Modification and Functionalization of Zeolites for Curcumin Uptake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
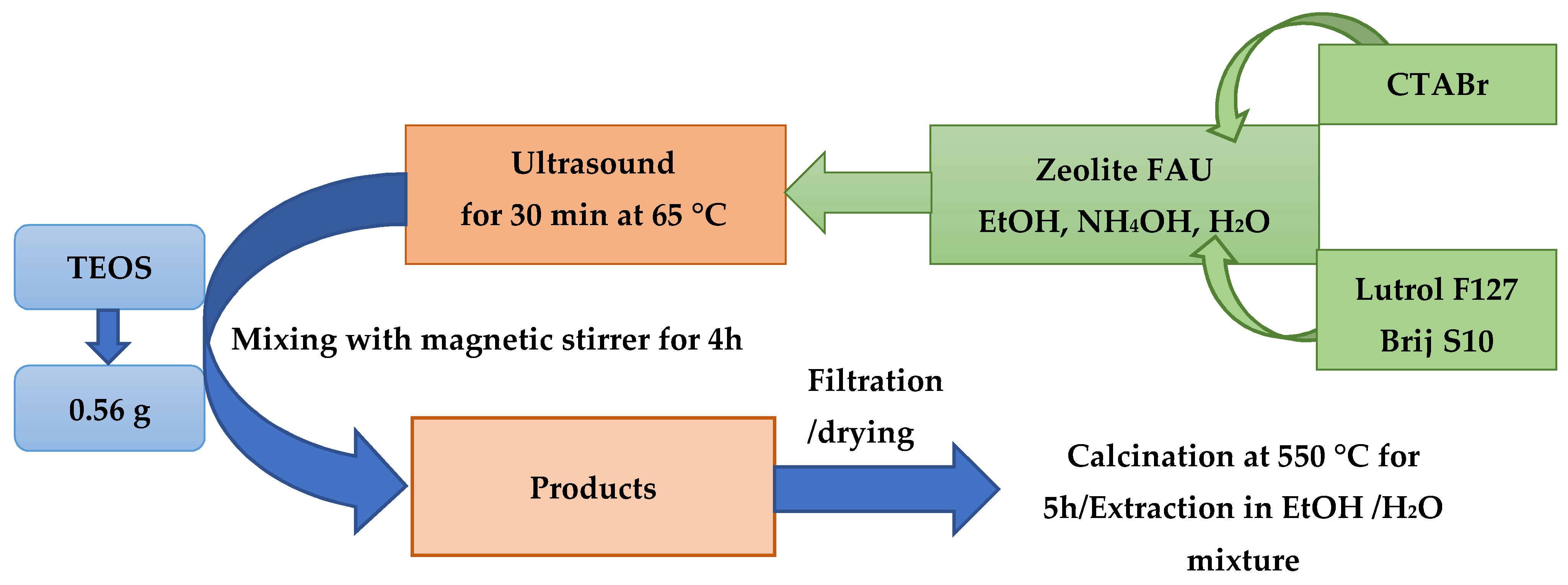
2.2. Methodology
Synthesis of Hierarchical Zeolites
2.3. Characterization of Hierarchical Materials
2.3.1. X-Ray Diffraction
2.3.2. Low-Temperature Nitrogen Adsorption/Desorption Isotherms
2.3.3. Differential Scanning Calorimetry
2.3.4. Elemental Analysis
2.3.5. Fourier Transform Infrared Spectroscopy
2.3.6. Transmission Electron Microscopy
2.3.7. Scanning Electron Microscopy
2.4. Optimization of Curcumin Deposition Conditions on Commercial Zeolite of FAU Type
2.4.1. The Process of Curcumin Loading to an FAU-Type Commercial Zeolite
2.4.2. Determination of the Percentage of Curcumin Loaded into Porous Material
2.4.3. pHZPC Analysis
2.4.4. Langmuir and Freundlich Adsorption Isotherms
3. Results and Discussion
3.1. Characterization of the Hierarchical Zeolites without and with Curcumin
3.1.1. X-Ray Diffraction Studies
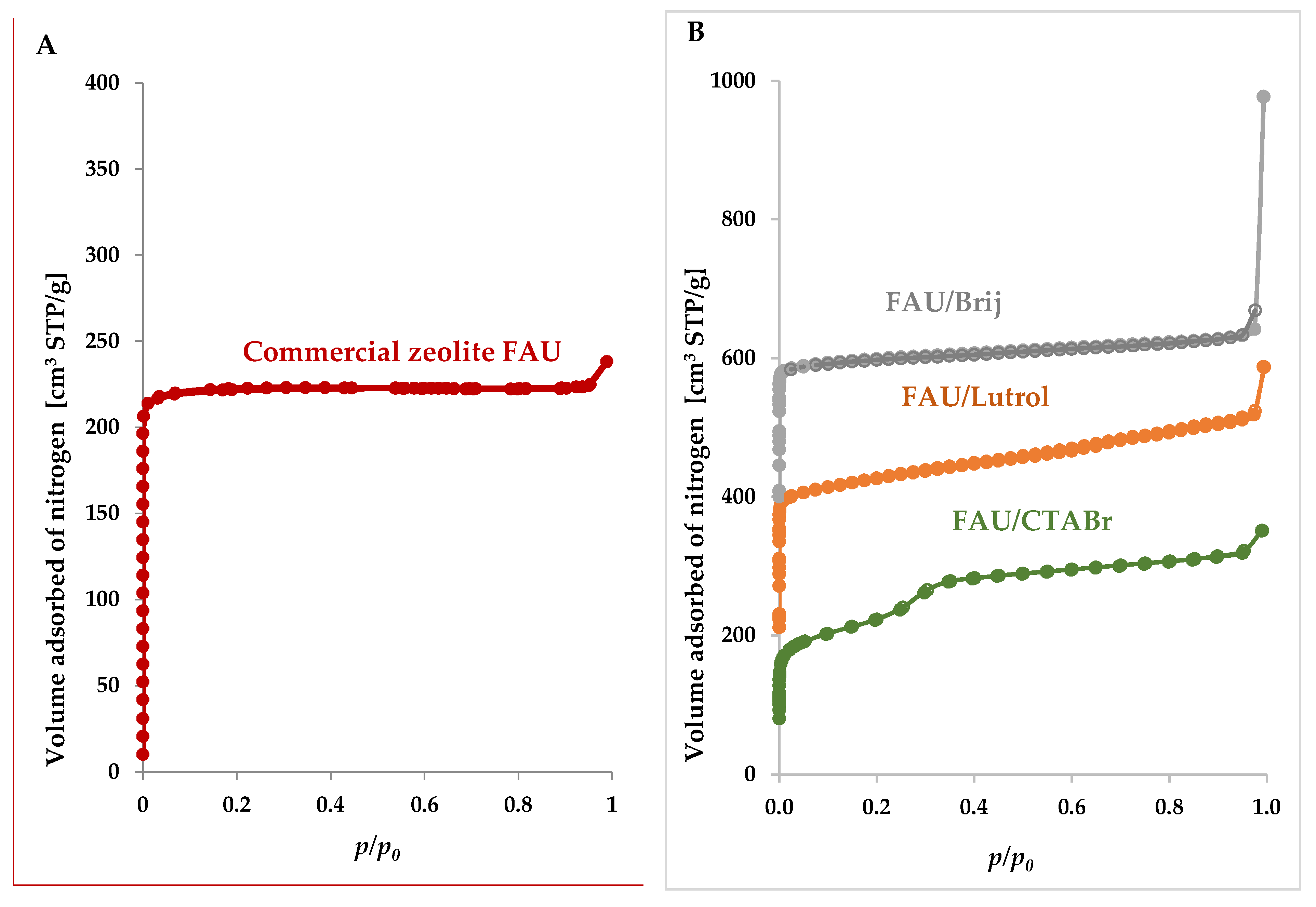
3.1.2. Nitrogen Adsorption/Desorption Studies
3.1.3. Differential Scanning Colorimetry Studies
3.1.4. Elemental Analysis Studies
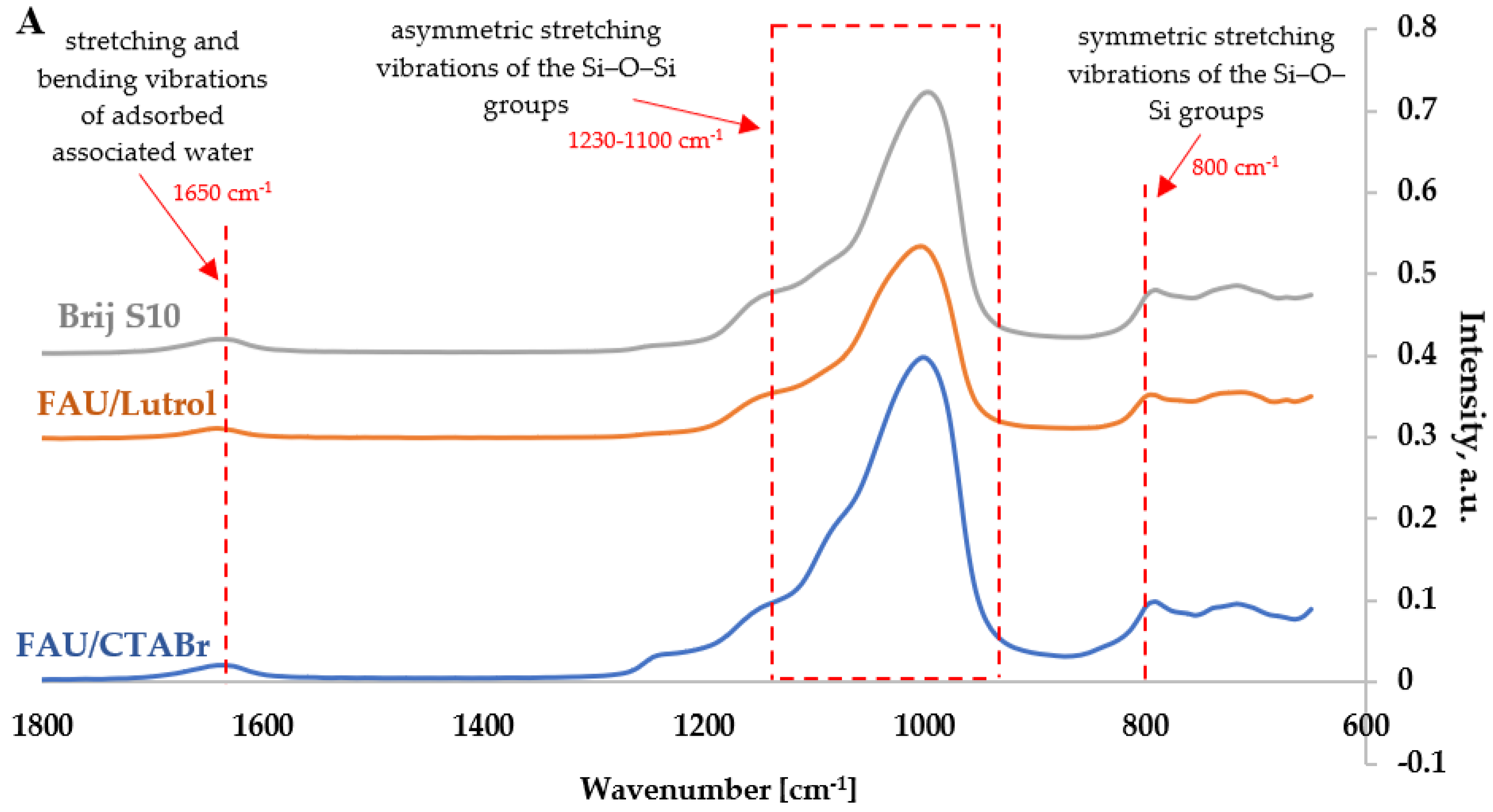
3.1.5. Fourier Transform Infrared Spectroscopy Studies
- about 1230 cm−1 and 1100 cm−1, which may indicate the presence of valence stretching vibrations for asymmetric Si-O-Si(Al) bonds; the band at ca. 1100 cm−1,
- slightly shifted toward higher wavenumbers, indicates an increase in the elemental cell size of the modified hierarchical zeolites,
- about 800 cm−1, which may originate from valence stretching vibrations for symmetric Si-O-Si bonds,
3.1.6. Transmission Electron Microscopy Studies
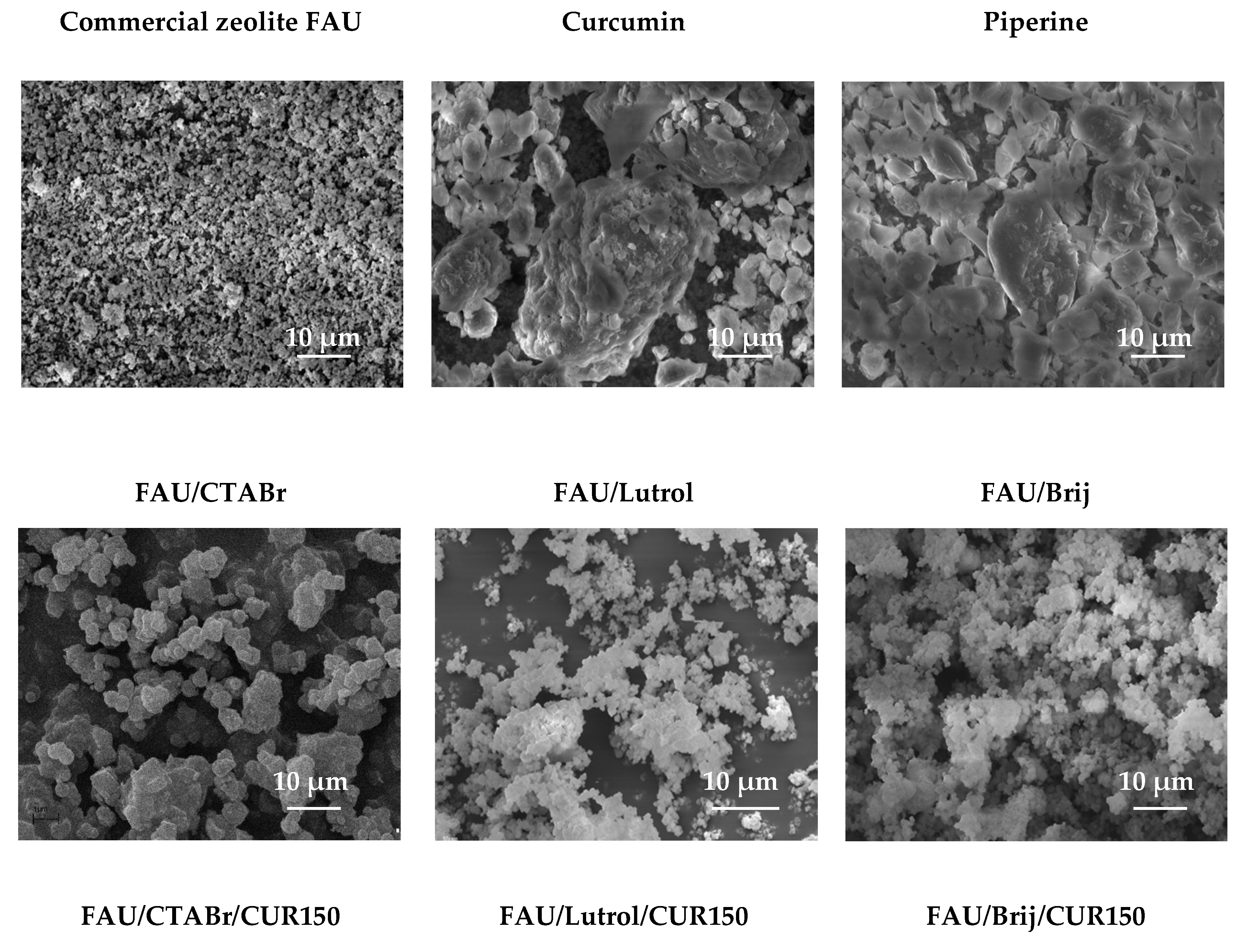
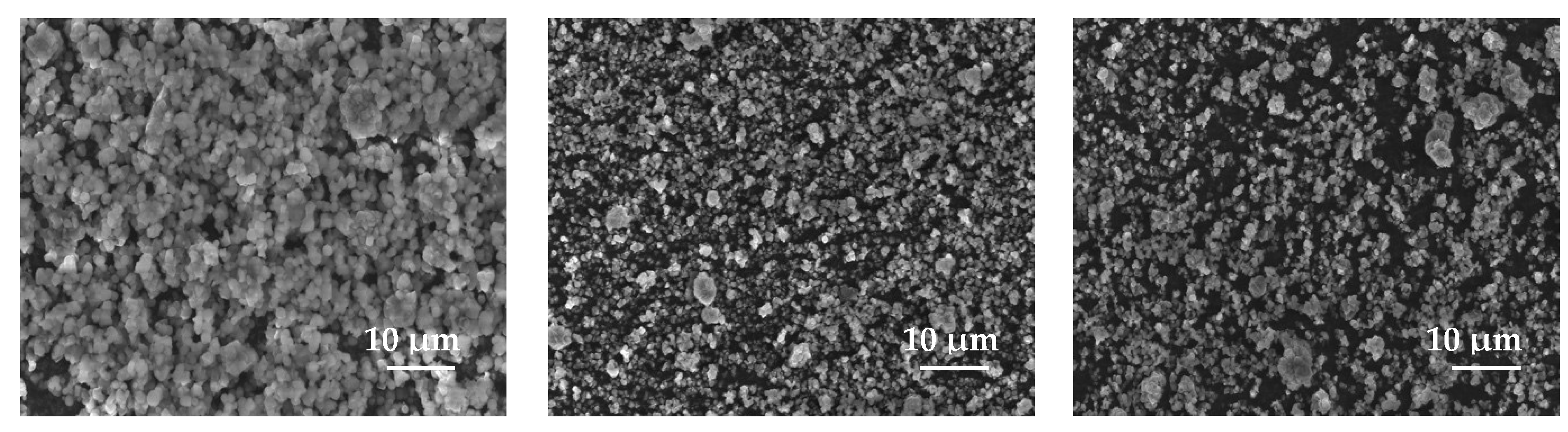
3.1.7. Scanning Electron Microscopy Studies
3.2. Adsorption of Curcumin
3.3. Determination of the Percentage of Curcumin Loading to FAU-Type Commercial Zeolite and Hierarchical Zeolites
3.4. pHZPC Analysis Zeolite Materials
3.5. Comparison of the Tested Materials with Other Curcumin Carriers
