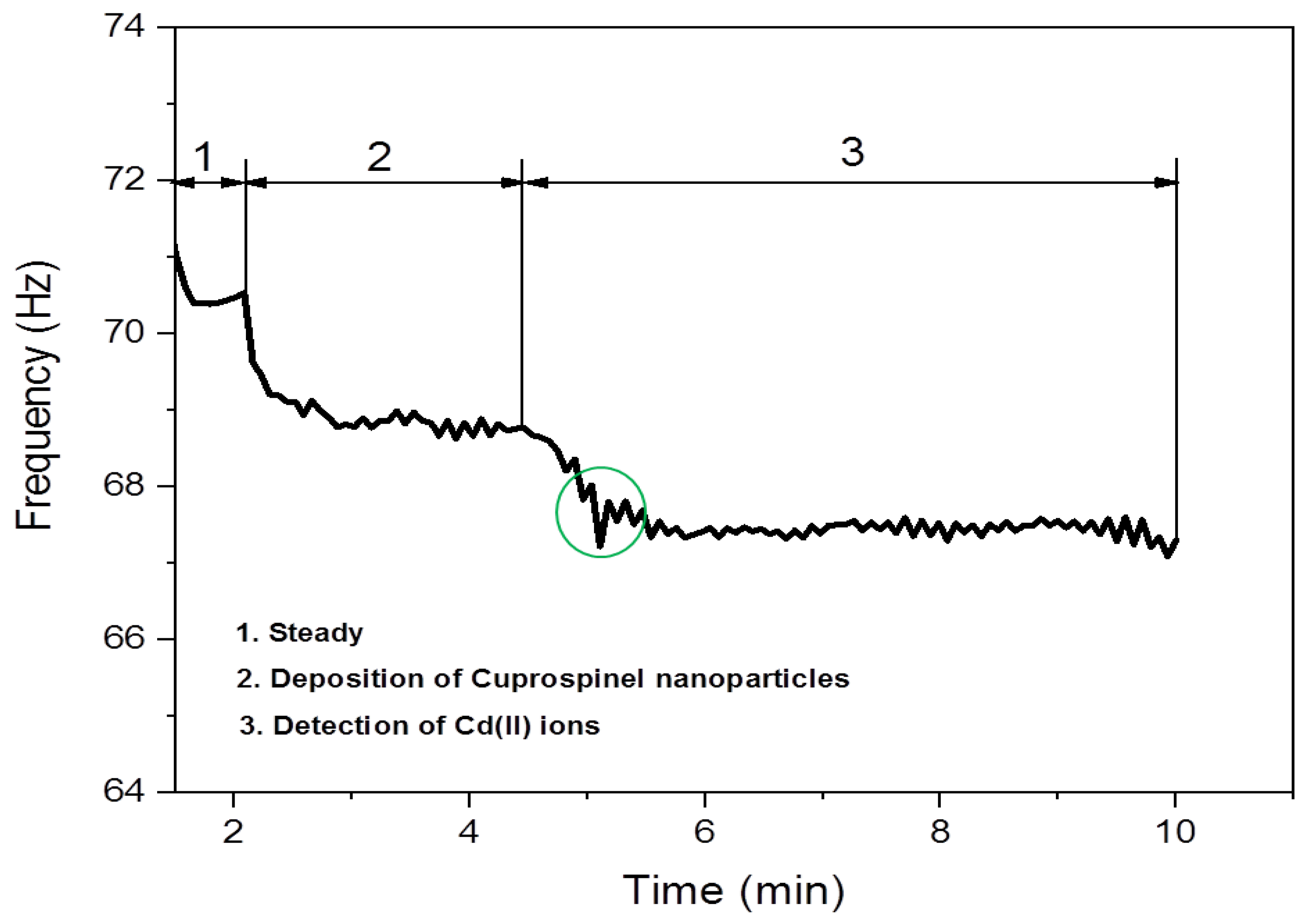
3.2. Detection of Cd(II) Ion by Cuprospinel Nanoparticles-Based QSM Nanosensor
3.3. Comparison of the Sensitivity of a Cuprospinel Nanoparticles-Based QSM Nanosensor with Currently Available Methods for the Detection of Cd(II) Ion in the Aqueous Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Germaninezhad, F.; Hosseinzadeh, R.; Tajbakhsh, M.; Beitollahi, A. Copper ferrite nanoparticles: An effective and recoverable nanomagnetic catalyst for the synthesis of N,N′,N″-trisubstituted guanidines from the addition reaction of anilines to carbodiimide. Micro Nano Lett. 2020, 15, 359–364. [Google Scholar] [CrossRef]
- Tran, C.V.; Quang, D.V.; Nguyen Thi, H.P.; Truong, T.N.; La, D.D. Effective removal of Pb(II) from aqueous media by a new design of Cu–Mg binary ferrite. Acs Omega 2020, 5, 7298–7306. [Google Scholar] [CrossRef] [PubMed]
- El-Wakeel, S.T.; Abdel-Karim, A.; Ismail, S.H.; Mohamed, G.G. Development of Ag-dendrites@Cu nanostructure for removal of selenium (IV) from aqueous solution. Water Environ. Res. 2022, 94, e10713. [Google Scholar] [CrossRef] [PubMed]
- Galvão, W.S.; Neto, D.; Freire, R.M.; Fechine, P.B. Super-paramagnetic nanoparticles with spinel structure: A review of synthesis and biomedical applications. In Solid State Phenomena; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2016. [Google Scholar]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Spinel ferrites nanoparticles: Synthesis methods and application in heterogeneous Fenton oxidation of organic pollutants–a review. Appl. Surf. Sci. Adv. 2021, 6, 100145. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Li, G.; Su, M.; Chen, X.; Zhang, H.; Zhang, Y.; Jiao, W.; He, Y.; Yi, J. Engineering ferrite nanoparticles with enhanced magnetic response for advanced biomedical applications. Mater. Today Adv. 2020, 8, 100119. [Google Scholar] [CrossRef]
- Masunga, N.; Mmelesi, O.K.; Kefeni, K.K.; Mamba, B.B. Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment. J. Environ. Chem. Eng. 2019, 7, 103179. [Google Scholar] [CrossRef]
- Henderson, C.; Charnock, J.; Plant, D. Cation occupancies in Mg, Co, Ni, Zn, Al ferrite spinels: A multi-element EXAFS study. J. Phys. Condens. Matter 2007, 19, 076214. [Google Scholar] [CrossRef]
- Prasad, S.; Deepty, M.; Ramesh, P.; Prasad, G.; Srinivasarao, K.; Srinivas, C.; Babu, K.V.; Kumar, E.R.; Mohan, N.K.; Sastry, D. Synthesis of MFe2O4 (M = Mg2+, Zn2+, Mn2+) spinel ferrites and their structural, elastic and electron magnetic resonance properties. Ceram. Int. 2018, 44, 10517–10524. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Bououdina, M.; Judith Vijaya, J.; John Kennedy, L. Spinel ferrite nanoparticles: Synthesis, crystal structure, properties, and perspective applications. In International Conference on Nanotechnology and Nanomaterials; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Anandan, S.; Selvamani, T.; Prasad, G.G.; Asiri, A.; Wu, J. Magnetic and catalytic properties of inverse spinel CuFe2O4. nanoparticles. J. Magn. Magn. Mater. 2017, 432, 437–443. [Google Scholar] [CrossRef]
