Enzyme-Triggered Crosslinked Hybrid Hydrogels for Bone Tissue Engineering
Abstract
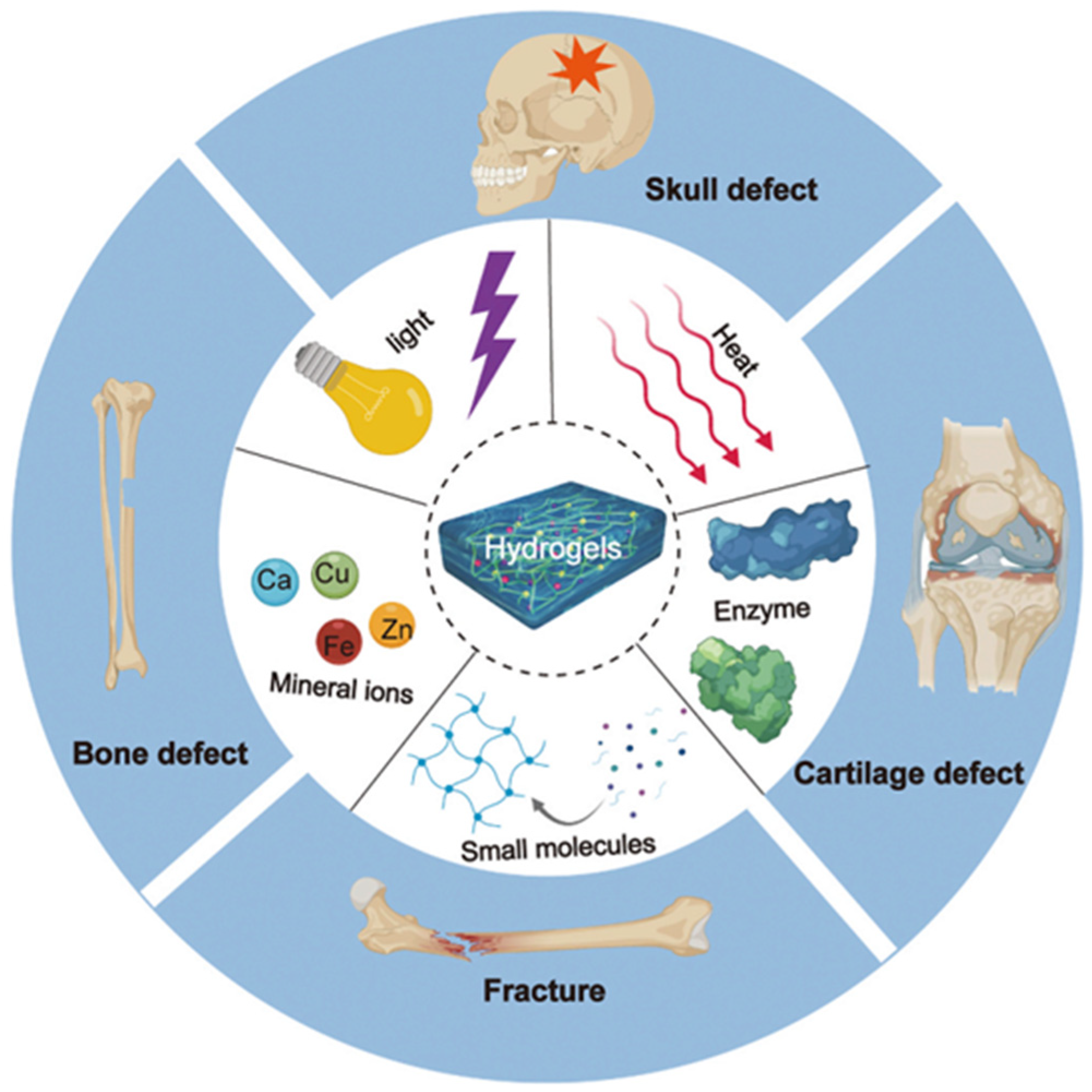
:1. Introduction
2. Hybrid Hydrogels
3. Various Crosslinking Approaches for Developing Hybrid Hydrogels
3.1. Physically Crosslinked Hydrogels
3.2. Chemically Crosslinked Hydrogels
4. Enzyme Crosslinking Approaches for Bone Tissue Engineering
4.1. Tyrosinase
4.2. Lysozyme
4.3. Horseradish Peroxidase
4.4. Transglutaminase (TG)
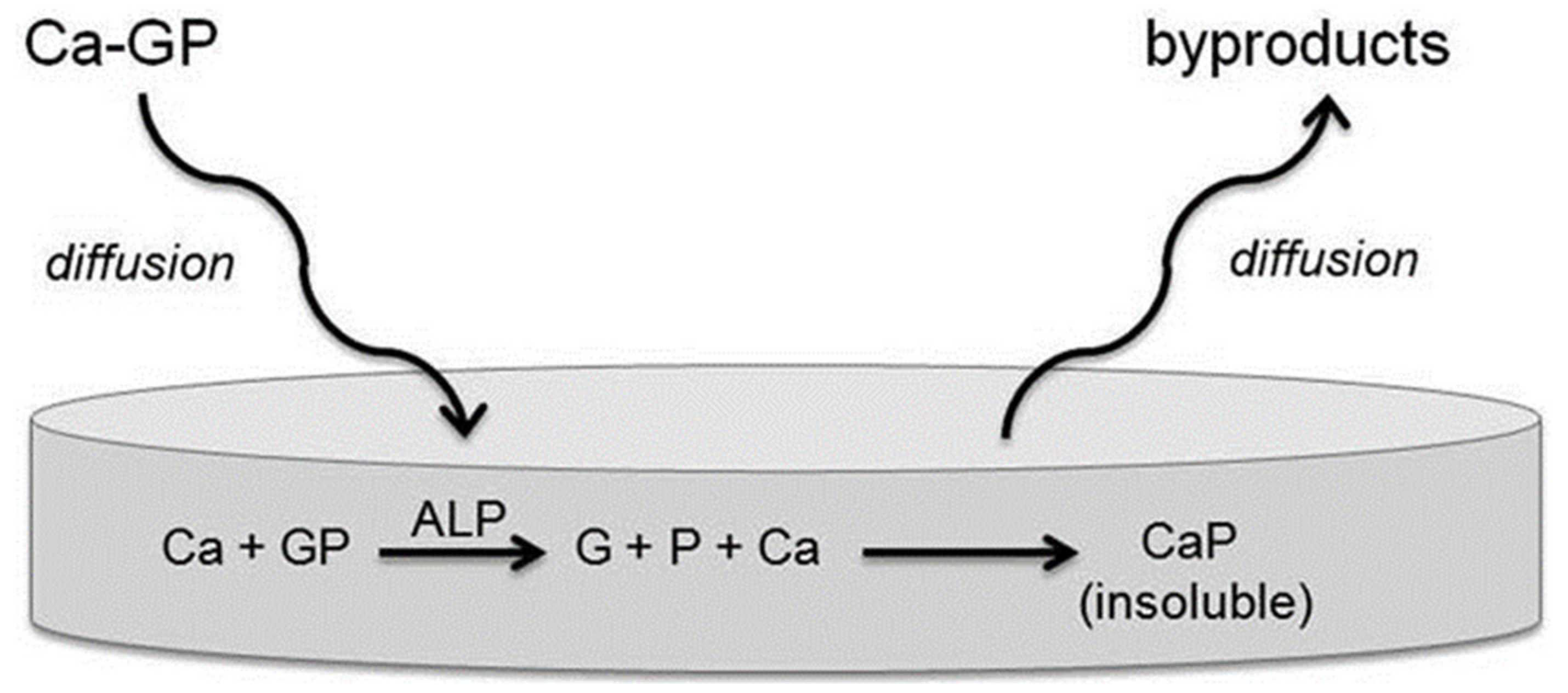
4.5. Alkaline Phosphatase (ALP)
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Echave, M.C.; Pimenta-Lopes, C.; Pedraz, J.L.; Mehrali, M.; Dolatshahi-Pirouz, A.; Ventura, F.; Orive, G. Enzymatic crosslinked gelatin 3D scaffolds for bone tissue engineering. Int. J. Pharm. 2019, 562, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Hayrapetyan, A.; Jansen, J.A.; van den Beucken, J.J. Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng. Part B Rev. 2015, 21, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Pathria, M.N.; Chung, C.B.; Resnick, D.L. Acute and Stress-related Injuries of Bone and Cartilage: Pertinent Anatomy, Basic Biomechanics, and Imaging Perspective. Radiology 2016, 280, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Rudert, M.; Hutmacher, D.W. Scaffold-based Bone Tissue Engineering]. Orthopade 2017, 46, 701–710. [Google Scholar] [CrossRef]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Xia, B.; Deng, Y.; Lv, Y.; Chen, G. Stem cell recruitment based on scaffold features for bone tissue engineering. Biomater. Sci. 2021, 9, 1189–1203. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Angelova Volponi, A.; Higgins, C.A.; Sharpe, P.T.; Celiz, A.D. Scaffold-based developmental tissue engineering strategies for ectodermal organ regeneration. Mater. Today Bio 2021, 10, 100107. [Google Scholar] [CrossRef]
- Lemos, R.; Maia, F.R.; Reis, R.L.; Oliveira, J.M. Engineering of Extracellular Matrix-Like Biomaterials at Nano- and Macroscale toward Fabrication of Hierarchical Scaffolds for Bone Tissue Engineering. Adv. NanoBiomed. Res. 2022, 2, 2100116. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, S.H.; Kim, M.G.; Park, S.H.; Yoo, T.H.; Kim, M.S. Biomimetic Scaffolds for Bone Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1064, 109–121. [Google Scholar] [PubMed]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive Nanoengineered Hydrogels for Bone Tissue Engineering: A Growth-Factor-Free Approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Guan, J.; Mao, Y.; Zhou, P. Hydrogel: A potential therapeutic material for bone tissue engineering. AIP Adv. 2021, 11, 010701. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Poly (vinyl alcohol)-alginate as potential matrix for various applications: A focused review. Carbohydr. Polym. 2022, 277, 118881. [Google Scholar] [CrossRef]
- Sood, A.; Kumar, A.; Dev, A.; Gupta, V.K.; Han, S.S. Advances in Hydrogel-Based Microfluidic Blood-Brain-Barrier Models in Oncology Research. Pharmaceutics 2022, 14, 993. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Mechanically viscoelastic nanoreinforced hybrid hydrogels composed of polyacrylamide, sodium carboxymethylcellulose, graphene oxide, and cellulose nanocrystals. Carbohyd. Polym. 2018, 193, 228–238. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Gupta, S.; Pramanik, A.K.; Kailath, A.; Mishra, T.; Guha, A.; Nayar, S.; Sinha, A. Composition dependent structural modulations in transparent poly(vinyl alcohol) hydrogels. Colloids Surf. B Biointerfaces 2009, 74, 186–190. [Google Scholar] [CrossRef]
