Customized Titanium Mesh for Guided Bone Regeneration with Autologous Bone and Xenograft
Abstract
:1. Introduction
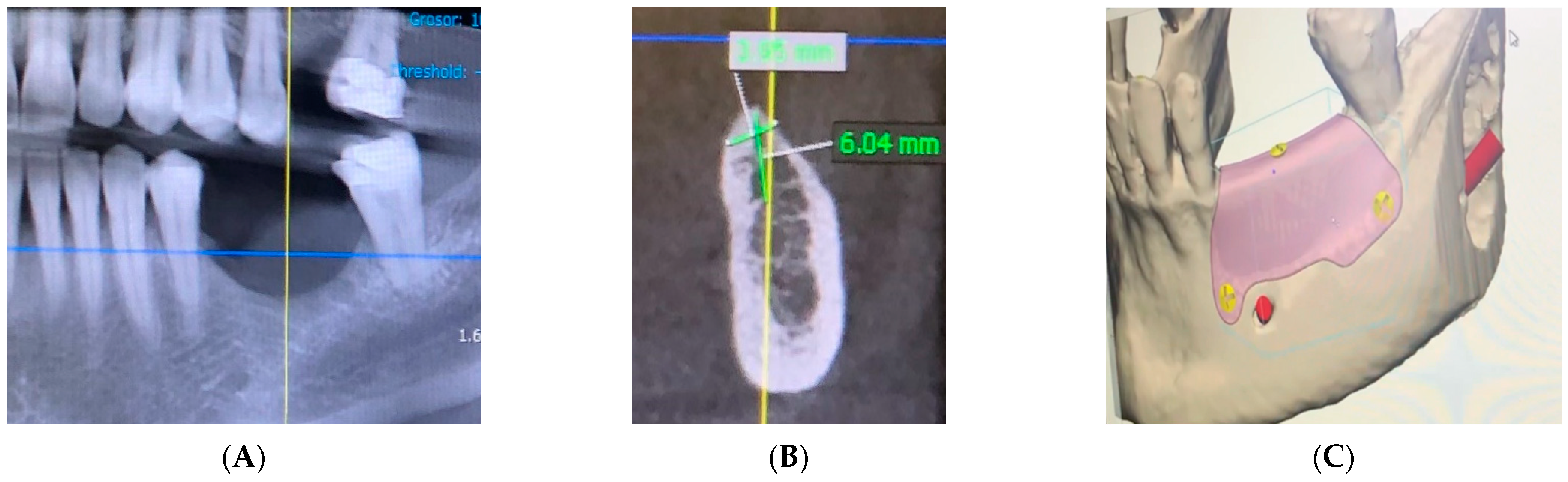
2. Materials and Methods
3. Case Report and Results
- i.
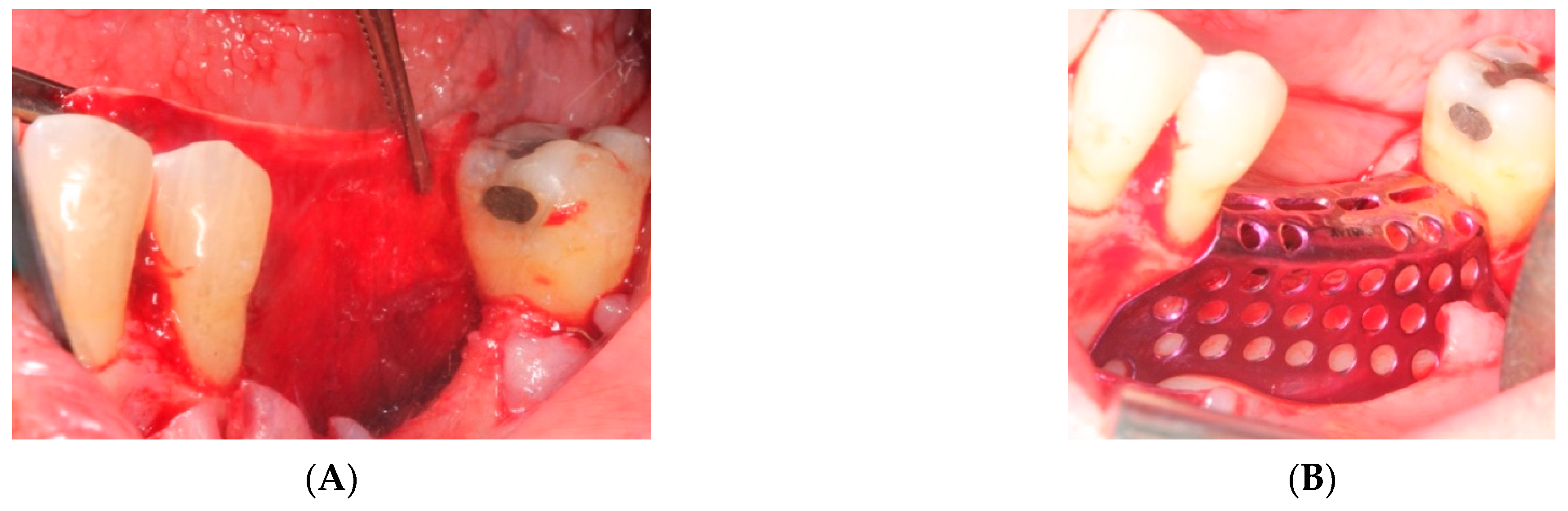
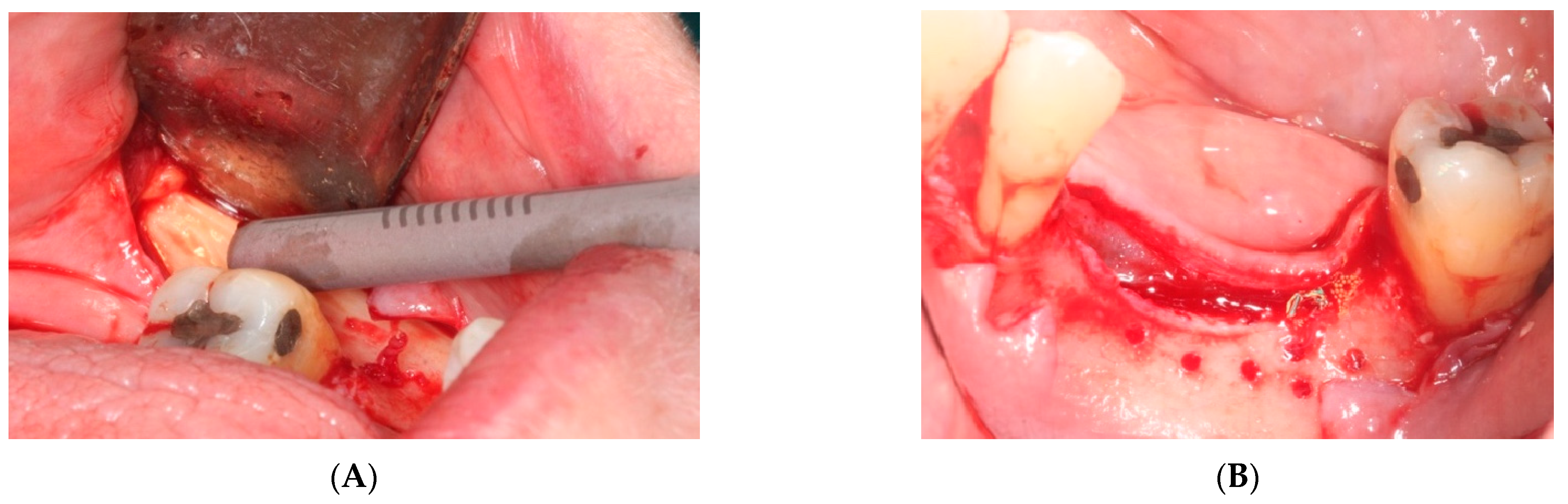
- First surgery: Local anesthesia Articaine 4% with epinephrine (1:100,000) was administered. An intrasulcular incision was made from the mesial of 3.3 to the distal of 3.7, continuing along the anterior border of the external ramus of the mandible. Periosteal incisions were made to mobilize the vestibular flap and the upper fibers of the mylohyoid muscle were disinserted to passivate the lingual flap and thus obtain a tension-free closure (Figure 3). We then checked the position of the mesh and proceeded to collect bone from the ascending branch using the bone scraper (Micross®, Selecdent, Barcelona, Spain) (Figure 4A). Cortical perforations were made to promote bleeding (Figure 4B). We mixed the autologous bone with the xenograft (Tioss®, Sanhigia, Bujaraloz, Spain) in a 70:30 ratio, inserted it into the mesh, and the mesh was placed in the defect.
- ii.
