Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review
Abstract
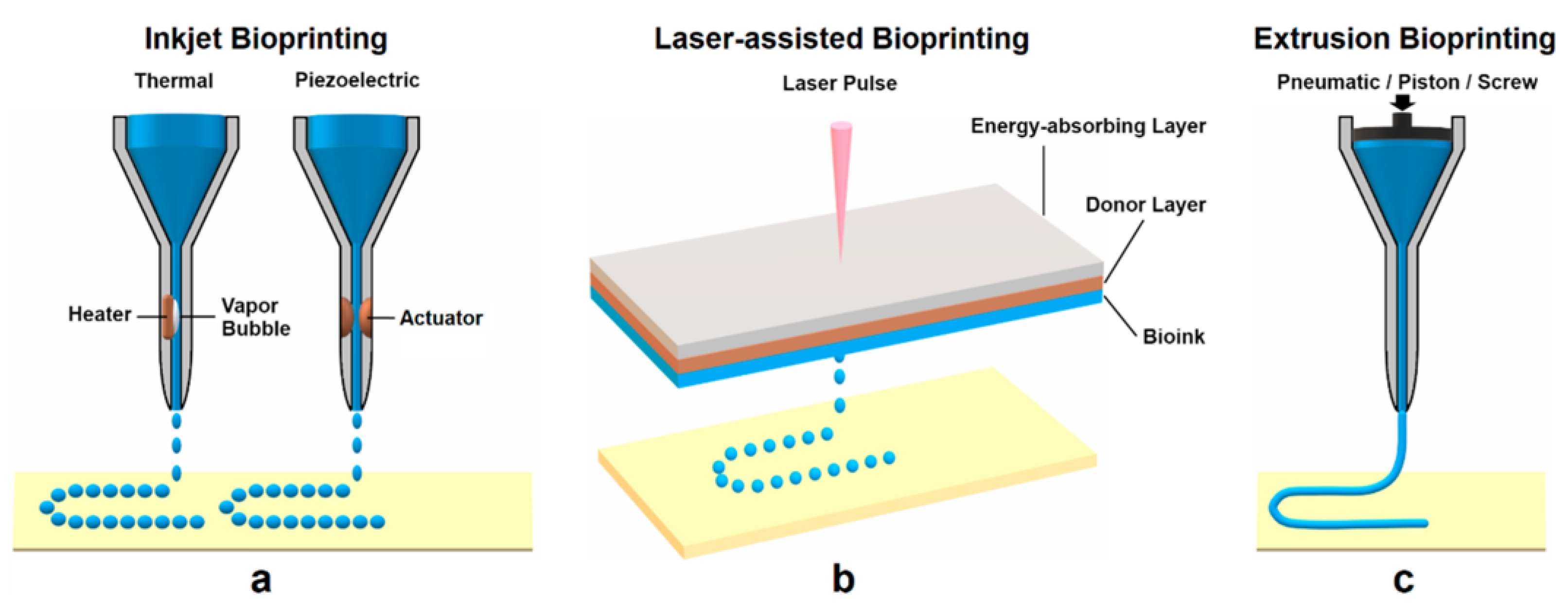
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. Characteristics of Included Studies
3.3. Three-Dimensional Bioprinting Strategy for Dental Application
3.4. Bioinks for 3D Bioprinting
3.5. Cells for 3D Bioprinting
3.6. In Vivo Application in Dental Tissue Engineering
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Marcenes, W.; Kassebaum, N.; Bernabe, E.; Flaxman, A.; Naghavi, M.; Lopez, A.D.; Murray, C. Global Burden of Oral Conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef] [PubMed]
- Nkenke, E.; Weisbach, V.; Winckler, E.; Kessler, P.; Schultze-Mosgau, S.; Wiltfang, J.; Neukam, F.W. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: A prospective study. Int. J. Oral Maxillofac. Surg. 2004, 33, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of Guided Bone Regeneration: A Review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. S3), S20–S27. [Google Scholar] [CrossRef]
- Young, C.; Terada, S.; Vacanti, J.; Honda, M.; Bartlett, J.; Yelick, P. Tissue Engineering of Complex Tooth Structures on Biodegradable Polymer Scaffolds. J. Dent. Res. 2002, 81, 695–700. [Google Scholar] [CrossRef]
- Sigaux, N.; Pourchet, L.; Breton, P.; Brosset, S.; Louvrier, A.; Marquette, C. 3D Bioprinting:principles, fantasies and prospects. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 128–132. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Yu, J.; Park, S.A.; Kim, W.D.; Ha, T.; Xin, Y.-Z.; Lee, J.; Lee, D. Current Advances in 3D Bioprinting Technology and Its Applications for Tissue Engineering. Polymers 2020, 12, 2958. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021, 9, 535–573. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Chang, S.-S.; Ng, H.Y.; Huang, Y.-X.; Chen, C.-C.; Shie, M.-Y. Additive Manufacturing of Astragaloside-Containing Polyurethane Nerve Conduits Influenced Schwann Cell Inflammation and Regeneration. Processes 2021, 9, 353. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017, 12, 1600671. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, Y.-J.; Shim, J.-H.; Park, J.H.; Cho, D.-W. Development of a 3D cell printed structure as an alternative to autologs cartilage for auricular reconstruction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1016–1028. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Monroy-García, A.; Ledesma-Martínez, E.; Mendoza-Núñez, V.M. Mesenchymal Stem Cells of Dental Origin for Inducing Tissue Regeneration in Periodontitis: A Mini-Review. Int. J. Mol. Sci. 2018, 19, 944. [Google Scholar] [CrossRef]
- Keating, A. Mesenchymal Stromal Cells: New Directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.; Yang, X. Mesenchymal stem cells and bone regeneration: Current status. Injury 2011, 42, 562–568. [Google Scholar] [CrossRef]