- Zhao, X.; Huebsch, N.; Mooney, D.J.; Suo, Z. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J. Appl. Phys. 2010, 107, 063509. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ko, J.; Choi, Y.H.; Hwang, N.S. Recent advancements in enzyme-mediated crosslinkable hydrogels: In vivo-mimicking strategies. APL Bioeng. 2021, 5, 021502. [Google Scholar] [CrossRef] [PubMed]
- Sofia, S.J.; Singh, A.; Kaplan, D.L. Peroxidase-catalyzed crosslinking of functionalized polyaspartic acid polymers. J. Macromol. Sci. Part A 2002, 39, 1151–1181. [Google Scholar] [CrossRef]
- Badali, E.; Hosseini, M.; Mohajer, M.; Hassanzadeh, S.; Saghati, S.; Hilborn, J.; Khanmohammadi, M. Enzymatic crosslinked hydrogels for biomedical application. Polym. Sci. Ser. A 2022, 63, S1–S22. [Google Scholar] [CrossRef]
- Nie, K.; Han, S.; Yang, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. Enzyme-Crosslinked Electrospun Fibrous Gelatin Hydrogel for Potential Soft Tissue Engineering. Polymers 2020, 12, 1977. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and development of hybrid hydrogels for biomedical applications: Recent trends in anticancer drug delivery and tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Jia, X.; Kiick, K.L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef]
- Patel, D.; Sharma, S.; Screen, H.R.; Bryant, S.J. Effects of cell adhesion motif, fiber stiffness, and cyclic strain on tenocyte gene expression in a tendon mimetic fiber composite hydrogel. Biochem. Biophys. Res. Commun. 2018, 499, 642–647. [Google Scholar] [CrossRef]
- Joseph, C.A.; McCarthy, C.W.; Tyo, A.G.; Hubbard, K.R.; Fisher, H.C.; Altscheffel, J.A.; He, W.; Pinnaratip, R.; Liu, Y.; Lee, B.P. Development of an injectable nitric oxide releasing poly (ethylene) glycol-Fibrin adhesive hydrogel. ACS Biomater. Sci. Eng. 2018, 5, 959–969. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Voorhaar, L.; Hoogenboom, R. Supramolecular polymer networks: Hydrogels and bulk materials. Chem. Soc. Rev. 2016, 45, 4013–4031. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, Y.; Zhang, S.; Yu, H.; Duan, P. A Hydrogen Bonds-Crosslinked Hydrogels with Self-Healing and Adhesive Properties for Hemostatic. Front. Bioeng. Biotechnol. 2022, 537, 855013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, H.; Wang, H.; Xu, Z.; Chen, X.; Liu, B.; Shi, Y.; Lu, Y.; Wen, L.; Li, Y. Radiopaque highly stiff and tough shape memory hydrogel microcoils for permanent embolization of arteries. Adv. Funct. Mater. 2018, 28, 1705962. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, S.; Zeng, H.; Liu, J.; Li, B.; Xu, Y.; Zhao, X.; Chen, G. Dynamic intermolecular interactions through hydrogen bonding of water promote heat conduction in hydrogels. Mater. Horiz. 2020, 7, 2936–2943. [Google Scholar] [CrossRef]
- Wanasingha, N.; Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Polyelectrolyte gels: Fundamentals, fabrication and applications. Gels 2021, 7, 148. [Google Scholar] [CrossRef]
- Song, H.; Sun, Y.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Hydrogen-bonded network enables polyelectrolyte complex hydrogels with high stretchability, excellent fatigue resistance and self-healability for human motion detection. Compos. Part B Eng. 2021, 217, 108901. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, Z.; Wei, Z.; Tian, L. Hydrophobic interaction: A promising driving force for the biomedical applications of nucleic acids. Adv. Sci. 2020, 7, 2001048. [Google Scholar] [CrossRef]
- Puza, F.; Zheng, Y.; Han, L.; Xue, L.; Cui, J. Physical entanglement hydrogels: Ultrahigh water content but good toughness and stretchability. Polym. Chem. 2020, 11, 2339–2345. [Google Scholar] [CrossRef]