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekambaram, P.; Sathali, A.A.H.; Priyanka, K. Solid lipid nanoparticles: A review. Sci. Rev. Chem. Commun. 2012, 2, 80–102. [Google Scholar]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN. NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Hafshejani, T.M.; Zamania, A.; Venugopal, J.R.; Rezvani, Z.; Sefat, F.; Saeb, M.R.; Vahabi, H.; Zarrintaj, P.; Mozafari, M. Antibacterial glass-ionomer cement restorative materials: A critical review on the current status of extended-release formulations. J. Control. Release. 2017, 262, 317–328. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Zarrintaj, P.; Oftadeh, M.O.; Keramati, F.; Fouladiha, H.; Jahromi, S.S.; Ziraksaz, Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. Genet. Eng. Rev. 2017, 33, 144–172. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Urbanska, A.M.; Gholizadeh, S.S.; Goodarzi, V.; Saeb, M.R.; Mozafari, M. A facile route to the synthesis of aniline electroactive colloidal hydrogels for neural tissue engineering applications. J. Colloid Interface Sci. 2018, 516, 57–66. [Google Scholar] [CrossRef]
- Devalapally, H.; Chakilam, A.; Amiji, M.M. Role of nanotechnology in pharmaceutical product development. J. Pharm. Sci. 2007, 96, 2547–2565. [Google Scholar] [CrossRef]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in biomedical engineering: A critical review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery-A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.; Saeb, M.R.; Mozafari, M. Photosensitizers in medicine: Does nanotechnology make a difference? Mater. Today Proc. 2018, 5, 15836–15844. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Khalili, R.; Vahabi, H.; Saeb, M.R.; Mozafari, M.; Mozafari, M. Polyaniline/metal oxides nanocomposites. Fund. Emer. Appl. Polyaniline 2019, 8, 131–141. [Google Scholar]
- Rámila, A.; Muňoz, B.; Pérez-Pariente, J.; Regí, M.V. Mesoporous MCM-41 as drug host system. J. Sol-Gel Sci. Technol. 2003, 26, 1199–1202. [Google Scholar] [CrossRef]
- Abd-Elsatar, A.G.; Farag, M.M.; Youssef, H.F.; Salih, S.A.; Mounier, M.M.; El-Meliegy, E. Different zeolite systems for colon cancer therapy: Monitoring of ion release, cytotoxicity, and drug release behavior. Prog. Biomat. 2019, 8, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Kralj, M.; Pavelic, K. Medicine on a small scale: How molecular medicine can benefit from self-assembled and nanostructured materials. EMBO Rep. 2003, 4, 1008–1012. [Google Scholar] [CrossRef]
