Micron-Scale Biogeography of Seawater Biofilm Colonies at Submersed Solid Substrata Affected by Organic Matter and Microbiome Transformation in the Baltic Sea
Abstract
1. Introduction
2. Materials and Methods
- biovolume (V) is the number of foreground pixels, N, in an image stack multiplied by the voxel volume [8];
- coverage area fraction (f); the fraction of pixels comprised by a biofilm material for each image cross section is calculated by PHLIP [7], thus the fraction f is derived as the ratio of foreground pixels to the total number of pixels for a given cross-section;
- area to volume ratio; its final value is determined by calculating the ratio A/V, where the surface area (A) of the said image stack represents the number of foreground pixels, which are attached to one neighboring background pixel [9];
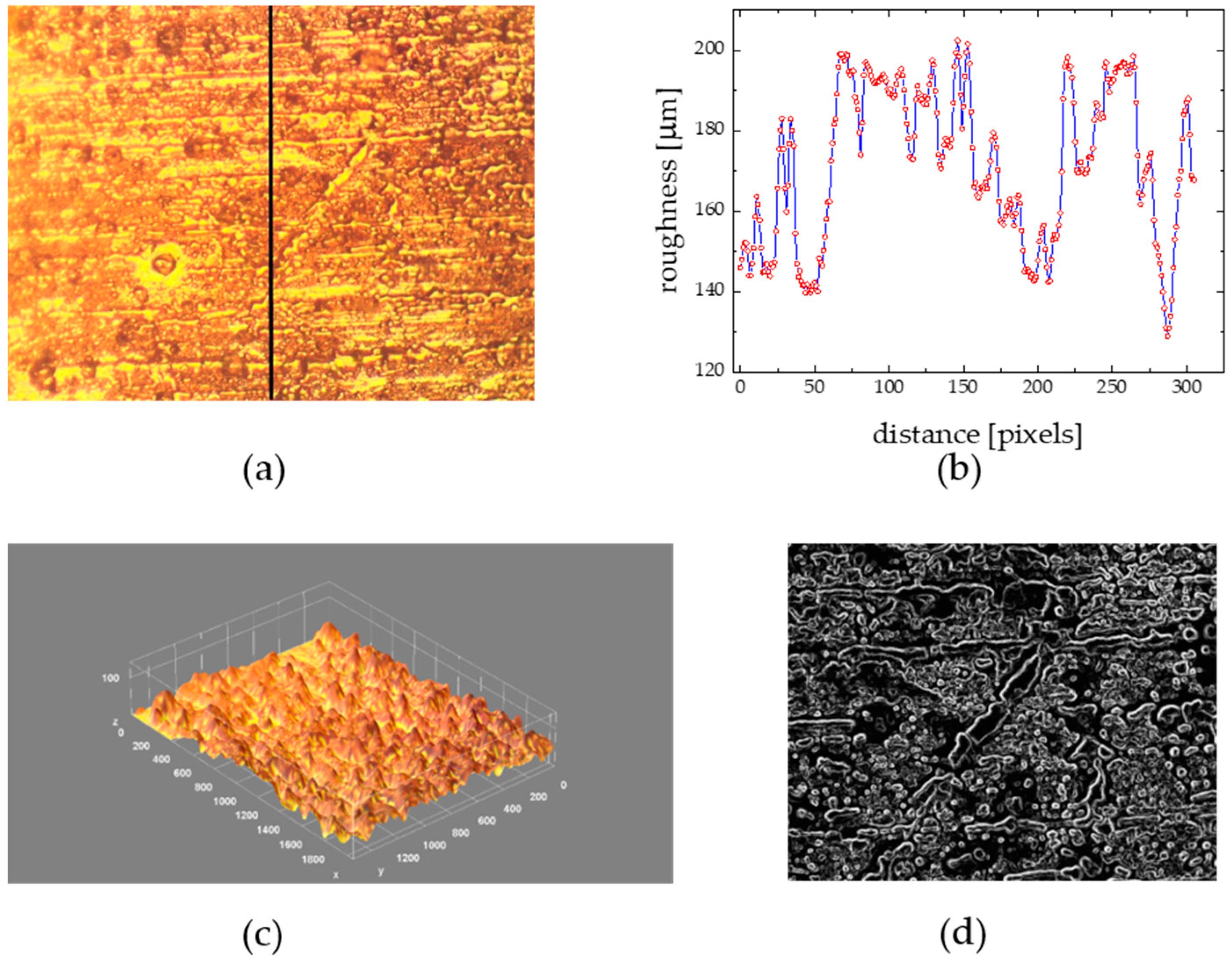
- mean thickness and roughness; are these parameters, which are often chosen to describe the biofilms topography. The average value of the resulting pixel height h distribution, for every point located in the x-y surface plane, stands for the mean thickness (M) [7]. Surface roughness fluctuation dimensionless coefficient (η) defined as: η = SD/Rrms (SD represents the standard deviation from the mean) was introduced to standardize the surface undulation degree [6]. Consequently, the roughness fluctuation coefficient (η) is obtained by dividing the standard deviation of the distribution by M [9];
- fractal dimension in a 2D plane: the fractal dimension parameter is a measure of the biofilm boundary roughness complexity (development degree) between foreground and background pixels in a cross-section at height z, with values varying between 1 and 2. Higher values of the fractal dimension parameter point to a wavier (roughened) biofilm boundary. The fractal dimension parameter is calculated, as described in [10];
- Hopkin’s aggregation index reflects the biofilm colony dispersion state and takes values <2, for randomly distributed biofilm species [11];
- aggregation coefficient (AC) to quantify the aggregation levels in a biofilm culture using 3D micrographs [12], reflecting the changes in average species aggregate dimensions and proportions.