- Ji, S.; Li, X.; Wang, S.; Li, H.; Duan, H.; Yang, X.; Lv, P. Physically Entangled Anti-Swelling Hydrogels with High Stiffness. Macromol. Rapid Commun. 2022, 2200272. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chang, X.; Mao, H.; Zhou, J.; Wu, Z.L.; Shan, G.; Bao, Y.; Pan, P. Stereocomplexed physical hydrogels with high strength and tunable crystallizability. Soft Matter 2017, 13, 8502–8510. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cao, H.; Yuan, W.; Bao, Y.; Shan, G.; Wu, Z.L.; Pan, P. Stereocomplexed and homocrystalline thermo-responsive physical hydrogels with a tunable network structure and thermo-responsiveness. J. Mater. Chem. B 2020, 8, 7947–7955. [Google Scholar] [CrossRef] [PubMed]
- Charlet, A.; Lutz-Bueno, V.; Mezzenga, R.; Amstad, E. Shape retaining self-healing metal-coordinated hydrogels. Nanoscale 2021, 13, 4073–4084. [Google Scholar] [CrossRef]
- Gabrielli, V.; Baretta, R.; Pilot, R.; Ferrarini, A.; Frasconi, M. Insights into the Gelation Mechanism of Metal-Coordinated Hydrogels by Paramagnetic NMR Spectroscopy and Molecular Dynamics. Macromolecules 2022, 55, 450–461. [Google Scholar] [CrossRef]
- Kim, S.; Regitsky, A.U.; Song, J.; Ilavsky, J.; McKinley, G.H.; Holten-Andersen, N. In situ mechanical reinforcement of polymer hydrogels via metal-coordinated crosslink mineralization. Nat. Commun. 2021, 12, 667. [Google Scholar] [CrossRef]
- Li, F.; Zhu, Y.; You, B.; Zhao, D.; Ruan, Q.; Zeng, Y.; Ding, C. Smart Hydrogels Co-switched by Hydrogen Bonds and π–π Stacking for Continuously Regulated Controlled-Release System. Adv. Funct. Mater. 2010, 20, 669–676. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Liu, J. π–π stacking interaction: A nondestructive and facile means in material engineering for bioapplications. Cryst. Growth Des. 2018, 18, 2765–2783. [Google Scholar] [CrossRef]
- Gaar, J.; Naffa, R.; Brimble, M. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org. Chem. Front. 2020, 7, 2789–2814. [Google Scholar] [CrossRef]
- Emami, Z.; Ehsani, M.; Zandi, M.; Daemi, H.; Ghanian, M.-H.; Foudazi, R. Modified hydroxyapatite nanoparticles reinforced nanocomposite hydrogels based on gelatin/oxidized alginate via Schiff base reaction. Carbohyd. Polym. Technol. Appl. 2021, 2, 100056. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Preparation of covalently bonded silica-alginate hybrid hydrogels by SCHIFF base and sol-gel reactions. Carbohyd. Polym. 2021, 267, 118186. [Google Scholar] [CrossRef] [PubMed]
- Pupkaite, J.; Rosenquist, J.; Hilborn, J.N.; Samanta, A. Injectable shape-holding collagen hydrogel for cell encapsulation and delivery cross-linked using thiol-michael addition click reaction. Biomacromolecules 2019, 20, 3475–3484. [Google Scholar] [CrossRef] [PubMed]
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated polymeric hydrogels for biomedical application: Cross-linking mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef]
- Wang, S.; Nawale, G.N.; Oommen, O.P.; Hilborn, J.; Varghese, O.P. Influence of ions to modulate hydrazone and oxime reaction kinetics to obtain dynamically cross-linked hyaluronic acid hydrogels. Polym. Chem. 2019, 10, 4322–4327. [Google Scholar] [CrossRef]
- Collins, J.; Xiao, Z.; Müllner, M.; Connal, L.A. The emergence of oxime click chemistry and its utility in polymer science. Polym. Chem. 2016, 7, 3812–3826. [Google Scholar] [CrossRef]
- Bai, X.; Lü, S.; Cao, Z.; Ni, B.; Wang, X.; Ning, P.; Ma, D.; Wei, H.; Liu, M. Dual crosslinked chondroitin sulfate injectable hydrogel formed via continuous Diels-Alder (DA) click chemistry for bone repair. Carbohyd. Polym. 2017, 166, 123–130. [Google Scholar] [CrossRef]