- Thom, D.C.; Davies, J.E.; Santerre, J.P.; Friedman, S. The hemolytic and cytotoxic properties of a zeolite-containing root filling material in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 101–108. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Soleimani, H.A.; Mohammadpour, F.; Hadizadeh, F. Synthetic zeolites as controlled-release delivery systems for antiinflammatory drugs. Chem Biol Drug Des. 2016, 87, 849–857. [Google Scholar] [CrossRef]
- Servatan, M.; Ghadiri, M.; Damanabi, A.T.; Bahadori, F.; Zarrintaj, P. Zeolite-based catalysts for exergy efficiency enhancement: The insights gained from nanotechnology. Mater. Today Proc. 2018, 5, 15868–15876. [Google Scholar] [CrossRef]
- Nemati, A.; Saghafi, M.; Khamseh, S.; Alibakhshi, E.; Zarrintaj, P.; Saeb, M.R. Magnetron-sputtered TixNy thin films applied on titanium-based alloys for biomedical applications: Composition-microstructure- property relationships. Surf. Coat. Technol. 2018, 349, 251–259. [Google Scholar] [CrossRef]
- Derakhshandeh, R.S.; Eshraghi, M.J. Diamond-like carbon-deposited films: A new class of biocorrosion protective coatings. Surf. Innovat. 2018, 6, 266–276. [Google Scholar] [CrossRef]
- Kiakhani, M.S.; Khamseh, S.; Rafie, A.; Fatemi, S.M. Thermally stable antibacterial wool fabrics surface-decorated by TiON and TiON/Cu thin films. Surf. Innovat. 2018, 6, 258–265. [Google Scholar] [CrossRef]
- Derakhshandeh, M.R.; Eshraghi, M.J.; Hadavi, M.; Javaheri, M. Diamond-like carbon thin films prepared by pulsed-DC PE-CVD for biomedical applications. Surf. Innovat. 2018, 6, 167–175. [Google Scholar] [CrossRef]
- Krajinik, D. Zeolites as potential drug carriers. In Modified Clay Zeolite Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–55. [Google Scholar]
- Ghiyasi, S.; Sari, M.G.; Saeb, M.R. Hyperbranched poly (ethyleneimine) physically attached to silica nanoparticles to facilitate curing of epoxy nanocomposite coatings. Progr. Organ. Coat. 2018, 120, 100–109. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Najafi, F.S.A.; Shahabadi, I.S.; Garmabi, H. A response surface study on microstructure and mechanical properties of poly (lactic acid)/thermoplastic starch/nanoclay nanocomposites. J. Composite Mater. 2016, 50, 269–278. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, Metals, Fibres and Dusts. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100C.) ERIONITE. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304368/ (accessed on 30 August 2022).