3. Results and Discussion
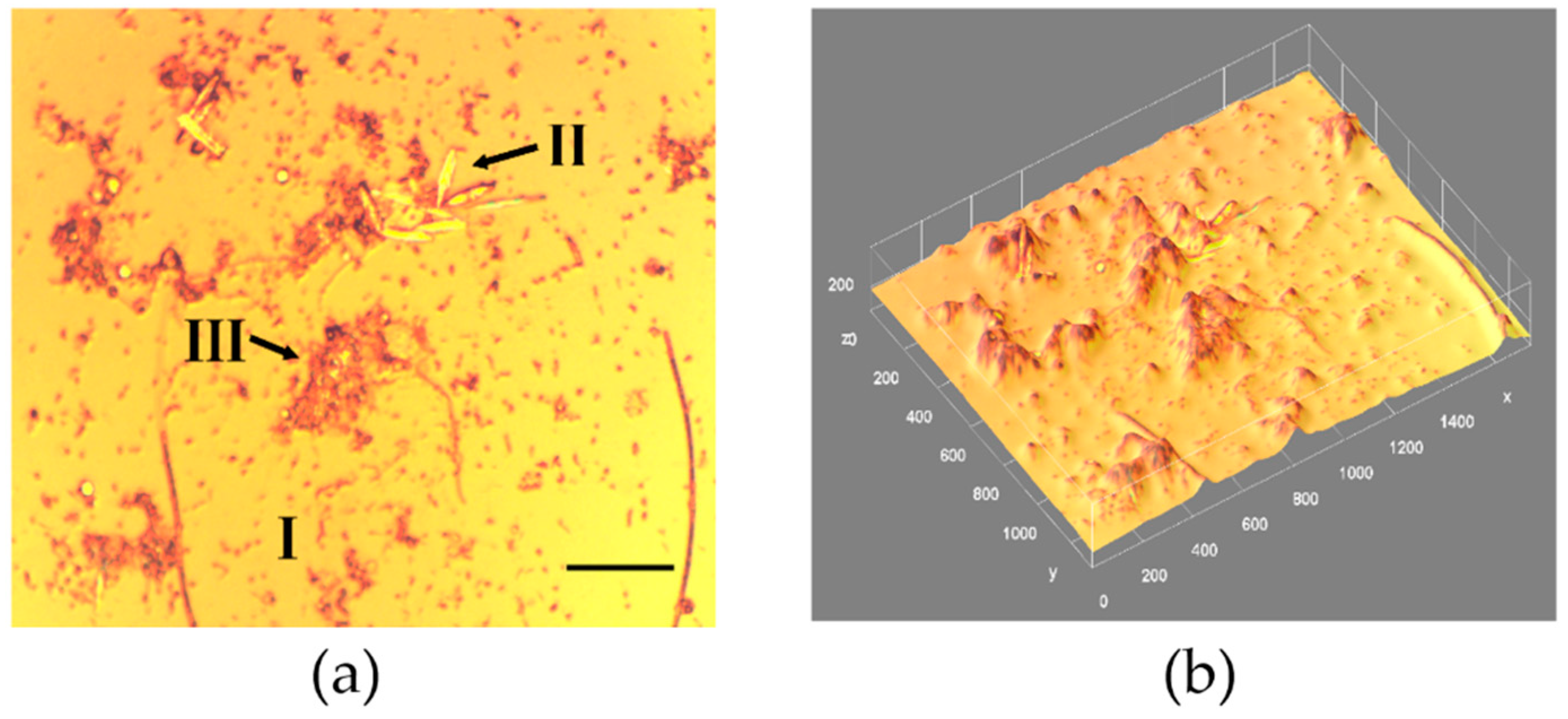
3.1. Biofilm Morphology
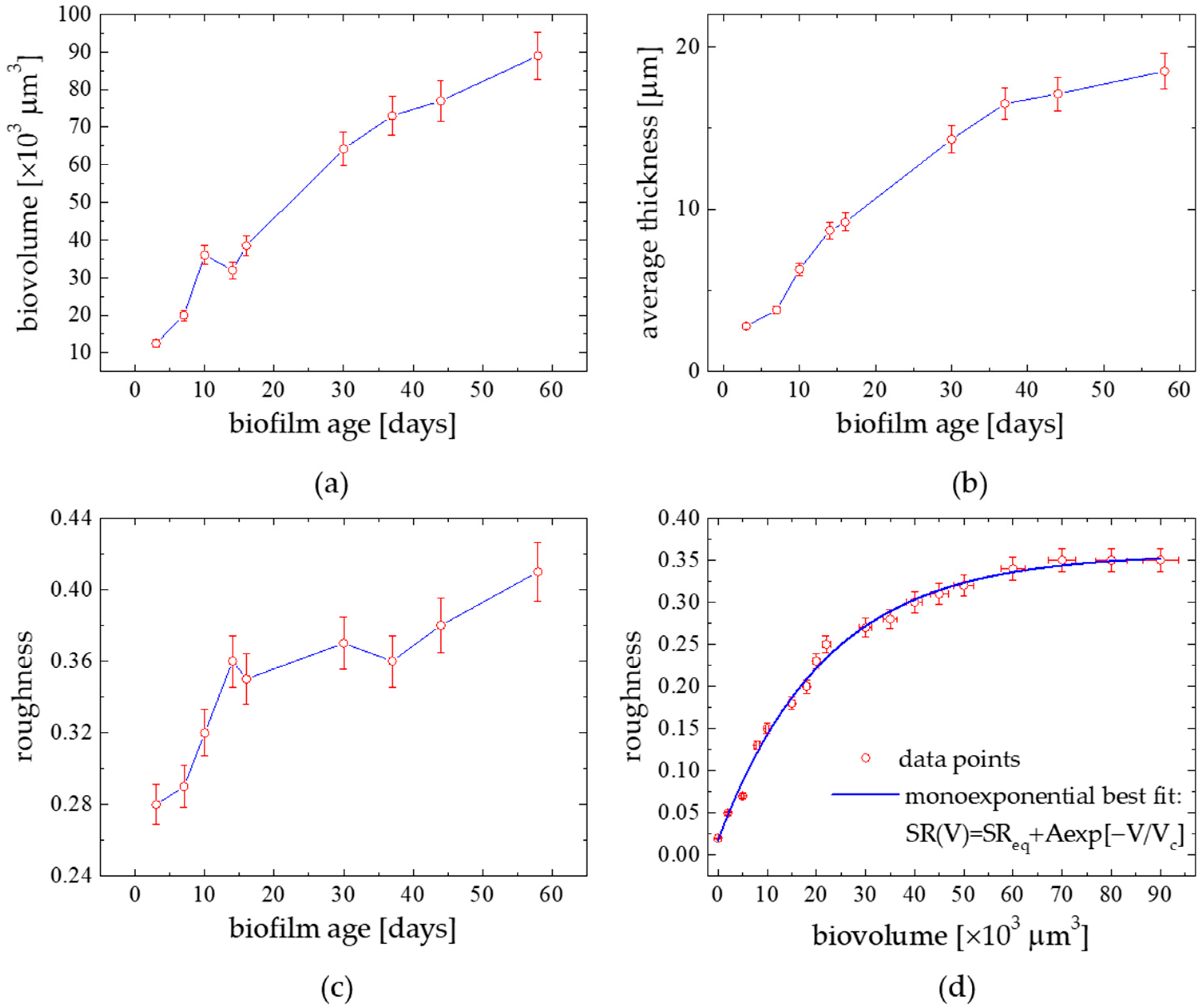
3.2. Temporal Structure Evolution
3.3. Seasonal Biofilm Morphology
3.4. Substratum Wettability Effect
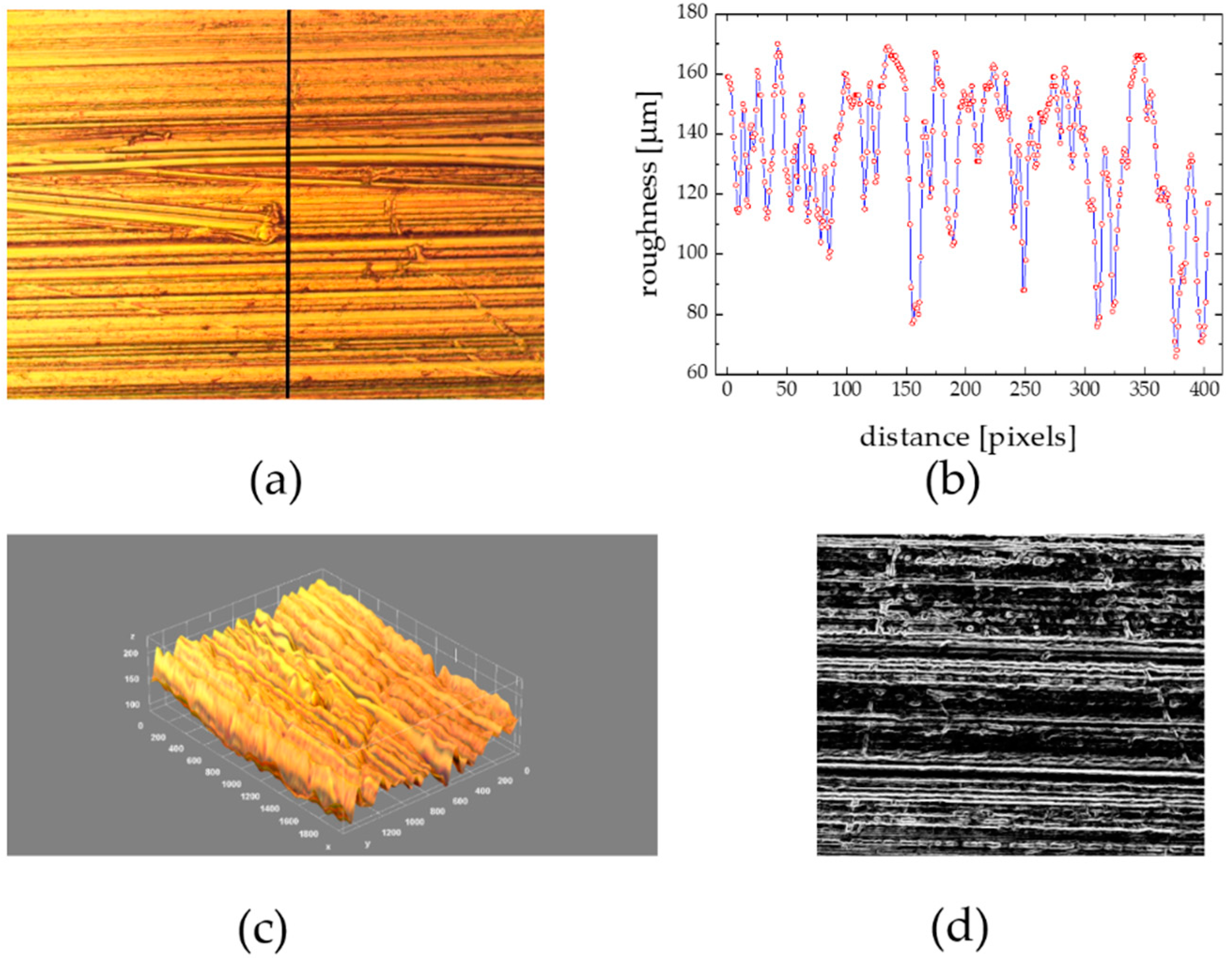
3.5. Substratum Roughness Effect
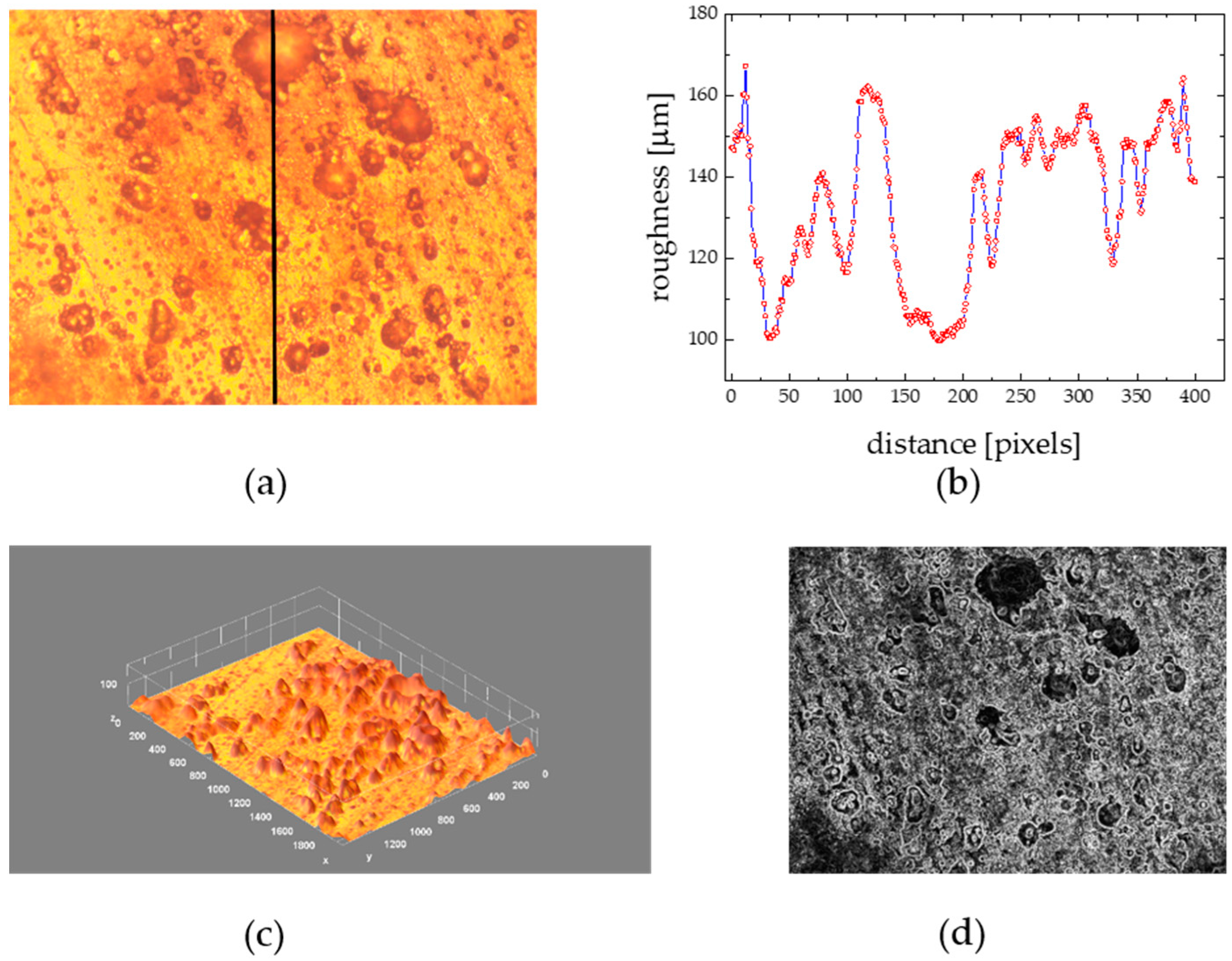
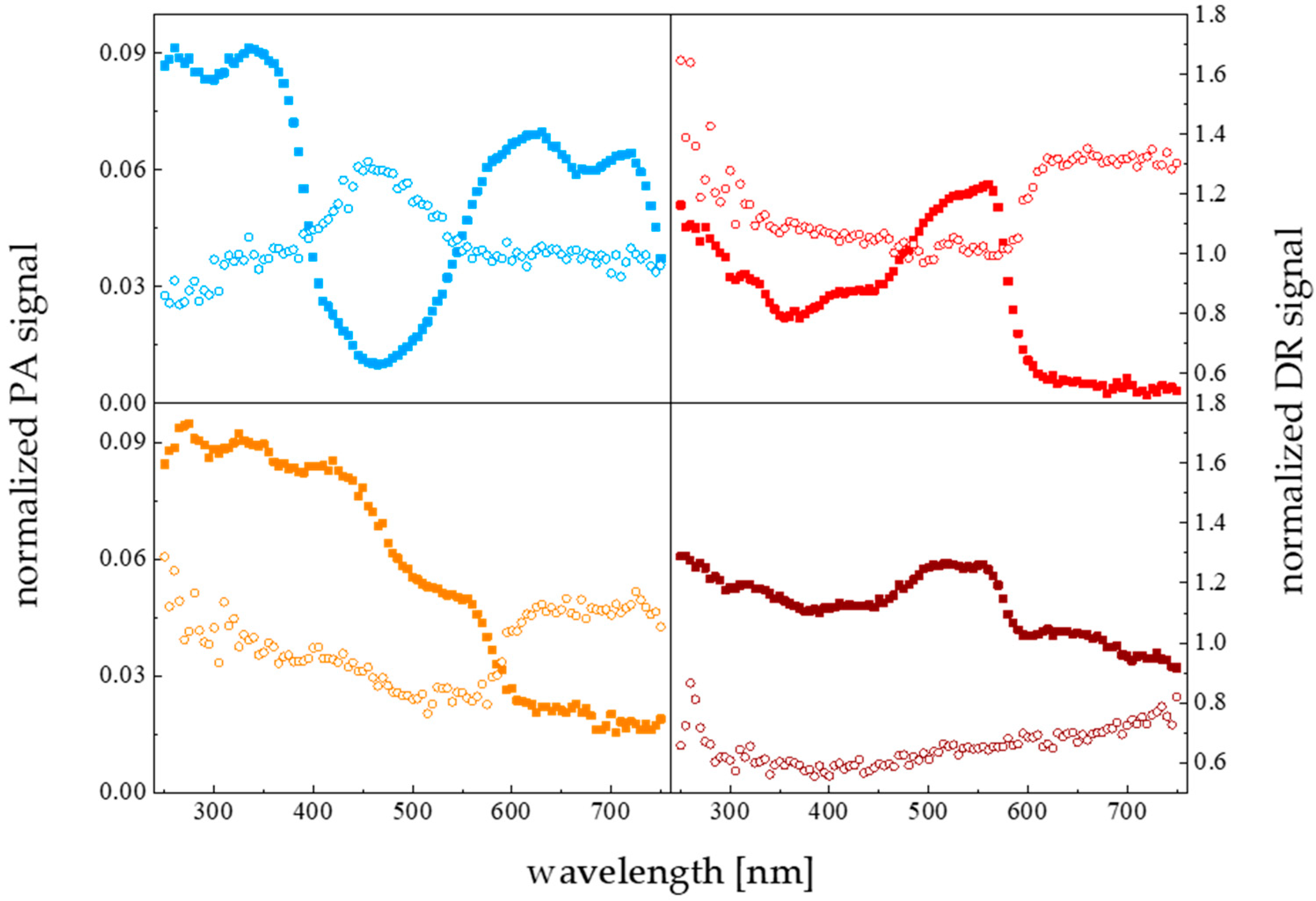
3.6. Substratum Color Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dang, H.; Lovell, C.R. Microbial Surface Colonization and Biofilm Development in Marine Environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef]
- Picioreanu, C.; van Loosdrecht, M.C.; Heijnen, J.J. Two-Dimensional Model of Biofilm Detachment Caused by Internal Stress from Liquid Flow. Biotechnol. Bioeng. 2001, 72, 205–218. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Pogorzelski, S.J.; Pospiech, A.; Boniewicz-Szmyt, K. Monitoring of Marine Biofilm Formation Dynamics at Submerged Solid Surfaces with Multitechnique Sensors. Front. Mar. Sci. 2018, 5, 363. [Google Scholar] [CrossRef]
- Inaba, T.; Ichihara, T.; Yawata, Y.; Toyofuku, M.; Uchiyama, H.; Nomura, N. Three-Dimensional Visualization of Mixed Species Biofilm Formation Together with Its Substratum. Microbiol. Immunol. 2013, 57, 589–593. [Google Scholar] [CrossRef]
- Boniewicz-Szmyt, K.; Pogorzelski, S. Surface Energy of Solids: Selection of Effective Substrates for Bioadhesion in Aqueous Media. Sci. J. Gdynia Marit. Univ. 2019, 112, 7–22. [Google Scholar] [CrossRef]
- Pogorzelski, S.; Boniewicz-Szmyt, K.; Grzegorczyk, M.; Rochowski, P. Wettability of Metal Surfaces Affected by Paint Layer Covering. Materials 2022, 15, 1830. [Google Scholar] [CrossRef]
