Designing a Biomaterial Approach to Control the Adaptive Response to a Skin Injury
Abstract
:1. Introduction
2. The Adaptive Response
2.1. Adaptive Responses to Injury
2.2. Regeneration
2.3. Graft Healing
2.4. Loss of Regeneration
2.4.1. Mammalian Regenerative Healing
2.4.2. Fetal Wound Healing
2.5. Developing Strategies to Reduce Scarring and Increase Regeneration
2.5.1. The Normal Adult Adaptive Response
2.5.2. Evolutionary Control of the Adaptive Response
3. Control Strategies
3.1. Inflammation Control
3.1.1. Causes of Inflammation
3.1.2. Inflammation Control Strategies
3.2. Repair Phase Control
4. Design Principles
4.1. General
4.2. Structure vs. Function
4.2.1. Relationship of Structure to Function
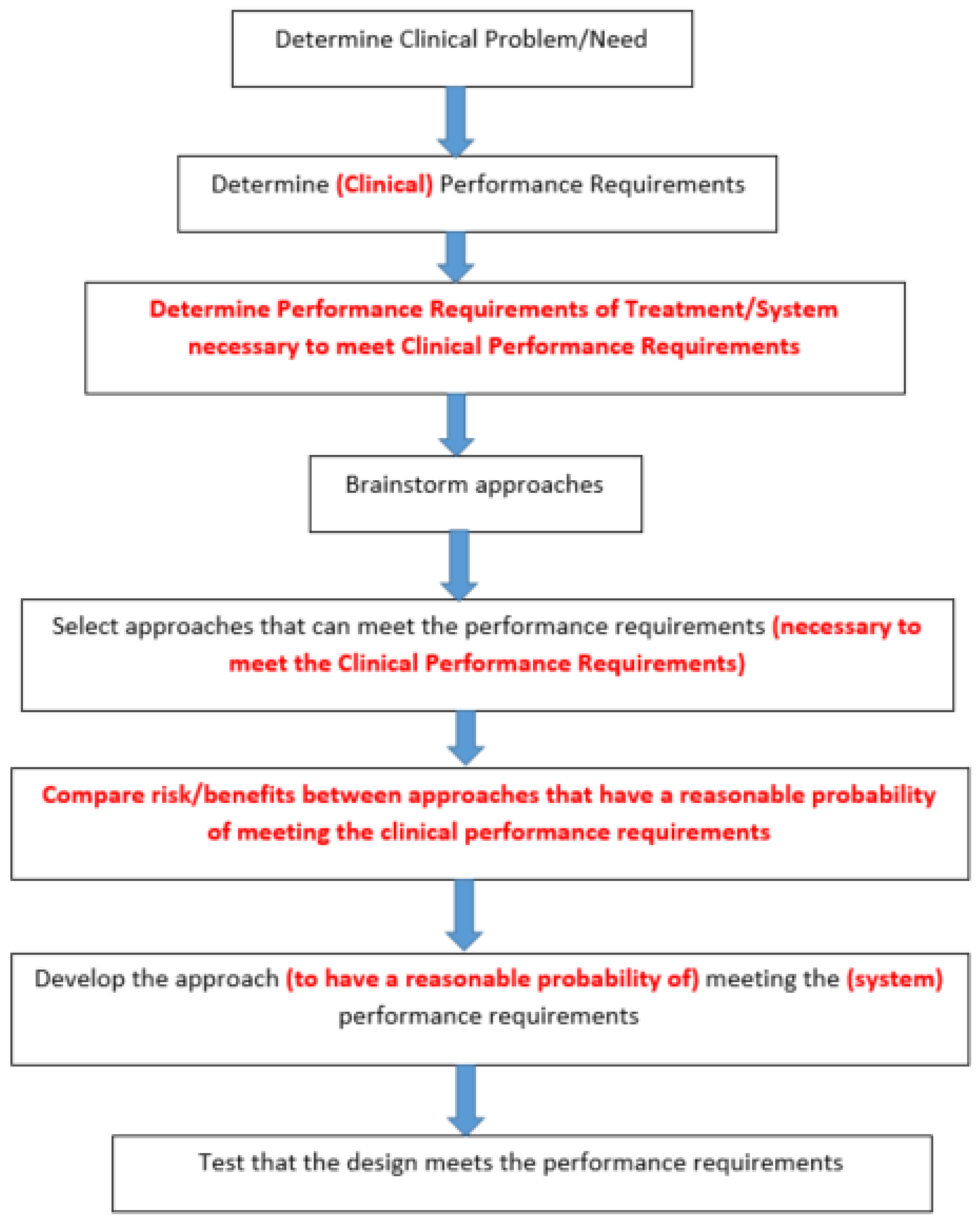
4.2.2. Clinical Performance
4.3. Skin Design Constraints
4.3.1. Clinical Performance Design Constraints
4.3.2. Approaches to Meet the Performance Design Constraints
Skin Grafts
Skin Graft Substitutes
Scaffold Systems
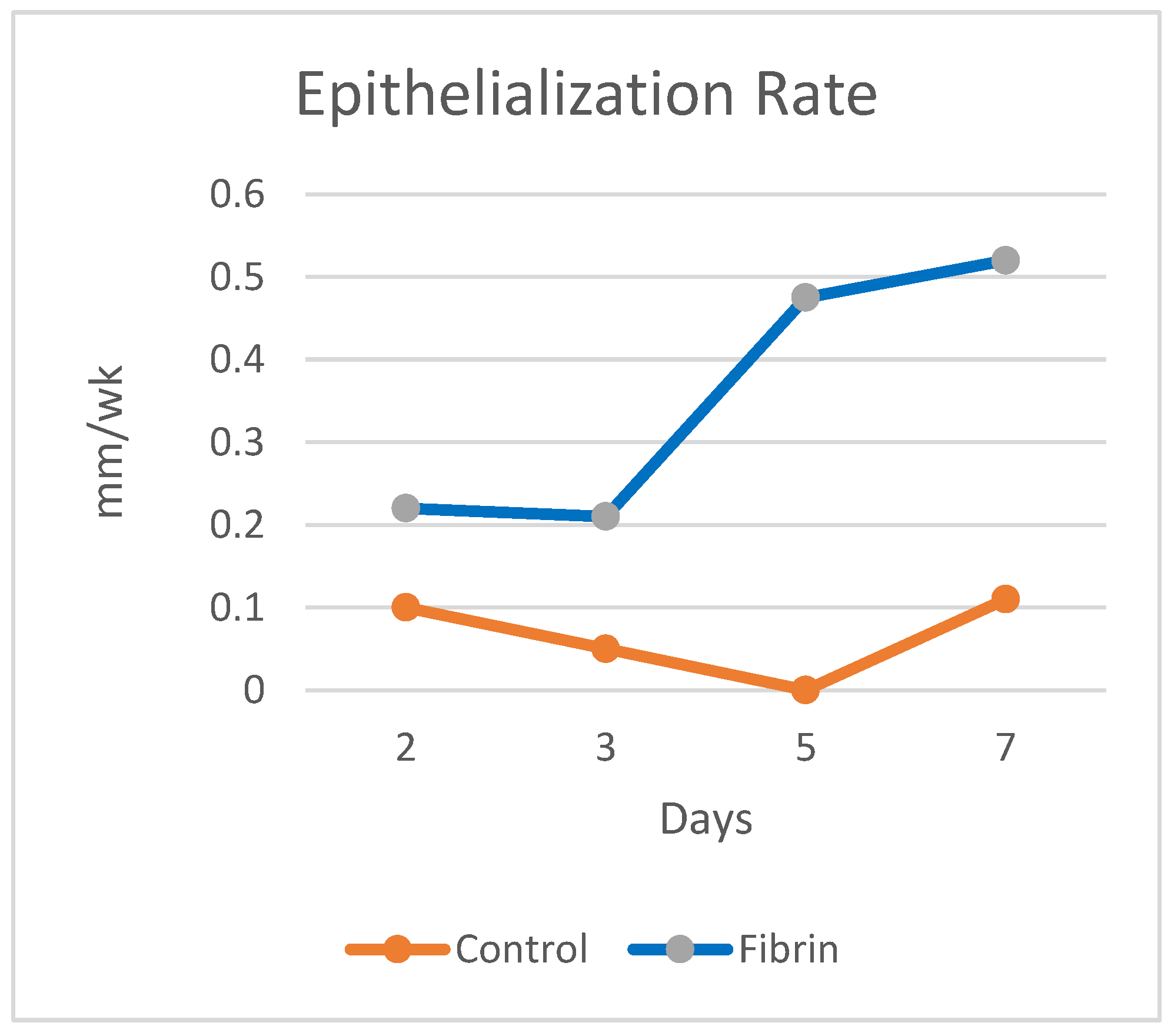
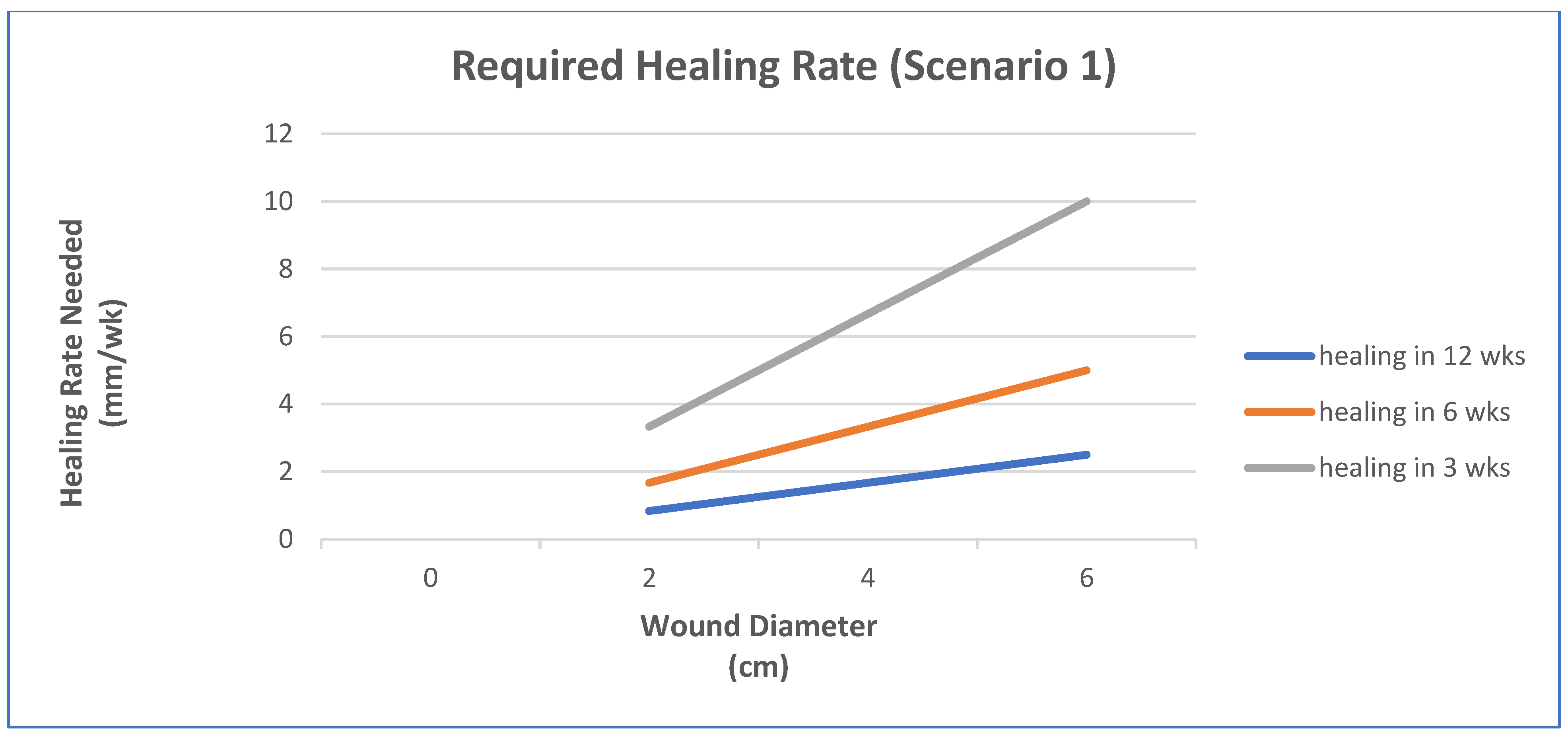
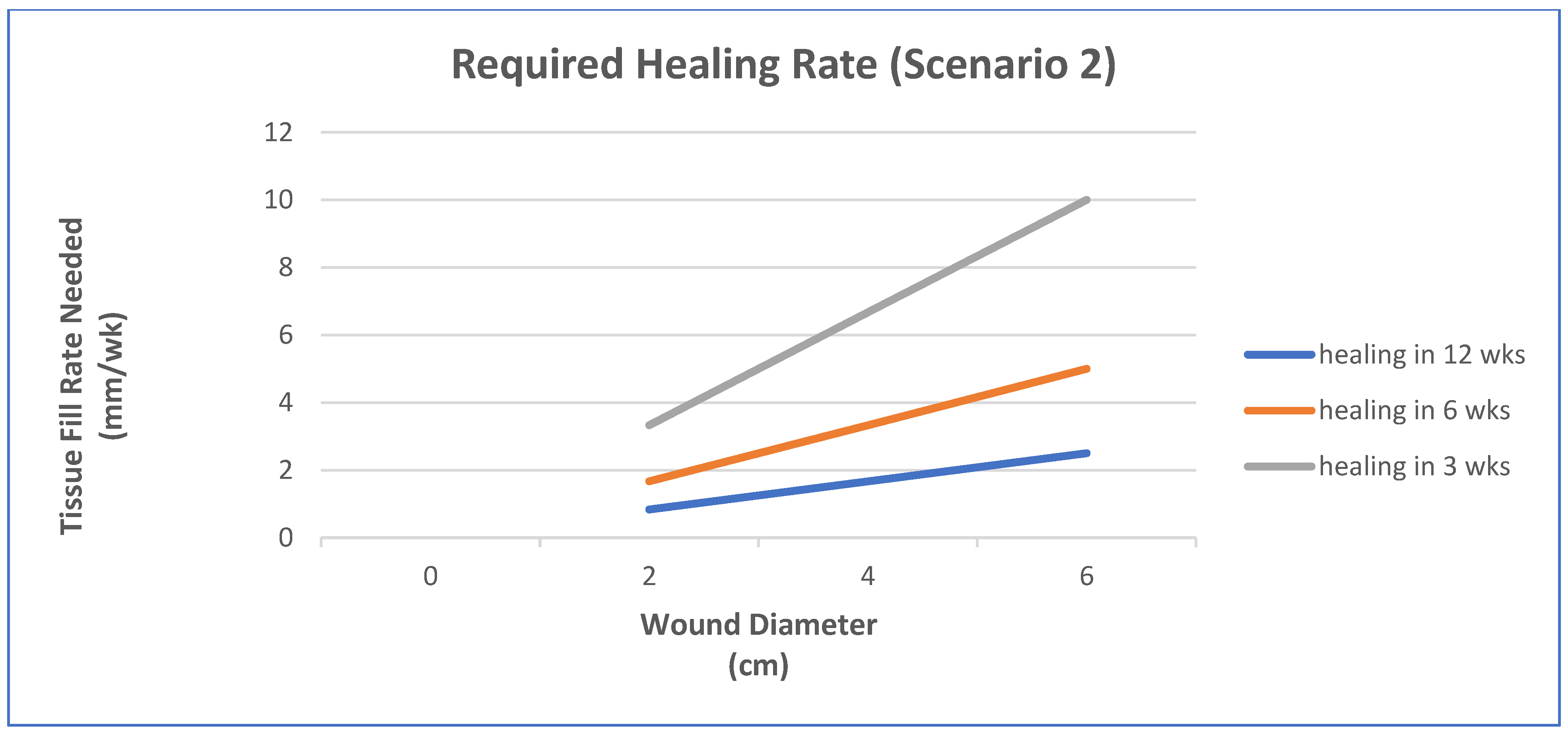
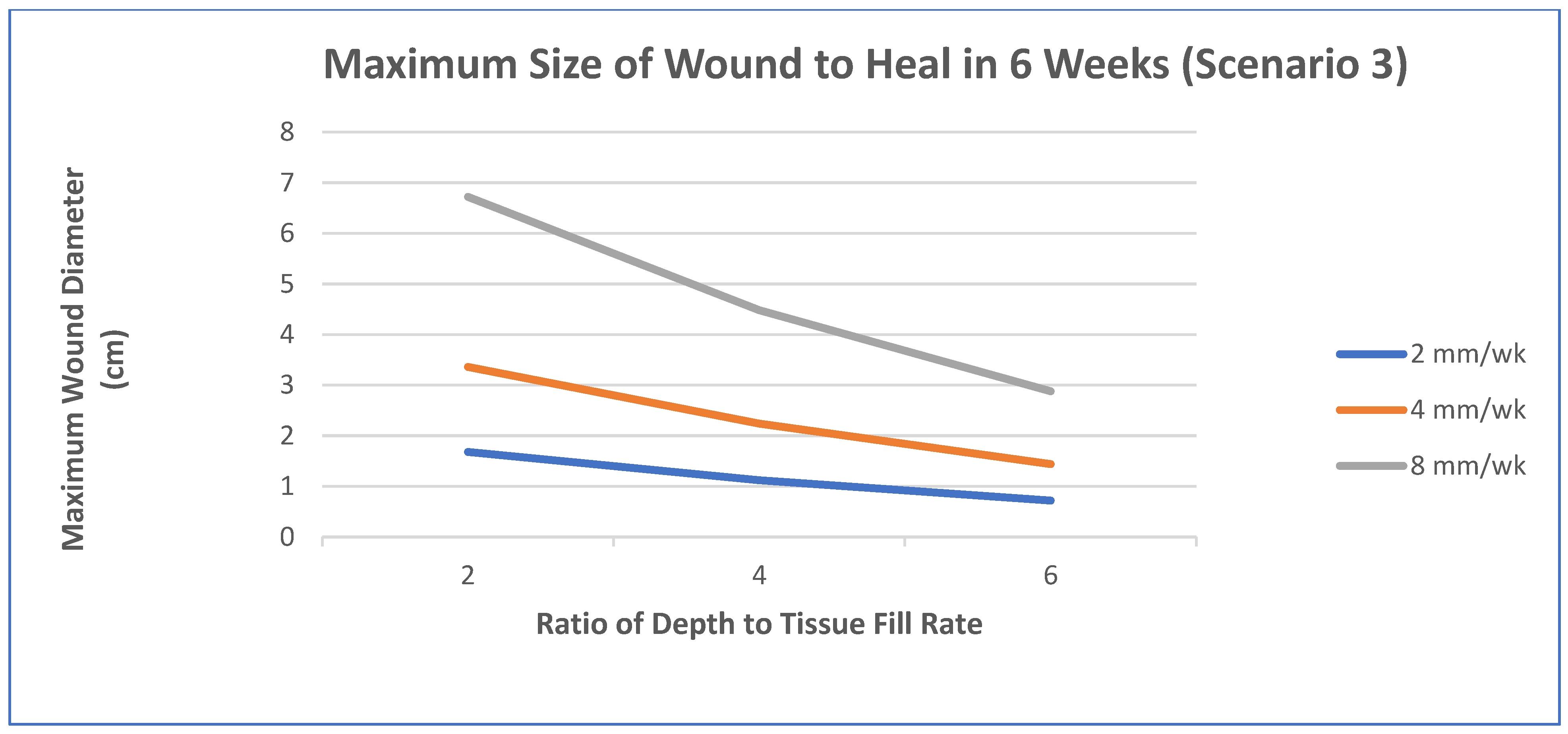
- Epithelialization rate (ER) and contraction rate (CR) occur independently of tissue fill rate (TFrate), as is often the case with shallower wounds;
- ER only occurs as fast as the tissue fill rate with CR probably dependent on TFrate, as is often seen in deeper wounds;
- Undermining and other factors require that wounds have higher percentages of tissue fill before significant epithelialization and contraction can occur.
5. Controlling the Adaptive Response to Skin Injury
5.1. General Strategies
5.2. Inflammation Control
5.2.1. Relationship to Clinical Outcome
5.2.2. Limiting the Inflammatory Response
5.3. Repair Control
Relationship to Clinical Outcome
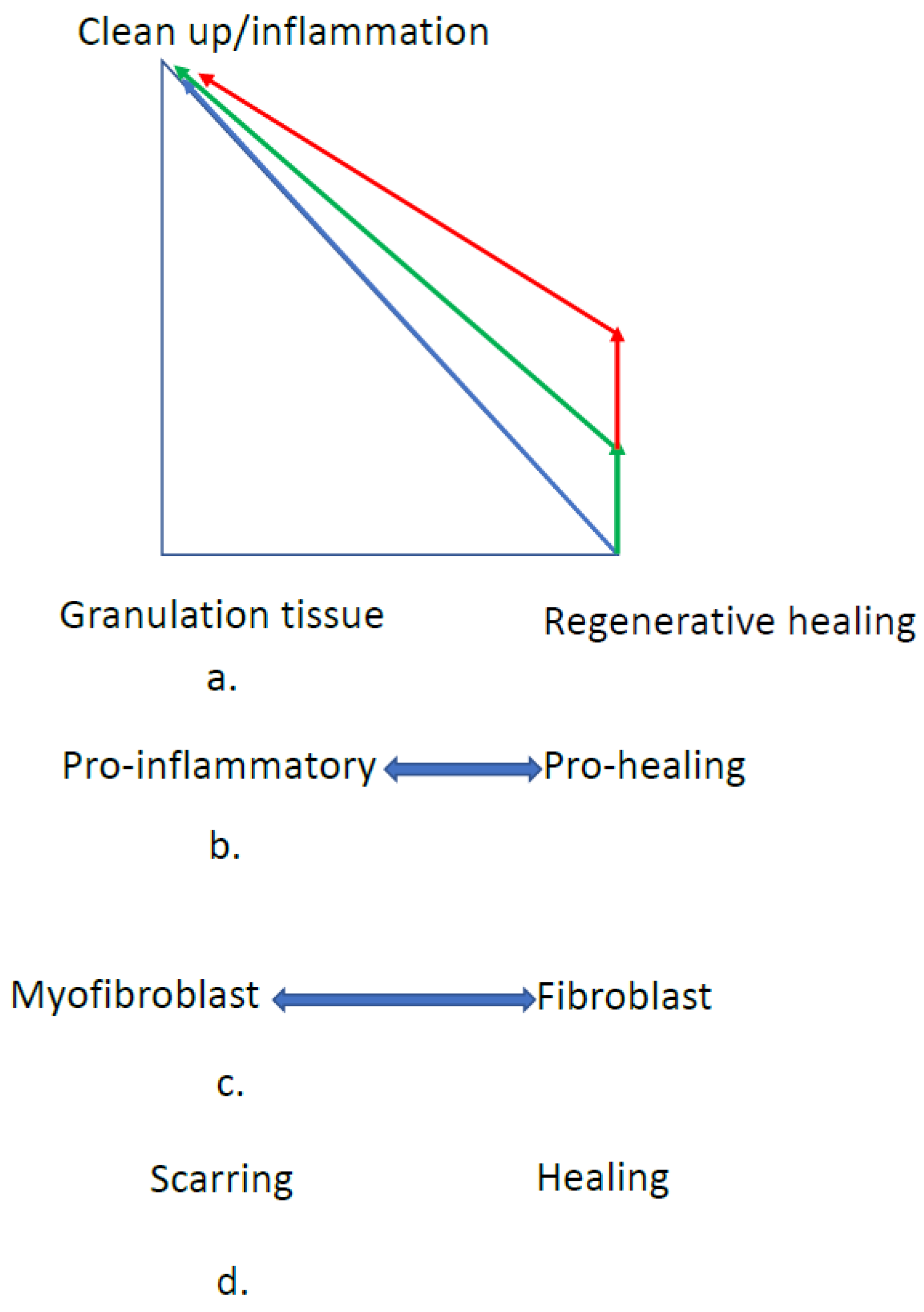
6. Skin Scaffolds
6.1. Variables to Control
6.1.1. Bioactivity
Cell Migration
Angiogenesis
Drug Delivery
Cell Seeding
Oxygen
Electrical Stimulation
Examples
6.1.2. Scaffolds
Fibrin
Albumin
6.2. Design Selection
6.2.1. Engineering Design Process vs. the Scientific Method
6.2.2. Real World Engineering Design Process for Skin Wounds
Real World Design Process
Iterative Nature of Design Process
7. System Design
7.1. Burns
7.2. Pressure Ulcers
7.2.1. Electrical Stimulation
7.2.2. Scaffold Systems
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Feldman, D. Quantification and Modeling of Biological Processes for Tissue Engineering and Regenerative Medicine. Biomed. J. Sci. Tech. Res. 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Feldman, D.S. Biomaterial Enhanced Regeneration Design Research for Skin and Load Bearing Applications. J. Funct. Biomater. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility; Marcel Dekker, Inc.: New York, NY, USA, 1981. [Google Scholar]
- Wound Healing. In An Introduction to Tissue Biomaterials Interactions; Dee, K.C.; Puleo, D.A.; Bizios, R. (Eds.) Wiley-Liss, Inc.: Hoboken, NJ, USA, 2002; pp. 127–147. [Google Scholar]
- Boyce, S.T.; Supp, D.M. Biologic skin substitutes. In Skin Tissue Engineering and Regenerative Medicine; Albanna, M.Z., Holmes, J.H., Eds.; Academic Press: New York, NY, USA, 2016; pp. 211–238. [Google Scholar]
- Feldman, D. The importance of engineering design constraints to justify a study, particularly in an applied bioengineering journal. J. Biomed. Imaging Bioeng 2017, 1, 5–6. [Google Scholar]
- Palsson, B.; Bhatia, S.N. Tissue Dynamics. In Tissue Engineering; Pearson Education: Upper Saddle River, NJ, USA, 2004; pp. 34–45. [Google Scholar]