- Zhao, J.; Xiao, P.; Han, S.; Zulhumar, M.; Wu, D. Preparation of magnetic copper ferrite nanoparticle as peroxymonosulfate activating catalyst for effective degradation of levofloxacin. Water Sci. Technol. 2022, 85, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Yang, A.; Vittoria, C.; Harris, V.G. Computational study of copper ferrite (CuFe2O4). J. Appl. Phys. 2006, 99, 08M909. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Recent advances in synthesis and applications of MFe2O4 (M = Co, Cu, Mn, Ni, Zn) nanoparticles. Nanomaterials 2021, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, G.K.; Bhardwaj, S.; Singh, M.; Batoo, K.M. Ferrites and Multiferroics: Fundamentals to Applications; Springer: Singapore, 2021. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017, 12, 908. [Google Scholar] [CrossRef]
- Benelli, G. Green synthesis of nanomaterials. Nanomaterials 2019, 9, 1275. [Google Scholar] [CrossRef]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ.-Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A.; Rao, R.A. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 1–28. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Hamdy, A.; Mostafa, M.K.; Nasr, M. Techno-economic estimation of electroplating wastewater treatment using zero-valent iron nanoparticles: Batch optimization, continuous feed, and scaling up studies. Environ. Sci. Pollut. Res. 2019, 26, 25372–25385. [Google Scholar] [CrossRef]
- Hamdy, A.; Mostafa, M.K.; Nasr, M. Zero-valent iron nanoparticles for methylene blue removal from aqueous solutions and textile wastewater treatment, with cost estimation. Water Sci. Technol. 2018, 78, 367–378. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Sadek, A.H.; Asker, M.S.; Abdelhamid, S.A. Bacteriostatic impact of nanoscale zero-valent iron against pathogenic bacteria in the municipal wastewater. Biologia 2021, 76, 2785–2809. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Hamdy, A.; Ismail, S.H.; Ebnalwaled, A.; Mohamed, G.G. Characterization of superparamagnetic/monodisperse PEG-coated magnetite nanoparticles Sonochemically prepared from the hematite ore for Cd(II) removal from aqueous solutions. J. Inorg. Organomet. Polym. Mater. 2021, 31, 397–414. [Google Scholar] [CrossRef]
- Pinot, F.; Kreps, S.E.; Bachelet, M.; Hainaut, P.; Bakonyi, M.; Polla, B.S. Cadmium in the environment: Sources, mechanisms of biotoxicity, and biomarkers. Rev. Environ. Health 2000, 15, 299–324. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.K.; Gautam, R.K.; Banerjee, S.; Chattopadhyaya, M.; Pandey, J. Heavy metals in the environment: Fate, transport, toxicity and remediation technologies. Nova Sci. Publ. 2016, 60, 101–130. [Google Scholar]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. Poisoning Mod. World-New Tricks Old Dog 2019, 10, 70–90. [Google Scholar]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.-a. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135. [Google Scholar]