- Xavier, J.B.; White, D.C.; Almeida, J.S. Automated Biofilm Morphology Quantification from Confocal Laser Scanning Microscopy Imaging. Water Sci. Technol. 2003, 47, 31–37. [Google Scholar] [CrossRef][Green Version]
- Kuehn, M.; Hausner, M.; Bungartz, H.-J.; Wagner, M.; Wilderer, P.A.; Wuertz, S. Automated Confocal Laser Scanning Microscopy and Semiautomated Image Processing for Analysis of Biofilms. Appl. Environ. Microbiol. 1998, 64, 4115–4127. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of Biofilm Structures by the Novel Computer Program Comstat. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef]
- Yang, X.; Beyenal, H.; Harkin, G.; Lewandowski, Z. Quantifying Biofilm Structure Using Image Analysis. J. Microbiol. Methods 2000, 39, 109–119. [Google Scholar] [CrossRef]
- Dazzo, F.B.; Niccum, B.C. Use of CMEIAS Image Analysis Software to Accurately Compute Attributes of Cell Size, Morphology, Spatial Aggregation and Color Segmentation That Signify in Situ Ecophysiological Adaptations in Microbial Biofilm Communities. Computation 2015, 3, 72–98. [Google Scholar] [CrossRef]
- Cai, Y.; Chatelet, D.S.; Howlin, R.P.; Wang, Z.-Z.; Webb, J.S. A Novel Application of Gini Coefficient for the Quantitative Measurement of Bacterial Aggregation. Sci. Rep. 2019, 9, 19002. [Google Scholar] [CrossRef]
- Mueller, L.N.; de Brouwer, J.F.; Almeida, J.S.; Stal, L.J.; Xavier, J.B. Analysis of a Marine Phototrophic Biofilm by Confocal Laser Scanning Microscopy Using the New Image Quantification Software PHLIP. BMC Ecol. 2006, 6, 1. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Pogorzelski, S.J.; Mazurek, A.Z.; Szczepanska, A. In-Situ Surface Wettability Parameters of Submerged in Brackish Water Surfaces Derived from Captive Bubble Contact Angle Studies as Indicators of Surface Condition Level. J. Mar. Syst. 2013, 119–120, 50–60. [Google Scholar] [CrossRef]