- Palsson, B.; Bhatia, S.N. Cellular-Fate Processes. In Tissue Engineering; Pearson Education: Upper Saddle River, NJ, USA, 2004; pp. 74–104. [Google Scholar]
- Palsson, B.; Bhatia, S.N. Coordination of Cellular-Fate Processes. In Tissue Engineering; Pearson Education: Upper Saddle River, NJ, USA, 2004; pp. 105–131. [Google Scholar]
- Wax, M.K. Split-Thickness Skin Grafts; Medscapes Online: New York, NY, USA, 2019. [Google Scholar]
- Goss, R. Regeneration Versus Repair. In Wound Healing: Biochemical and Clinical Aspects; Cohen, I., Diegelmann, R., Lindblad, W., Eds.; W.B. Saunders, Co.: Philadelphia, PA, USA, 1992; pp. 20–29. [Google Scholar]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Duranceau, L.; Genest, H.; Bortoluzzi, P.; Moulin, V.; Auger, F.A.; Germain, L. Successful grafting of a novel autologous tissue-engineered skin substitutes (dermis and epidermis) on twelve burn patients. J. Burn Care Res. 2014, 35, S121. [Google Scholar]
- Feldman, D. The Role of Macrophages in Controlling the Adaptive Response to Injury: Regeneration Vs. Scarring. In Macrophages-140 Years of Their Discovery; Vijay, K., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Varkey, M.; Ding, J.; Tredget, E.E. Advances in skin substitutes—Potential of tissue engineered skin for facilitating anti-fibrotic healing. J. Funct. Biomater. 2015, 6, 547–563. [Google Scholar] [CrossRef]
- Thomas-Virnig, C.L.; Allen-Hoffmann, B.L. A bioengineered human skin tissue for the treatment of infected wounds. Adv. Wound Care 2012, 1, 88–94. [Google Scholar] [CrossRef]
- Boyce, S.T.; Kagan, R.J.; Greenhalgh, D.G.; Warner, P.; Yakuboff, K.P.; Palmieri, T.; Warden, G.D. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J. Trauma Acute Care Surg. 2006, 60, 821–829. [Google Scholar]
- Kagan, R.J.; Peck, M.D.; Ahrenholz, D.H.; Hickerson, W.L.; Holmes, I.V.J.; Korentager, R.; Kraatz, J.; Pollock, K.; Kotoski, G. Surgical management of the burn wound and use of skin substitutes: An expert panel white paper. J. Burn Care Res. 2013, 34, e60–e79. [Google Scholar] [CrossRef]
- Heimbach, D.M.; Warden, G.D.; Luterman, A.; Jordan, M.H.; Ozobia, N.; Ryan, C.M.; Voigt, D.W.; Hickerson, W.L.; Saffle, J.R.; DeClement, F.A. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J. Burn Care Rehabil. 2003, 24, 42–48. [Google Scholar] [CrossRef]
- Yannas, I.V.; Burke, J.F.; Gordon, P.L.; Huang, C.; Rubenstein, R. Design of an artificial skin. II. Control Chem. Compos. J. Biomed. Mater. Res. 1981, 14, 107–131. [Google Scholar]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, S.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed]
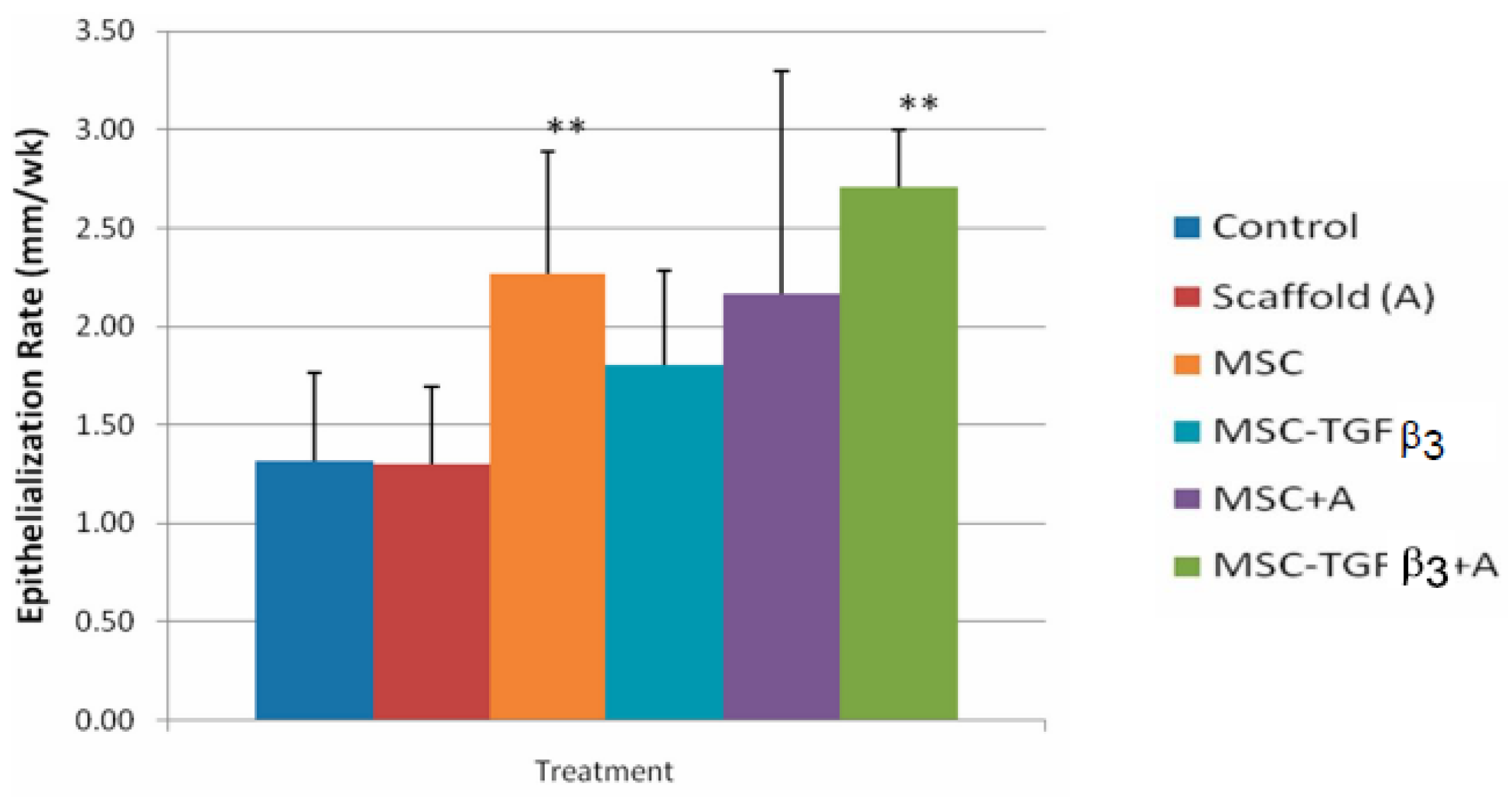
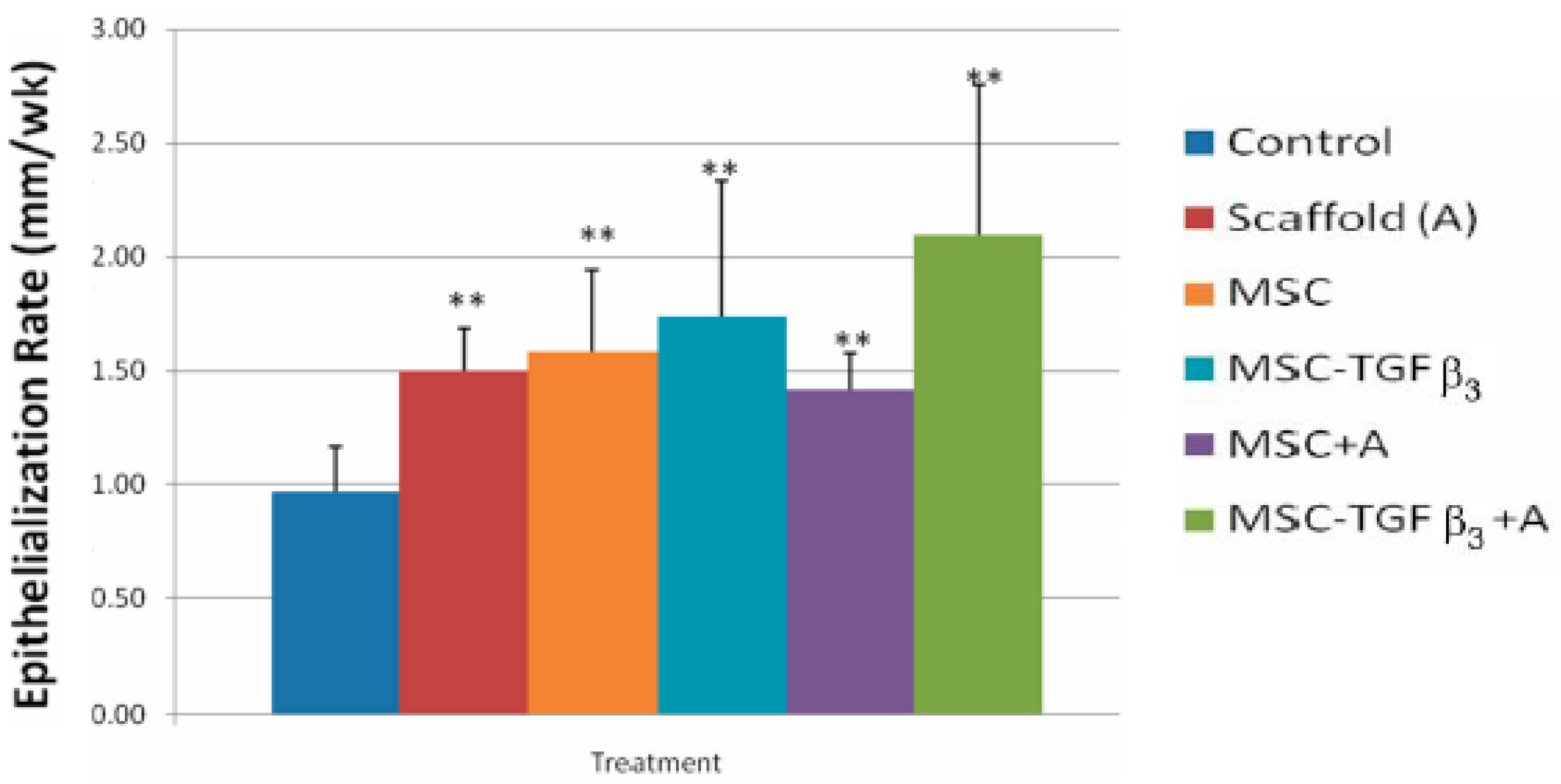
- Feldman, D.S.; McCauley, J.F. Mesenchymal Stem Cells and Transforming Growth Factor-β3 (TGF-β3) to Enhance the Regenerative Ability of an Albumin Scaffold in Full Thickness Wound Healing. J. Funct. Biomater. 2018, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Atala, A.; Irvine, D.J.; Moses, M.; Shaunak, S. Wound Healing vs. Regeneration: Role of the Tissue Environment in Regenerative Medicine. MRS Bull. 2010, 35, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Bely, A.E.; Nyberg, K.G. Evolution of Animal Regeneration: Reemergence of a Field. Trends Ecol. Evol. 2009, 25, 131–198. [Google Scholar]
- Morrell, V. Why Do Animals Sometimes Kill Their Babies? 28 March 2014. Available online: https://www.nationalgeographic.com/ (accessed on 3 April 2022).
- Shieh, S.J.; Cheng, T.C. Regeneration and repair of human digits and limbs: Fact and fiction. Regeneration 2015, 2, 149–168. [Google Scholar] [CrossRef]