- Cadamuro, F.; Russo, L.; Nicotra, F. Biomedical Hydrogels Fabricated Using Diels–Alder Crosslinking. Eur. J. Org. Chem. 2021, 2021, 374–382. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Synthesis of mechanically stiff and bioactive hybrid hydrogels for bone tissue engineering applications. Chem. Eng. J. 2017, 317, 119–131. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. Enhanced mechanical, biomineralization, and cellular response of nanocomposite hydrogels by bioactive glass and halloysite nanotubes for bone tissue regeneration. Mater. Sci. Eng. C 2021, 128, 112236. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Hedstrom, L. Enzyme Specificity and Selectivity. eLS 2010. [Google Scholar] [CrossRef]
- Choi, S.; Ahn, H.; Kim, S.-H. Tyrosinase-mediated hydrogel crosslinking for tissue engineering. J. Appl. Polym. Sci. 2022, 139, 51887. [Google Scholar] [CrossRef]
- Chen, T.; Embree, H.D.; Brown, E.M.; Taylor, M.M.; Payne, G.F. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003, 24, 2831–2841. [Google Scholar] [CrossRef]
- Chen, S.; Gil, C.J.; Ning, L.; Jin, L.; Perez, L.; Kabboul, G.; Tomov, M.L.; Serpooshan, V. Adhesive Tissue Engineered Scaffolds: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 683079. [Google Scholar] [CrossRef]
- Sousa, M.P.; Mano, J.F. Cell-Adhesive Bioinspired and Catechol-Based Multilayer Freestanding Membranes for Bone Tissue Engineering. Biomimetics 2017, 2, 19. [Google Scholar] [CrossRef]
- Mishra, D.; Bhunia, B.; Banerjee, I.; Datta, P.; Dhara, S.; Maiti, T.K. Enzymatically crosslinked carboxymethyl–chitosan/gelatin/nano-hydroxyapatite injectable gels for in situ bone tissue engineering application. Mater. Sci. Eng. C 2011, 31, 1295–1304. [Google Scholar] [CrossRef]
- Sharma, A.; Desando, G.; Petretta, M.; Chawla, S.; Bartolotti, I.; Manferdini, C.; Paolella, F.; Gabusi, E.; Trucco, D.; Ghosh, S.; et al. Investigating the Role of Sustained Calcium Release in Silk-Gelatin-Based Three-Dimensional Bioprinted Constructs for Enhancing the Osteogenic Differentiation of Human Bone Marrow Derived Mesenchymal Stromal Cells. ACS Biomater. Sci. Eng. 2019, 5, 1518–1533. [Google Scholar] [CrossRef]
- Chameettachal, S.; Midha, S.; Ghosh, S. Regulation of Chondrogenesis and Hypertrophy in Silk Fibroin-Gelatin-Based 3D Bioprinted Constructs. ACS Biomater. Sci. Eng. 2016, 2, 1450–1463. [Google Scholar] [CrossRef]
- Das, S.; Pati, F.; Chameettachal, S.; Pahwa, S.; Ray, A.R.; Dhara, S.; Ghosh, S. Enhanced Redifferentiation of Chondrocytes on Microperiodic Silk/Gelatin Scaffolds: Toward Tailor-Made Tissue Engineering. Biomacromolecules 2013, 14, 311–321. [Google Scholar] [CrossRef]
- Lim, S.; Jeong, D.; Ki, M.-R.; Pack, S.P.; Choi, Y.S. Tyrosinase-mediated rapid and permanent chitosan/gelatin and chitosan/gelatin/nanohydroxyapatite hydrogel. Korean J. Chem. Eng. 2021, 38, 98–103. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Rasoulianboroujeni, M.; Yadegari, A.; Tajik, S.; Moharamzadeh, K.; Tayebi, L. Osteo-mucosal engineered construct: In situ adhesion of hard-soft tissues. Mater. Sci. Eng. C 2021, 128, 112255. [Google Scholar] [CrossRef] [PubMed]
- Pangburn, S.H.; Trescony, P.V.; Heller, J. Lysozyme degradation of partially deacetylated chitin, its films and hydrogels. Biomaterials 1982, 3, 105–108. [Google Scholar] [CrossRef]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Okuda, D.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 2005, 40, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef]
- Kim, S.; Fan, J.; Lee, C.S.; Lee, M. Dual Functional Lysozyme-Chitosan Conjugate for Tunable Degradation and Antibacterial Activity. ACS Appl. Bio. Mater. 2020, 3, 2334–2343. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.K.; Koo, B.; Zheng, J.; Aghaloo, T.; Lee, M. Chitosan-Lysozyme Conjugates for Enzyme-Triggered Hydrogel Degradation in Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 41138–41145. [Google Scholar] [CrossRef]