- Pogorzelski, S.; Rochowski, P.; Grzegorczyk, M. Photosynthetic Energy Storage Efficiency in Biofilms Determined by Photoacoustics. In Proceedings of the Fourteenth School on Acousto-Optics and Applications, Torun, Poland, 24–27 June 2019; Grulkowski, I., Linde, B.B.J., Duocastella, M., Eds.; SPIE: Bellingham, WA, USA, 2019; p. 16. [Google Scholar]
- Grzegorczyk, M.; Pogorzelski, S.J. Photoacoustic Spectroscopy Studies of Biofilm-Covered Solid Substrata Submerged in the Sea. In Proceedings of the 2018 Joint Conference—Acoustics, Ustka, Poland, 11–14 September 2018; pp. 1–5. [Google Scholar]
- Buswell, C.M.; Herlihy, Y.M.; Marsh, P.D.; Keevil, C.W.; Leach, S.A. Coaggregation amongst Aquatic Biofilm Bacteria. J. Appl. Microbiol. 1997, 83, 477–484. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R. Marine Biofilms: A Successful Microbial Strategy With Economic Implications. Front. Mar. Sci. 2018, 5, 126. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular Polymeric Substances (EPS) of Microbial Aggregates in Biological Wastewater Treatment Systems: A Review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Finlay, J.A.; Callow, M.E.; Ista, L.K.; Lopez, G.P.; Callow, J.A. The Influence of Surface Wettability on the Adhesion Strength of Settled Spores of the Green Alga Enteromorpha and the Diatom Amphora. Integr. Comp. Biol. 2002, 42, 1116–1122. [Google Scholar] [CrossRef]