- Ehrampoush, M.H.; Miria, M.; Salmani, M.H.; Mahvi, A.H. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract. J. Environ. Health Sci. Eng. 2015, 13, 84. [Google Scholar] [CrossRef]
- Goyer, R.A.; Clarkson, T.W. Toxic effects of metals. Casarett Doull’s Toxicol. Basic Sci. Poisons 1996, 5, 691–736. [Google Scholar]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Cotruvo, J.A. 2017 WHO guidelines for drinking water quality: First addendum to the fourth edition. J. Am. Water Work Assoc. 2017, 109, 44–51. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Vidu, R.; Matei, E.; Predescu, A.M.; Alhalaili, B.; Pantilimon, C.; Tarcea, C.; Predescu, C. Removal of heavy metals from wastewaters: A challenge from current treatment methods to nanotechnology applications. Toxics 2020, 8, 101. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Anandan, S.; Ashokkumar, M. Removal of heavy metal from wastewater. Handb. Ultrason. Sonochem. 2016, 92, 813–839. [Google Scholar]
- Yang, L.; Hu, W.; Chang, Z.; Liu, T.; Fang, D.; Shao, P.; Shi, H.; Luo, X. Electrochemical recovery and high value-added reutilization of heavy metal ions from wastewater: Recent advances and future trends. Environ. Int. 2021, 152, 106512. [Google Scholar] [CrossRef]
- Türkmen, D.; Bakhshpour, M.; Akgönüllü, S.; Aşır, S.; Denizli, A. Heavy Metal Ions Removal From Wastewater Using Cryogels: A Review. Front. Sustain. 2022, 3, 765592. [Google Scholar] [CrossRef]
- Hussain, A.; Madan, S.; Madan, R. Removal of heavy metals from wastewater by adsorption. In Heavy Metals—Their Environmental Impacts and Mitigation; Intechopen: London, UK, 2021. [Google Scholar]
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Nallakukkala, S.; Rehman, A.u.; Zaini, D.B.; Lal, B. Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review. Water 2022, 14, 1171. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol. 2016, 158, 124–136. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.; Chintalpudi, V.K.; Muddada, S. Application of biosorption for removal of heavy metals from wastewater. Biosorption 2018, 18, 70–116. [Google Scholar]
- Hamdy, A. Experimental study of the relationship between dissolved iron, turbidity, and removal of Cu(II) ion from aqueous solutions using zero-valent iron nanoparticles. Arab. J. Sci. Eng. 2021, 46, 5543–5565. [Google Scholar] [CrossRef]
- Abdelmigeed, M.O.; Sadek, A.H.; Ahmed, T.S. Novel easily separable core–shell Fe3O4/PVP/ZIF-8 nanostructure adsorbent: Optimization of phosphorus removal from Fosfomycin pharmaceutical wastewater. RSC Adv. 2022, 12, 12823–12842. [Google Scholar] [CrossRef]