- Proctor, V.A.; Cunningham, F.E. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit. Rev. Food Sci. Nutr. 1988, 26, 359–395. [Google Scholar] [CrossRef]
- Tan, H.; Jin, D.; Qu, X.; Liu, H.; Chen, X.; Yin, M.; Liu, C. A PEG-Lysozyme hydrogel harvests multiple functions as a fit-to-shape tissue sealant for internal-use of body. Biomaterials 2019, 192, 392–404. [Google Scholar] [CrossRef]
- Hu, S.; Lu, Q.; Xu, Y. Biosensors based on direct electron transfer of protein. In Electrochemical Sensors, Biosensors and Their Biomedical Applications; Zhang, X., Ju, H., Wang, J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 531–581. [Google Scholar]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Lee, F.; Bae, K.H.; Kurisawa, M. Injectable hydrogel systems crosslinked by horseradish peroxidase. Biomed. Mater. 2015, 11, 014101. [Google Scholar] [CrossRef] [PubMed]
- Carnes, M.E.; Gonyea, C.R.; Mooney, R.G.; Njihia, J.W.; Coburn, J.M.; Pins, G.D. Horseradish Peroxidase-Catalyzed Crosslinking of Fibrin Microthread Scaffolds. Tissue Eng. Part C Methods 2020, 26, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Uchida, K.; Satio, W.; Sekiguchi, H.; Inoue, G.; Miyagi, M.; Takata, K.; Yokozeki, Y.; Takaso, M. Acceleration of bone union by in situ-formed hydrogel containing bone morphogenetic protein-2 in a mouse refractory fracture model. J. Orthop. Surg. Res 2020, 15, 426. [Google Scholar] [CrossRef] [PubMed]
- Jo, B.S.; Lee, Y.; Suh, J.S.; Park, Y.S.; Lee, H.J.; Lee, J.-Y.; Cho, J.; Lee, G.; Chung, C.P.; Park, K.D.; et al. A novel calcium-accumulating peptide/gelatin in situ forming hydrogel for enhanced bone regeneration. J. Biomed. Mater. Res. Part A 2018, 106, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Gonçalves, C.; Shin, M.E.; Lee, S.; Reis, R.L.; Khang, G.; Oliveira, J.M. Enzymatically crosslinked tyramine-gellan gum hydrogels as drug delivery system for rheumatoid arthritis treatment. Drug Deliv. Transl. Res. 2021, 11, 1288–1300. [Google Scholar] [CrossRef]
- Yokoyama, K.; Nio, N.; Kikuchi, Y. Properties and applications of microbial transglutaminase. Appl. Microbiol. Biotechnol. 2004, 64, 447–454. [Google Scholar] [CrossRef]
- Savoca, M.P.; Tonoli, E.; Atobatele, A.G.; Verderio, E.A.M. Biocatalysis by Transglutaminases: A Review of Biotechnological Applications. Micromachines 2018, 9, 562. [Google Scholar] [CrossRef]
- Kuwahara, K.; Yang, Z.; Slack, G.C.; Nimni, M.E.; Han, B. Cell delivery using an injectable and adhesive transglutaminase-gelatin gel. Tissue Eng. Part C Methods 2010, 16, 609–618. [Google Scholar] [CrossRef]
- Spurlin, T.A.; Bhadriraju, K.; Chung, K.H.; Tona, A.; Plant, A.L. The treatment of collagen fibrils by tissue transglutaminase to promote vascular smooth muscle cell contractile signaling. Biomaterials 2009, 30, 5486–5496. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ 2016, 4, e2497. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-K.; Ke, C.-J.; Lin, Y.-W.; Lin, F.-H.; Tsai, T.-H.; Sun, J.-S. Transglutaminase Cross-Linked Gelatin-Alginate-Antibacterial Hydrogel as the Drug Delivery-Coatings for Implant-Related Infections. Polymers 2021, 13, 414. [Google Scholar] [CrossRef]
- La Gatta, A.; Tirino, V.; Cammarota, M.; La Noce, M.; Stellavato, A.; Pirozzi, A.V.A.; Portaccio, M.; Diano, N.; Laino, L.; Papaccio, G.; et al. Gelatin-biofermentative unsulfated glycosaminoglycans semi-interpenetrating hydrogels via microbial-transglutaminase crosslinking enhance osteogenic potential of dental pulp stem cells. Regen. Biomater. 2021, 8, rbaa052. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.E.; Esterly, K.; Awadallah, A.; Parrish, C.R.; Poynter, G.M.; Goltry, K.L. Clinical-scale expansion of a mixed population of bone-marrow-derived stem and progenitor cells for potential use in bone-tissue regeneration. Stem Cells 2007, 25, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef]