- Feldman, D. Regeneration vs. Scarring: Did Evolution Get it Right? Curr. Trends Biomed. Eng. Biosci. 2018, 12, 55. [Google Scholar] [CrossRef]
- Goss, R. Tissue Repair in the Mammalian Fetus. In Wound Healing: Biochemical and Clinical Aspects; Cohen, I., Diegelmann, R., Lindblad, W., Eds.; W.B. Saunders, Co.: Philadelphia, PA, USA, 1992; pp. 326–343. [Google Scholar]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Rep. Reg. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanism of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Shanbhag, A.; Jacobs, J.; Black, J.; Galante, J.; Glant, T. Macrophage/particle interaction: Effect of size, composition, and surface area. J. Biomed. Mater. Res. 1994, 28, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, O.; Smith, R.; Goodman, S. Effect of size, concentration, surface area, and volume of polymethylmethacrylate particles on human macrophages in vitro. J. Biomed. Mater. Res. 1996, 30, 463–473. [Google Scholar] [CrossRef]
- Nagse, M. Host reactions to particulate biomaterials. In Encyclopedic Handbook of Biomaterials and Bioengineering; Wise, D., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 269–303. [Google Scholar]
- Mooney, D.; Langer, S. Engineering Biomaterials for Tissue Engineering: The 10–100 Micron Size Scale. In The Biomedical Engineering Handbook; Bronzino, J., Ed.; CRC Press: Hartford, CT, USA, 1995; pp. 1609–1617. [Google Scholar]
- Tenenhaus, M.; Rennekamoff, H.O. Current Concepts in Tissue Engineering: Skin Wound Healing. Plast. Reconstruct. Surg. 2016, 138 (Suppl. S3), 42S–50S. [Google Scholar] [CrossRef] [PubMed]
- Whitneey, J.A. Physiologic effects of tissue oxygenation on wound healing. Heart Lung 1989, 18, 466–467. [Google Scholar]
- Knighton, D.R.; Fiegel, V. Macrophage-derived growth factors in wound healing: Regulation of growth factor production by the oxygen microenvironment. Am. Rev. Respir. Dis. 1989, 140, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.K.; Pai, M.P. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg. Gynecol. Obstet. 1972, 135, 561. [Google Scholar] [PubMed]
- Winter, G. Oxygen and epidermal wound healing. Adv. Expl. Med. Biol. 1977, 94, 673–674. [Google Scholar]
- Winter, G.W. Epidermal regeneration studied in the domestic pig. In Epidermal Wound Healing; Maibach, H.I., Rovee, D.T., Eds.; Year Book Publishers: Chicago, IL, USA, 1972; pp. 71–112. [Google Scholar]
- Feldman, D.; Jennings, A. Healing equations. Wounds 2003, 15, 385. [Google Scholar]
- Baranoski, S.; Ayello, E.A. Wound Care Essentials: Practice Principles; Lippincott, Williams, and Wilkins: Philadelphia, PA, USA, 2004; pp. 62–70. [Google Scholar]
- Gibran, N.S.; Boyce, S.; Greenhalgh, D.G. Cutaneous wound healing. J. Burn Care Res. 2007, 28, 577–579. [Google Scholar] [CrossRef]
- Tatara, A.M.; Mikos, A.G. Tissue Engineering in Orthopaedics. J. Bone Jt. Surg. 2016, 98, 1132–1139. [Google Scholar] [CrossRef]
- Fishero, B.A.; Kohli, N.; Das, A.; Christophel, J.J.; Cui, Q. Current Concepts of Bone Tissue Engineering for Craniofacial Bone Defect Repair. J. Bone Jt. Surg. Am. 2016, 98, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Simian, M.; Bissel, M.J. Organoids: A historical perspective of thinking in three dimension. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Feldman, D. Determining Optimal Treatment Design for Full Thickness Wounds. Wound Repair Regen. 2003, 11, 21. [Google Scholar]
- Bowman, J.; Feldman, D. Tissue Adhesives for Growth Factor Delivery, Biomaterials and Bioengineering Handbook; Wise, D., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 261–312. [Google Scholar]