- Lakshmi, K.; Muthukumar, T.; Doble, M.; Vedaprakash, L.; Kruparathnam; Dineshram, R.; Jayaraj, K.; Venkatesan, R. Influence of Surface Characteristics on Biofouling Formed on Polymers Exposed to Coastal Sea Waters of India. Colloids Surf. B Biointerfaces 2012, 91, 205–211. [Google Scholar] [CrossRef]
- Schmidt, D.L.; Brady, R.F.; Lam, K.; Schmidt, D.C.; Chaudhury, M.K. Contact Angle Hysteresis, Adhesion, and Marine Biofouling. Langmuir 2004, 20, 2830–2836. [Google Scholar] [CrossRef]
- Baier, R.E. Surface Behaviour of Biomaterials: The Theta Surface for Biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef]
- Akuzov, D.; Franca, L.; Grunwald, I.; Vladkova, T. Sharply Reduced Biofilm Formation from Cobetia Marina and in Black Sea Water on Modified Siloxane Coatings. Coatings 2018, 8, 136. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley and Sons: New York, NY, USA, 1997; ISBN 0471148733. [Google Scholar]
- Wang, X.; Ma, M.; Liu, J. Biological Characteristics of Biofilms Formed on Different Substrata in a Shallow Lake in Haihe Basin (China). Bull. Environ. Contam. Toxicol. 2013, 90, 414–420. [Google Scholar] [CrossRef]
- Jones, P.R.; Cottrell, M.T.; Kirchman, D.L.; Dexter, S.C. Bacterial Community Structure of Biofilms on Artificial Surfaces in an Estuary. Microb. Ecol. 2007, 53, 153–162. [Google Scholar] [CrossRef]
- Conradi, M.; Sever, T.; Gregorčič, P.; Kocijan, A. Short-and Long-Term Wettability Evolution and Corrosion Resistance of Uncoated and Polymer-Coated Laser-Textured Steel Surface. Coatings 2019, 9, 592. [Google Scholar] [CrossRef]
- Abbas, M.; Mehran, M.T.; Moon, M.-W.; Byun, J.Y.; Kim, S.H. Wettability Control of Modified Stainless Steel Surfaces for Oxide Catalyst Carrier Slurry Coating. J. Ind. Eng. Chem. 2020, 91, 330–339. [Google Scholar] [CrossRef]
