Thermally Insulating, Thermal Shock Resistant Calcium Aluminate Phosphate Cement Composites for Reservoir Thermal Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Cement Formulas and Samples Preparation
2.3. Measurements
3. Results and Discussion
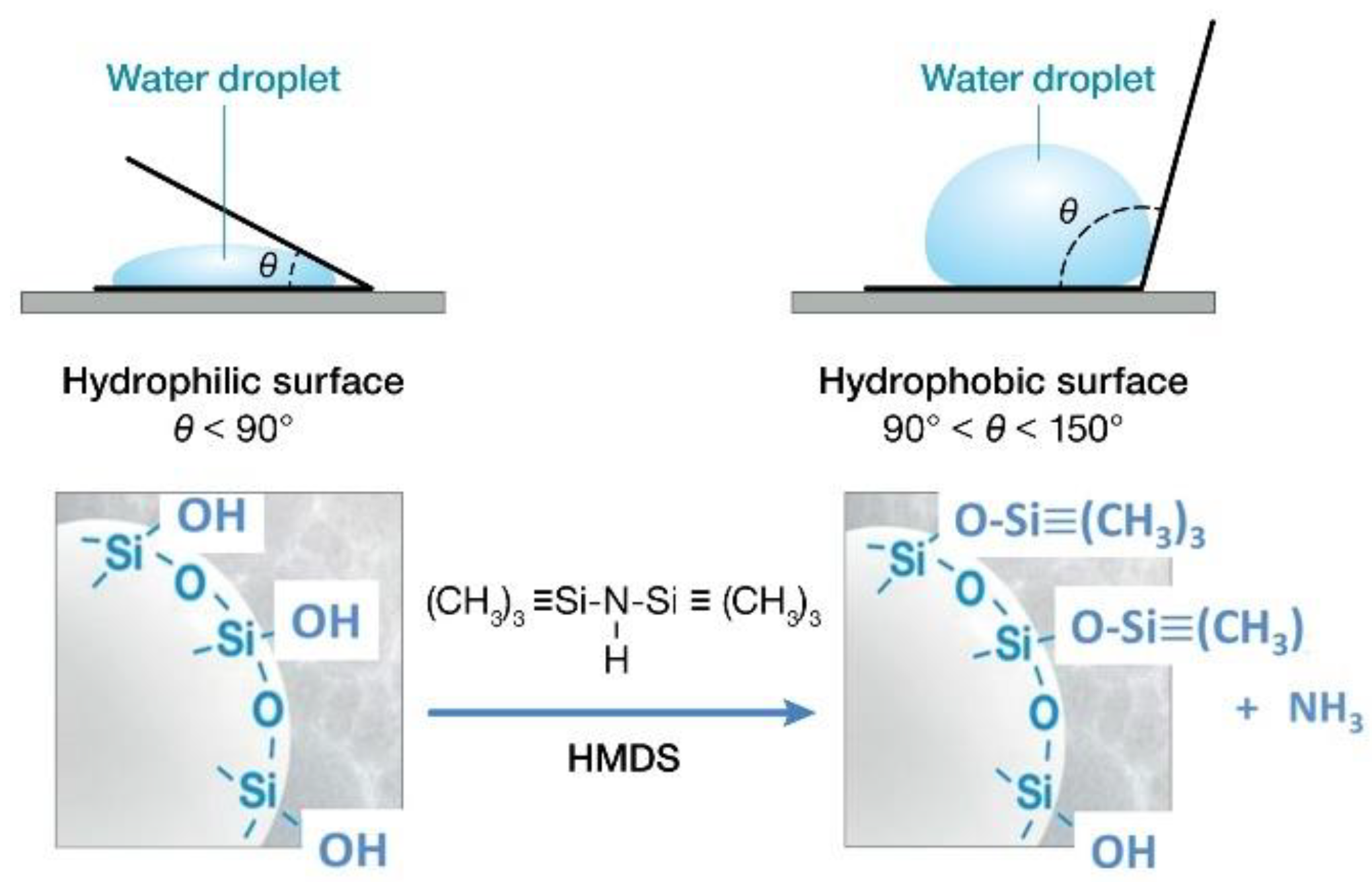
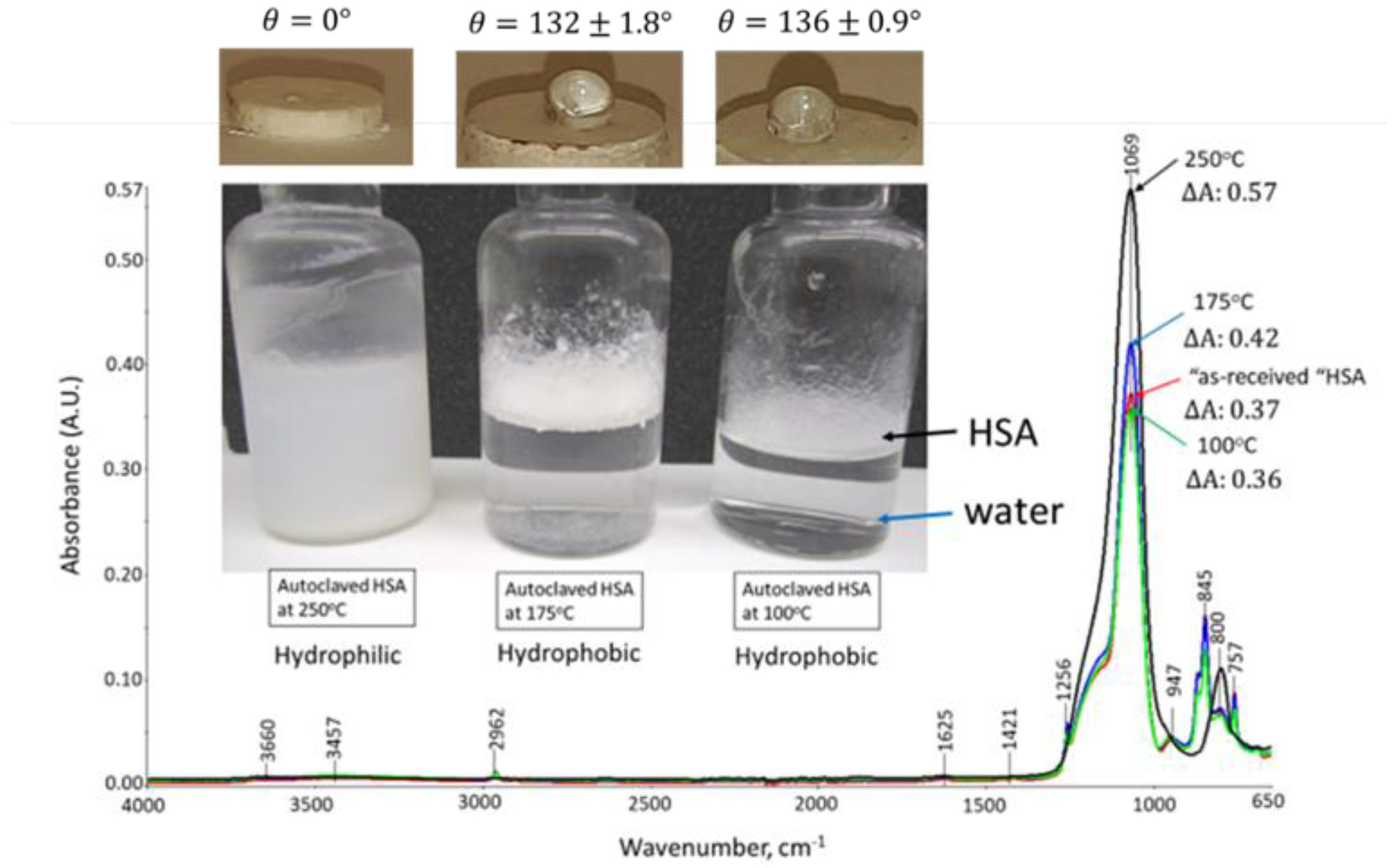
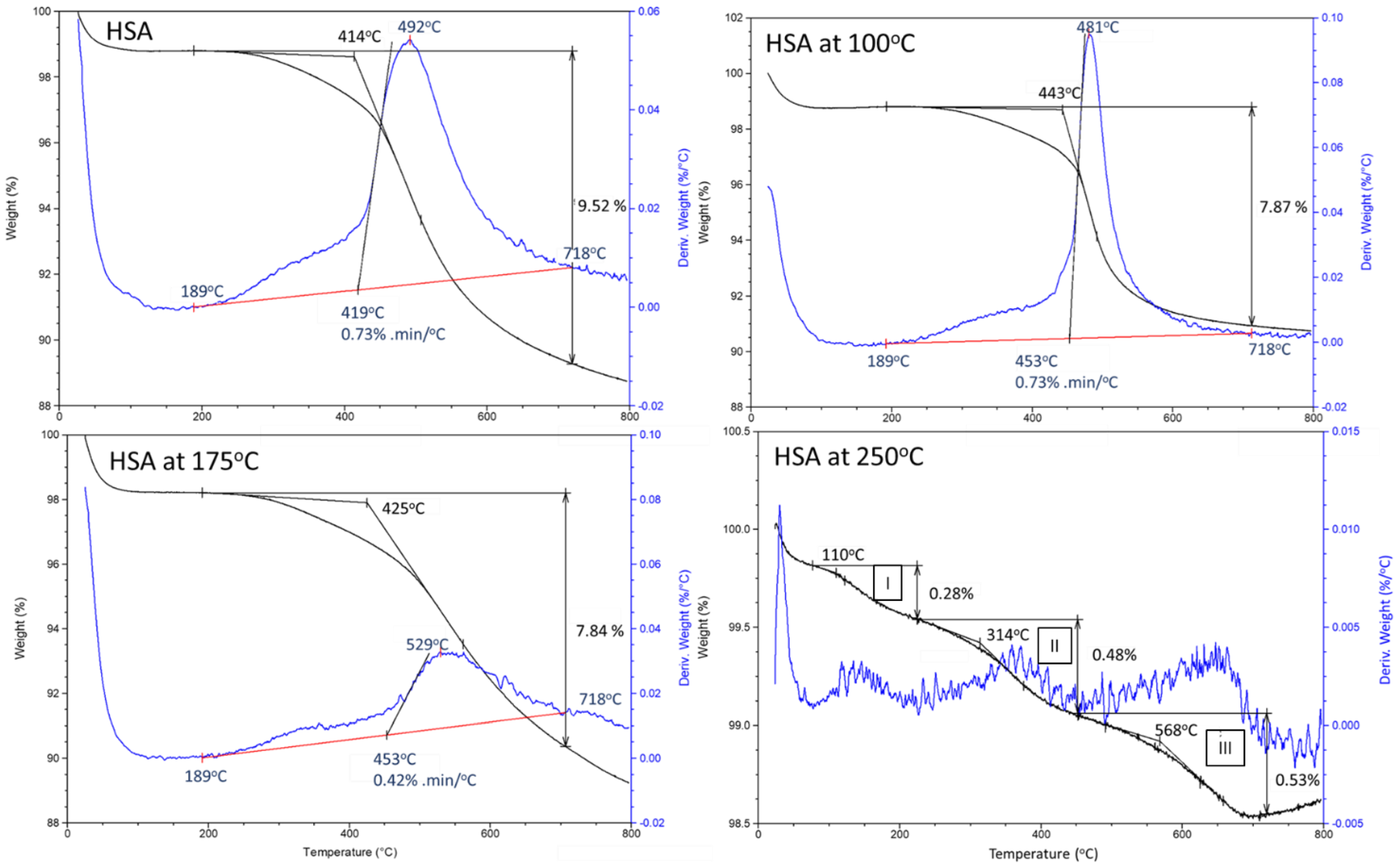
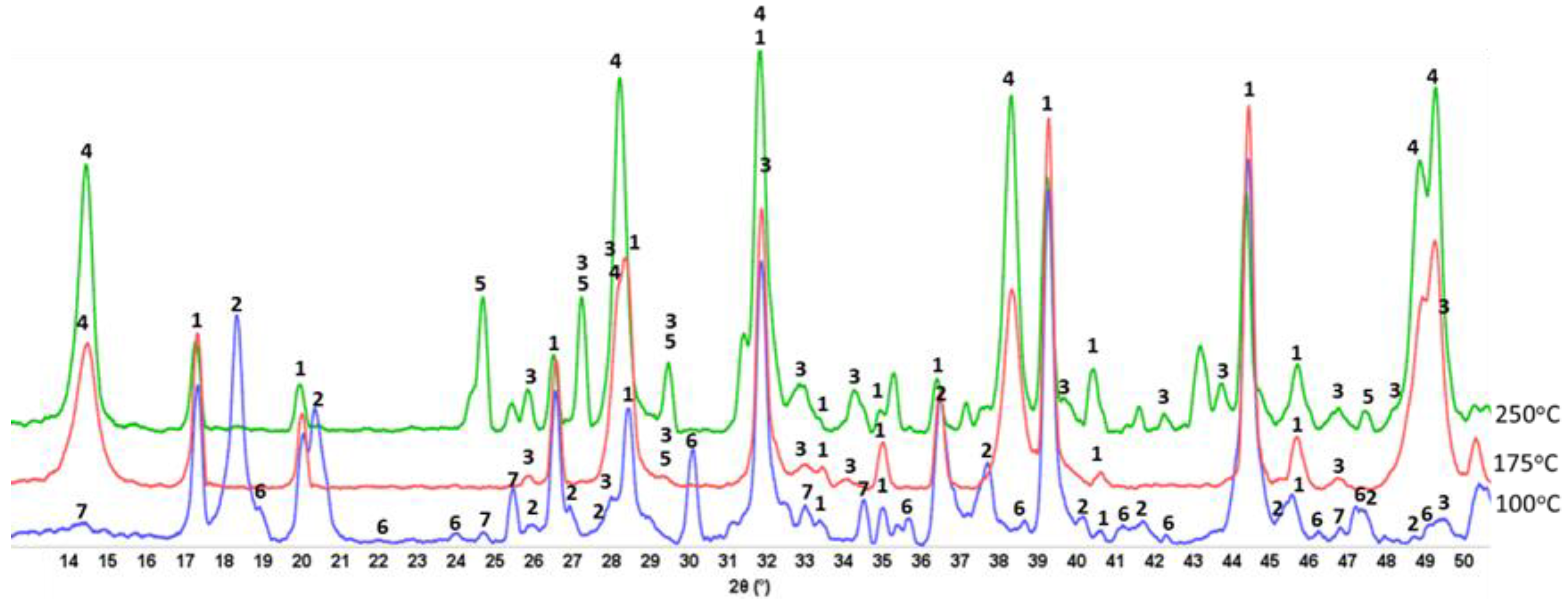
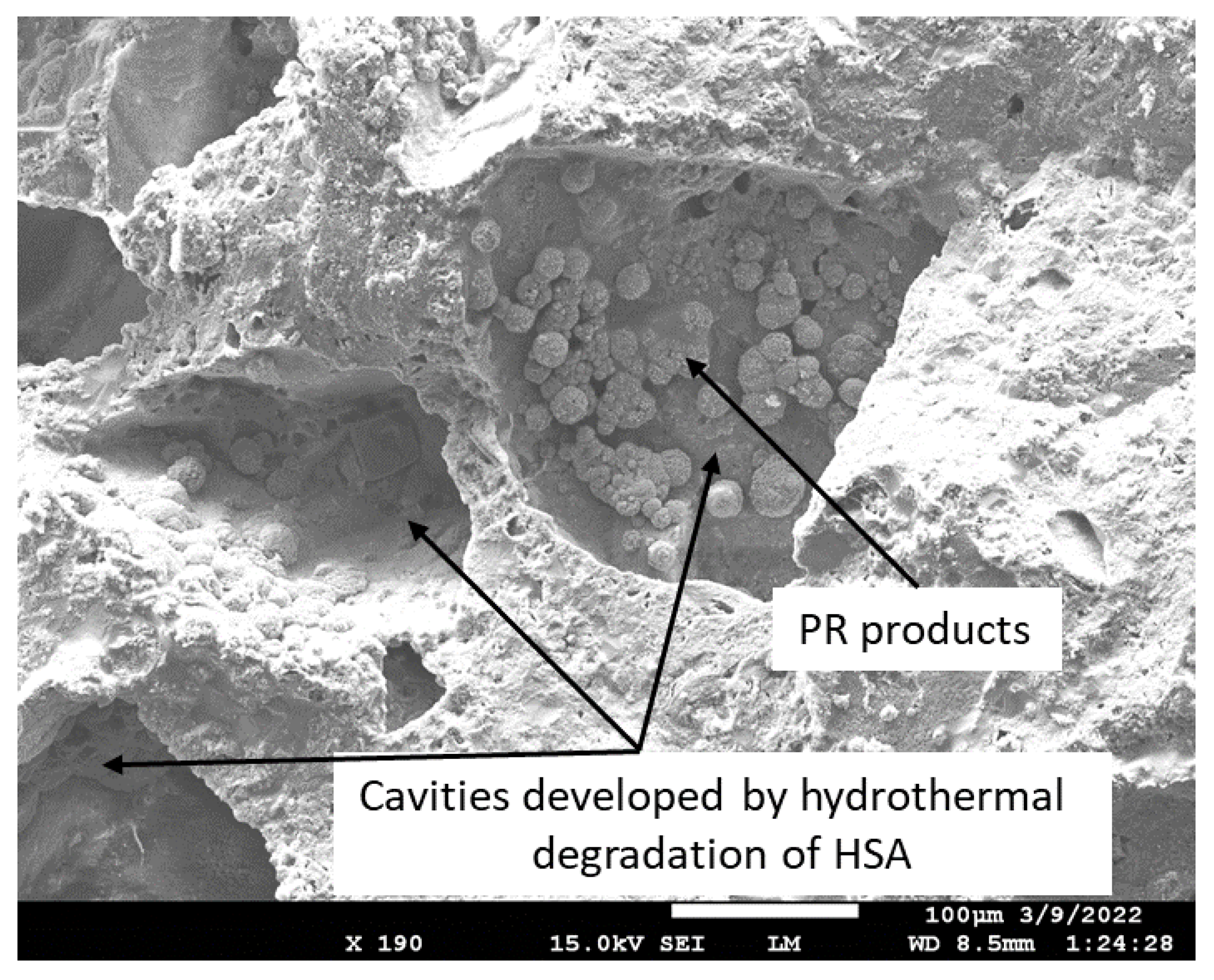
3.1. Hydrothermal Stability of HSA
3.2. Slurry Properties
3.3. Pozzolanic and Mechanical Behaviors of Aggregates and Reinforcements
3.3.1. Neat CaP Cement
3.3.2. CaP/FCS System
3.3.3. CaP/HSA System
3.3.4. CaP/MGF System
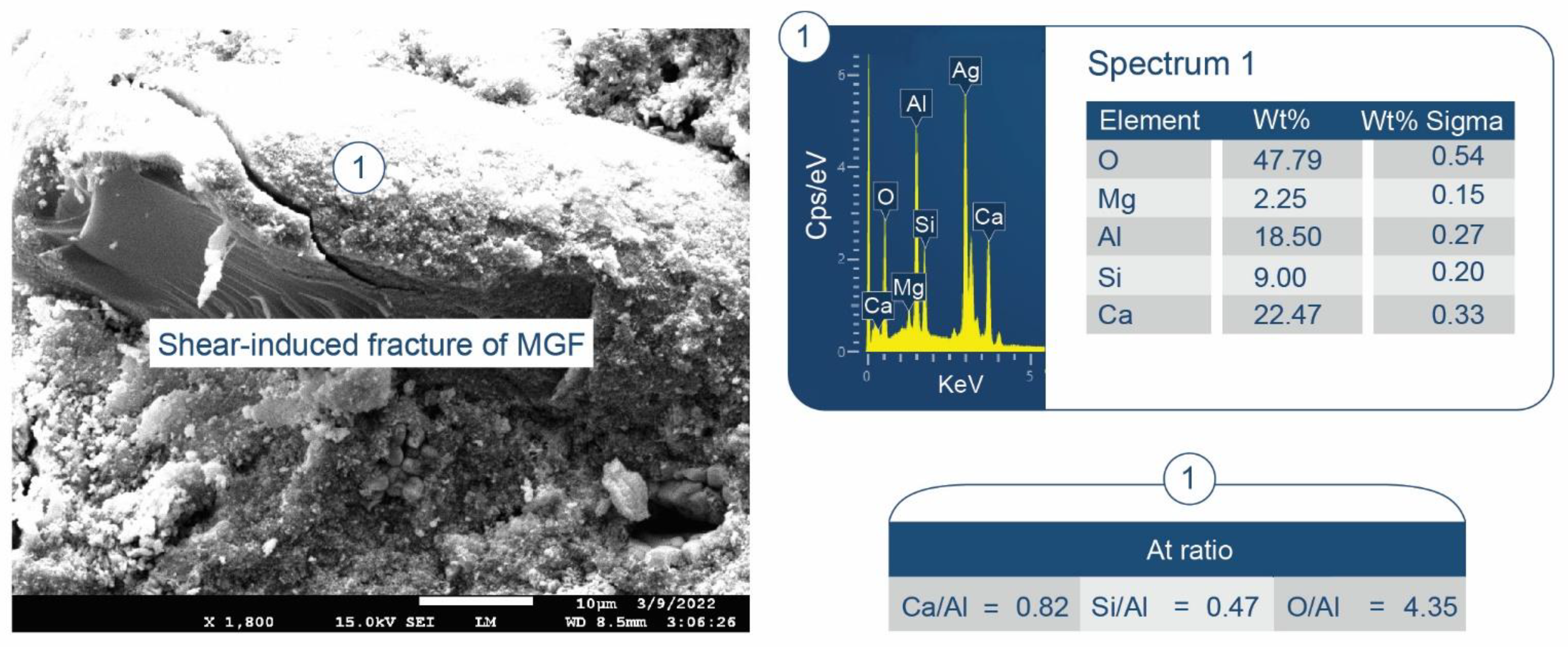
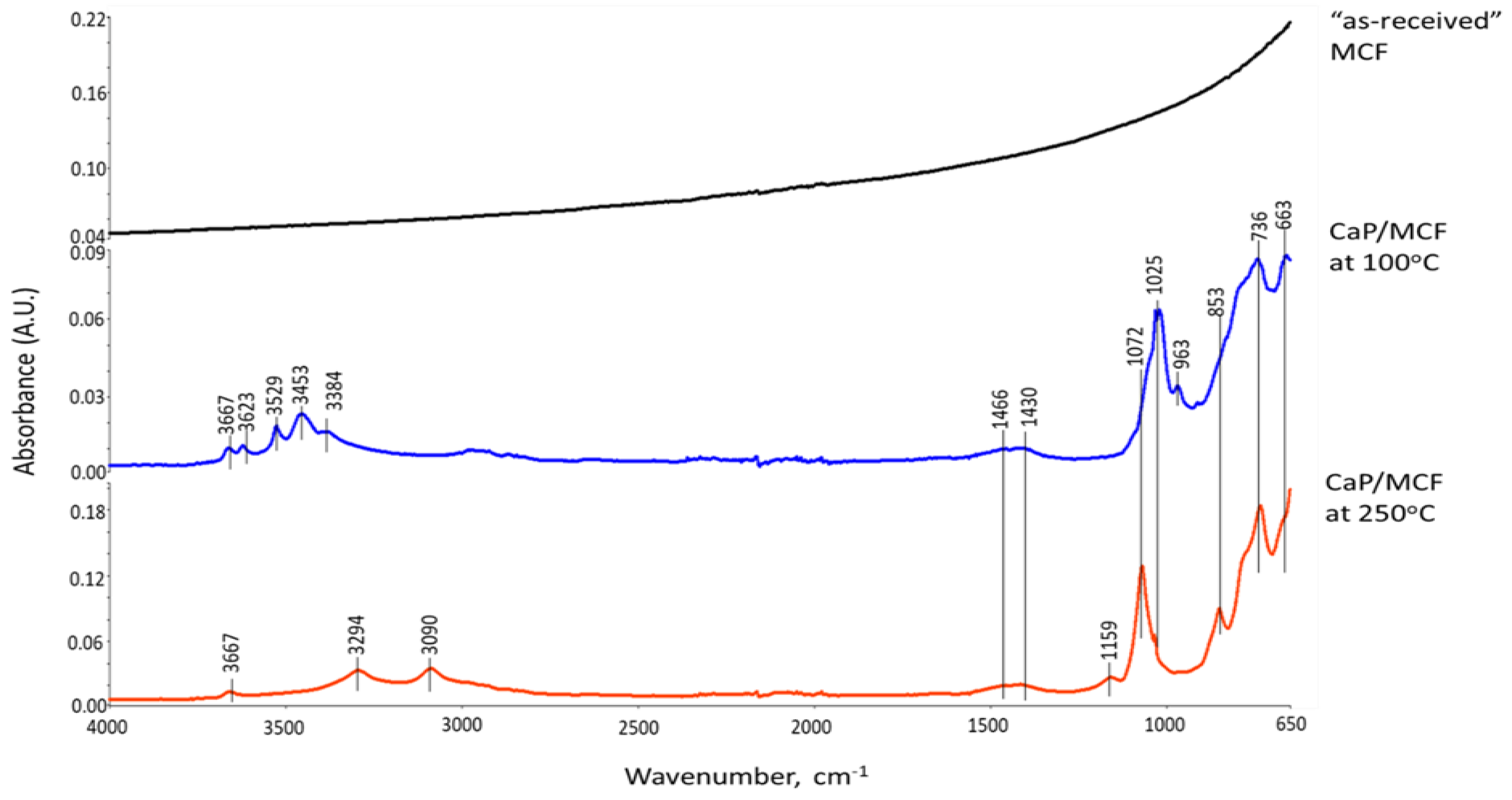
3.3.5. CaP/MCF System
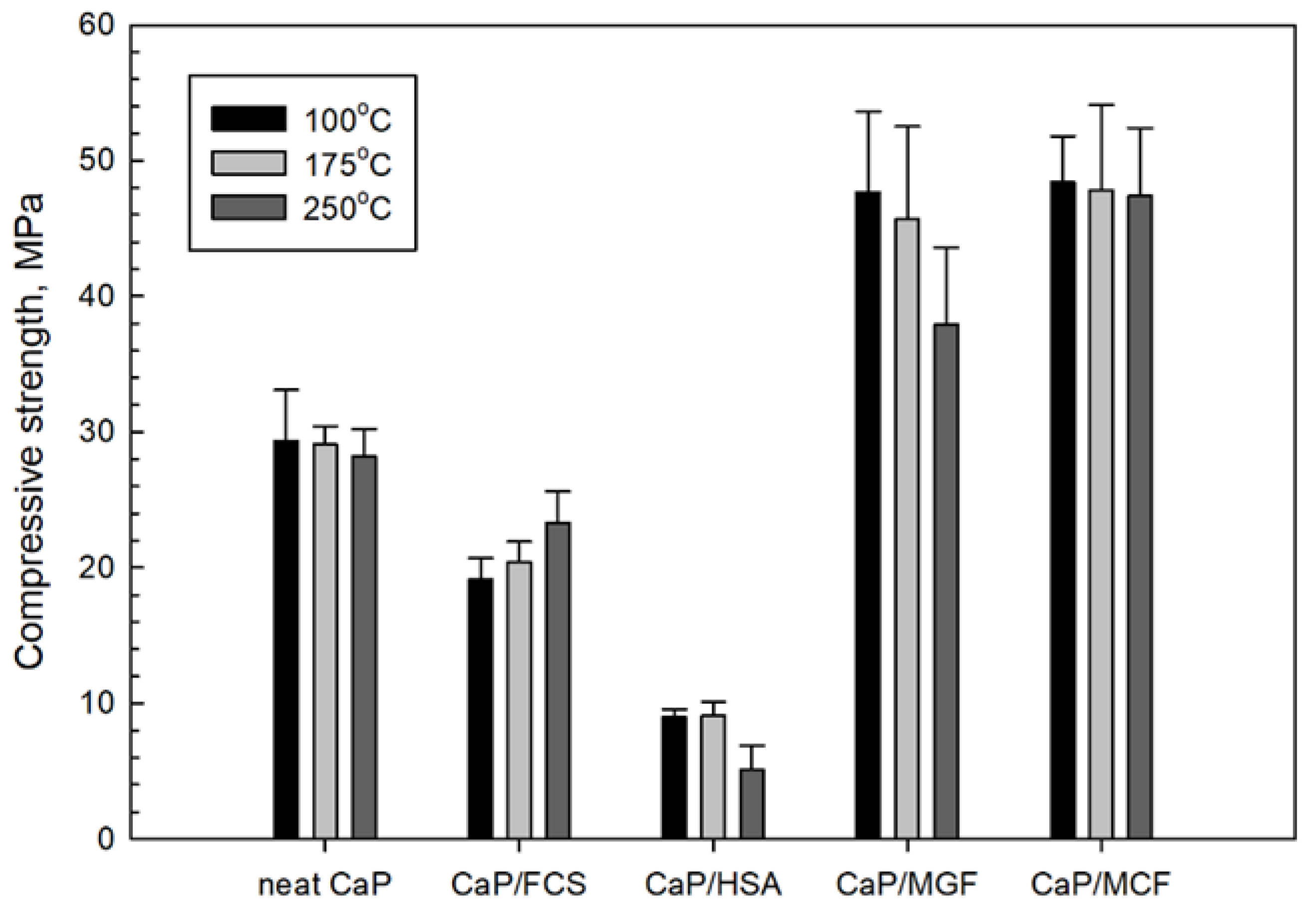
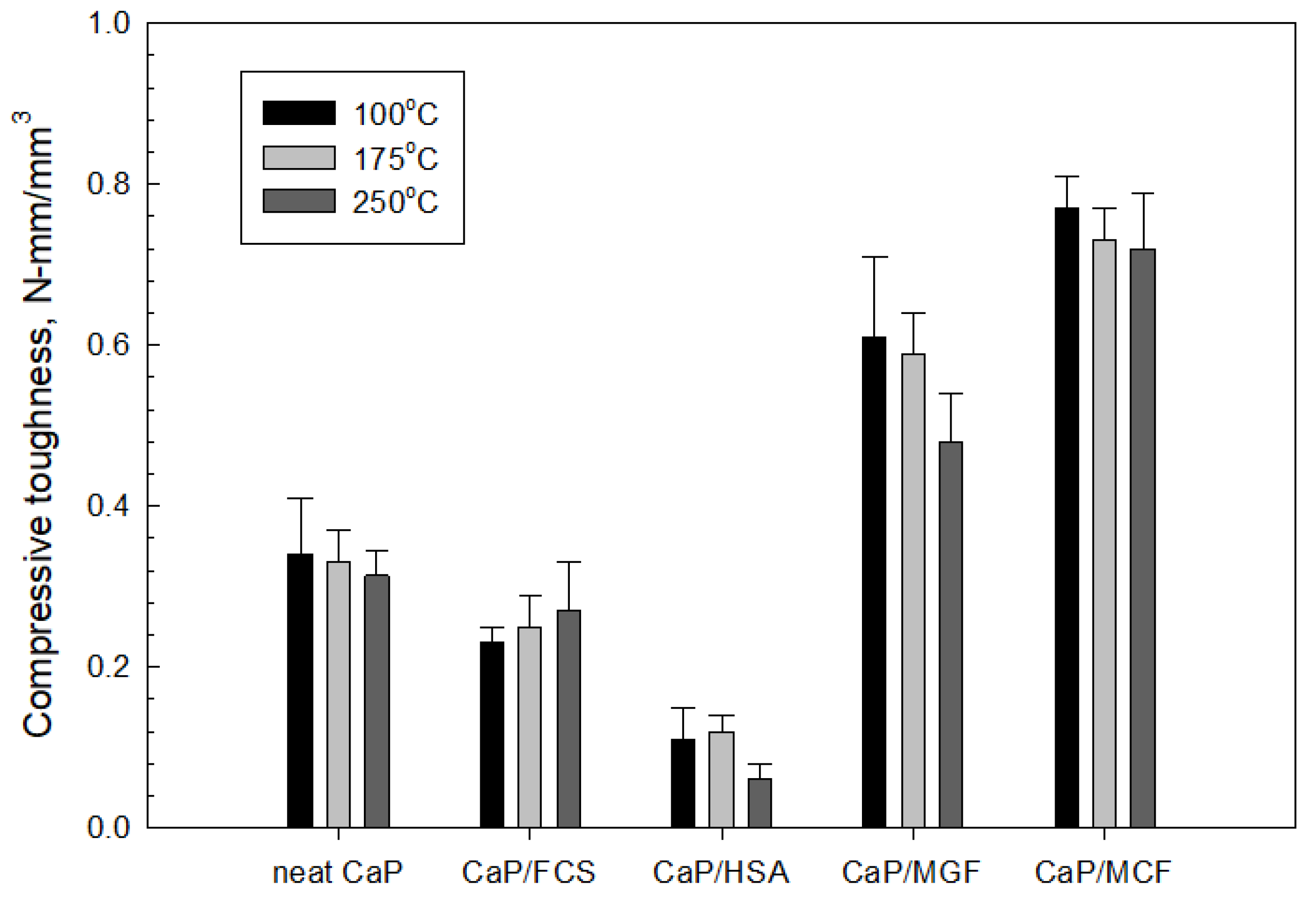
3.3.6. Mechanic Properties
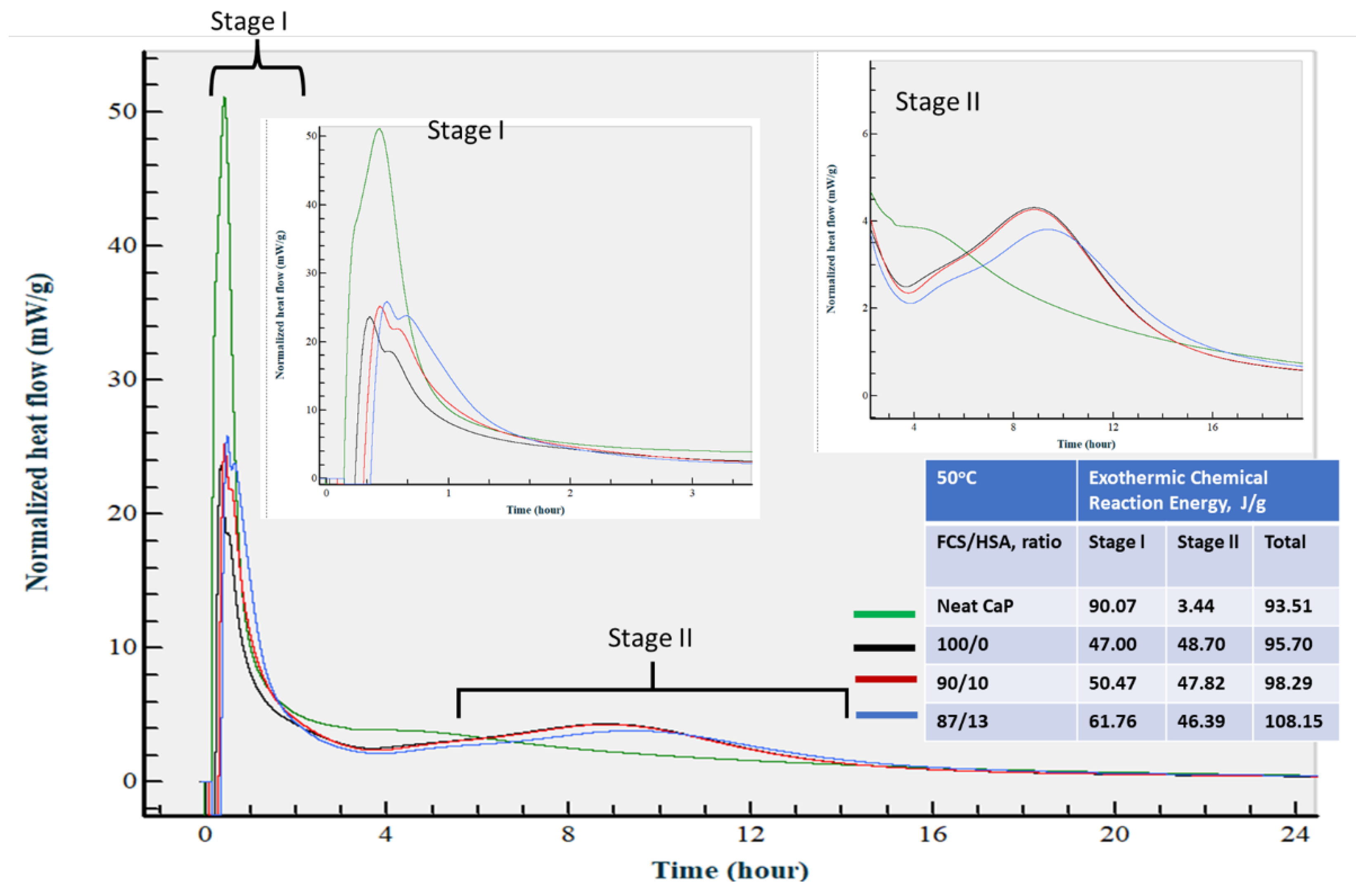
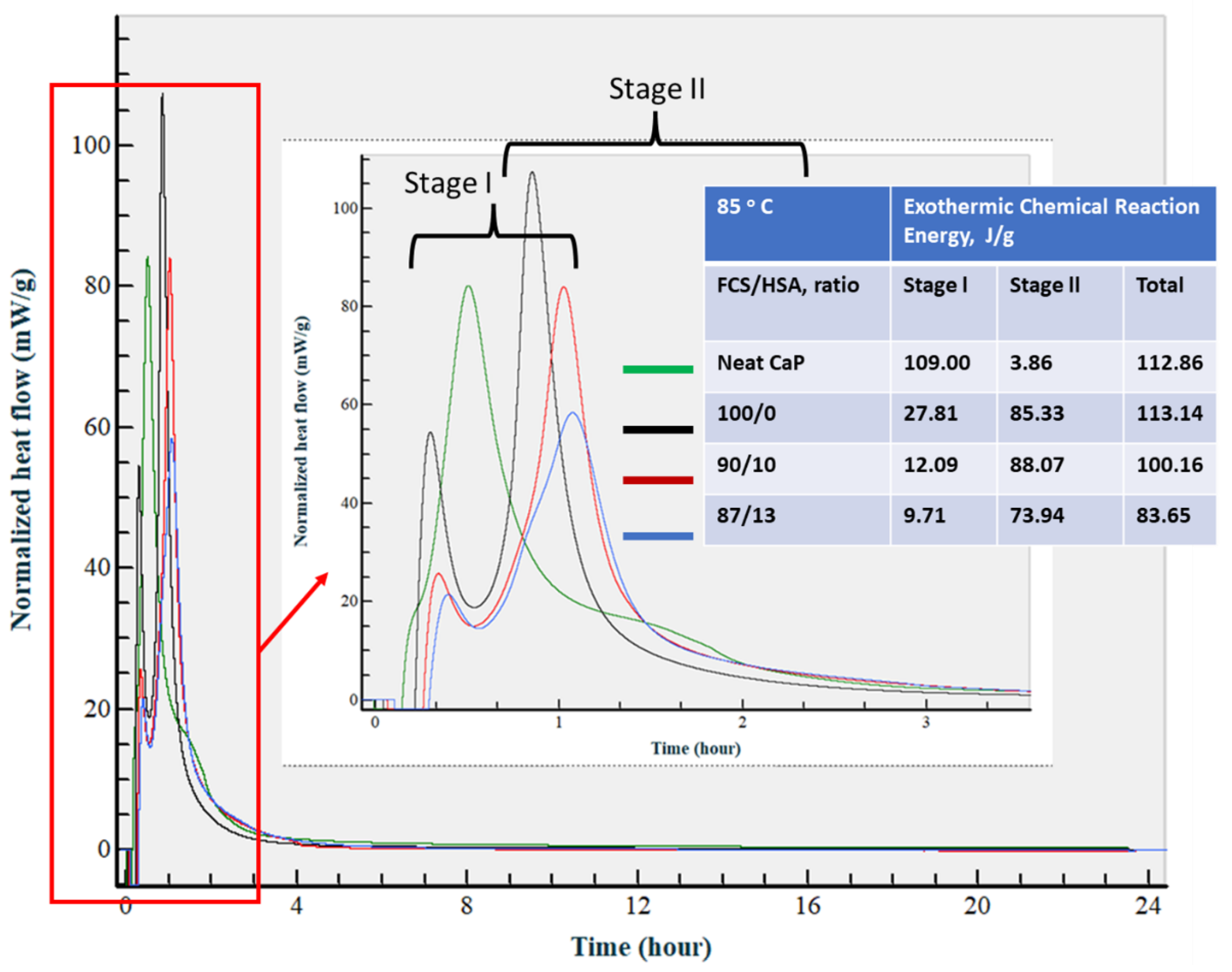