- Feldman, D. Adhesion and Hemostasis in Surgery, Encyclopedia of Materials: Science and Technology; Williams, D., Ed.; Elsevier Science Ltd.: London, UK, 2002; pp. 38–43. [Google Scholar]
- White, R.; McIntosh, C. A review of the literature on topical therapies for diabetic foot ulcers. Part 2: Advanced treatments. J. Wound Care 2009, 18, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Estridge, T.; Feldman, D. The effects of chelated silver on the success of percutaneous devices. In Digest of Papers of the Seventh Southern Biomedical Engineering Conference; Moyle, D., Ed.; McGregor and Werner: Washington, DC, USA, 1988; pp. 192–193. [Google Scholar]
- Feldman, D.; Colaizzo, R.; von Recum, A.; Hultman, S. Electron Microscope Analysis of Tissue Ingrowth into Dacron Velour, Biomedical Engineering II. Recent Developments; Hall, C., Ed.; Pergamon Press: San Antonio, TX, USA, 1983; pp. 275–278. [Google Scholar]
- Feldman, D. Percutaneous Implants: Histological Interface Study. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 1982. [Google Scholar]
- Saltz, R.; Sierra, D.; Feldman, D.; Saltz, M.; Dimick, A.; Vasconez, L. Experimental and clinical applications of fibrin glue. Plast. Reconstruct. Surg. 1991, 88, 1005–1015. [Google Scholar] [CrossRef]
- Feldman, D.S.; Osborne, D. Fibrin as a Tissue Adhesive and Scaffold with an Angiogenic Agent (FGF-1) to Enhance Burn Graft Healing In Vivo and Clinically. J. Funct. Biomater. 2018, 9, 68. [Google Scholar] [CrossRef]
- von Recum, A.; Park, J. Permanent percutaneous devices. CRC Crit. Rev. Bioeng. 1981, 11, 37. [Google Scholar]
- McCullars, J.; Moore, S.; Jennings, A.; Feldman, D. Local versus systemic delivery of endothelial progenitor cells for a tissue scaffold. Trans. Soc. Biomater. 2006, 31, 588. [Google Scholar]
- Feldman, D.; Sierra, D. Tissue adhesives in wound healing. In Encyclopedic Handbook of Biomaterials and Bioengineering; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Greisler, H.; Kim, D. Aspects of biodegradable vascular prosthesis. In Vascular Graft Update; Kahalic, H., Kantrowitz, A., Sung, P., Eds.; ASTM: Fairfield, PA, USA, 1986; pp. 197–218. [Google Scholar]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- Nakagawa, H.; Akita, S.; Fukui, M.; Fujii, T.; Akino, K. Human mesenchymal stem cells successfully improve skin- substitute wound healing. Br. J. Dermatol. 2005, 153, 29–36. [Google Scholar] [CrossRef]
- McFarlin, K.; Gao, X.; Liu, Y.B.; Dulchavsky, D.S.; Kwon, D.; Arbab, A.S.; Bansal, M.; Li, Y.; Chopp, M.; Dulchavsky, S.A.; et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Rep. Reg. 2006, 14, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Badiavas, E.V.; Falanga, V. Treatment of chronic wounds with bone marrow-derived cells. Arch. Dermatol. 2003, 139, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.I.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell- based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 80, 2581–2587. [Google Scholar] [CrossRef]
- Fathke, C.; Wilson, L.; Hutter, J.; Kapoor, V.; Smith, A.; Hocking, A.; Isik, F. Contribution of bone marrow-derived cells to skin: Collagen deposition and wound repair. Stem Cells 2004, 22, 812–822. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Phys. 2007, 213, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Weinand, C.; Xu, J.W.; Peretti, G.M.; Bonassar, L.J.; Gill, T.J. Conditions affecting cell seeding onto three-dimensional scaffolds for cellular-based biodegradable implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 80–87. [Google Scholar] [CrossRef]