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 140–141. [Google Scholar]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef]
- Hartmann, M.; Machoke, A.G.; Schwieger, W. Catalytic test reactions for the evaluation of hierarchical zeolites. Chem. Soc. Rev. 2016, 45, 3313–3330. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Micropor. Mesopor. Mat. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Xi, D.; Sun, Q.; Xu, J.; Cho, M.; Cho, H.S.; Asahina, S.; Li, Y.; Deng, F.; Terasaki, O.; Yu, J. In situ growth-etching approach to the preparation of hierarchically macroporous zeolites with high MTO catalytic activity and selectivity. J. Mater. Chem. A 2014, 2, 17994–18004. [Google Scholar] [CrossRef]
- Besser, B.; Tajiri, H.A.; Mikolajczyk, G.; Mollmer, J.; Schumacher, T.C.; Odenbach, S.; Glaser, R.; Kroll, S.; Rezwan, K. Hierarchical porous zeolite structure for pressure swing adsorption applications. ACS Appl. Mater. Interfaces 2016, 8, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zha, X.; Wu, Y.; Ke, Q.; Yu, W. Visible-light-mediated radical oxydifluoromethylation of olefinic amides for the synthesis of CF2H-containing heterocycles. Chem. Commun. 2016, 52, 6367–6370. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin and Cancer: A Review of It’s Effects on Human Health. Foods 2017, 10, 92. [Google Scholar] [CrossRef]
- Lestari, M.L.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar]
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar] [PubMed]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Ramirez, L.V.; Lopez, P.P.; Lopez, A.V.; Tortosa, M.R.; Battino, M.; Quiles, J.L. Curcumin and liver disease. Biofactors 2013, 39, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.E.; Frye, J.B.; Gorti, B.; Timmermann, B.N.; Funk, J.L. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Curr. Pharm. Des. 2013, 19, 6218–6225. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A. Evaluation of Release Kinetics and Mechanisms of Curcumin and Curcumin-β-Cyclodextrin Inclusion Complex Incorporated in Electrospun Almond Gum/PVA Nanofibers in Simulated Saliva and Simulated Gastrointestinal Conditions. BioNanoScience 2019, 9, 438–445. [Google Scholar] [CrossRef]