- Jia, X. Wettability of Rough Polymer, Metal and Oxide Surfaces as Well as of Composite Surfaces. J. Adhes. Sci. Technol. 2008, 22, 1893–1905. [Google Scholar] [CrossRef]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards Understanding Interactions between Biofilms and Metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef]
- Dang, Y.T.H.; Power, A.; Cozzolino, D.; Dinh, K.B.; Ha, B.S.; Kolobaric, A.; Vongsvivut, J.; Truong, V.K.; Chapman, J. Analytical Characterisation of Material Corrosion by Biofilms. J. Bio-Tribo-Corros. 2022, 8, 50. [Google Scholar] [CrossRef]
- Wanner, O.; Eberl, H.; Morgenroth, E.; Noguera, D.; Picioreanu, C.; Rittmann, B.; van Loosdrecht, M. Mathematical Modeling of Biofilms; IWA Publishing: London, UK, 2006; Volume 18. [Google Scholar]
- Callow, M.E.; Jennings, A.R.; Brennan, A.B.; Seegert, C.E.; Gibson, A.; Wilson, L.; Feinberg, A.; Baney, R.; Callow, J.A. Microtopographic Cues for Settlement of Zoospores of the Green Fouling Alga Enteromorpha. Biofouling 2002, 18, 229–236. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus Roughness of Engineering Surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.M.; Voolstra, C.R. The Effect of Surface Colour on the Formation of Marine Micro and Macrofouling Communities. Biofouling 2013, 29, 617–627. [Google Scholar] [CrossRef]
- Robson, M.A.; Williams, D.; Wolff, K.; Thomason, J.C. The Effect of Surface Colour on the Adhesion Strength of Elminius Modestus Darwin on a Commercial Non-Biocidal Antifouling Coating at Two Locations in the UK. Biofouling 2009, 25, 215–227. [Google Scholar] [CrossRef]