- Horwitz, E.M. MSC: A coming of age in regenerative medicine. Cytotherapy 2006, 8, 194–195. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Vallmajo-Martin, Q.; Broguiere, N.; Millan, C.; Zenobi-Wong, M.; Ehrbar, M. PEG/HA Hybrid Hydrogels for Biologically and Mechanically Tailorable Bone Marrow Organoids. Adv. Funct. Mater. 2020, 30, 1910282. [Google Scholar] [CrossRef]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef]
- Schnepp, Z.A.C.; Gonzalez-McQuire, R.; Mann, S. Hybrid Biocomposites Based on Calcium Phosphate Mineralization of Self-Assembled Supramolecular Hydrogels. Adv. Mater. 2006, 18, 1869–1872. [Google Scholar] [CrossRef]
- Douglas, T.E.; Messersmith, P.B.; Chasan, S.; Mikos, A.G.; de Mulder, E.L.; Dickson, G.; Schaubroeck, D.; Balcaen, L.; Vanhaecke, F.; Dubruel, P.; et al. Enzymatic mineralization of hydrogels for bone tissue engineering by incorporation of alkaline phosphatase. Macromol. Biosci. 2012, 12, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Koo, J.M.; Cui, H. One-component nanomedicine. J. Control. Release 2015, 219, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Shi, J.; Du, X.; Huang, Y.; Gao, Y.; Baoum, A.A.; Xu, B. The enzyme-instructed assembly of the core of yeast prion Sup35 to form supramolecular hydrogels. J. Mater. Chem. B 2016, 4, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.R.; Fernandes, P.R.; Guedes, J.M.; Hollister, S.J. Permeability analysis of scaffolds for bone tissue engineering. J. Biomech. 2012, 45, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Akbar, I. Permeability study of functionally graded scaffold based on morphology of cancellous bone. Malays. J. Med. Health Sci. 2021, 17, 60–66. [Google Scholar]







| Type of Crosslinking | Mechanism/Response | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Physical approaches | ||||
| Hydrogen bonding | Interaction between electropositive (H) and electronegative (O, N, F) atoms | Safe and less toxic, non-covalent bonds (sacrificial), highly dynamic and reversible character, excellent toughening effect, lowest toxicity, tunable properties, and allows deviations in ion amounts | Weak mechanical characteristics, highly detrimental in aqueous medium, easily dissociate upon heating, low degree of crosslinking | [33,34,35] |
| Electrostatic (ionic) interactions | Interaction between positively charged ions (Ca2+, Mg2+, Cu2+, etc.) and negatively charged functional groups or interaction between anionic and cationic molecules or polyelectrolytes under physiological environment | Safe and less toxic, In-situ formation, highly dynamic and reversible character, excellent tunability, stimuli-sensitive behavior | Weak mechanical characteristics, low degree of crosslinking | [36,37] |
| Hydrophobic/hydrophilic interactions | Interaction between chemical groups located close to one another | Safe and less toxic, sol-gel transition (i.e., thermal gelation), highly dynamic and reversible character, and tunable properties | Low mechanical properties, hydrophobic/hydrophilic balance is sensitive to polar solvents and temperature, incapability to control the reaction growth, low degree of crosslinking, | [38,39] |
| Entanglements of polymeric chains | Complication of intramolecular or intermolecular chains. | Safe and less toxic, enhanced interchain connectivity | Too weak, depends on molecular weight fraction of polymers | [40,41] |