3.3.7. Calorimetric Study of CaP Composites in Hydration
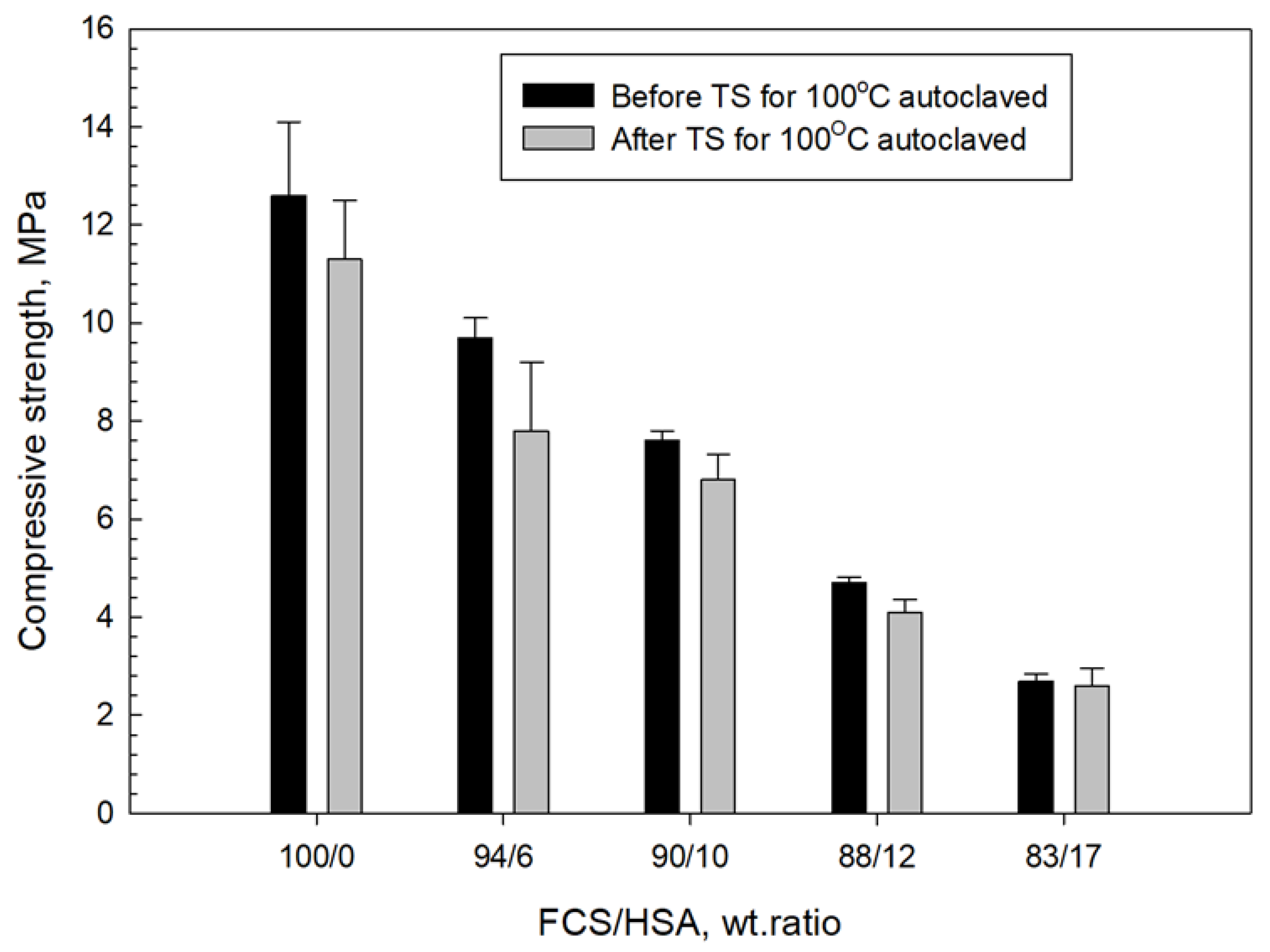
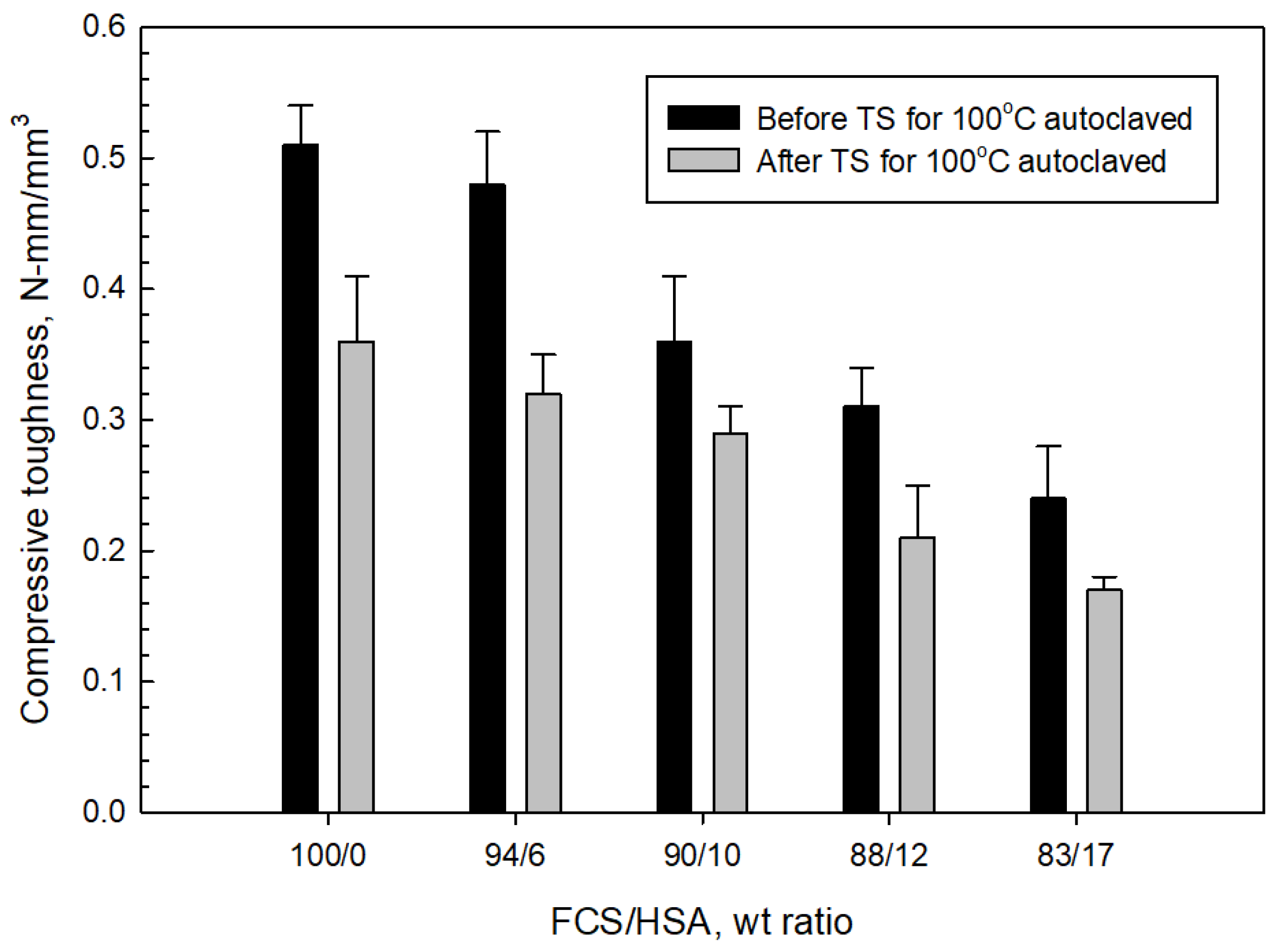
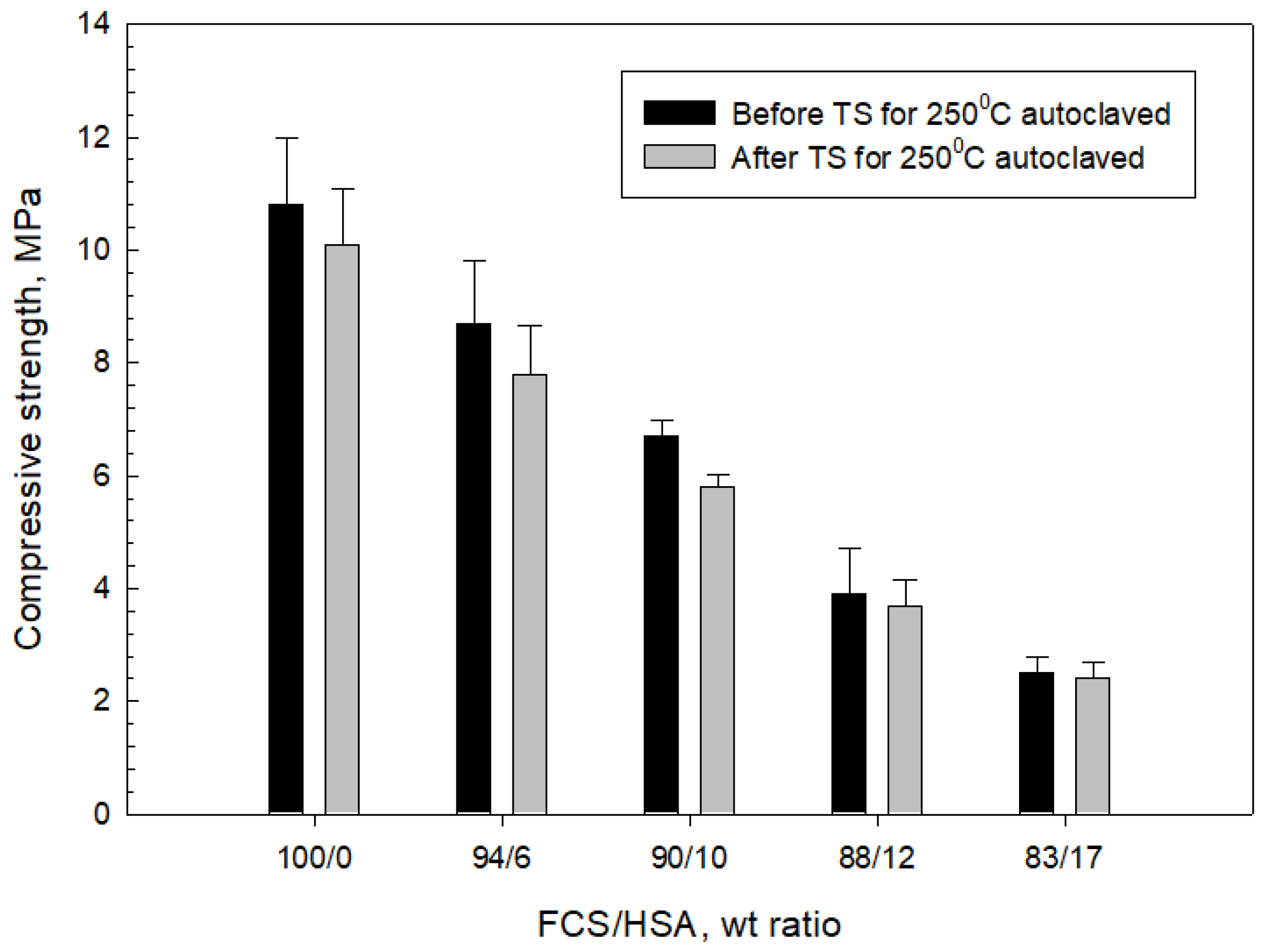
3.4. Compressive Strength and Toughness of 100 and 250 °C-Autoclaved CaP Cement Composites before and after Thermal Shock (TS) Tests
3.5. Thermal Conductivity
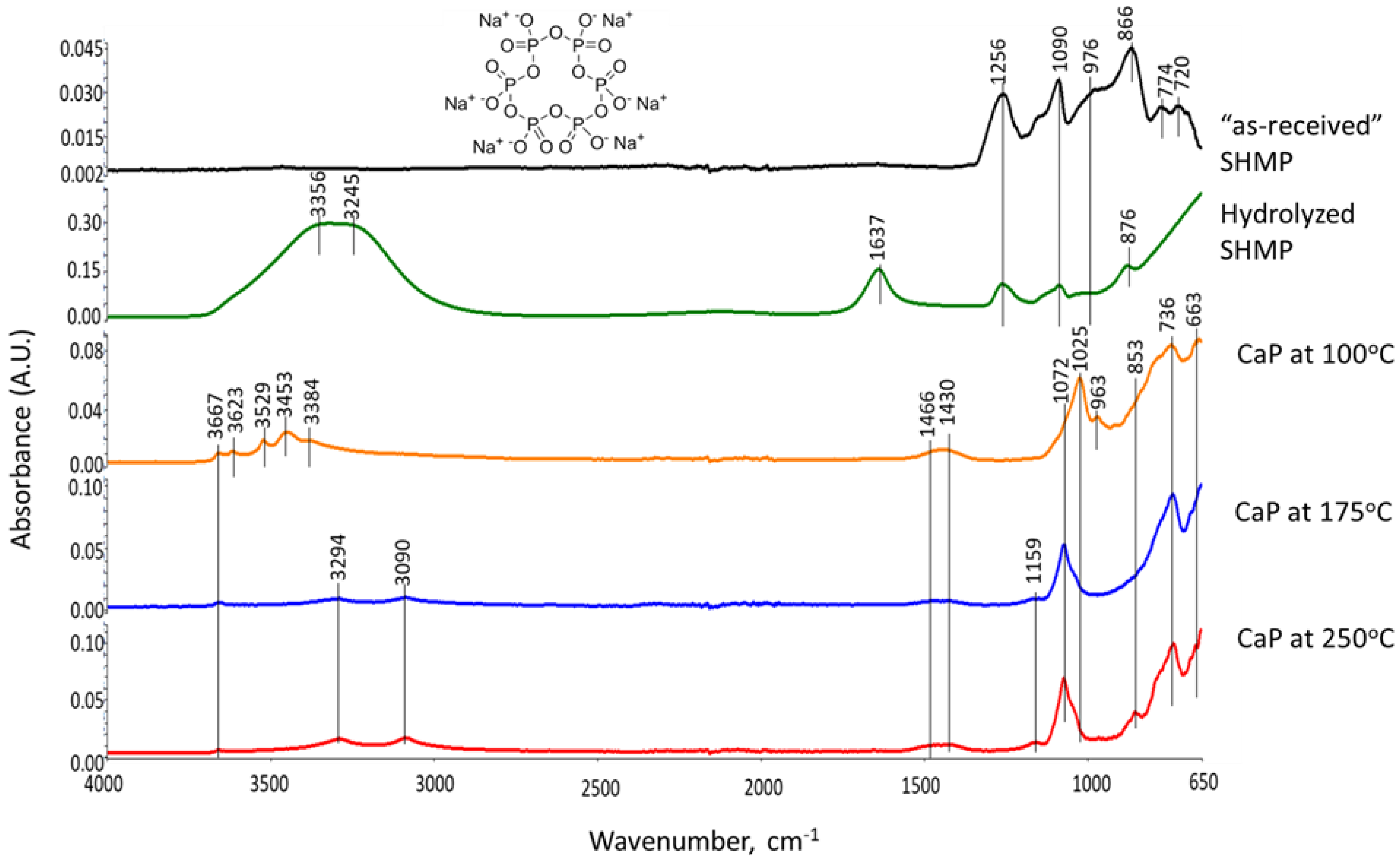
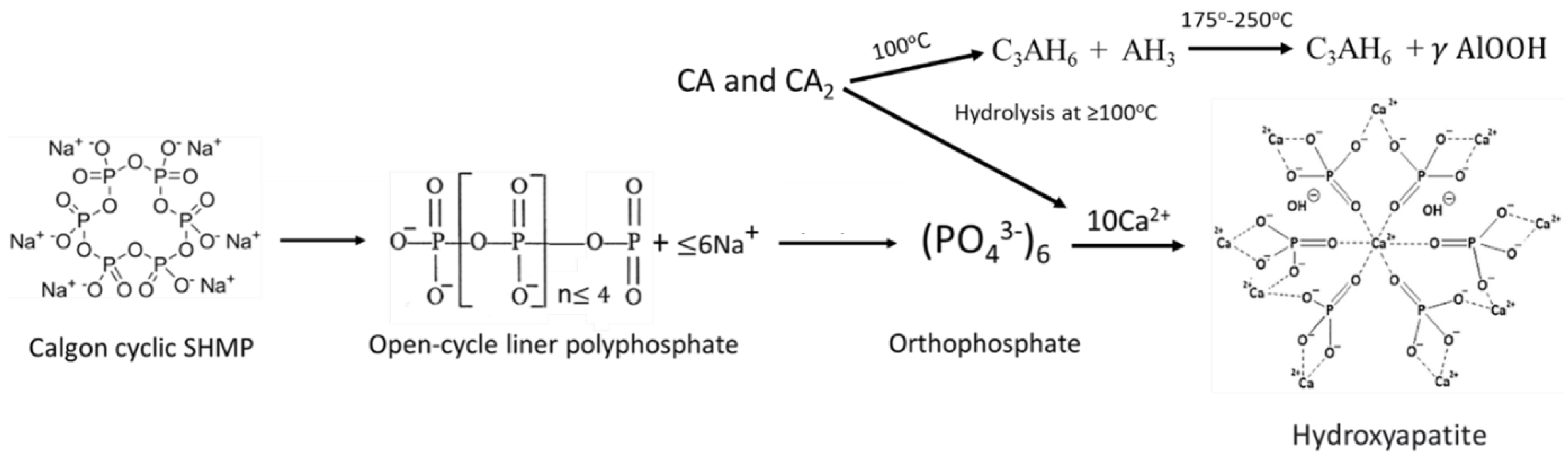
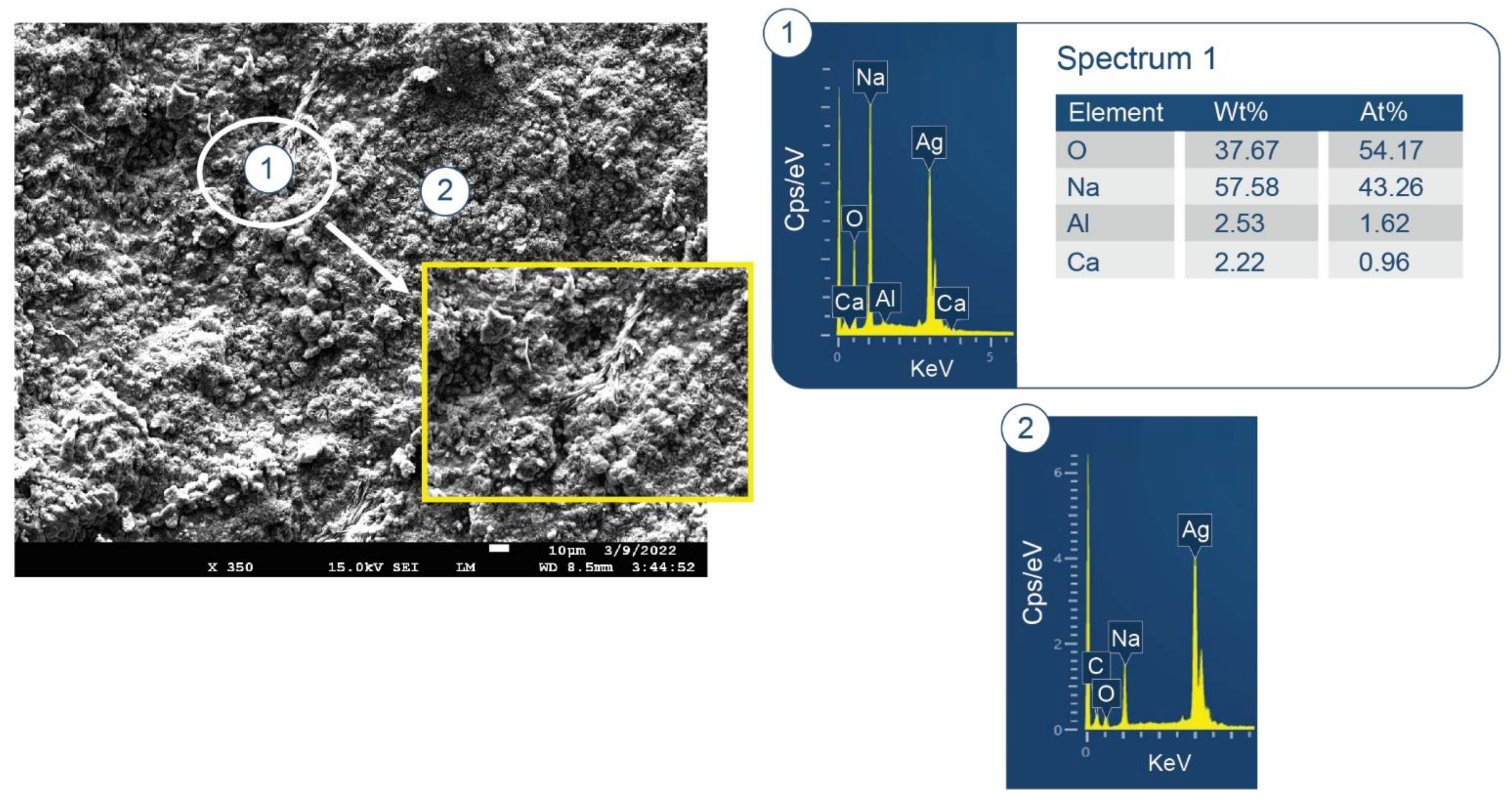
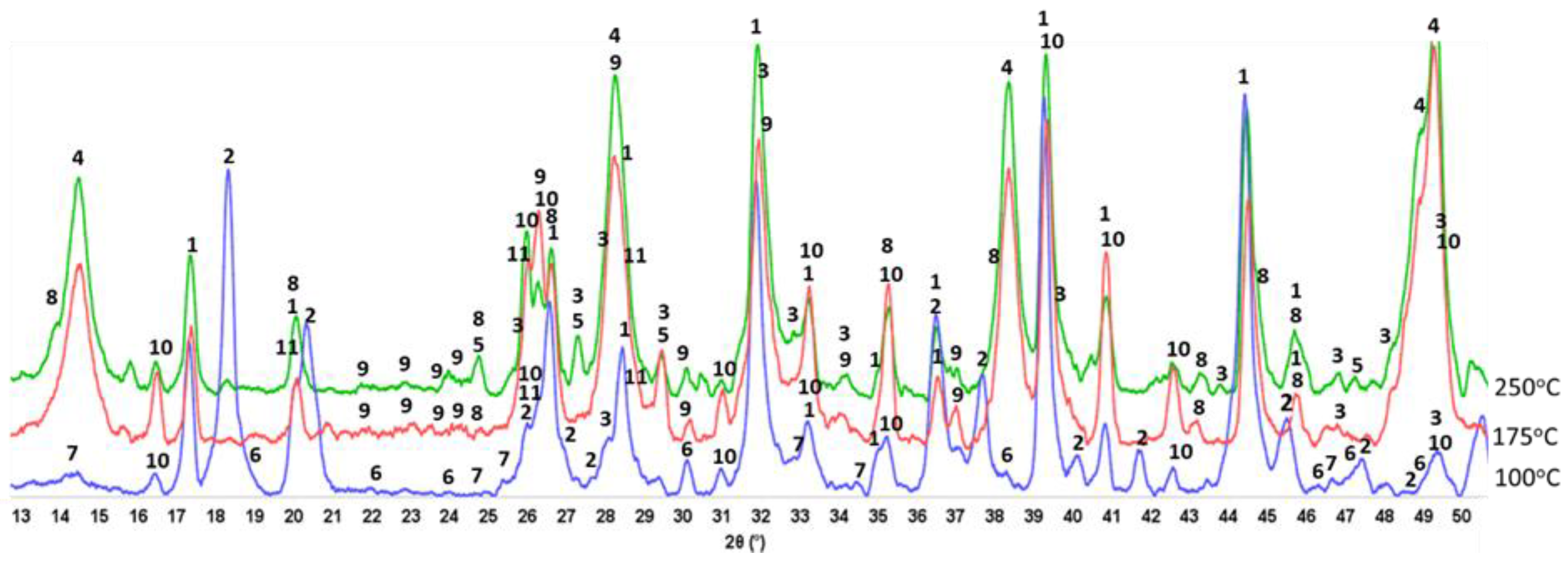
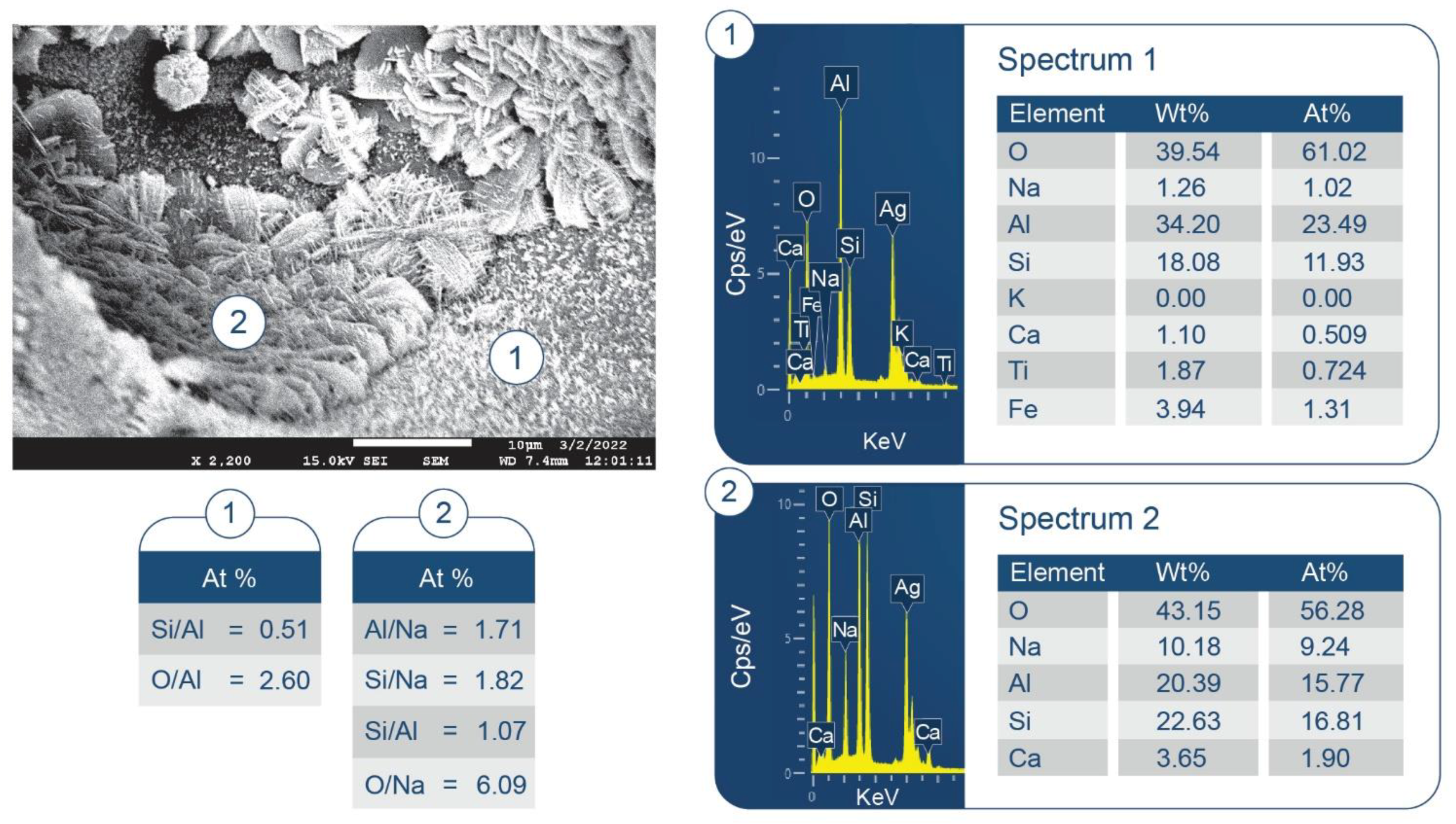
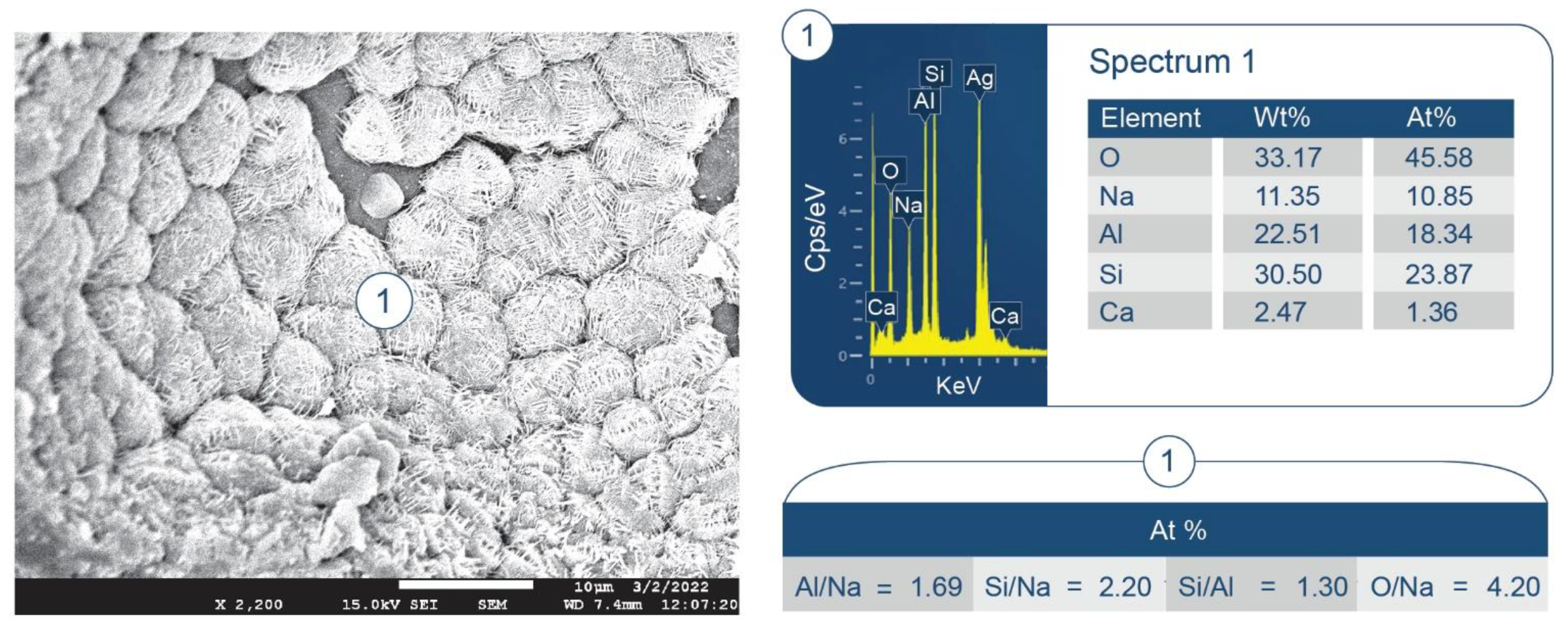
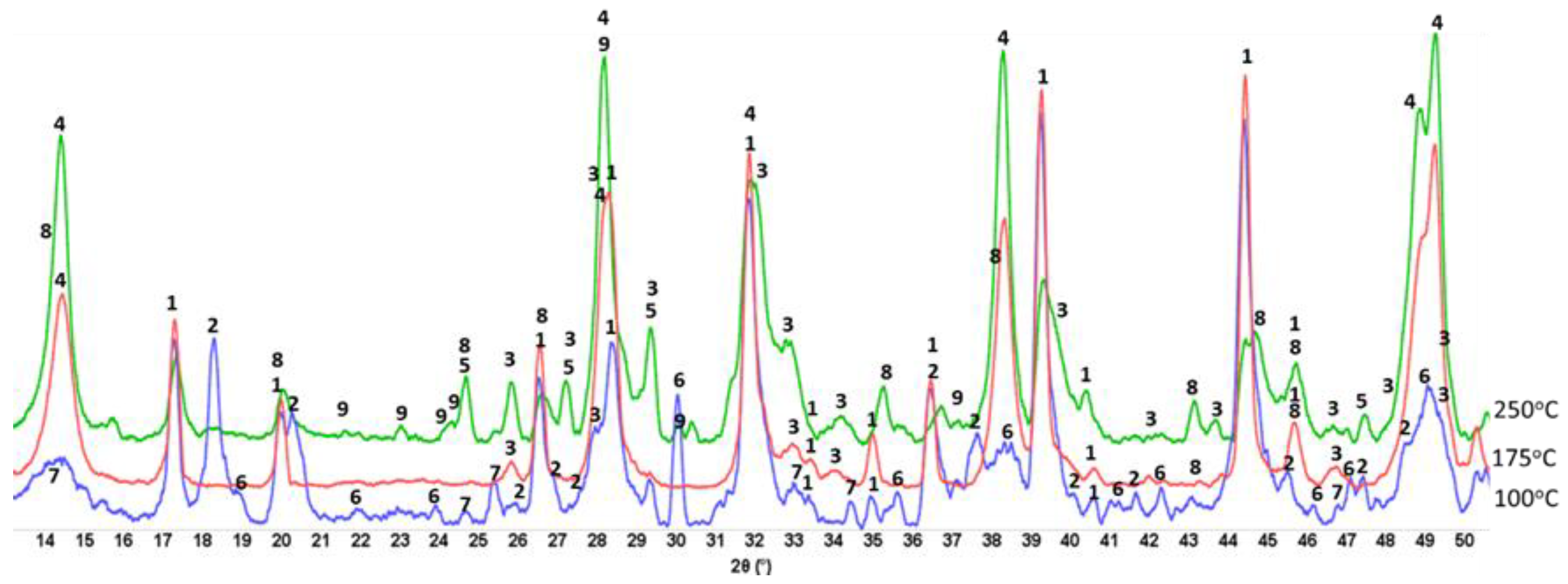
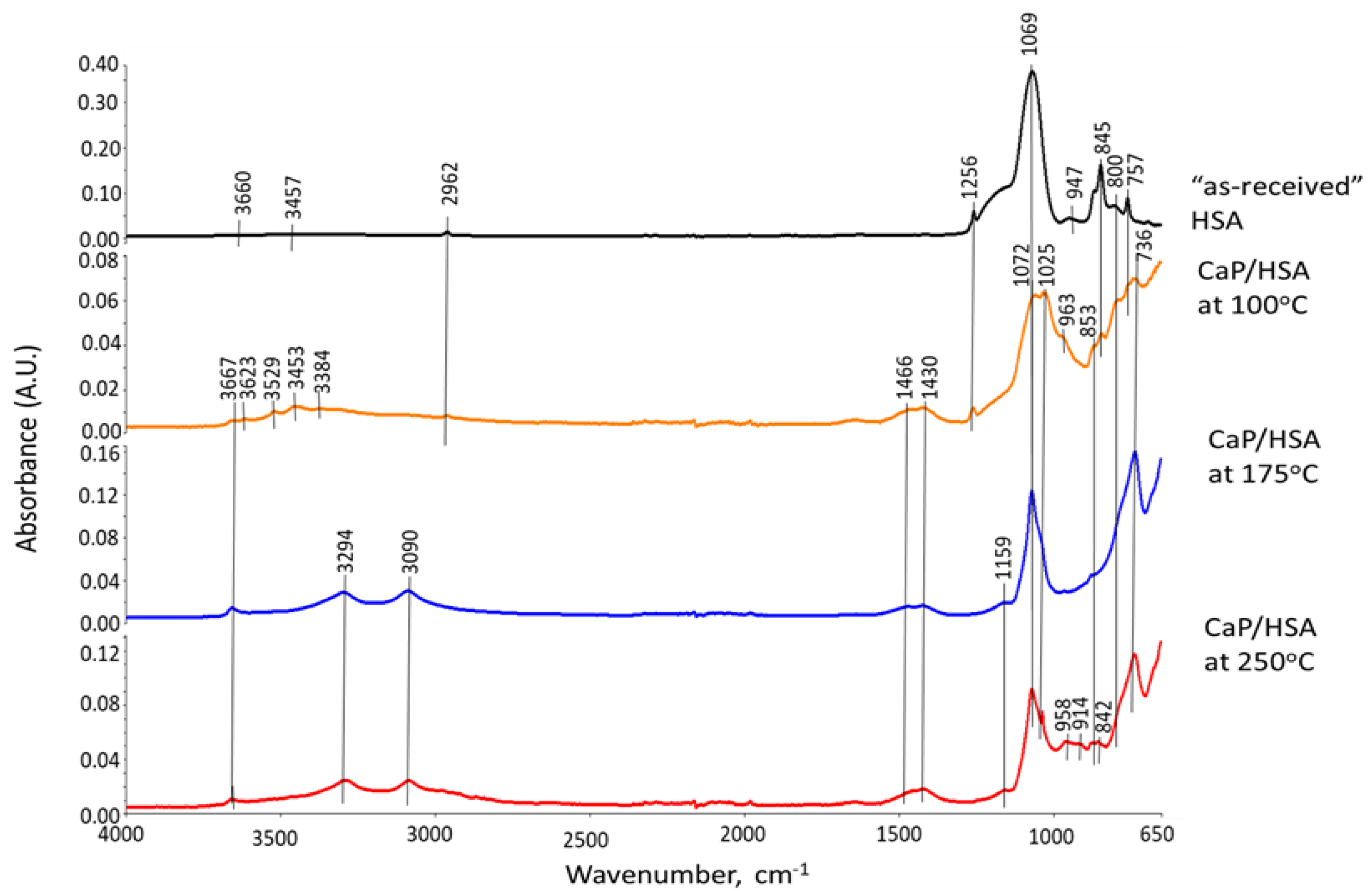
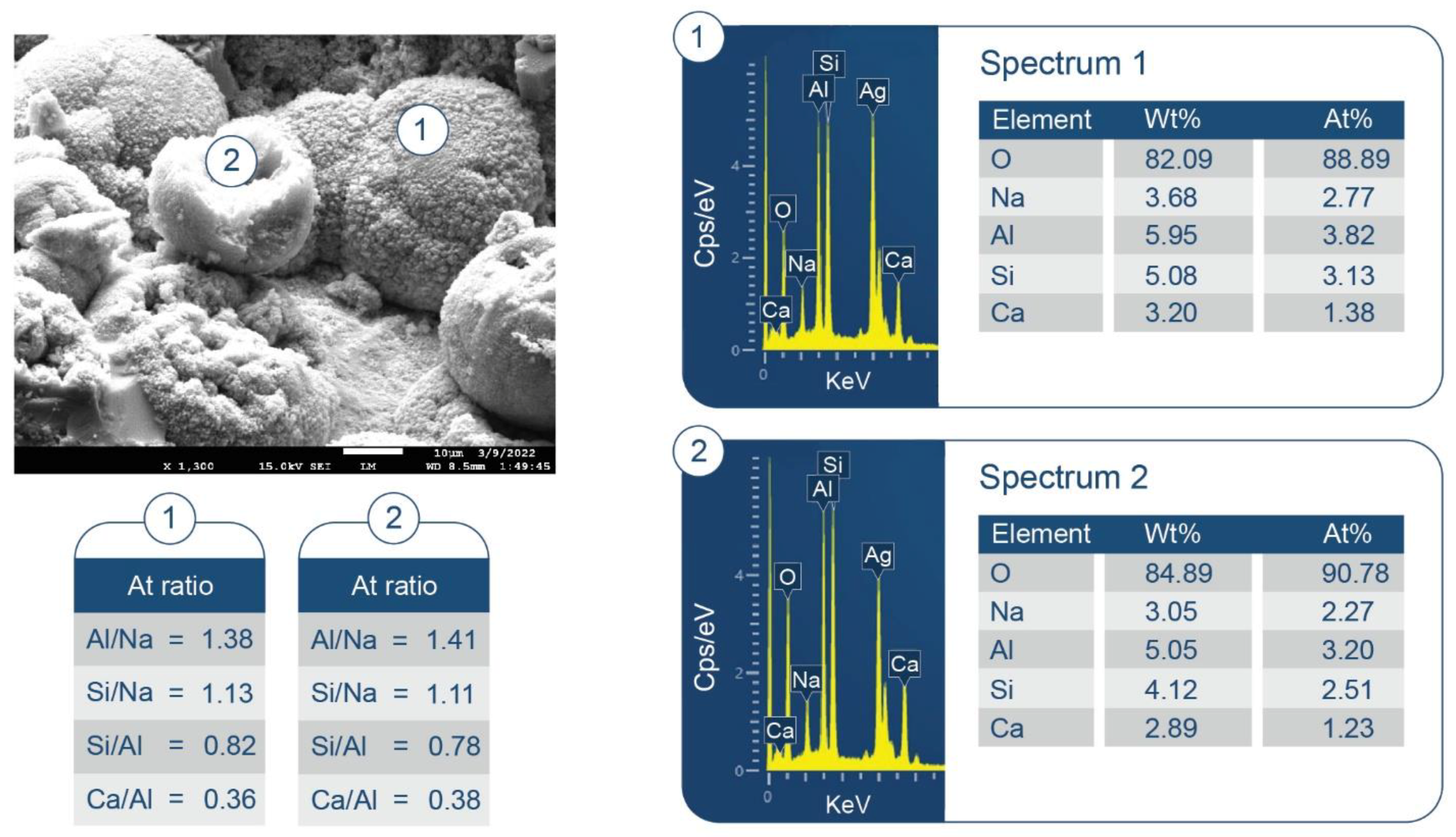
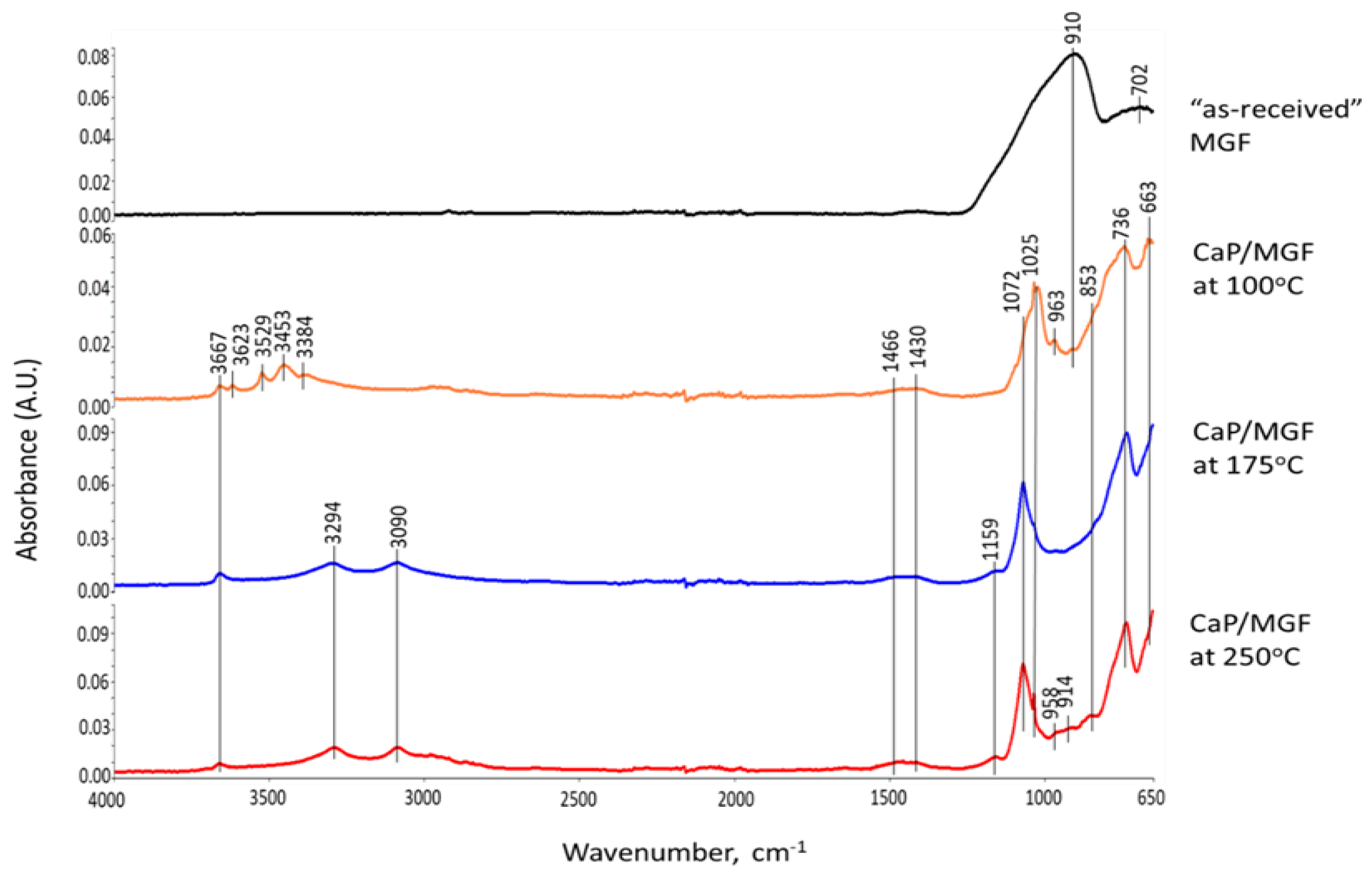
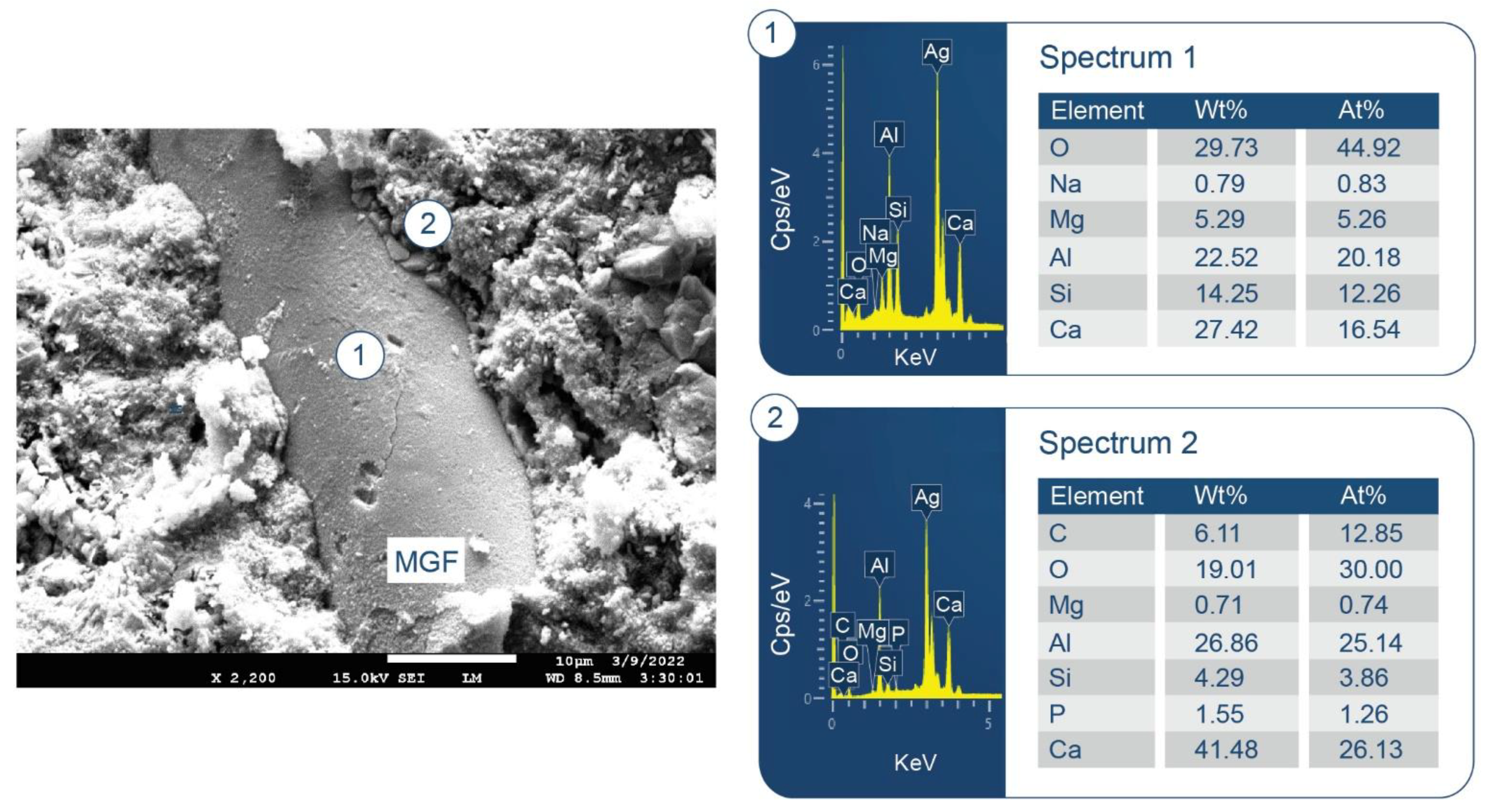
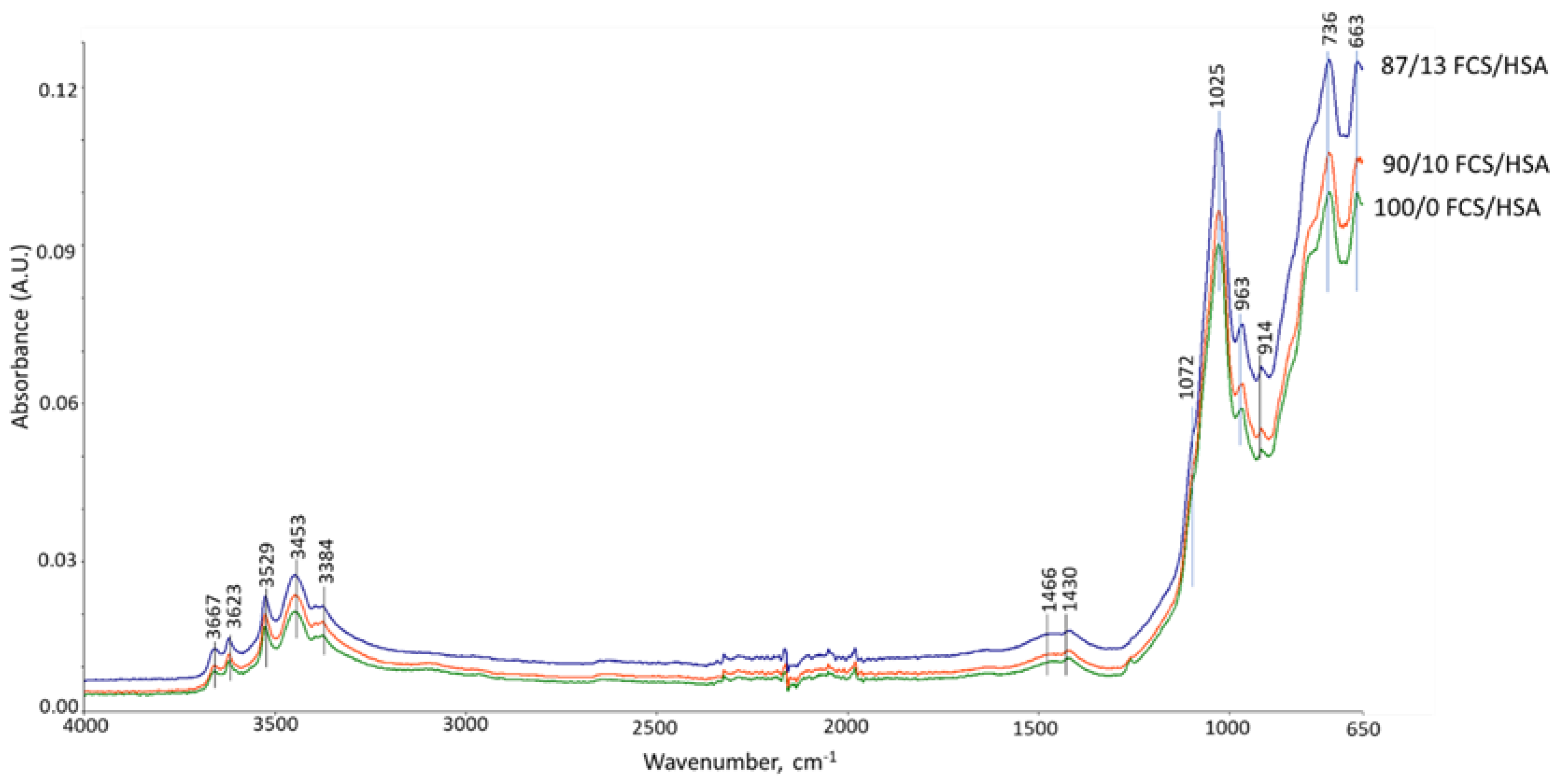
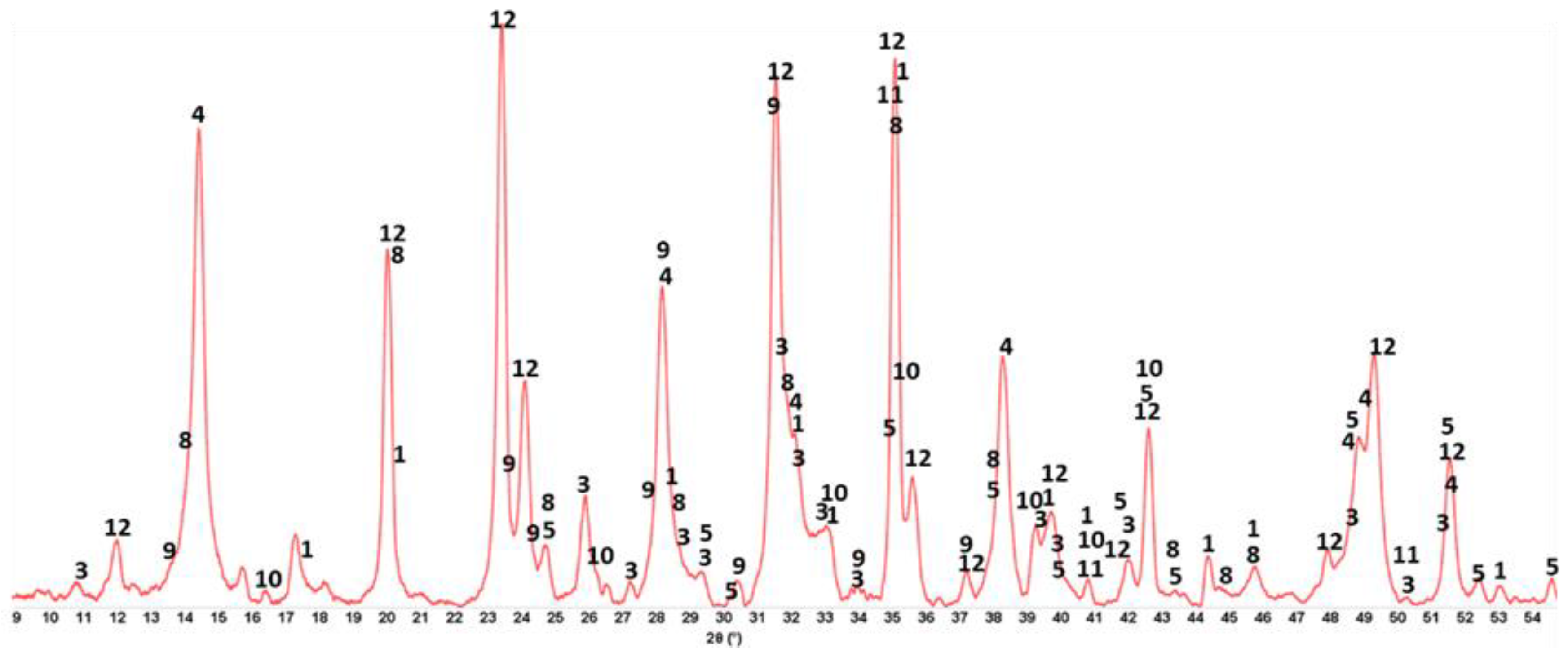
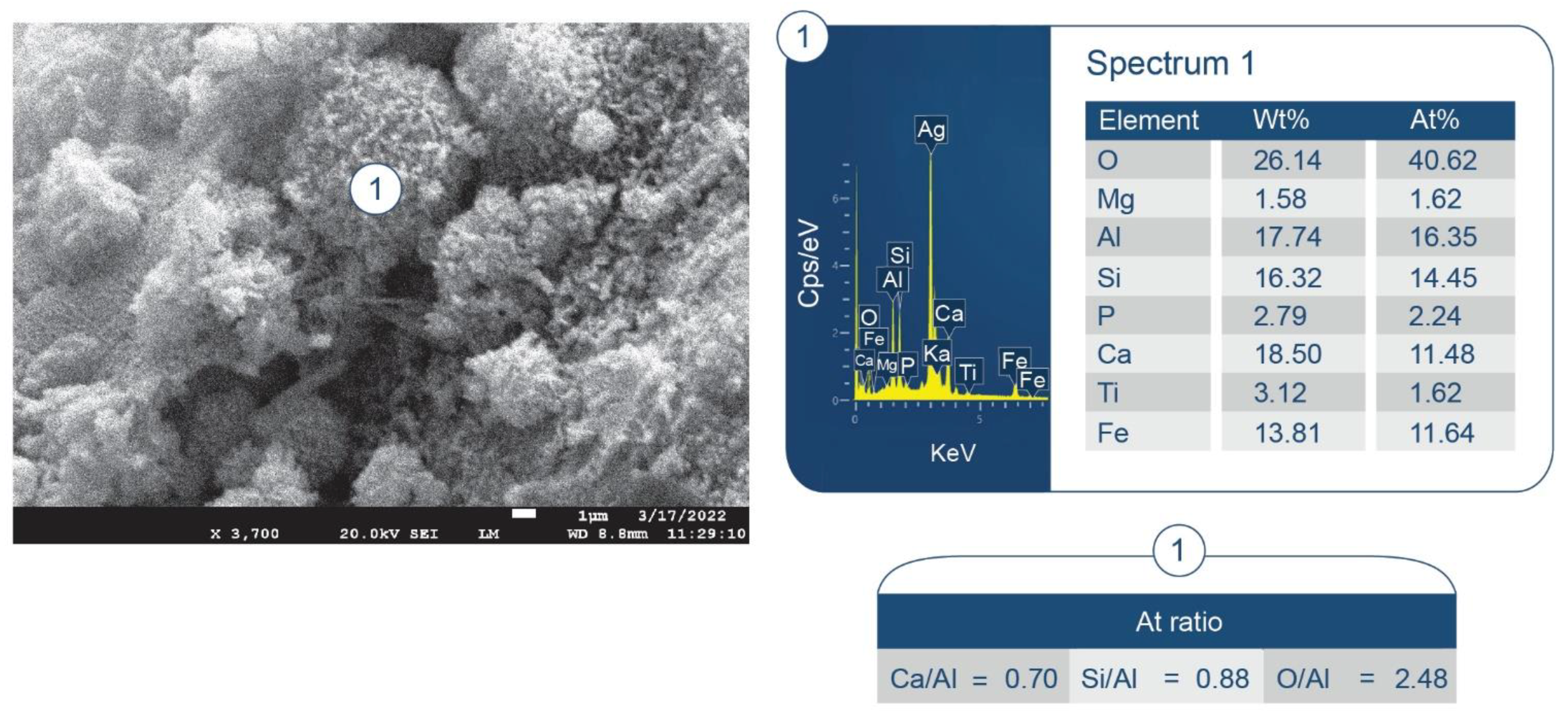