- Kyburz, K.A.; Anseth, K.S. Synthetic Mimics of the Extracellular Matrix: How Simple is Complex Enough? Ann. Biomed. Eng. 2015, 43, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L.; et al. Natural hydrogels for cartilage regeneration: Modification, preparation and application. J. Orthop. Transl. 2019, 17, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Busra, M.F.M. Recent Development in the Fabrication of Collagen Scaffolds for Tissue Engineering Applications: A Review. Curr. Pharm. Biotechnol. 2019, 20, 992–1003. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J. Dent. Res. 2015, 94, 143S–152S. [Google Scholar] [CrossRef]
- Peters, M.D.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Évid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Harms, R.; Wu, S.; Wang, Y.; Untrauer, J.B.; Carlson, M.A.; Duan, B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017, 7, 29312–29320. [Google Scholar] [CrossRef]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; Franca, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.N.; Smith, L.J.; Olivos, D.J., 3rd; Chu, T.-M.G.; Kacena, M.A.; Wagner, D.R. Scaffold-free bioprinting of mesenchymal stem cells with the regenova printer: Optimization of printing parameters. Bioprinting 2019, 15, e00048. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.N.; Olivos, D.J.; Brinker, A.; Alvarez, M.B.; Smith, L.J.; Chu, T.-M.G.; Kacena, M.A.; Wagner, D.R. Scaffold-free bioprinting of mesenchymal stem cells using the Regenova printer: Spheroid characterization and osteogenic differentiation. Bioprinting 2019, 15, e00050. [Google Scholar] [CrossRef]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered Osteoinductive Bioink for 3D Bioprinting Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Extracellular Matrix/Amorphous Magnesium Phosphate Bioink for 3D Bioprinting of Craniomaxillofacial Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 23752–23763. [Google Scholar] [CrossRef]
- Moncal, K.K.; Gudapati, H.; Godzik, K.P.; Heo, D.N.; Kang, Y.; Rizk, E.; Ravnic, D.J.; Wee, H.; Pepley, D.F.; Ozbolat, V.; et al. Intra-Operative Bioprinting of Hard, Soft, and Hard/Soft Composite Tissues for Craniomaxillofacial Reconstruction. Adv. Funct. Mater. 2021, 31, 2010858. [Google Scholar] [CrossRef]
- Moncal, K.K.; Aydın, R.S.T.; Godzik, K.P.; Acri, T.M.; Heo, D.N.; Rizk, E.; Wee, H.; Lewis, G.S.; Salem, A.K.; Ozbolat, I.T. Controlled Co-delivery of pPDGF-B and pBMP-2 from intraoperatively bioprinted bone constructs improves the repair of calvarial defects in rats. Biomaterials 2022, 281, 121333. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.S.; Jang, H., II; Kim, H.-R.; Kang, H.-W. Bioprinting of three-dimensional dentin–pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jeong, W.; Kim, M.-K.; Nam, S.-H.; Park, E.-K.; Kang, H.-W. Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration. Polymers 2021, 13, 1294. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.-L.; Yun, S.; Cao, H.-L.; Ahn, G.; Shim, J.-H.; Woo, S.-H.; Choung, P.-H. Bioprinting on 3D Printed Titanium Scaffolds for Periodontal Ligament Regeneration. Cells 2021, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Bin, J.; Ganguly, K.; Patel, D.K.; Lim, K.-T. Electromagnetic field-assisted cell-laden 3D printed poloxamer-407 hydrogel for enhanced osteogenesis. RSC Adv. 2021, 11, 20342–20354. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Lee, G.; Hoang, T.; Kim, H.; Kim, G.H. Fabrication of Bone-derived decellularized extracellular matrix/Ceramic-based Biocomposites and Their Osteo/Odontogenic Differentiation Ability for Dentin Regeneration. Bioeng. Transl. Med. 2022, 7, e10317. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef]