| Stereocomplex crystallization | Highly organized form of atoms or molecules/parallel or antiparallel interactions between two independent helices or co-crystallization between polymers with complementary configurations | Safe and less toxic, alternative packing of different polymer chains, and tunable and better mechanical properties | [42,43] | |
| Metal coordination | A coordination complex of a central atom/ions (typically metallic) and surrounding arrangement of bound ligands | Safe and less toxic, complexation between ligand-adorned polymers and metal ions | Low degree of crosslinking | [44,45,46] |
| Stacking of π–π bonds | Speculative attractive and noncovalent (orbital overlapping) interface between the π bonds in aromatic rings | Strong physical interaction (noncovalent), non-destructive and reversible, can provide hydrophobic domain | Low degree of crosslinking, not very weak interactions, not very directional, generally aromaticity is essential π–π stacking interactions, sensitive to polar solvents, non-negligible cytotoxicity | [47,48,49] |
| Chemical approaches | ||||
| Enzymatic effect | Enzyme catalyzes the functional groups of the substrate in close proximity | Highly biocompatible, high specificity (chemo-, region-, and stereo-selectivity), high crosslinking density, and minimal requirement | High processing cost, negligible recovery, high sterile condition required, high degree of crosslinking, | [24,50] |
| Schiff-base reaction (imine formation) | Shortening of primary amines and active carbonyl groups (aldehyde or ketone) to form imine bonds | Dynamic reversibility, potential pH-sensitive linker, in situ formation of hydrogels | Required washing to remove remaining unreacted substances, high degree of crosslinking | [51,52] |
| Michael type addition reaction | Reaction between nucleophile and crosslinked unsaturated carbonyl systems (e.g., olefins, alkynes) to form (-C–C-) bond | In situ formation of hydrogels | Required washing to remove remaining unreacted substances, high degree of crosslinking | [53,54] |
| Oxime bond formation | Reaction between an aldehyde or ketone and hydroxylamine | Dynamic covalent character, high efficiency, chemo-selectivity, formation in aqueous solvents and water being the only side-product | Complex synthetic methods, Required washing to remove remaining unreacted substances, high degree of crosslinking | [55,56] |
| Diels–Alder “click” reaction | No catalyst needed | Dynamic covalent character, mild and copper-free reaction, facile for in vitro and in vivo applications, | Long gelation time relatively, Required washing to remove remaining unreacted substances, high degree of crosslinking | [57,58] |
| Free-radical polymerization | Production of free radicals in monomers using particular initiator and then they form long polymeric chains by joining them together through covalent bonding | Rapid and simple method, no sophisticated and expensive instruments needed | Difficult to control chain propagation, wide particle size distribution, required washing to remove remaining unreacted substances, high degree of crosslinking | [59,60] |
| S. No | Enzyme Involved/ Mimicked | Composition | Key Features | Ref. |
|---|---|---|---|---|
| 1. | Tyrosinase | Hyaluronic acid, Chitosan, Dopamine hydrochloride | Multilayer membrane formation, Enhanced cell adhesion, viability, proliferation, and density of immortalized murine calvarial cell line (MC3T3-E1) | [66] |
| 2. | Tyrosinase | Carboxymethyl–chitosan (CMC), Gelatin, Nano-hydroxyapatite | Assessment of CMC-dependent crosslinking and strength; support in proliferation and differentiation of osteoblast; gel stability in vivo | [67] |