3.6. ATR-FTIR, XRD, and SEM-EDX Studies of CaP Cement Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sugama, T.; Pyatina, T. Hydrophobic Lightweight Cement with Thermal Shock Resistance and Thermal Insulating Properties for Energy-Storage Geothermal Well Systems. Materials 2021, 14, 6679. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, Q.; Sun, F. Numerical simulation of CO2 circulating in a retrofitted geothermal well. J. Pet. Sci. Eng. 2019, 172, 217–227. [Google Scholar] [CrossRef]
- Lanahan, M.; Tabares-Velasco, P.C. Seasonal Thermal-Energy Storage: A Critical Review on BTES Systems, Modeling, and System Design for Higher System Efficiency. Energies 2017, 10, 743. [Google Scholar] [CrossRef]
- Rao, P.; Momayez, M.; Runge, K.; Muralidharan, K. Recent Developments in Thermally Insulating Materials Based on Geopolymers—A Review Article. Min. Metall. Explor. 2020, 37, 995–1014. [Google Scholar] [CrossRef]
- Kremieniewski, M.; Jasí, B.; Zima, G.; Kut, Ł. Reduction of Fractionation of Lightweight Slurry to Geothermal Boreholes. Energies 2021, 14, 3686. [Google Scholar] [CrossRef]