- Katowah, D.F.; Alsulami, Q.A.; Alam, M.; Ismail, S.H.; Asiri, A.M.; Mohamed, G.G.; Rahman, M.M.; Hussein, M.A. The Performance of Various SWCNT Loading into CuO–PMMA Nanocomposites Towards the Detection of Mn2+ Ions. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5024–5041. [Google Scholar] [CrossRef]
- Ismail, S.H.; Hamdy, A.; Ismail, T.A.; Mahboub, H.H.; Mahmoud, W.H.; Daoush, W.M. Synthesis and characterization of antibacterial carbopol/ZnO hybrid nanoparticles gel. Crystals 2021, 11, 1092. [Google Scholar] [CrossRef]
- Lu, C.; Czanderna, A.W. Applications of Piezoelectric Quartz Crystal Microbalances; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Capparelli, R.; De Chiara, F.; Nocerino, N.; Montella, R.C.; Iannaccone, M.; Fulgione, A.; Romanelli, A.; Avitabile, C.; Blaiotta, G.; Capuano, F. New perspectives for natural antimicrobial peptides: Application as antinflammatory drugs in a murine model. BMC Immunol. 2012, 13, 61. [Google Scholar] [CrossRef]
- Al-Qasmi, N. Facial eco-friendly synthesis of copper oxide nanoparticles using chia seeds extract and evaluation of its electrochemical activity. Processes 2021, 9, 2027. [Google Scholar] [CrossRef]
- Saade, A.J.; Indrajit, P.; Maria, J.C.O.; Kadda, H.; Rosario, M.; Mohammad, J.; Abduladheem, T.; Mahin, N.; Reza, A. Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity. Nanotechnol. Rev. 2022, 11, 2483–2492. [Google Scholar]
- Verwey, E.J.W.; Heilmann, E.L. Physical properties and cation arrangement of oxides with spinel structures Locality: Synthetic. J. Chem. Phys. 1947, 15, 174–180. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Broekhoff, J.C.P. Mesopore determination from nitrogen sorption isotherms: Fundamentals, scope, limitations. Stud. Surf. Sci. Catal. 1979, 3, 663–684. [Google Scholar]
- Palchoudhury, S.; Baalousha, M.; Lead, J.R. Methods for measuring concentration (mass, surface area and number) of nanomaterials. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2015; Volume 8, pp. 153–181. [Google Scholar]
- He, M.-Y.; Lin, Y.-J.; Kao, Y.-L.; Kuo, P.; Grauffel, C.; Lim, C.; Cheng, Y.-S.; Chou, H.-H.D. Sensitive and specific cadmium biosensor developed by reconfiguring metal transport and leveraging natural gene repositories. ACS Sens. 2021, 6, 995–1002. [Google Scholar] [CrossRef]
- Marguí, E.; Queralt, I.; Hidalgo, M. Determination of cadmium at ultratrace levels in environmental water samples by means of total reflection X-ray spectrometry after dispersive liquid–liquid microextraction. J. Anal. At. Spectrom. 2013, 28, 266–273. [Google Scholar] [CrossRef]