- Estridge, T.D. The Use of Oxygen, Growth Factors and Implants to Effect Cellular Activity In Vitro. Ph.D. Thesis, University of Alabama at Birmingham, Birmingham, AL, USA, 1991. [Google Scholar]
- Estridge, T.; Feldman, D. The use of oxygen for optimal fibroblast activation. Trans. FASEB 1991, 75, 7230. [Google Scholar]
- Pandit, A.; Feldman, D. The effect of oxygen permeability on full-thickness skin defects. Wound Heal. Regen. 1994, 2, 130–137. [Google Scholar] [CrossRef]
- Pandit, A.; Wilson, D.; Feldman, D.; Thompson, J. Fibrin scaffold as an effective vehicle for the delivery of acidic fibroblast growth factor (FGF-1). J. Biomater. Appl. 2000, 14, 229–242. [Google Scholar] [CrossRef]
- Andino, R. The Use of a Low Frequency PEMF in the Treatment of Full Thickness Skin Defects in the Rabbit Model. Master’s Thesis, University of Alabama at Birmingham, Birmingham, AL, USA, 1991. [Google Scholar]
- Andino, R.; Feldman, D. Pulsating electromagnetic fields used to treat full thickness defects in the rabbit model. Trans. FASEB 1991, 5, 5. [Google Scholar]
- Kelpke, S. Pulsed Electromagnetic Field to Accelerate Wound Healing. Master’s Thesis, University of Alabama at Birmingham, Birmingham, AL, USA, 1994. [Google Scholar]
- Feldman, D.S. The Feasibility of Using Pulsatile Electromagnetic Fields (PEMFs) to Enhance the Regenerative Ability of Dermal Biomaterial Scaffolds. J. Funct. Biomater. 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, A.; Chen, D.; Feldman, D. Upregulation of chemokine (C-C motif) ligand 20 in adult epidermal keratinocytes in direct current electric fields. Arch. Dermatol. Res. 2009, 302, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Chen, D.; Feldman, D. Transcriptional response of dermal fibroblasts in direct current electric fields. Bioelectromagnetics 2008, 29, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Feldman, D. Electric Field-induced gene expression in skin cells. Wound Repair Regen. 2006, 14, A52. [Google Scholar]
- Jennings, A.; Andino, R.; Feldman, D. Preliminary evaluation of an electrical stimulation bandage (Posifect Dressing). Trans. Congr. Ger. Soc. Wound Heal. Wound Treat. 2005, 9, 224. [Google Scholar]
- Jennings, A.; Andino, R.; Feldman, D. Clinical evaluation of an electrical stimulation bandage (Posifect Dressing). Wound Repair Regen. 2005, 13, A13. [Google Scholar]
- Assimacopoulos, D. Wound healing promotion by the use of negative electrical current. Am. Surg. 1968, 34, 423–431. [Google Scholar]
- Alvarez, O.M.; Mertz, P.M.; Smerbeck, R.V.; Eaglstein, W.H. The healing of superficial skin wounds is stimulated by the external electrical current. J. Investig. Dermatol. 1983, 81, 144–148. [Google Scholar] [CrossRef]
- Assimacopoulos, D. Low intensity negative electric current in the treatment of ulcers of the leg due to chronic venous insufficiency. Am. J. Surg. 1968, 115, 683–687. [Google Scholar] [CrossRef]
- Wolcott, L.E.; Wheeler, P.C.; Hardwicke, H.M.; Rowley, B.S. Accelerated healing of skin ulcers by electrotherapy: Preliminary clinical results. South. Med. J. 1969, 62, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Gault, W.R.; Gatens, P.F. Use of low intensity direct current in management of ischemic skin ulcers. Phys. Ther. 1976, 56, 265. [Google Scholar] [CrossRef] [PubMed]
- Carley, P.F.J.; Wainapel, S.F. Electrotherapy for acceleration of wound healing: Low intensity direct current. Arch. Phys. Med. Rehabil. 1985, 66, 443–446. [Google Scholar]