- Ignacio Velasco, J.; Traven, K.; Wisniewski, W.; Ducman, V. Academic Editors: Ildiko Merta and Microstructural Characterization of Alkali-Activated Composites of Lightweight Aggregates (LWAs) Embedded in Alkali-Activated Foam (AAF) Matrices. Polymers 2022, 14, 1729. [Google Scholar] [CrossRef]
- Shen, L.; Tan, H.; Ye, Y.; He, W. Using Fumed Silica to Develop Thermal Insulation Cement for Medium–Low Temperature Geothermal Wells. Materials 2022, 15, 5087. [Google Scholar] [CrossRef]
- Sliwa, T.; Ciepielowska, M. Cement Slurries with Modified Thermal Conductivity for Geothermal Applications. In Proceedings of the 47th Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 7–9 February 2022. [Google Scholar]
- US Department of Energy. Energy Storage Grand Challenge Roadmap; US Department of Energy: Washington, DC, USA, 2020.
- Wendt, D.; Huang, H.; Zhu, G.; Kitz, K.; Green, S.; McLennan, J.; McTigue, J.; Neupane, G. Flexible Geothermal Power Generation Utilizing Geologic Thermal Energy Storage; Idaho National Lab.(INL): Idaho Falls, ID, USA, 2019.
- Wang, K.S.; Tseng, C.J.; Chiou, I.J.; Shih, M.H. The thermal conductivity mechanism of sewage sludge ash lightweight materials. Cem. Concr. Res. 2005, 35, 803–809. [Google Scholar] [CrossRef]
- Notaria, B.; Pinto, J.; Solorzano, E.; de Sajaa, J.A.; Dumont, M.; Rodrigues-Perez, M.A. Experimental validation of the Knudsen effect in nanocellular polymeric foams. Polymer 2015, 56, 57–67. [Google Scholar] [CrossRef]
- Bhagat, S.; Kim, Y.-H.; Moon, M.-J.; Ahn, Y.-S.; Yeo, J.-G. A cost-effective and fast synthesis of nanoporous SiO2 aerogel powders using water-glass via ambient pressure drying route. Solid State Sci. 2007, 9, 628–635. [Google Scholar] [CrossRef]
- Hair, M.; Hertl, W. Reaction of hexamethyldisilazane with silica. J. Phys. Chem. 1971, 75, 2181–2185. [Google Scholar] [CrossRef]