- Wang, D.; Gao, F.; Wang, X.; Ning, X.; Wang, K.; Wang, X.; Wei, Y.; Fujita, T. Detection of Cd2+ in Aqueous Solution by the Fluorescent Probe of CdSe/CdS QDs Based on OFF–ON Mode. Toxics 2022, 10, 367. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, Y.; Yu, Z.; Lu, L.; Wang, X. Simultaneous determination of Cd2+ and Pb2+ by an electrochemical sensor based on Fe3O4/Bi2O3/C3N4 nanocomposites. Talanta Open 2021, 3, 100024. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Denizli, A. Highly sensitive detection of Cd(II) ions using ion-imprinted surface plasmon resonance sensors. Microchem. J. 2020, 159, 105572. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R.; Baishya, U. Ultrasensitive trace determination of cadmium through a green synthesized hybrid PVA-Chitosan nanocomposite. Plasmonics 2020, 15, 1903–1912. [Google Scholar] [CrossRef]
- Golbedaghi, R.; Jafari, S.; Yaftian, M.R.; Azadbakht, R.; Salehzadeh, S.; Jaleh, B. Determination of cadmium (II) ion by atomic absorption spectrometry after cloud point extraction. J. Iran. Chem. Soc. 2012, 9, 251–256. [Google Scholar] [CrossRef]
- González-Albarrán, R.; de Gyves, J.; Rodríguez de San Miguel, E. Determination of Cadmium (II) in Aqueous Solutions by In Situ MID-FTIR-PLS Analysis Using a Polymer Inclusion Membrane-Based Sensor: First Considerations. Molecules 2020, 25, 3436. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Khosropour, Z.; Najarian, S. Determination of cadmium and lead ions in water sample by AAS after preconcentration by XAD-4/GBHD system. In Proceedings of the 15th International Conference on Heavy Metals in the Environment, Gdańsk, Poland, 19–23 September 2010. [Google Scholar]
- Tian, H.; Jiao, L.; Dong, D. Rapid determination of trace cadmium in drinking water using laser-induced breakdown spectroscopy coupled with chelating resin enrichment. Sci. Rep. 2019, 9, 10443. [Google Scholar]
- Ghorbani, M.; Akbarzade, S.; Aghamohammadhasan, M.; Seyedin, O.; Lahoori, N.A. Pre-concentration and determination of cadmium and lead ions in real water, soil and food samples using a simple and sensitive green solvent-based ultrasonic assisted dispersive liquid–liquid microextraction and graphite furnace atomic absorption spectrometry. Anal. Methods 2018, 10, 2041–2047. [Google Scholar]
- Adamczyk, M.; Grabarczyk, M. Simple, insensitive to environmental matrix interferences method of trace cadmium determination in natural water samples. Ionics 2019, 25, 1959–1966. [Google Scholar] [CrossRef]
- Hassan, S. Single and simultaneous voltammetric sensing of lead(II), cadmium(II) and zinc(II) using a bimetallic Hg-Bi supported on poly (1,2-diaminoanthraquinone)/glassy carbon modified electrode. Sens. Bio-Sens. Res. 2020, 29, 100369. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Feng, W. Cadmium-ion detection: A comparative study for a SnO2, MoS2, SnO2/MoS2, SnO2-MoS2 sensing membrane combination with a fiber-optic Mach–Zehnder interferometer. Appl. Opt. 2021, 60, 799–804. [Google Scholar] [CrossRef]
- Şolomonea, B.-G.; Jinga, L.-I.; Antohe, V.-A.; Socol, G.; Antohe, I. Cadmium Ions’ Trace-Level Detection Using a Portable Fiber Optic—Surface Plasmon Resonance Sensor. Biosensors 2022, 12, 573. [Google Scholar] [CrossRef]



| No. | Method | LOD Value | Reference |
|---|---|---|---|