- Abadeh, Z.A.; Saviano, G.; Ballirano, P.; Santonicola, M.G. Curcumin-loaded zeolite as anticancer drug carrier: Effect of curcumin adsorption on zeolite structure. Pure Appl. Chem. 2019, 92, 461–471. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Reinoso, F.R.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evalua-tion of Surface Area and Pore Size Distribu-tion (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Prouzet, E.; Pinnavaia, T.J. Assembly of Mesoporous Molecular Sieves Containing Wormhole Motifs by a Nonionic Surfactant Pathway: Control of Pore Size by Synthesis Temperature. Angew. Chem. Int. Ed. 1997, 36, 516–518. [Google Scholar] [CrossRef]
- Nowak, I.; Kilos, B.; Ziolek, M.; Lewandowska, A. Epoxidation of cyclohexene on Nb-containing meso- and macroporous materials. Catalysis Today. 2003, 78, 487–498. [Google Scholar] [CrossRef]
- Klancnik, G.; Medved, G.J.; Mrvar, P. Differential thermal analysis (DTA) and differential scanning calorimetry (DSC) as a method of material investigation. RMZ–Mater. Geoenviron. 2010, 57, 127–142. [Google Scholar]
- Estanqueiro, M.; Conceição, J.; Amaral, M.; Lobo, J.S. Characterization, sensorial evaluation and moisturizing efficacy of nanolipidgel formulations. Int. J. Cosmet. Sci. 2014, 36, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.D.; Surianarayanan, M.; Vijayaraghavan, R.; Mandal, A.B.; MacFarlane, D.R. Curcumin loaded poly (2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid--in vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur. J. Pharm. Sci. 2014, 51, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Jayandran, M.; Haneefa, M.M.; Balasubramanian, V. Characterization and comparative studies of turmeric oleoresin derived from selected turmeric plants. Asian J. Pharm. Sci. Technol. 2015, 5, 18–21. [Google Scholar]