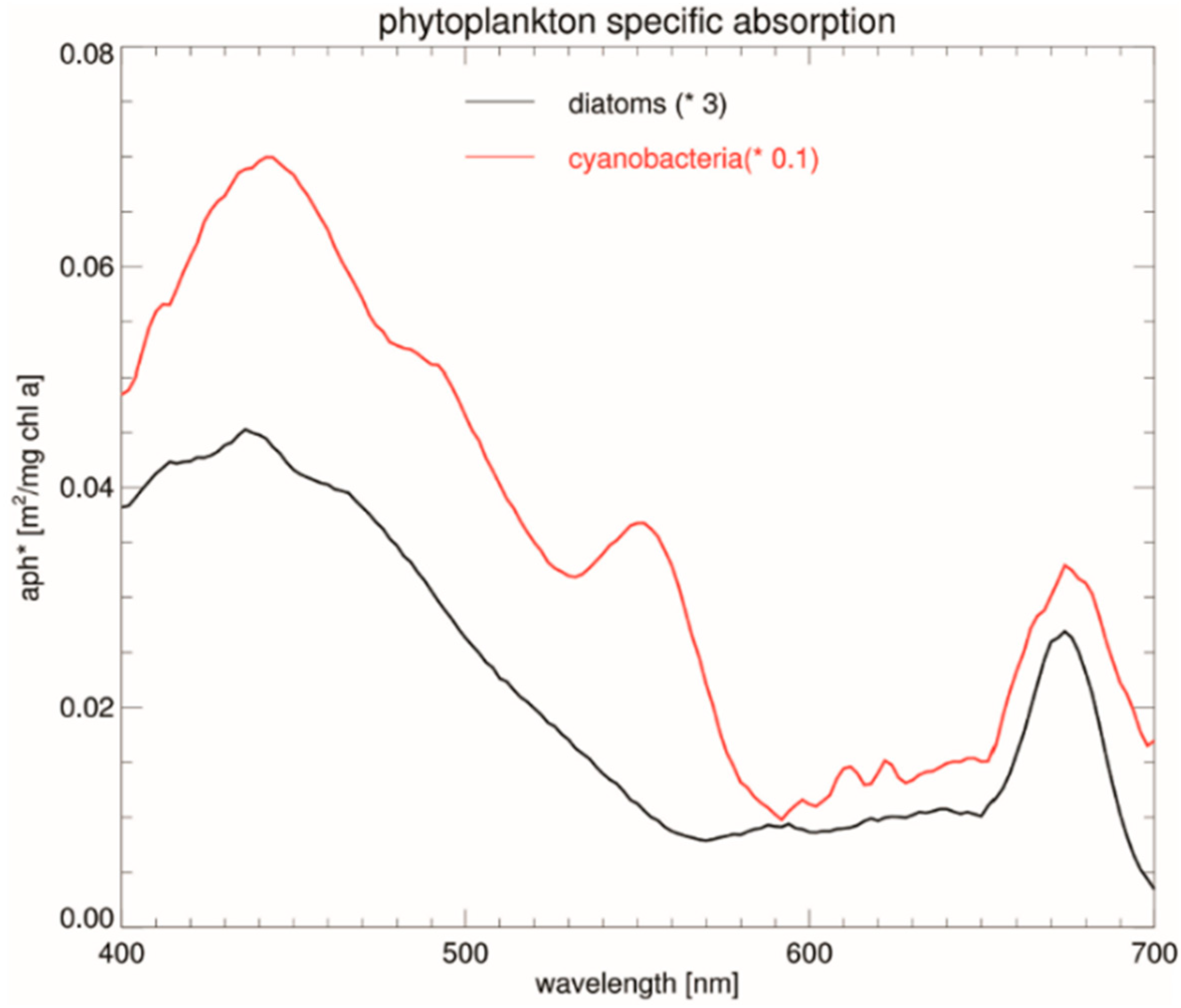
- Ficek, D.; Kaczmarek, S.; Stoń-Egiert, J.; Woźniak, B.; Majchrowski, R.; Dera, J. Spectra of Light Absorption by Phytoplankton Pigments in the Baltic; Conclusions to Be Drawn from a Gaussian Analysis of Empirical Data. Oceanologia 2004, 46, 533–555. [Google Scholar]
- Eich, A.; Mildenberger, T.; Laforsch, C.; Weber, M. Biofilm and Diatom Succession on Polyethylene (PE) and Biodegradable Plastic Bags in Two Marine Habitats: Early Signs of Degradation in the Pelagic and Benthic Zone? PLoS ONE 2015, 10, e0137201. [Google Scholar] [CrossRef] [PubMed]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic Pigments in Diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef]
- Bracher, A.; Vountas, M.; Dinter, T.; Burrows, J.P.; Röttgers, R.; Peeken, I. Quantitative Observation of Cyanobacteria and Diatoms from Space Using PhytoDOAS on SCIAMACHY Data. Biogeosciences 2009, 6, 751–764. [Google Scholar] [CrossRef]
- Rochowski, P.; Niedziałkowski, P.; Pogorzelski, S.J. The Benefits of Photoacoustics for the Monitoring of Drug Stability and Penetration through Tissue-Mimicking Membranes. Int. J. Pharm. 2020, 580, 119233. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Pogorzelski, S.; Rochowski, P. Towards a Novel Class of Photoacoustics-Based Water Contamination Sensors. J. Environ. Chem. Eng. 2022, 10, 107983. [Google Scholar] [CrossRef]


| Month | N | P | O | Chl. a | PP |
|---|---|---|---|---|---|
| IV | 228.7 (35.2) | 97.7 (12.4) | 13.1 (2.6) | 6.0 (1.7) | 751 (69.4) |
| VIII | 39.3 (2.8) | 62.4 (5.8) | 11.3 (3.4) | 4.1 (1.5) | 972 (88.5) |
| XI | 75.5 (6.9) | 45.0 (3.9) | 10.4 (1.9) | 4.6 (2.0) | 255 (30.1) |