- Kérourédan, O.; Ribot, E.J.; Fricain, J.-C.; Devillard, R.; Miraux, S. Magnetic Resonance Imaging for tracking cellular patterns obtained by Laser-Assisted Bioprinting. Sci. Rep. 2018, 8, 15777. [Google Scholar] [CrossRef]
- Kérourédan, O.; Hakobyan, D.; Rémy, M.; Ziane, S.; Dusserre, N.; Fricain, J.-C.; Delmond, S.; Thébaud, N.B.; Devillard, R. In situ prevascularization designed by laser-assisted bioprinting: Effect on bone regeneration. Biofabrication 2019, 11, 045002. [Google Scholar] [CrossRef]
- Touya, N.; Devun, M.; Handschin, C.; Casenave, S.; Omar, N.A.; Gaubert, A.; Dusserre, N.; De Oliveira, H.; Kérourédan, O.; Devillard, R. In vitro and in vivo characterization of a novel tricalcium silicate-based ink for bone regeneration using laser-assisted bioprinting. Biofabrication 2022, 14, 024104. [Google Scholar] [CrossRef]
- Campos, D.F.D.; Zhang, S.; Kreimendahl, F.; Köpf, M.; Fischer, H.; Vogt, M.; Blaeser, A.; Apel, C.; Esteves-Oliveira, M. Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration. Connect. Tissue Res. 2020, 61, 205–215. [Google Scholar] [CrossRef]
- Amler, A.-K.; Thomas, A.; Tüzüner, S.; Lam, T.; Geiger, M.-A.; Kreuder, A.-E.; Palmer, C.; Nahles, S.; Lauster, R.; Kloke, L. 3D bioprinting of tissue-specific osteoblasts and endothelial cells to model the human jawbone. Sci. Rep. 2021, 11, 4876. [Google Scholar] [CrossRef] [PubMed]
- Amler, A.-K.; Dinkelborg, P.; Schlauch, D.; Spinnen, J.; Stich, S.; Lauster, R.; Sittinger, M.; Nahles, S.; Heiland, M.; Kloke, L.; et al. Comparison of the Translational Potential of Human Mesenchymal Progenitor Cells from Different Bone Entities for Autologous 3D Bioprinted Bone Grafts. Int. J. Mol. Sci. 2021, 22, 796. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ji, Y.; Huang, G.; Ling, K.; Zhang, X.; Xu, F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication 2015, 7, 044105. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ji, Y.; Zhong, T.; Wan, W.; Yang, Q.; Li, A.; Zhang, X.; Lin, M. Bioprinting-Based PDLSC-ECM Screening for in Vivo Repair of Alveolar Bone Defect Using Cell-Laden, Injectable and Photocrosslinkable Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3534–3545. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, M.; Liu, Y.; Shi, C.; Wang, Y.; Liu, T.; Huang, Y.; Zhong, P.; Dai, J.; Liu, X. The performance of 3D bioscaffolding based on a human periodontal ligament stem cell printing technique. J. Biomed. Mater. Res. Part A 2021, 109, 1209–1219. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chiu, Y.-C.; Lee, A.K.-X.; Lin, Y.-A.; Lin, P.-Y.; Shie, M.-Y. Biofabrication of Gingival Fibroblast Cell-Laden Collagen/Strontium-Doped Calcium Silicate 3D-Printed Bi-Layered Scaffold for Osteoporotic Periodontal Regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Hsu, T.-T.; Liu, Y.-W.; Kao, C.-T.; Huang, T.-H. Bidirectional Differentiation of Human-Derived Stem Cells Induced by Biomimetic Calcium Silicate-Reinforced Gelatin Methacrylate Bioink for Odontogenic Regeneration. Biomedicines 2021, 9, 929. [Google Scholar] [CrossRef]