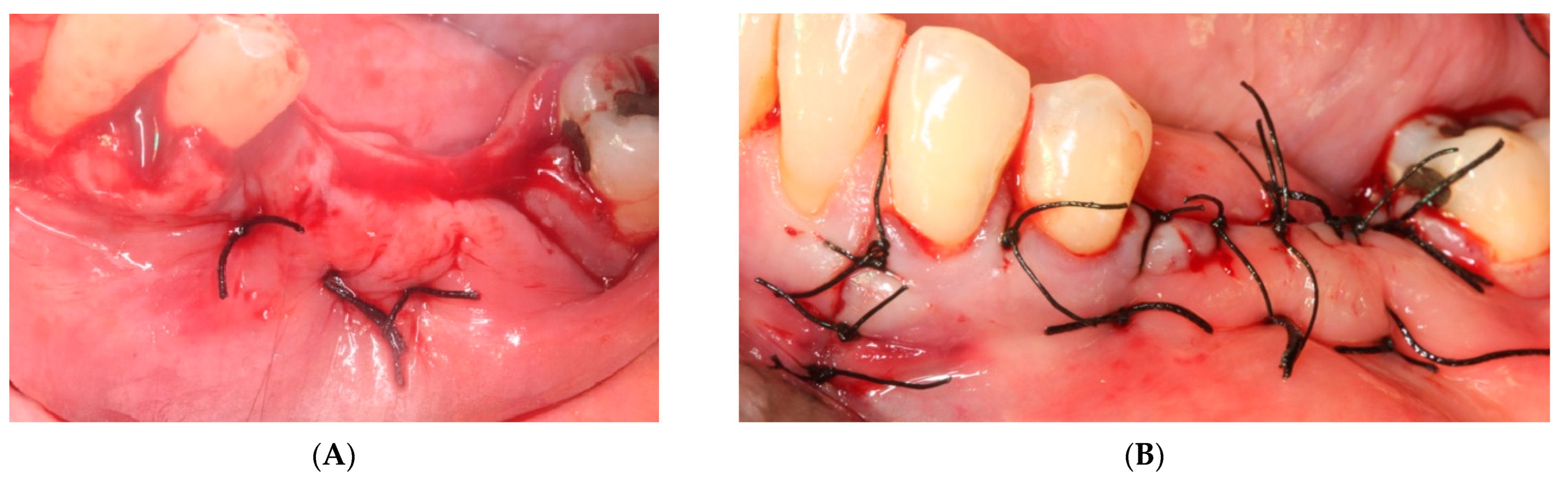
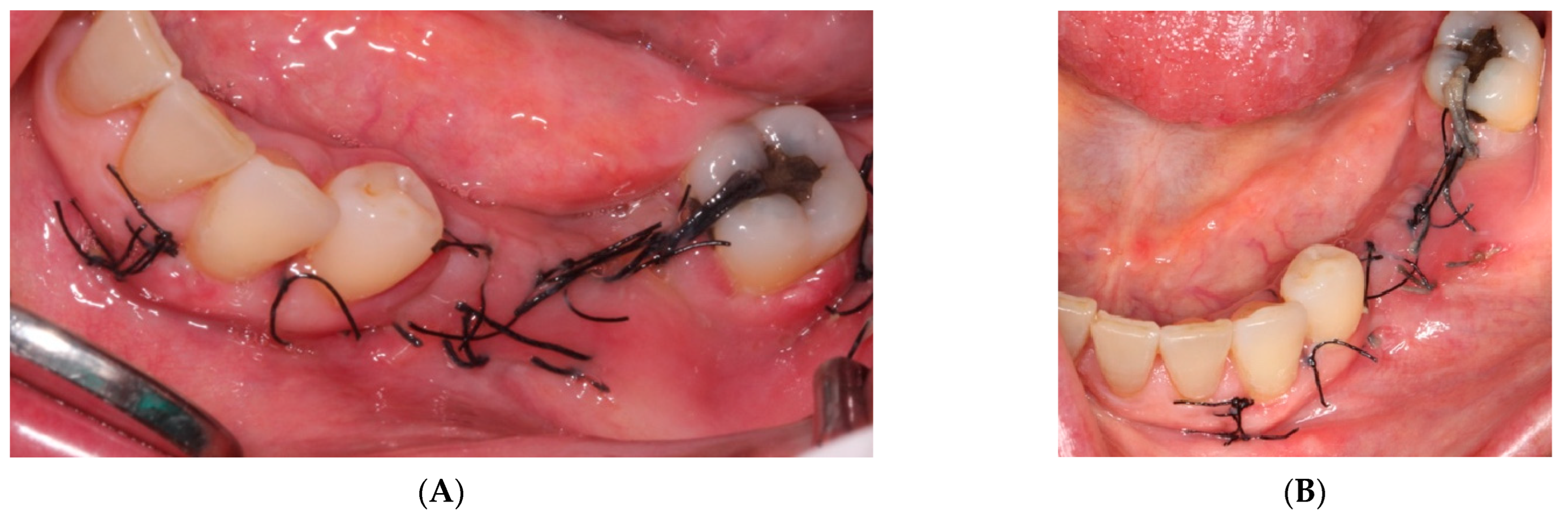
- The sutures were removed on day 21. A panoramic radiograph was taken after surgery. (Figure 8). Periodic controls were scheduled; every week during the first 2 months, every two weeks in the third and fourth months, and once a month up to 6 months.