| Substratum | Surface Coverage [%] | BWM [mg cm−2] | AC |
|---|---|---|---|
| PVC blue | 8.36 (2.19) | 1.18 (0.24) | 0.62 (0.07) |
| PVC orange | 22.25 (2.87) | 10.78 (2.21) | 0.32 (0.05) |
| PVC red | 20.46 (5.85) | 13.69 (3.14) | 0.29 (0.04) |
| PVC brown | 16.84 (8.97) | 6.85 (2.23) | 0.53 (0.08) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzegorczyk, M.; Pogorzelski, S.; Janowicz, P.; Boniewicz-Szmyt, K.; Rochowski, P. Micron-Scale Biogeography of Seawater Biofilm Colonies at Submersed Solid Substrata Affected by Organic Matter and Microbiome Transformation in the Baltic Sea. Materials 2022, 15, 6351. https://doi.org/10.3390/ma15186351
Grzegorczyk M, Pogorzelski S, Janowicz P, Boniewicz-Szmyt K, Rochowski P. Micron-Scale Biogeography of Seawater Biofilm Colonies at Submersed Solid Substrata Affected by Organic Matter and Microbiome Transformation in the Baltic Sea. Materials. 2022; 15(18):6351. https://doi.org/10.3390/ma15186351
Chicago/Turabian StyleGrzegorczyk, Maciej, Stanislaw Pogorzelski, Paulina Janowicz, Katarzyna Boniewicz-Szmyt, and Pawel Rochowski. 2022. "Micron-Scale Biogeography of Seawater Biofilm Colonies at Submersed Solid Substrata Affected by Organic Matter and Microbiome Transformation in the Baltic Sea" Materials 15, no. 18: 6351. https://doi.org/10.3390/ma15186351
APA StyleGrzegorczyk, M., Pogorzelski, S., Janowicz, P., Boniewicz-Szmyt, K., & Rochowski, P. (2022). Micron-Scale Biogeography of Seawater Biofilm Colonies at Submersed Solid Substrata Affected by Organic Matter and Microbiome Transformation in the Baltic Sea. Materials, 15(18), 6351. https://doi.org/10.3390/ma15186351