- Gurav, J.; Jung, I.; Park, H.; Kang, E.; Nadargi, D. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Julio, M.; Ilharco, L. Ambient Pressure Hybrid Silica Monoliths with Hexamethyldisilazane_ From Vitreous Hydrophilic Xerogels to Superhydrophobic Aerogels _ Enhanced Reader.pdf. ACS Omega 2017, 2, 5060–5070. [Google Scholar] [CrossRef]
- Wagh, P.; Ingale, S. Comparison of some physico-chemical properties of hydrophilic and hydrophobic silica aerogels. Ceram. Int. 2002, 28, 43–50. [Google Scholar] [CrossRef]
- Koyano, K.; Tatsumi, T.; Yanaka, Y.; Nakata, S. Stabilization of Mesoporous Molecular Sieves by Trimethylsilylation. J. Phys. Chem. B 1997, 101, 9436–9440. [Google Scholar] [CrossRef]
- Park, D.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Enhancement of Hydrothermal Stability and Hydrophobicity of a Silica MCM-48 Membrane by Silylation. Ind. Eng. Chem. Res. 2001, 40, 6105–6110. [Google Scholar] [CrossRef]
- Castricum, H.; Sah, A.; Kreiter, R.; Blank, D.; Vente, J.; ten Elshof, J. Hydrothermally stable molecular separation membranes from organically linked silica. J. Mater. Chem. 2008, 18, 2150–2158. [Google Scholar] [CrossRef]
- Castricum, H.; Kreiter, R.; Veen, H.; Blank, D.; Vente, J.; ten Elshof, J. High-performance hybrid pervaporation membranes with superior hydrothermal and acid stability. J. Membr. Sci. 2008, 324, 111–118. [Google Scholar] [CrossRef]
- Jung, J.S.; Park, H.C.; Stevens, R. Mullite ceramics derived from coal fly ash. J. Mater. Sci. Lett. 2001, 20, 1089–1091. [Google Scholar] [CrossRef]
- Ignaszak, Z.; Baranowski, A.; Hycnar, J.; Zak, M. Heat-Insulating, High-Temperature Materials on Cenosphere Base. In Insulation Materials, Testing and Applications; ASTM International: West Conshohocken, PA, USA, 1990; pp. 741–747. [Google Scholar]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Data Sheet of EconoStar 500 Cenospheres. Available online: https://www.cenostar.com/pages/cenospheres (accessed on 20 August 2022).