- iii.
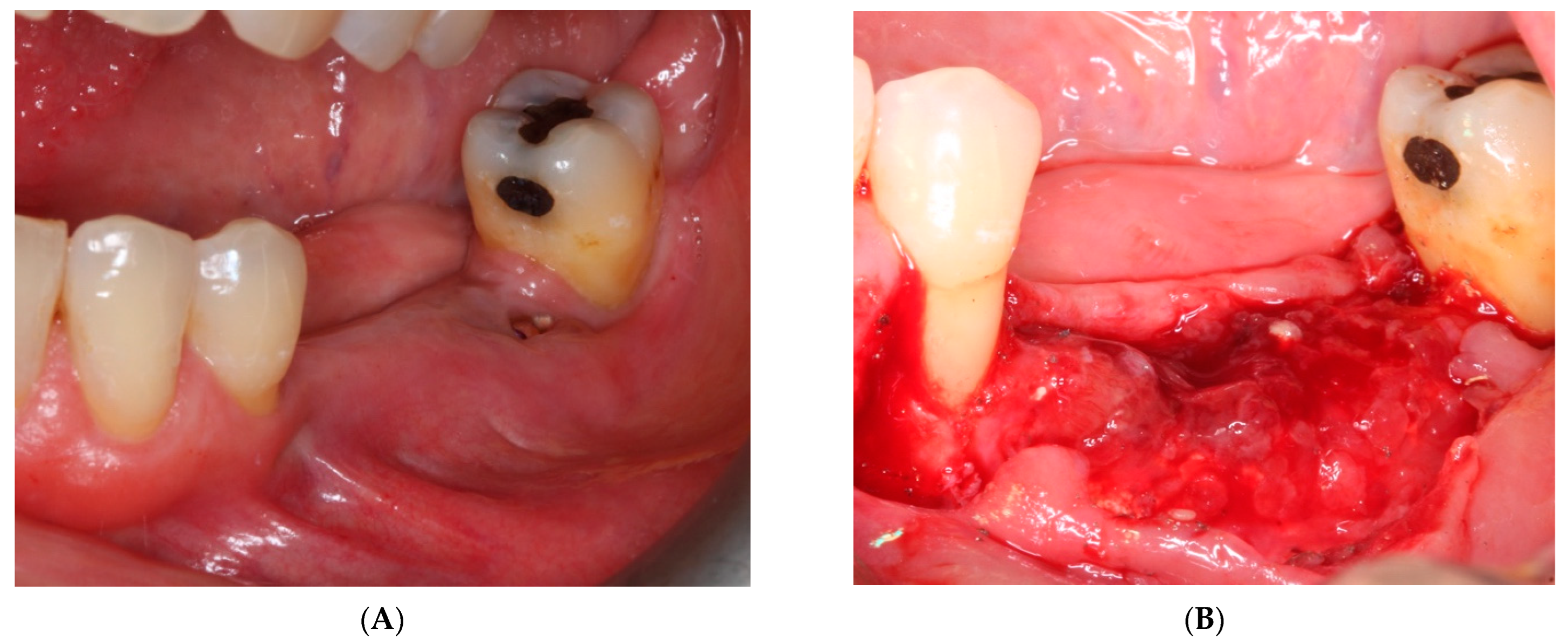
- On the day of surgery, the titanium mesh and the microscrews were removed (Figure 9). When the mesh was removed, a soft consistency was observed in the most coronally newly formed bone and it was decided to postpone the placement of the implants and allow it to ossify for another month and a half. A panoramic radiograph was taken after surgery (Figure 10).
- iv.