| 3. | Tyrosinase | Silk fibroin, Gelatin, Calcium chloride | Enhanced the osteogenesis of bone marrow derived progenitor cells (hMSCs); upregulating the gene expression of osteogenic markers (RUNX2, COL I, ALP, OPN, ON); osteocytic markers (DMP 1, PDPN, SOST), and β-catenin, BMP-2, and BMP4; enhancing mineralization processes | [68] |
| 4. | Tyrosinase | Chitosan, Gelatin, Nanohydroxyapatite | Pore size greater than 150 µm; high swelling ratio; acceptable biodegradability and biocompatibility | [71] |
| 5. | Tyrosinase | Polycaprolactane, beta-tricalcium phosphate | Enhanced viability and proliferation of osteoblast cells; development of an osteo-mucosal model; stable adhesive strength in wet conditions | [72] |
| 6. | Lysozyme | Polyethylene Glycol (PEG) | Facile clinical process; good biocompatibility; tight adherence to tissues | [79] |
| 7. | Horseradish peroxidase (HRP) | Hyaluronic acid, Tyramine | In situ-formed hydrogels; sustained release of BMP-2 in vitro; enhanced gene expressions of osteogenic makers, Alpl, Bglap, and Osx; callus formation in fractured bone in vivo | [84] |
| 8. | Horseradish peroxidase (HRP) | Calcium-accumulating peptide, Collagen | Induced bone mineralization around the human periodontal ligament stem cells (PDLSCs) loaded in hydrogel; increased osteogenic marker expressions in vitro; formation of bone layer post implantation in vivo | [85] |
| 9. | Horseradish peroxidase (HRP) | Silk fibroin, Gelatin | Enhanced gelation kinetics; improved mechanical attributes; slow enzymatic degradation; imparted stiffness and β sheet formation; superior morphology and metabolic activity of human mesenchymal stem cells (hMSCs) | [86] |
| 10. | Horseradish peroxidase (HRP) | Tyramine, Gellan gum | High degree of substitution (~30%); sustained release of betamethasone; high mechanical strength; negligible cytotoxicity and non-significant change in the metabolic activity of chondrogenic primary cells | [87] |
| 11. | Transglutaminase | Gelatin, Alginate | Cost-effectiveness and adequate safety profile; offered antibacterial properties; effective in reducing implant-related infection; upon treatment in vivo, substantial higher bone volume with intact bony architect was observed | [93] |
| 12. | Transglutaminase | Gelatin, Hyaluronan, Bacteriological chondroitin, | Enhanced expression of osteocalcin, and osteopontin at gene and protein level in human dental pluripotent stem cells (hDPSCs); effective in accelerated bone regeneration | [94] |
| 13. | Alkaline phosphatase | Calcium phosphate, Collagen type-1, catechol substituted PEG (cPEG), Fumaric acid/PEG copolymer (OPF) | The effectiveness in mineral formation decreases in the order cPEG > collagen > OPF; highest increment of Young’s modulus in cPEG was demonstrated | [102] |
| 14. | Alkaline phosphatase | Yeast prion Sup35 with the insertion of peptide segment (GNNQQNY) | Self-assembled nanofibers formation of hydrogelators; cytocompatible upon the insertion of the peptide segment along with enhancement in self-assembly | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sood, A.; Ji, S.M.; Kumar, A.; Han, S.S. Enzyme-Triggered Crosslinked Hybrid Hydrogels for Bone Tissue Engineering. Materials 2022, 15, 6383. https://doi.org/10.3390/ma15186383
Sood A, Ji SM, Kumar A, Han SS. Enzyme-Triggered Crosslinked Hybrid Hydrogels for Bone Tissue Engineering. Materials. 2022; 15(18):6383. https://doi.org/10.3390/ma15186383
Chicago/Turabian StyleSood, Ankur, Seong Min Ji, Anuj Kumar, and Sung Soo Han. 2022. "Enzyme-Triggered Crosslinked Hybrid Hydrogels for Bone Tissue Engineering" Materials 15, no. 18: 6383. https://doi.org/10.3390/ma15186383