- Gardner, S.E.; Frantz, R.A.; Schmidt, F.L. Effect of electrical stimulation on chronic wound healing: A meta-analysis. Wound Repair Regen. 1997, 7, 95–503. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.C.; Lepley, D. Effect of continuous direct electric current on healing wounds. Surg. Forum 1962, 13, 33. [Google Scholar] [PubMed]
- Goldin, J.H.; Broadbent, N.; Nancarrow, J.D.; Marshall, T. The effects of Diapulse on the healing of wounds: A double-blind randomized controlled trial in man. Br. J. Plast. Surg. 1981, 34, 267–270. [Google Scholar] [CrossRef]
- Ieran, M.; Zaffuto, S.; Bagnacani, M.; Annovi, M.; Moratti, A.; Cadossi, R. Effect of low frequency pulsing electromagnetic fields on skin ulcers of venous origin in humans: A double blind study. J. Orthop. Res. 1990, 8, 276–282. [Google Scholar] [CrossRef]
- Ieran, M.; Zaffuto, S.; Moratti, A.; Bagnacani, M.; Cadossi, R. PEMF stimulation of skin ulcers of venous origin in humans: Preliminary report of a double blind study. J. Bioelectr. 1987, 6, 181–188. [Google Scholar]
- Glassman, L.S.; McGrath, M.H.; Bassett, C.A. Effect of external pulsing electromagnetic fields on the healing of soft tissue. Annu. Plas. Surg. 1986, 16, 287–295. [Google Scholar] [CrossRef]
- Gentzkow, G.D. Electrical stimulation to heal dermal wounds. J. Derm. Surg. Onco. 1993, 19, 753–758. [Google Scholar] [CrossRef]
- Weiss, D.S.; Eaglestein, W.H.; Falanga, V. Pulsed electrical stimulation decreases scar thickness at split-thickness graft donor sites. J. Investig. Dermatol. 1989, 92, 539. [Google Scholar]
- Ashar, R. The Use of FGF-1 and TGF-β in the Treatment of Full-Thickness Skin Defects in the Rabbit Model; UAB: Birmingham, AL, USA, 1993. [Google Scholar]
- Pandit, A.; Ashar, R.; Feldman, D. The effect of TGF-β delivered through a collagen scaffold in stimulation of healing in full-thickness defects. J. Investig. Surg. 1999, 12, 89–100. [Google Scholar]
- Pandit, A.; Ashar, R.; Feldman, D.; Thompson, J. Investigation of acidic fibroblast growth factor delivered through a collagen scaffold for the treatment of full-thickness skin defects, in a rabbit model. Plast. Reconstruct. Surg. 1998, 101, 766–775. [Google Scholar] [CrossRef]
- Pandit, A.; Ashar, R.; Feldman, D. Acidic fibroblast growth factor and transforming growth factor beta in stimulation of healing in full thickness skin defects. In Proceedings of the Annual Meeting of the Wound Healing Society and the European Tissue Repair Society, Amsterdam, The Netherlands, 22–25 August 1993. [Google Scholar]
- Feldman, D. Wound healing applications of fibrin sealants. In Surgical Tissue Adhesives and Sealants; Sierra, D., Saltz, R., Eds.; Technomic: Lancaster, PA, USA, 1996; pp. 99–108. [Google Scholar]
- Ronfard, V.; Broly, H.; Mitchell, V.; Galizia, J.P.; Hochart, D.; Chambon, E.; Pellerin, P.; Huart, J.J. Use of human keratinocytes cultured on fibrin glue in the treatment of burn wounds. Burns 1991, 17, 181–184. [Google Scholar] [CrossRef]
- Dahlstrom, K.K.; Weis-Fogh, U.S.; Medgyesi, S.; Rostgaard, J.; Sorenson, H. The use of autologous fibrin adhesive in skin transplantation. Plast. Reconstr. 1992, 89, 968–972. [Google Scholar] [CrossRef]
- Brown, D.M.; Barton, B.R.; Young, V.L.; Pruitt, B.A. Decreased wound contraction with fibrin glue-treated skin grafts. Arch. Surg. 1992, 127, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Redden, R.; Blum, B.; Osborne, S. Porous fibrin as a degradable adhesive and drug delivery system. Trans. Soc. Biomater. 1997, 23, 185. [Google Scholar]