- Ichikawa, T.; Miura, M. MOdified model of alakli-silica reaction. Cem. Concr. Res. 2007, 37, 1291–1297. [Google Scholar] [CrossRef]
- Mindess, S. Resistance of Concrete to Destructive Agencies. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Hewlett, P., Liska, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 251–283. [Google Scholar]
- Wang, J.Y.; Zhang, M.H.; Li, W.; Chia, K.S.; Liew, R.J.Y. Stability of cenospheres in lightweight cement composites in terms of alkali-silica reaction. Cem. Concr. Res. 2012, 42, 721–727. [Google Scholar] [CrossRef]
- Manders, P.; Bader, M. The strength of hybrid glass/carbon fibre composites. J. Mater. Sci. 1981, 16, 2233–2245. [Google Scholar] [CrossRef]
- Sudarisman, R.; de San Miguel, B.; Davies, I. The effect of partial substitution of E-glass fibre for carbon fibre on the mechanical properties of CFRP composites. In Proceedings of the International Conference on Materials and Metallurgical Technology, ICONNET 2009, Surabaya, Indonesia, 24–25 June 2009; pp. 125–128. [Google Scholar]
- Dong, C.; Ranaweera-Jayawardena, H.; Davies, I. Flexural properties of hybrid composites reinforced by S-2 glass and T700S carbon fibres. Compos. Part B Eng. 2012, 43, 573–581. [Google Scholar] [CrossRef]
- Hung, P.; Lau, K.; Cheng, L.; Leng, J.; Hui, D. Impact response of hybrid carbon/glass fibre reinforced polymer composites designed for engineering applications. Compos. Part B Eng. 2018, 133, 86–90. [Google Scholar] [CrossRef]
- McIvor, S.; Darby, M.; Wostenholm, G.; Yates, B.; Banfield, L.; King, R.; Webb, A. Thermal conductivity measurements of some glass fibre- and carbon fibre-reinforced plastics. J. Mater. Sci. 1990, 25, 3127–3132. [Google Scholar] [CrossRef]
- Cao, X.; Liu, J.; Li, Q.; Hu, E.; Fan, F. Study of the thermal insulation properties of the glass fiber board used for interior building envelope.pdf. Energy Build. 2015, 107, 49–58. [Google Scholar] [CrossRef]
- Purnell, P.; Short, N.; Page, C.; Majumdar, A. Microstructural observations in new matrix glass fibre reinforced cement. Cem. Concr. Res. 2000, 30, 1747–1753. [Google Scholar] [CrossRef]
- He, P.; Zhang, B.; Lu, J.; Poon, C. Reaction mechanisms of alkali-activated glass powder-ggbs-CAC composites. Cem. Concr. Compos. 2021, 122, 104143. [Google Scholar] [CrossRef]
- Ranjbar, N.; Zhang, M. Fiber-reinforced geopolymer composites: A review. Cem. Concr. Compos. 2020, 107, 103498. [Google Scholar] [CrossRef]
- Teixeira Marvila, M.; Rangel Garcez De Azevedo, A.; De Matos, P.R.; Monteiro, S.N.; Maurício, C.; Vieira, F.; Zichella, L. Materials for Production of High and Ultra-High Performance Concrete: Review and Perspective of Possible Novel Materials. Materials 2021, 14, 4304. [Google Scholar] [CrossRef]
- Barluenga, G. Fiber-matrix interaction at early ages of concrete with short fibers. Cem. Concr. Res. 2010, 40, 802–809. [Google Scholar] [CrossRef]
- Butler, M.; Hempel, S.; Mechtcherine, V. Modelling of ageing effects on crack-bridging behavior of AR-glass multifilament yarns embedded in cement-based matrix. Cem. Concr. Res. 2011, 41, 403–411. [Google Scholar] [CrossRef]
- Kartal, A.; Erkey, C. Surface Modification of Silica Aerogels by Hexamethyldisilazane carbondioxide mixtures and their phase behavior. J. Supercrit. Fluids 2010, 53, 115–120. [Google Scholar] [CrossRef]
- Gun’ko, V.; Vedamuthu, M.; Henderson, G.; Blitz, J. Mechanism and Kinetics of Hexamethyldisilazane Reaction with a Fumed Silica Surface. J. Colloid Interface Sci. 2000, 228, 157–170. [Google Scholar] [CrossRef]
- Slavov, S.; Sanger, A.; Chuang, K. Mechanism of Silation of Silica with Hexamethyldisilazane. J. Phys. Chem. B 2000, 104, 903–989. [Google Scholar] [CrossRef]
- Haukka, S.; Root, A. The reaction of hexamethyldisilazane and subsequent oxidation of trimethylsilyl groups on silica studied by solid-state NMR and FTIR. J. Phys. Chem. 1994, 98, 1695–1703. [Google Scholar] [CrossRef]
- Nagappan, S.; Ha, C.-S. Effect of Sodium Hydroxide on the Fast Synthesis of Superhydro-phobic Powder from Polymethylhydrosiloxane. J. Coat. Sci. Technol. 2014, 1, 151–160. [Google Scholar] [CrossRef]
- Groza, A.; Surmeian, A. Characterization of the oxides present in a polydimethylsiloxane layer obtained by polymerisation of its liquid precursor in corona discharge. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Warring, S.; Beattie, D.; McQuillan, J. Surficial Siloxane-to-Silanol Interconversion during Room-Temperature Hydration/Dehydration of Amorphous Silica Films Observed by ATR-IR and TIR-Raman Spectroscopy. Langmuir 2016, 32, 1568–1576. [Google Scholar] [CrossRef]
- Efimov, A.M.; Pogareva, V.G.; Shashkin, A.V. Water-related bands in the IR absorption spectra of silicate glasses. J. Non-Cryst. Solids 2003, 332, 93–114. [Google Scholar] [CrossRef]
- Cheng, F.; Cao, Q.; Guan, Y.; Cheng, H.; Wang, X.; Miller, J. FTIR analysis of water structure and its influence on the flotation of arcanite (K2SO4) and epsomite (MgSO4·7H2O). Int. J. Miner. Process. 2013, 122, 36–42. [Google Scholar] [CrossRef]
- Iacob, C.; Sangoro, J.; Papadopoulos, P.; Schubert, T.; Naumov, S.; Valiullin, R.; Karger, J.; Kremer, F. Charge transport and diffusion of ionic liquids in nanoporous silica membranes. Phys. Chem. Chem. Phys. 2010, 12, 13798–13803. [Google Scholar] [CrossRef]
- Kytokivi, A.; Haukka, S. Reactions of HMDS, TiCl4, ZrCl4, and AlCl3 with Silica As Interpreted from Low-Frequency Diffuse Reflectance Infrared Spectra. J. Phys. Chem. B 1997, 101, 10365–10372. [Google Scholar] [CrossRef]
- Flores-Vivian, I.; Hejazi, V.; Kozhukhova, M.I.; Nosonovsky, M.; Sobolev, K. Self-assembling particle-siloxane coatings for superhydrophobic concrete. ACS Appl. Mater. Interfaces 2013, 5, 13284–13294. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polymethyl silozane thermal degradation: Part I. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Singh, U.B.; Gupta, S.C.; Flerchinger, G.N.; Moncrief, J.F.; Lehmann, R.G.; Fendinger, N.J.; Traina, S.J.; Logan, T.J. Modeling polydimethylsiloxane degradation based on soil water content. Environ. Sci. Technol. 2000, 34, 266–273. [Google Scholar] [CrossRef]
- Purkayastha, A.; Baruah, J.B. Synthetic methodologies in siloxanes. Appl. Organomet. Chem. 2004, 18, 166–175. [Google Scholar] [CrossRef]
- Varaprath, S.; Stutts, D.H.; Kozerski, G.E. A primer on the analytical aspects of silicones at trace levels-challenges and artifacts-A review. Silicon Chem. 2006, 3, 79–102. [Google Scholar] [CrossRef]
- Chainet, F.; Meur, L.L.; Lienemann, C.P.; Ponthus, J.; Courtiade, M.; Donard, O.F.X. Characterization of silicon species issued from PDMS degradation under thermal cracking of hydrocarbons: Part 1-Gas samples analysis by gas chromatography-time of flight mass spectrometry. Fuel 2013, 111, 519–527. [Google Scholar] [CrossRef]
- Ducom, G.; Laubie, B.; Ohannessian, A.; Germain, P.; Chatain, V. Hydrolysis of polydimethylsiloxane fluids in controlled aqueous solutions. Water Sci. Technol. 2013, 68, 813–820. [Google Scholar] [CrossRef]
- Perez, M.; Vazquez, T.; Trivino, F. Study of stabilized phases in high alumina cement mortars Part I Hydration at elevated temperatures followed by carbonation. Cem. Concr. Res. 1983, 13, 759–770. [Google Scholar] [CrossRef]
- Klaus, S.; Neubauer, J.; Goetz-Neuhoeffer, F. Hydration kinetics of CA2 and CA—Investigations performed on a synthetic calcium aluminate cement. Cem. Concr. Res. 2013, 43, 62–69. [Google Scholar] [CrossRef]
- Reardon, E. An ion interaction model for the determination of chemical equilibria in cement/water systems. Cem. Concr. Res. 1990, 20, 175–192. [Google Scholar] [CrossRef]
- Corbridge, D.; Lowe, E. The infra-red spectra of inorganic phosphorus compounds. Part II. Some salts of phosphorus oxy-acids. J. Chem. Soc. 1954, 0, 4555–4564. [Google Scholar] [CrossRef]
- Sugama, T.; Weber, L.; Brother, L. Sodium-polyphosphate-modified fly ash/calcium aluminate blend cement: Durability in wet, harsh geothermal environments. Mater. Lett. 2000, 44, 45–53. [Google Scholar] [CrossRef]
- Moustafa, Y.; El-Egili, K. Infrared spectra of sodium phosphate glasses. J. Non-Cryst. Solids 1998, 240, 144–153. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Irisawa, K.; Jin, F.; Meguro, Y.; Kinoshita, H. Reduction of water content in calcium aluminate cement with/out phosphate modification for alternative cementation technique. Cem. Concr. Res. 2018, 109, 243–253. [Google Scholar] [CrossRef]
- Galameau, E.; Gehr, R. Phosphorus removal from wastewaters: Experimental and theoretical support for alternative mechanisms. Water Res. 1997, 31, 328–338. [Google Scholar] [CrossRef]
- Holm, T.; Edwards, M. Metaphosphate reversion in Laboratory and Pipe-Rig Experiments. J. Am. Water Work Assoc. 2003, 95, 172–178. [Google Scholar] [CrossRef]
- Georgantas, D.; Grigoropoulou, H. Orthophosphate and metaphosphate ion removal from aqueous solution using alum and aluminum hydroxide. J. Colloid Interface Sci. 2007, 315, 70–79. [Google Scholar] [CrossRef]
- Lu, J.; Sun, M.; Yuan, Z.; Qi, S.; Tong, Z.; Li, L.; Meng, Q. Innovative insight for sodium hexametaphosphate interaction with serpentine. Colloinds Surf. A Physicochem. Eng. Asp. 2019, 560, 35–41. [Google Scholar] [CrossRef]
- Glonek, T. Did Cyclic Metaphosphates Have a Role in the Origin of Life? Orig. Life Evol. Biosph. 2021, 51, 1–60. [Google Scholar] [CrossRef]
- Tarte, P. Infra-red spectra of inorganic aluminates and characteristic vibrational frequencies of AlO4 tetrahedra and AlO6 octahedra. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 2127–2143. [Google Scholar] [CrossRef]
- Fernandez-Carrasco, L.; Torrens-Martin, D.; Morales, L.M.; Martinez-Ramirez, S. Infrared spectroscopy in the analysis of building and construction materials. In Infrared Spectroscopy-Materials Science, Engineering and Technology; Theophile, T., Ed.; Intechopen: London, UK, 2012; pp. 370–382. [Google Scholar]
- Xyla, A.G.; Koutsoukos, P.G. Quantitative Analysis of Calcium Carbonate Polymorphs by Infrared Spectroscopy. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 3165–3172. [Google Scholar] [CrossRef]
- Ylmen, R.; Jaglid, U. Carbonation of Portland cement studied by diffuse reflection Fourier transform infrared spectroscopy. Int. J. Concr. Struct. Mater. 2013, 7, 119–125. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy-Materials Science, Engineering and Technology; Theophanides, T., Ed.; Intechopen: London, UK, 2012; pp. 123–148. [Google Scholar]
- Fujita, S.; Suzuki, K.; Shibasaki, Y. The mild hydrothermal synthesis of hydrogrossular from coal ash. J. Mater. Cycles Waste Manag. Vol. 2002, 4, 41–45. [Google Scholar]
- Kolesov, B.; Geiger, C. The vibrational spectrum of synthetic hydrogrossular (katoite) Ca3Al2(O4H4)3: A low-temperature IR and Raman spectroscopic study. Am. Mineral. 2005, 90, 1335–1341. [Google Scholar] [CrossRef]
- Eisinas, A.; Dambrauskas, T.; Baltakys, K.; Ruginyte, K. The peculiarities of mayenite formation from synthetic katoite and calcium monocarboaluminate samples in temperature range 25–1150 °C. J. Therm. Anal. Calorim. 2019, 138, 2275–2282. [Google Scholar] [CrossRef]
- Kiss, A.B.; Keresztury, G.; Farkas, L. Raman and ir spectra and structure of boehmite (γ-AlOOH). Evidence for the recently discarded D 17 2h space group. Spectrochim. Acta Part A Mol. Spectrosc. 1980, 36, 653–658. [Google Scholar] [CrossRef]
- Morterra, C.; Emanuel, C. Infrared Study of some Surface Properties of Boehmite (y-AlO2H). J. Chem. Soc. Faraday 1992, 88, 339–348. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Ubaidah, A.; Takeya, M.; Shimokawara, M.; Okano, H. Effect of Electrokinetics and Thermodynamic Equilibrium on LowSalinity Water Flooding for Enhanced Oil Recovery in Sandstone Reservoirs. ACS Omega 2021, 6, 3727–3735. [Google Scholar] [CrossRef]
- Bertoluzza, A.; Fagnano, C.; Antonietta Morelli, M.; Gottardi, V.; Guglielmi, M. Raman and infrared spectra on silica gel evolving toward glass. J. Non-Cryst. Solids 1982, 48, 117–128. [Google Scholar] [CrossRef]
- Padmaja, P.; Anilkumar, G.M.; Mukundan, P.; Aruldhas, G.; Warrier, K.G.K. Characterisation of stoichiometric sol-gel mullite by fourier transform infrared spectroscopy. Int. J. Inorg. Mater. 2001, 3, 693–698. [Google Scholar] [CrossRef]
- Saikia, B.J.; Parthasarathy, G.; Sarmah, N.C. Fourier transform infrared spectroscopie etimation of crystallinity in SiO2 based rocks. Bull. Mater. Sci. 2008, 31, 775–779. [Google Scholar] [CrossRef]
- Guan, W.; Ji, F.; Fang, Z.; Fang, D.; Cheng, Y.; Yan, P.; Chen, Q. Low hydrothermal temperature synthesis of porous calcium silicate hydrate with enhanced reactivity SiO2. Ceram. Int. 2014, 40, 4415–4420. [Google Scholar] [CrossRef]
- Urhan, S. Alkali silica and pozzolanic reactions in concrete. Part 1: Interpretation of published results and an hypothesis concerning the mechanism. Cem. Concr. Res. 1987, 17, 141–152. [Google Scholar] [CrossRef]
- Mertens, G.; Snellings, R.; Van Balen, K.; Bicer-Simsir, B.; Verlooy, P.; Elsen, J. Pozzolanic reactions of commn natural zeolites with lime and parameters affecting their reactivity. Cem. Concr. Res. 2009, 39, 233–240. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Almeida, R.; Pantano, C. Structural investigation of silica gel films by infrared spectroscopy. J. Appl. Phys. 1990, 68, 4225–4232. [Google Scholar] [CrossRef]
- Henrik, B.; Ernest, K.; Bo, C. Thermal conductivity of a microporous particulate medium: Moist silica gel. Int. J. Heat Mass Transf. 1984, 27, 2025–2036. [Google Scholar]
- Griesinger, A.; Spindler, K.; Hahne, E. Measurements and theoretical modelling of the effective thermal conductivity of zeolites. Int. J. Heat Mass Transf. 1999, 42, 4363–4374. [Google Scholar] [CrossRef]
- Linvill, M.L.; Vandersande, J.W.; Pohl, R.O. Thermal conductivity of feldspars. Bull. Minéral. 1984, 107, 521–527. [Google Scholar] [CrossRef]
- Bento, A.C.; Almond, D.P.; Brown, S.R.; Turner, I.G. Thermal and optical characterization of the calcium phosphate biomaterial hydroxyapatite. J. Appl. Phys. 1996, 79, 20053. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Matusinović, T. Thermal properties of hydrating calcium aluminate cement pastes. Cem. Concr. Res. 2010, 40, 128–136. [Google Scholar] [CrossRef]
- Duwe, S.; Arlt, C.; Aranda, S.; Riedel, U.; Ziegmann, G. A detailed thermal analysis of nanocomposites filled with SiO 2, AlN or boehmite at varied contents and a review of selected rules of mixture. Compos. Sci. Technol. 2012, 72, 1324–1330. [Google Scholar] [CrossRef]











| Component | Oxide Composition, wt% | |||||||
|---|---|---|---|---|---|---|---|---|
| Al2O3 | CaO | SiO2 | Fe2O3 | Na2O | K2O | TiO2 | MgO | |
| CAC, #71 | 55.8 | 44.0 | - | 0.2 | - | - | - | - |
| HSA | - | - | 100 | - | - | - | - | - |
| FCS | 35.0 | 2.7 | 50.1 | 7.1 | 0.30 | 3.1 | 1.6 | - |
| E-type MGF | 11.4 | 28.6 | 55.0 | 0.9 | 0.6 | - | 0.7 | 2.8 |
| Property | FCS/HSA Weight Ratio | ||||
|---|---|---|---|---|---|
| 100/0 | 94/6 | 90/10 | 88/12 | 83/17 | |
| W/B ratio | 0.53 | 0.49 | 0.5 | 0.54 | 0.55 |
| Density, g/cm3 | 1.29 | 1.21 | 1.18 | 1.08 | 1.07 |
| Slump, mm | 78 | 82 | 83 | 80 | 79 |
| pH | 10.15 | 10.23 | 10.30 | 10.42 | 10.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugama, T.; Pyatina, T. Thermally Insulating, Thermal Shock Resistant Calcium Aluminate Phosphate Cement Composites for Reservoir Thermal Energy Storage. Materials 2022, 15, 6328. https://doi.org/10.3390/ma15186328
Sugama T, Pyatina T. Thermally Insulating, Thermal Shock Resistant Calcium Aluminate Phosphate Cement Composites for Reservoir Thermal Energy Storage. Materials. 2022; 15(18):6328. https://doi.org/10.3390/ma15186328
Chicago/Turabian StyleSugama, Toshifumi, and Tatiana Pyatina. 2022. "Thermally Insulating, Thermal Shock Resistant Calcium Aluminate Phosphate Cement Composites for Reservoir Thermal Energy Storage" Materials 15, no. 18: 6328. https://doi.org/10.3390/ma15186328