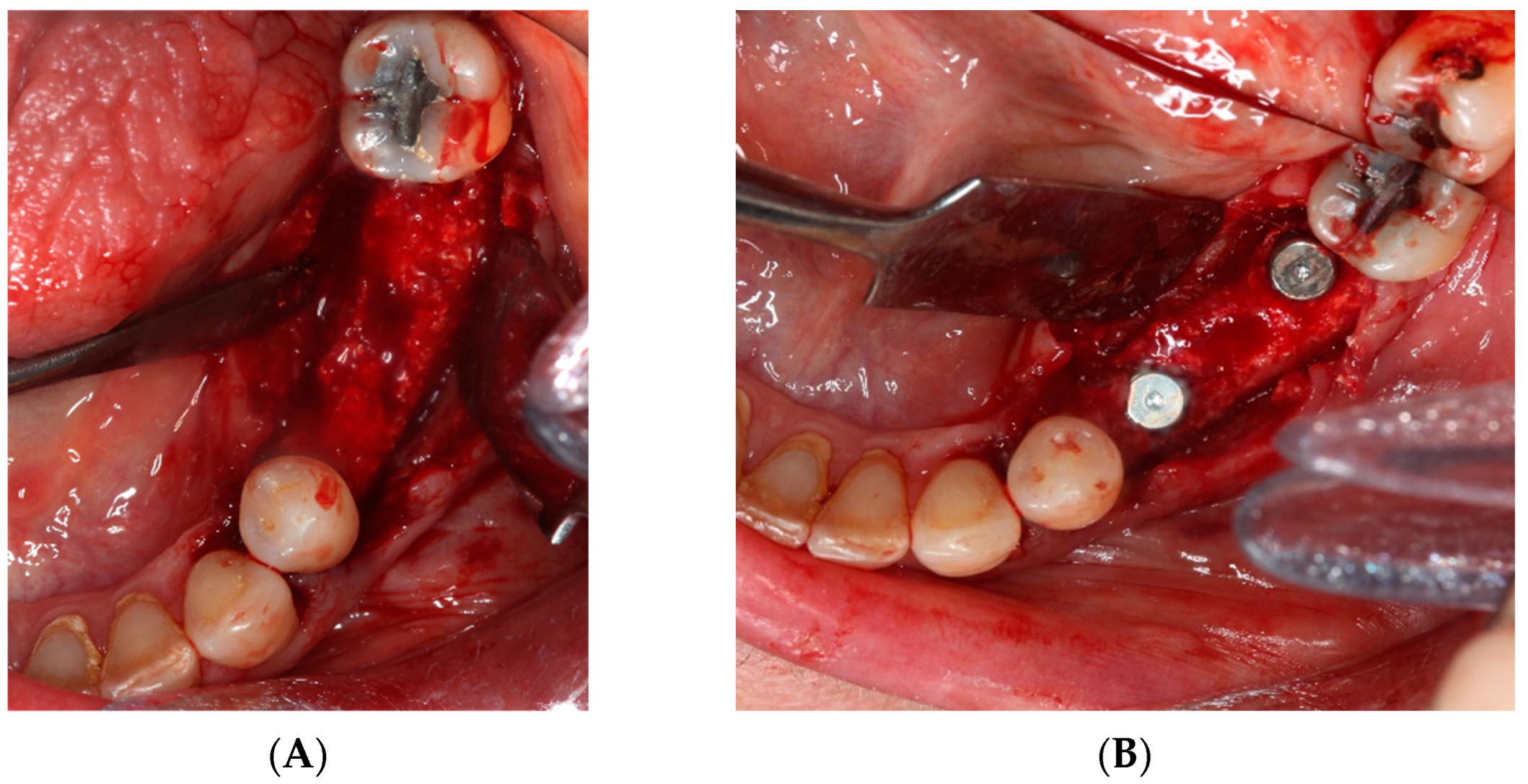
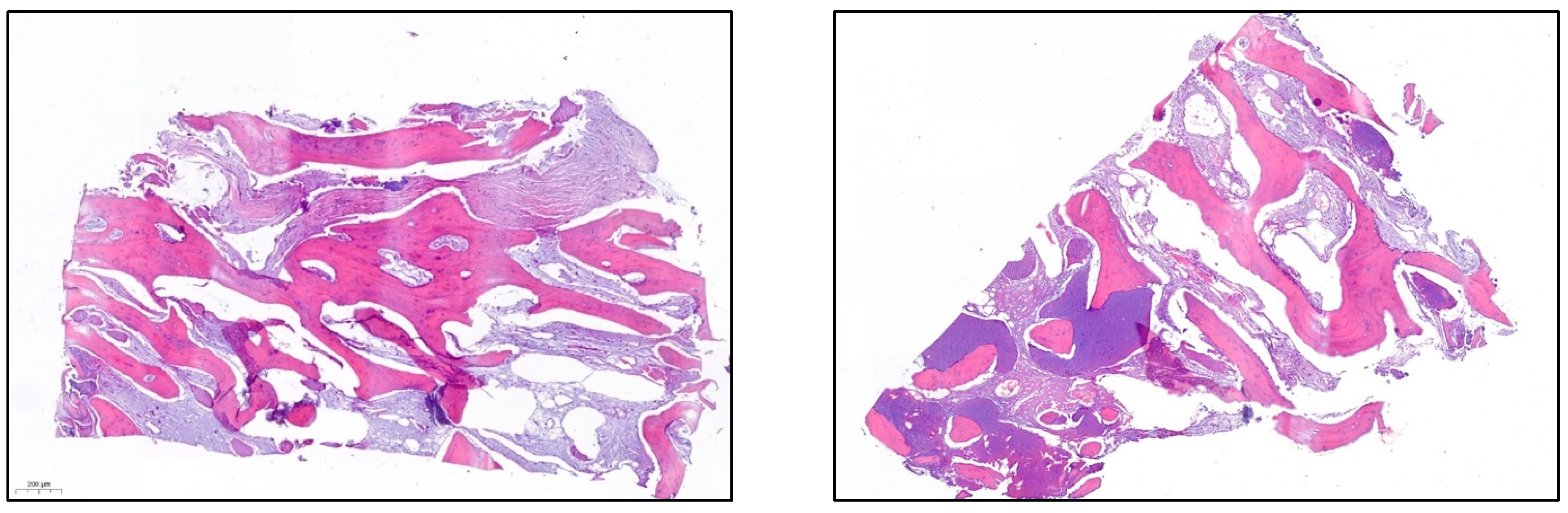
- After 7 and a half months, the Avinent® 3.8 × 8.5 implants were placed in position 3.5 and 4 × 8.5 in position 3.6 (Figure 11). The torque of the implants was greater than 45 N/cm. The ISQ of both implants was taken, being 82 buccal and palatal for the implant in position 3.5 and an ISQ of 57 buccal and palatal for the implant in position 3.6. The bone gain obtained was 1.84 and 1.92 mm in width and 4.2 and 3.78 mm in height for positions 3.5 and 3.6. Simultaneously with the placement of the implants, a bone biopsy was performed between the implants, using a 2 mm bone trephine (Sanhigia, Bujaraloz, Spain) (Figure 12). Three months after the placement of the implants, the implants were rehabilitated using metal-ceramic screw-retained crowns.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trombelli, L.; Farina, R.; Marzola, A.; Bozzi, L.; Liljenberg, B.; Lindhe, J. Modeling and remodeling of human extraction sockets. J. Clin. Periodontol. 2008, 35, 630–639. [Google Scholar] [CrossRef]
- Yamada, M.; Egusa, H. Current Bone Substitutes for Implant Dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Giuliani, A.; De Tullio, I.; Raspanti, M.; Piattelli, A.; Iezzi, G. Case Report: Histological and Histomorphometrical Results of a 3-D Printed Biphasic Calcium Phosphate Ceramic 7 Years after Insertion in a Human Maxillary Alveolar Ridge. Front. Bioeng. Biotechnol. 2021, 9, 614325. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Amaral Valladão, C.A., Jr.; Freitas Monteiro, M.; Joly, J.C. Guided Bone Regeneration in Staged Vertical and Horizontal Bone Augmentation Using Platelet-Rich Fibrin Associated with Bone Grafts: A Retrospective Clinical Study. Int. J. Implant Dent. 2020, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef]
- Angelo, T.; Marcel, W.; Andreas, K.; Izabela, S. Biomechanical Stability of Dental Implants in Augmented Maxillary Sites: Results of a Randomized Clinical Study with Four Different Biomaterials and Prf and a Biological View on Guided Bone Regeneration. BioMed Res. Int. 2015, 2015, 850340. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.Y.; Chung, H.M.; Song, Y.W.; Strauss, F.J. Vertical Ridge Augmentation Feasibility Using Unfixed Collagen Membranes and Particulate Bone Substitutes: A 1- to 7-year Retrospective Single-cohort Observational Study. Clin. Implant Dent. Relat. Res. 2022, 24, 372–381. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Ding, X.; Hu, Z.; Cen, L.; Zhang, X. Fabrication and Characterization of Collagen/Pva Dual-Layer Membranes for Periodontal Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 630977. [Google Scholar] [CrossRef]