- Pandit, A.; Feldman, D.; Caufield, J.; Thompson, J. Stimulation of angiogenesis by FGF-1 delivered through a modified fibrin scaffold. Growth Factors 1998, 15, 113–123. [Google Scholar] [CrossRef]
- Pandit, A.; Caufield, J.; Feldman, D. In vivo wound healing response to a modified degradable fibrin scaffold. J. Biomater. Appl. 1997, 12, 222–236. [Google Scholar] [CrossRef]
- Pandit, A.; Feldman, D.; Listinsky, K.; Thompson, J. The effect on wound healing by a modified fibrin scaffold delivering acidic fibroblast growth factor (FGF-1). J. Bioact. Compat. Polym. 1997, 12, 99. [Google Scholar] [CrossRef]
- Pandit, A. Fibrin as a matrix for FGF-1 delivery in vivo. Ph.D Thesis, University of Alabama at Birmingham, Birmingham, AL, USA, 1998. [Google Scholar]
- Flahiff, C.; Feldman, D.; Saltz, R.; Huang, S. Mechanical testing of fibrin adhesives for blood vessel anastomosis. J. Biomater. Mater. Res. 1992, 26, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Sierra, D.; Feldman, D.; Saltz, R. A method to determine the shear adhesive strength of fibrin sealants. Appl. Biomater. 1992, 3, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Reddy, M.; Geurs, N.; Palcanis, K.; Lemons, J.; Rahemtulla, F.; Ho, K.; Chen, D.J.; Davis, C.; Feldman, D. Efficacy of platelet-rich plasma on wound healing in rabbits. J. Peridontol. 2008, 25, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, C. Fibrin sealants for haemostasis and drug delivery. Lancet 1997, 349, 334. [Google Scholar] [CrossRef]
- Huang, S.; Kilpadi, D.; Feldman, D. A comparison of the shear strength of fibrin and albumin glues. Trans. Wound Health Soc. 1997, 7, 63. [Google Scholar]
- Slimane, S.B.; Guidoin, R.; Mourad, W.; Hebert, J.; King, M.W.; Sigot-Luizard, M.F. Polyester arterial grafts impregnated with cross-linked albumin: The rate of degradation of the coating in vivo. Eur. J. Surg. Res. 1998, 20, 12–17. [Google Scholar] [CrossRef]
- Slimane, S.B.; Guidoin, R.; Marceau, D.; Merhi, Y.; King, M.W.; Sigot-Luizard, M.F. Characteristics of polyester arterial grafts coated with albumin: The role and importance of the cross-linking chemicals. Eur. J. Surg. Res. 1998, 20, 18–28. [Google Scholar] [CrossRef]
- Slimane, S.B.; Guidoin, R.; Merhi, Y.; King, M.W.; Domurado, D.; Sigot-Luizard, M.F. In vivo evaluation of polyester arterial grafts coated with albumin: The role and importance of cross-linking agents. Eur. J. Surg. Res. 1988, 20, 66–74. [Google Scholar] [CrossRef]
- Chafke, N.; Gasser, B.; Lindner, V.; Rouyer, N.; Rooke, R.; Kretz, J.G.; Nicolini, P.; Eisenmann, B. Albumin as a sealant for a polyester vascular prosthesis: Its impact on the healing sequence in humans. J. Cardiovasc. Surg. 1996, 37, 431–440. [Google Scholar]
- An, Y.H.; Stuart, G.W.; McDowell, S.J.; McDaniel, S.E.; Kang, Q.; Friedman, R.J. Prevention of bacterial adherence to implant surfaces with a crosslinked albumin coating in vivo. J. Orthop. Res. 1996, 14, 846–849. [Google Scholar] [CrossRef]
- An, H.; Bradley, J.; Powers, D.L.; Friedman, R.J. Preventing prosthetic infection using a crosslinked albumin coating in vivo. In Proceedings of the 5th World Biomaterials Conference, Toronto, ON, Canada, 29 May–2 June 1996. [Google Scholar]
- Overby, R.J.; Feldman, D.S. Influence of Poly(Ethylene Glycol) End Groups on Poly(Ethylene Glycol)-Albumin System Properties as a Potential Degradable Tissue Scaffold. J. Funct. Biomater. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Becker, R. The bioelectric factors in amphibian limb regeneration. J. Bone Jt. Surg. 1961, 43, 643–656. [Google Scholar] [CrossRef]
- Regeneration: Better In Vitro or In Vivo, Panel Discussion; SFB: Mount Laurel, NJ, USA, 2019.