- Feliczak-Guzik, A.; Sprynskyy, M.; Nowak, I.; Jaroniec, M.; Buszewski, B. Application of novel hierarchical niobium-containing zeolites for synthesis of alkyl lactate and lactic acid. J. Col. Int. Sci. 2015, 516, 379–383. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Kuncoro, E.P.; Dwi Ratri Mitha Isnadina, D.R.M.; Darmokoesoemo, H.; Fauziah, O.R.; Kusuma, H.S. Characterization, kinetic, and isotherm data for adsorption of Pb2+ from aqueous solution by adsorbent from mixture of bagasse-bentonite. Data. Brief. 2018, 16, 622–629. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Nowicki, P.; Pietrzak, R. Production of new activated bio-carbons by chemical activation of residue left after supercritical extraction of hops. Environ. Res. 2018, 161, 456–463. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Dash, S.; Ghorai, S.; Pal, S.; Sarkar, A. SBA-16: Application for the removal of neutral, cationic, and anionic dyes from aqueous medium. J. Environ. Chem. Eng. 2016, 4, 157–166. [Google Scholar] [CrossRef]
- Jiménez, S.M.R.; González, S.M.; Maldonado, A.H. Metal (M = Co2+, Ni2+, and Cu2+) grafted mesoporous SBA-15: Effect of transition metal incorporation and pH conditions on the adsorption of Naproxen from water. Microporous Mesoporous Mater. 2010, 132, 470–479. [Google Scholar] [CrossRef]
- Anitha, A.; Deepagan, V.; Rani, V.D.; Menon, D.; Nair, S.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Martinho, O.; Vilaça, N.; Castro, P.J.G.; Amorim, R.; Fonseca, A.M.; Baltazar, F.; Reis, R.M.; Neves, I.C. In vitro and in vivo studies of temozolomide loading in zeolite structures as drug delivery systems for glioblastoma. RSC Adv. 2015, 5, 28219–28227. [Google Scholar] [CrossRef]
- Mavrodinova, V.; Popova, M.; Yoncheva, K.; Mihály, J.; Szegedi, A. Solid-state encapsulation of Ag and sulfadiazine on zeolite Y carrier. J. Colloid Interface Sci. 2015, 458, 32–38. [Google Scholar] [CrossRef]
- Janssen, A.H.; Koster, A.J.; de Jong, K.P. On the shape of the mesopores in zeolite Y: A three-dimensional transmission electron microscopy study combined with texture analysis. J. Phys. Chem. 2002, 106, 11905–11909. [Google Scholar] [CrossRef] [Green Version]
- Amosa, M.K.; Al Khatib, M.; Jami, M.; Jimat, D.; Uthman, O.; Muyibi, S. Morphological synthesis and environmental application of ZSM-5 zeolite crystals from combined low-water and fluoride syntheses routes. Adv. Environ. Biol. 2014, 8, 613–625. [Google Scholar]