- Simion, M.; Jovanovic, S.A.; Tinti, C.; Benfenati, S.P. Long-term evaluation of osseointegrated implants inserted at the time or after vertical ridge augmentation. A retrospective study on 123 implants with 1–5-year follow-up. Clin. Oral Implant. Res. 2001, 12, 35–45. [Google Scholar] [CrossRef]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265–273. [Google Scholar]
- Jung, G.U.; Jeon, J.Y.; Hwang, K.G.; Park, C.J. Preliminary Evaluation of a Three-Dimensional, Customized, and Preformed Titanium Mesh in Peri-Implant Alveolar Bone Regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3d Printing-Encompassing the Facets of Dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, T.; Shigeta, Y.; Hirabayashi, R.; Hirai, S.; Hirai, K.; Harada, N.; Kawamura, N.; Ogawa, T. Computer Assisted Mandibular Reconstruction Using a Custom-Made Titan Mesh Tray and Removable Denture Based on the Top-Down Treatment Technique. J. Prosthodont. Res. 2016, 60, 321–331. [Google Scholar] [CrossRef] [PubMed]
- El Morsy, O.A.; Barakat, A.; Mekhemer, S.; Mounir, M. Assessment of 3-dimensional Bone Augmentation of Severely Atrophied Maxillary Alveolar Ridges Using Patient-specific Poly Ether-ether Ketone (PEEK) Sheets. Clin. Implant Dent. Relat. Res. 2020, 22, 148–155. [Google Scholar] [CrossRef]
- Matsuo, A.; Chiba, H.; Takahashi, H.; Toyoda, J.; Abukawa, H. Clinical application of a custom-made bioresorbable raw particulate hydroxyapatite/poly-L-lactide mesh tray for mandibular reconstruction. Odontology 2010, 98, 85–88. [Google Scholar] [CrossRef]
- Vaquette, C.; Mitchell, J.; Ivanovski, S. Recent Advances in Vertical Alveolar Bone Augmentation Using Additive Manufacturing Technologies. Front. Bioeng. Biotechnol. 2022, 9, 798393. [Google Scholar] [CrossRef]
- Roca-Millan, E.; Jané-Salas, E.; Estrugo-Devesa, A.; López-López, J. Evaluation of Bone Gain and Complication Rates after Guided Bone Regeneration with Titanium Foils: A Systematic Review. Materials 2020, 13, 5346. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Du, M.; Zhang, S.; Liu, Y.; Yang, H.; Shi, R.; Guo, Y.; Song, F.; Zhao, Y.; et al. Customized Barrier Membrane (Titanium Alloy, Poly Ether-Ether Ketone and Unsintered Hydroxyapatite/Poly-l-Lactide) for Guided Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 28, 916967. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral. Sci. 2020, 12, 37. [Google Scholar] [CrossRef]