| 1 | Whole-cell biosensors | 3 nM | [62] |
| 2 | Dispersive liquid-liquid microextraction approach (DLLME) combined with total reflection X-ray spectrometry (TXRF) | 0.04 μg/L | [63] |
| 3 | Fluorescent probe of CdSe/CdS core–shell quantum dots (QDs) | 6 nmol/L | [64] |
| 4 | Electrochemical sensor based on Fe3O4/Bi2O3/C3N4 nanocomposites | 3 × 10−9 mol/L | [65] |
| 5 | Electrochemical sensor using a Fe3O4/G composite | 0.08 μg/L | [66] |
| 6 | Ion-imprinted surface plasmon resonance sensor | 0.01 µg/L | [67] |
| 7 | Green synthesized hybrid PVA-chitosan nanocomposite sensor probe | 800 ppt | [68] |
| 8 | Atomic absorption spectrometry after cloud point extraction | 0.44 ng/mL | [69] |
| 9 | In situ MID-FTIR-PLS analysis using a polymer inclusion membrane-based sensor | 0.45 × 10−4 mol/dm3 | [70] |
| 10 | Atomic absorption spectroscopy (AAS) after preconcentration by the XAD-4/GBHD system | 0.06–0.50 µg/L | [71] |
| 11 | Laser-induced breakdown spectroscopy coupled with chelating resin enrichment | 3.6 µg/L | [72] |
| 12 | Green solvent-based ultrasonic-assisted dispersive liquid–liquid microextraction and graphite furnace atomic absorption spectrometry | 0.2 ng/L | [73] |
| 13 | Mercury film silver-based electrode (Hg(Ag)FE) and anodic stripping voltammetric analysis | 1.3 × 10−8 mol/L | [74] |
| 14 | Voltammetric sensing using a bimetallic Hg–Bi supported on poly(1,2 diaminoanthraquinone)/glassy carbon-modified electrode | 0.107 μg/L | [75] |
| 15 | SnO2, MoS2, SnO2/MoS2, SnO2–MoS2 sensing membrane combination with a fiber-optic Mach–Zehnder interferometer | 0–100 µM | [76] |
| 16 | Gold-coated reflection-type fiber optic-surface plasmon resonance (Au-coated FO-SPR) sensor | [77] | |
| BSA/Au | 7.1 nM | ||
| PANI/Au | 8.8 nM | ||
| Chitosan/Au | 9.4 nM | ||
| 17 | Cuprospinel nanoparticles-based QCM nanosensor | 3.6 ng/L | The current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qasmi, N.; Al-Gethami, W.; Alhashmialameer, D.; Ismail, S.H.; Sadek, A.H. Evaluation of Green-Synthesized Cuprospinel Nanoparticles as a Nanosensor for Detection of Low-Concentration Cd(II) Ion in the Aqueous Solutions by the Quartz Crystal Microbalance Method. Materials 2022, 15, 6240. https://doi.org/10.3390/ma15186240
Al-Qasmi N, Al-Gethami W, Alhashmialameer D, Ismail SH, Sadek AH. Evaluation of Green-Synthesized Cuprospinel Nanoparticles as a Nanosensor for Detection of Low-Concentration Cd(II) Ion in the Aqueous Solutions by the Quartz Crystal Microbalance Method. Materials. 2022; 15(18):6240. https://doi.org/10.3390/ma15186240
Chicago/Turabian StyleAl-Qasmi, Noha, Wafa Al-Gethami, Dalal Alhashmialameer, Sameh H. Ismail, and Ahmed H. Sadek. 2022. "Evaluation of Green-Synthesized Cuprospinel Nanoparticles as a Nanosensor for Detection of Low-Concentration Cd(II) Ion in the Aqueous Solutions by the Quartz Crystal Microbalance Method" Materials 15, no. 18: 6240. https://doi.org/10.3390/ma15186240