- Ameri, A.; Taghizadeh, T.; Kiakalaieh, A.T.; Forootanfar, H.; Mojtabavi, S.; Jahandar, H.; Tarighi, S.; Faramarzi, M.A. Bio-removal of phenol by the immobilized laccase on the fabricated parent and hierarchical NaY and ZSM-5 zeolites. J. Taiwan Inst. Chem. Eng. 2021, 120, 300–312. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of Curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Preisig, D.; Haid, D.; Varum, F.J.O.; Bravo, R.; Alles, R.; Huwyler, J.; Puchkov, M. Drug loading into porous calcium carbonate microparticles by solvent evaporation. Eur. J. Pharm. Biopharm. 2014, 87, 548–558. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.; Adnan, R.; Ngah, W.S.W.; Norita Mohamed, N. Iron Impregnated Activated Carbon as an Efficient Adsorbent for the Removal of Methylene Blue: Regeneration and Kinetics Studies. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Pradhan, M.; Patel, R.J.; Singhvi, G.; Alexander, A. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed. Pharmacother. 2021, 141, 111919. [Google Scholar] [CrossRef] [PubMed]



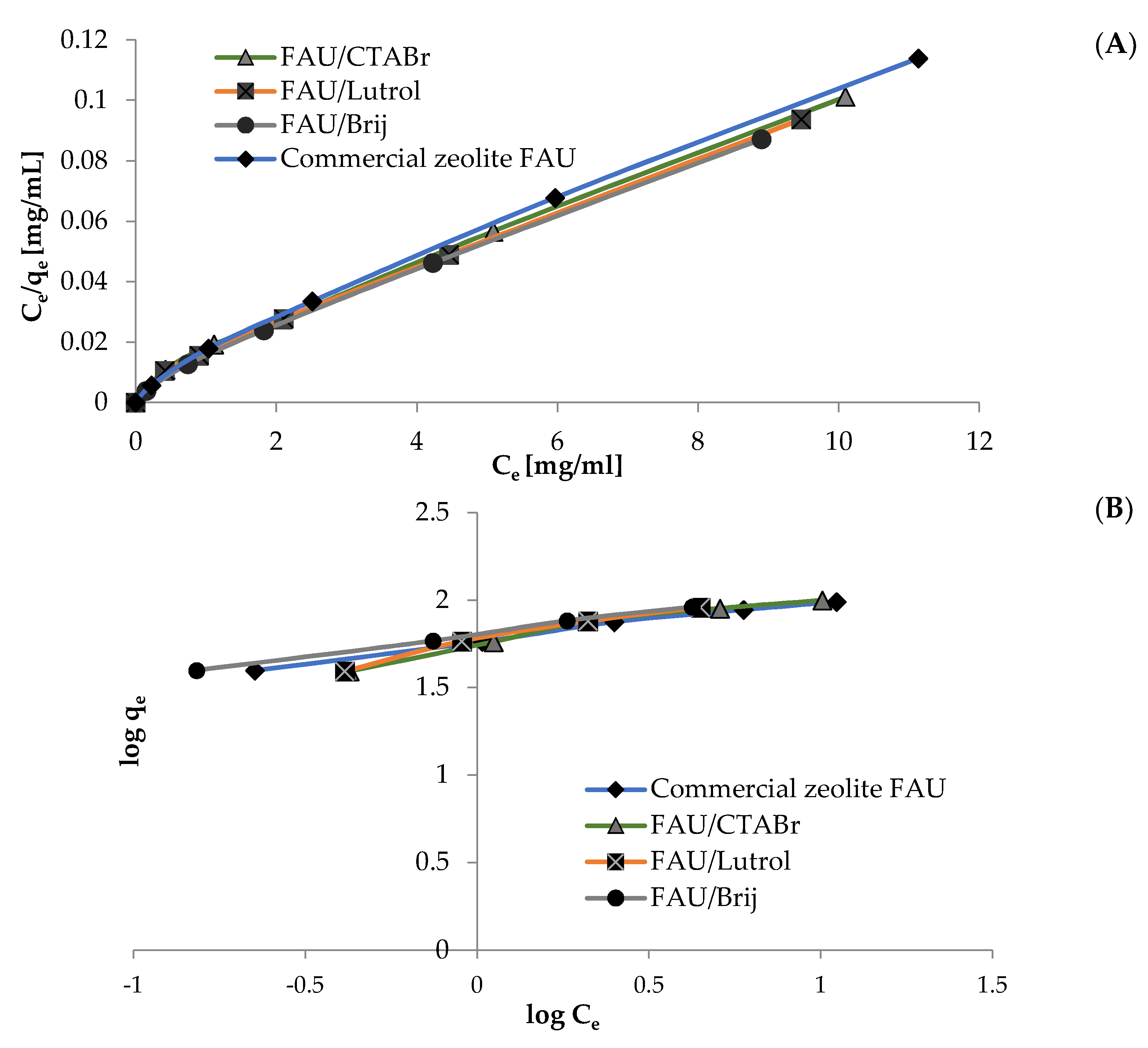
| Name | Curcumin [mg] | Piperine [mg] |
|---|---|---|
| FAU/CTABr/CUR50 | 50.00 | - |
| FAU/CTABr/CUR150 | 150.00 | - |
| FAU/Lutrol/CUR50 | 50.00 | - |
| FAU/Lutrol/CUR150 | 150.00 | - |
| FAU/Brij/CUR50 | 50.00 | - |
| FAU/Brij/CUR150 | 150.00 | - |
| FAU/CTABr/CUR50/PIP0.25 | 50.00 | 0.25 |
| FAU/CTABr/CUR150/PIP0.25 | 150.00 | 0.25 |
| FAU/Lutrol/CUR50/PIP0.25 | 50.00 | 0.25 |
| FAU/Lutrol/CUR150/PIP0.25 | 150.00 | 0.25 |
| FAU/Brij/CUR50/PIP0.25 | 50.00 | 0.25 |
| FAU/Brij/CUR150/PIP0.25 | 150.00 | 0.25 |
| Materials | a0, [nm] | BET Surface Area [m2/g] SBET | Pore Volume [cm3/g] | Average Mesopore Size [nm] | ||
|---|---|---|---|---|---|---|
| Total Pore Volume | Volume of Micropores | Mesopore Volume | ||||
| Commercial zeolite FAU | 2.467 | 718 | 0.37 | 0.30 | 0.05 | - |
| FAU/CTABr | 2.474 | 892 | 0.49 | 0.19 | 0.30 | 3.40 |
| FAU/Lutrol | 2.469 | 696 | 0.46 | 0.19 | 0.27 | 3.10 |
| FAU/Brij | 2.479 | 588 | 0.43 | 0.17 | 0.26 | 3.20 |
| Material | qe [mg/g] | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | qmax [mg/g] | KL | RL | R2 | KF | 1/n | ||