- Hasegawa, H.; Masui, S.; Ishihata, H.; Kaneko, T.; Ishida, D.; Endo, M.; Kanno, C.; Yamazaki, M.; Kitabatake, T.; Utsunomiya, S.; et al. Evaluation of a Newly Designed Microperforated Pure Titanium Membrane for Guided Bone Regeneration. Int. J. Oral Maxillofac. Implant. 2019, 34, 411–422. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yun, P.Y.; Kim, S.G.; Oh, D.S. In vitro scanning electron microscopic comparison of inner surface of exposed and unexposed nonresorbable mem- branes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Kang, T.; Fien, M.J. Titanium mesh as an alternative to a membrane for ridge augmentation. J. Oral Maxillofac. Surg. 2012, 70, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, L.; Lizio, G.; Baldissara, P.; Sambuco, A.; Scotti, R.; Corinaldesi, G. Prosthetically CAD-CAM-Guided Bone Augmentation of Atrophic Jaws Using Customized Titanium Mesh: Preliminary Results of an Open Prospective Study. J. Oral Implantol. 2018, 44, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, C.; Santoro, F.; Rabagliati, M.; Salina, S. Evaluation of the use of iliac cancellous bone and anorganic bovine bone in the reconstruction of the atrophic maxilla with titanium mesh: A clinical and histologic investigation. Int. J. Oral Maxillofac. Implant. 2001, 16, 427–432. [Google Scholar]
- Corinaldesi, G.; Pieri, F.; Sapigni, L.; Marchetti, C. Evaluation of survival and success rates of dental implants placed at the time of or after alveolar ridge augmentation with an autogenous mandibular bone graft and titanium mesh: A 3- to 8-year retrospective study. Int. J. Oral Maxillofac. Implant. 2009, 24, 1119–1128. [Google Scholar]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Ruiz, C.; Toledano, M.; Osorio, R. Testing ctive membranes for bone regeneration: A review. J. Dent. 2021, 105, 103580. [Google Scholar] [CrossRef]
- Poli, P.P.; Beretta, M.; Cicciu, M.; Maiorana, C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent. J. 2014, 8, 148–158. [Google Scholar]
- Pieri, F.; Corinaldesi, G.; Fini, M.; Aldini, N.N.; Giardino, R.; Marchetti, C. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: A 2-year prospective study. J. Periodontol. 2008, 79, 2093–2103. [Google Scholar] [CrossRef]
- Serrano Méndez, C.A.; Lang, N.P.; Caneva, M.; Ramírez Lemus, G.; Mora Solano, G.; Botticelli, D. Comparison of allografts and xenografts used for alveolar ridge preservation. A clinical and histomorphometric RCT in humans. Clin. Implant Dent. Relat. Res. 2017, 19, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Poulias, E.; Greenwell, H.; Hill, M.; Morton, D.; Vidal, R.; Shumway, B.; Peterson, T.L. Ridge preservation comparing socket allograft alone to socket allograft plus facial overlay xenograft: A clinical and histologic study in humans. J. Periodontol. 