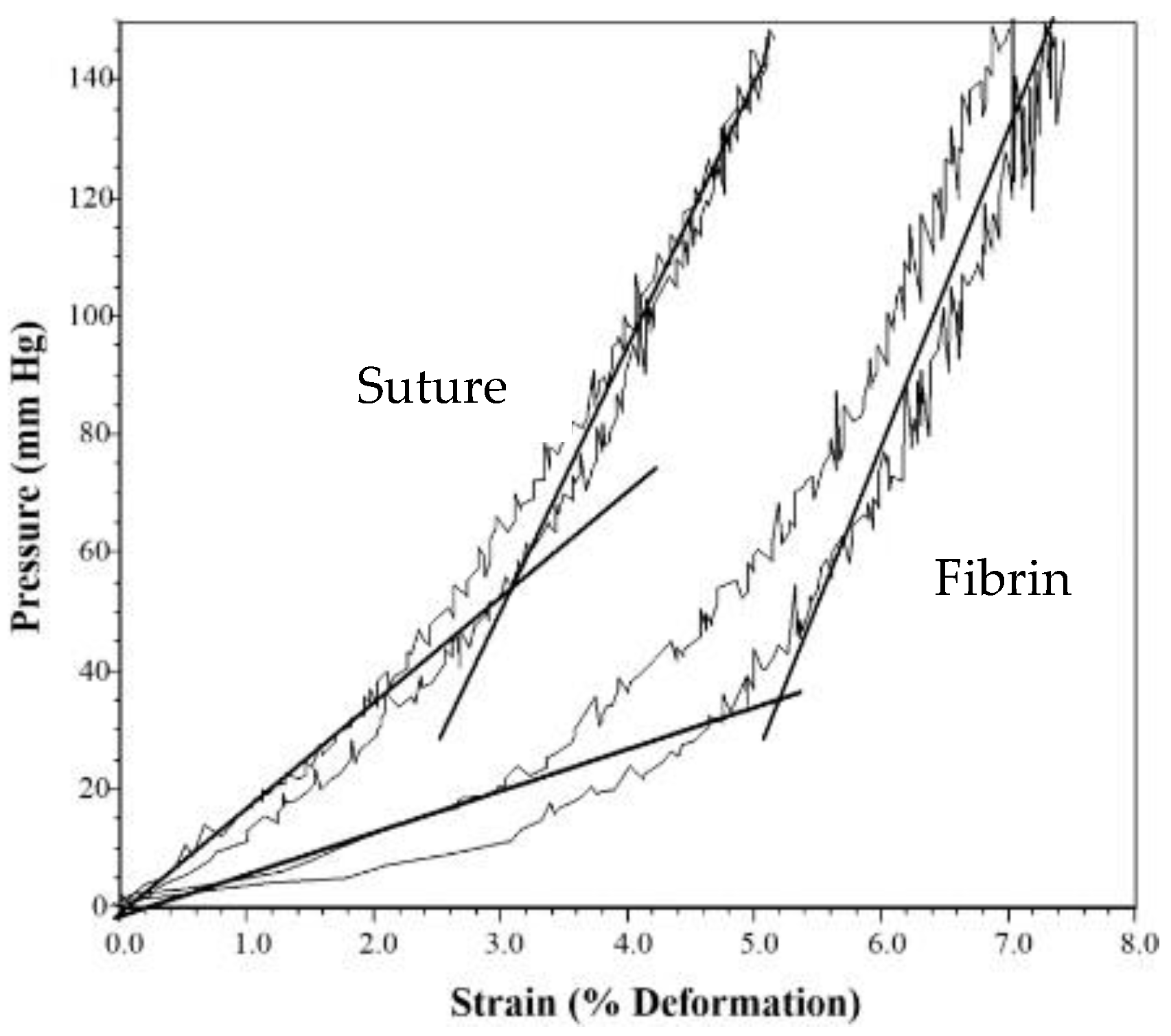
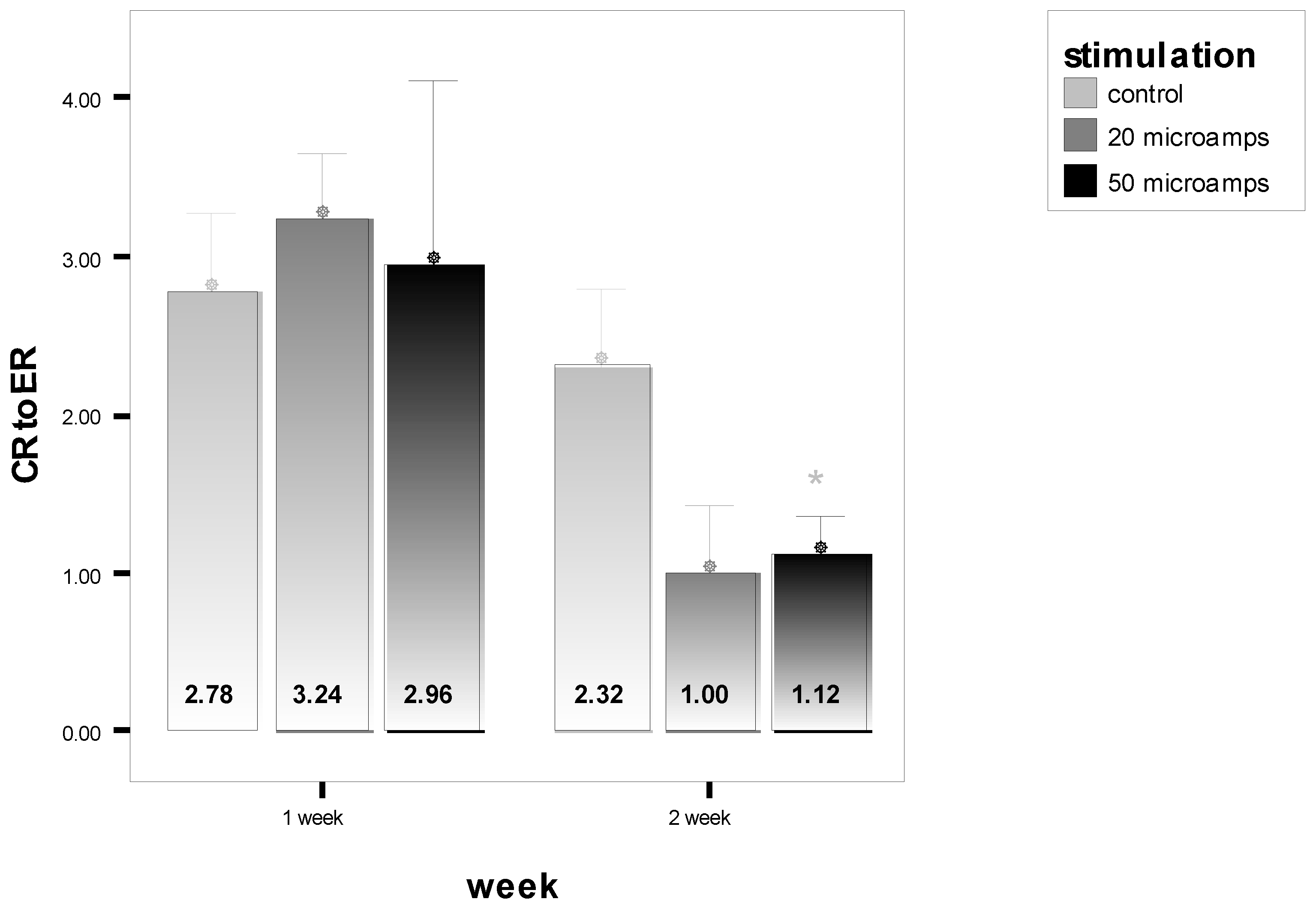

| 3 Day | 10 Day | |||
|---|---|---|---|---|
| F | N = 5 | 63.0 ± 28.5 | N = 5 | 57.4 ± 43.9 |
| FW | N = 5 | 34.4 ± 15.7 * | N = 5 | 51.6 ± 29.6 |
| PF | N = 4 | 46.34 ± 32.1 | N = 5 | 30.5 ± 21.6 |
| PFW | N = 5 | 54.1 ± 32.1 | N = 5 | 54.4 ± 35.2 |
| S | N = 5 | 101.5 ± 41.8 * | N = 5 | 123.3 ± 142.5 |
| NL | N = 3 | 56.0 ± 23.9 | N = 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldman, D. Designing a Biomaterial Approach to Control the Adaptive Response to a Skin Injury. Materials 2022, 15, 6366. https://doi.org/10.3390/ma15186366
Feldman D. Designing a Biomaterial Approach to Control the Adaptive Response to a Skin Injury. Materials. 2022; 15(18):6366. https://doi.org/10.3390/ma15186366
Chicago/Turabian StyleFeldman, Dale. 2022. "Designing a Biomaterial Approach to Control the Adaptive Response to a Skin Injury" Materials 15, no. 18: 6366. https://doi.org/10.3390/ma15186366
APA StyleFeldman, D. (2022). Designing a Biomaterial Approach to Control the Adaptive Response to a Skin Injury. Materials, 15(18), 6366. https://doi.org/10.3390/ma15186366