| Commercial zeolite FAU | 97.93 | 0.993 | 98.04 | 0.027 | 0.2784–0.7851 | 0.962 | 57.40 | 0.24 |
| FAU/CTABr | 99.80 | 0.990 | 102.04 | 0.022 | 0.4330–0.8208 | 0.955 | 54.51 | 0.30 |
| FAU/Lutrol | 101.06 | 0.990 | 103.09 | 0.023 | 0.4207–0.8133 | 0.934 | 56.48 | 0.35 |
| FAU/Brij | 102.19 | 0.993 | 103.09 | 0.032 | 0.3394–0.7550 | 0.928 | 64.02 | 0.25 |
| Name | % Load | ±SD |
|---|---|---|
| FAU/M/CUR50 | 29.30 | ±0.27 |
| FAU/M/CUR100 | 47.30 | ±0.70 |
| FAU/M/CUR150 | 77.60 | ±0.89 |
| FAU/E/CUR50 | 30.00 | ±0.21 |
| FAU/E/CUR100 | 48.10 | ±0.82 |
| FAU/E/CUR150 | 54.20 | ±0.43 |
| FAU/A/CUR50 | 39.20 | ±0.18 |
| FAU/A/CUR100 | 61.80 | ±0.65 |
| FAU/A/CUR150 | 79.60 | ±0.65 |
| FAU/A/CUR50/PIP0.25 | 25.10 | ±0.26 |
| FAU/A/CUR50/PIP0.50 | 32.10 | ±0.36 |
| FAU/A/CUR50/PIP1.00 | 25.40 | ±0.70 |
| FAU/A/CUR50/PIP2.00 | 22.10 | ±0.36 |
| Name | % Load | ±SD |
|---|---|---|
| FAU/CTABr/CUR50 | 39.20 | ±0.40 |
| FAU/CTABr/CUR150 | 89.60 | ±0.40 |
| FAU/Lutrol/CUR50 | 32.60 | ±0.45 |
| FAU/Lutrol/CUR150 | 87.90 | ±0.65 |
| FAU/Brij/CUR50 | 41.50 | ±0.60 |
| FAU/Brij/CUR150 | 89.60 | ±0.80 |
| FAU/CTABr/CUR50/PIP0.25 | 39.50 | ±0.36 |
| FAU/CTABr/CUR150/PIP0.25 | 89.40 | ±0.45 |
| FAU/Lutrol/CUR50/PIP0.25 | 41.30 | ±0.62 |
| FAU/Lutrol/CUR150/PIP0.25 | 81.90 | ±0.66 |
| FAU/Brij/CUR50/PIP0.25 | 42.30 | ±0.43 |
| FAU/Brij /CUR150/PIP0.25 | 88.80 | ±0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musielak, E.; Feliczak-Guzik, A.; Jaroniec, M.; Nowak, I. Modification and Functionalization of Zeolites for Curcumin Uptake. Materials 2022, 15, 6316. https://doi.org/10.3390/ma15186316
Musielak E, Feliczak-Guzik A, Jaroniec M, Nowak I. Modification and Functionalization of Zeolites for Curcumin Uptake. Materials. 2022; 15(18):6316. https://doi.org/10.3390/ma15186316
Chicago/Turabian StyleMusielak, Ewelina, Agnieszka Feliczak-Guzik, Mietek Jaroniec, and Izabela Nowak. 2022. "Modification and Functionalization of Zeolites for Curcumin Uptake" Materials 15, no. 18: 6316. https://doi.org/10.3390/ma15186316
APA StyleMusielak, E., Feliczak-Guzik, A., Jaroniec, M., & Nowak, I. (2022). Modification and Functionalization of Zeolites for Curcumin Uptake. Materials, 15(18), 6316. https://doi.org/10.3390/ma15186316