2013, 84, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, I.; Funaki, K.; Yamauchi, K.; Kodama, T.; Takahashi, T. Alveolar ridge reconstruction with titanium mesh and autogenous particulate bone graft: Computed tomography-based evaluations of augmented bone quality and quantity. Clin. Implant Dent. Relat. Res. 2012, 14, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Lizio, G.; Corinaldesi, G.; Marchetti, C. Alveolar ridge reconstruction with titanium mesh: A three-dimensional evaluation of factors affecting bone augmentation. Int. J. Oral Maxillofac. Implant. 2014, 29, 1354–1363. [Google Scholar] [CrossRef]
- Lim, H.C.; Lee, J.S.; Choi, S.H.; Jung, U.W. The effect of overlaying titanium mesh with collagen membrane for ridge preservation. J. Periodontal Implant Sci. 2015, 45, 128–135. [Google Scholar] [CrossRef]
- Sumida, T.; Otawa, N.; Kamata, Y.U.; Kamakura, S.; Mtsushita, T.; Kitagaki, H.; Mori, S.; Sasaki, K.; Fujibayashi, S.; Takemoto, M.; et al. Custom-made titanium devices as membranes for bone augmentation in implant treatment: Clinical application and the comparison with conventional titanium mesh. J. Craniomaxillofac. Surg. 2015, 43, 2183–2188. [Google Scholar] [CrossRef]
- Al-Ardah, A.J.; Alqahtani, N.; AlHelal, A.; Goodacre, B.J.; Swamidass, R.; Garbacea, A.; Lozada, J. Using Virtual Ridge Augmentation and 3-Dimensional Printing to Fabricate a Titanium Mesh Positioning Device: A Novel Technique Letter. J. Oral Implantol. 2018, 44, 293–299. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertran Faus, A.; Cordero Bayo, J.; Velasco-Ortega, E.; Torrejon-Moya, A.; Fernández-Velilla, F.; García, F.; López-López, J. Customized Titanium Mesh for Guided Bone Regeneration with Autologous Bone and Xenograft. Materials 2022, 15, 6271. https://doi.org/10.3390/ma15186271
Bertran Faus A, Cordero Bayo J, Velasco-Ortega E, Torrejon-Moya A, Fernández-Velilla F, García F, López-López J. Customized Titanium Mesh for Guided Bone Regeneration with Autologous Bone and Xenograft. Materials. 2022; 15(18):6271. https://doi.org/10.3390/ma15186271
Chicago/Turabian StyleBertran Faus, Anna, José Cordero Bayo, Eugenio Velasco-Ortega, Aina Torrejon-Moya, Francesca Fernández-Velilla, Fernando García, and José López-López. 2022. "Customized Titanium Mesh for Guided Bone Regeneration with Autologous Bone and Xenograft" Materials 15, no. 18: 6271. https://doi.org/10.3390/ma15186271
APA StyleBertran Faus, A., Cordero Bayo, J., Velasco-Ortega, E., Torrejon-Moya, A., Fernández-Velilla, F., García, F., & López-López, J. (2022). Customized Titanium Mesh for Guided Bone Regeneration with Autologous Bone and Xenograft. Materials, 15(18), 6271. https://doi.org/10.3390/ma15186271