- Kort-Mascort, J.; Bao, G.; Elkashty, O.; Flores-Torres, S.; Munguia-Lopez, J.G.; Jiang, T.; Ehrlicher, A.J.; Mongeau, L.; Tran, S.D.; Kinsella, J.M. Decellularized Extracellular Matrix Composite Hydrogel Bioinks for the Development of 3D Bioprinted Head and Neck in Vitro Tumor Models. ACS Biomater. Sci. Eng. 2021, 7, 5288–5300. [Google Scholar] [CrossRef]
- Raveendran, N.T.; Vaquette, C.; Meinert, C.; Ipe, D.S.; Ivanovski, S. Optimization of 3D bioprinting of periodontal ligament cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef]
- Walladbegi, J.; Schaefer, C.; Pernevik, E.; Sämfors, S.; Kjeller, G.; Gatenholm, P.; Sándor, G.K.; Rasmusson, L. Three-dimensional bioprinting using a coaxial needle with viscous inks in bone tissue engineering—An In vitro study. Ann. Maxillofac. Surg. 2020, 10, 370–376. [Google Scholar] [CrossRef]
- Ono, T.; Tomokiyo, A.; Ipposhi, K.; Yamashita, K.; Alhasan, M.A.; Miyazaki, Y.; Kunitomi, Y.; Tsuchiya, A.; Ishikawa, K.; Maeda, H. Generation of biohybrid implants using a multipotent human periodontal ligament cell line and bioactive core materials. J. Cell. Physiol. 2021, 236, 6742–6753. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yi, H.-G.; Cho, D.-W. 3D Printed Tissue Models: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1722–1731. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Zorlutuna, P.; Vrana, N.E.; Khademhosseini, A. The Expanding World of Tissue Engineering: The Building Blocks and New Applications of Tissue Engineered Constructs. IEEE Rev. Biomed. Eng. 2013, 6, 47–62. [Google Scholar] [CrossRef]
- Walters, B.; Stegemann, J. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef]
- Mori, H.; Shimizu, K.; Hara, M. Dynamic viscoelastic properties of collagen gels with high mechanical strength. Mater. Sci. Eng. C 2013, 33, 3230–3236. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Daly, A.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sarvestani, S.K.; Moeinzadeh, S.; He, X.; Jabbari, E. Three-Dimensional-Engineered Matrix to Study Cancer Stem Cells and Tumorsphere Formation: Effect of Matrix Modulus. Tissue Eng. Part A 2013, 19, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tharayil, A.; Thomas, S. 3D Bioprinting of Nature-Inspired Hydrogel Inks Based on Synthetic Polymers. ACS Appl. Polym. Mater. 2021, 3, 3685–3701. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Kang, H.-W.; Mead, I.; Bishop, C.; Shupe, T.; Lee, S.J.; Jackson, J.; Yoo, J.; Soker, S.; et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015, 25, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, A.M.; Kokini, K.; Vaughn, L.C.; Waisner, B.Z.; Voytik-Harbin, S.L. Extracellular matrix (ECM) microstructural composition regulates local cell-ECM biomechanics and fundamental fibroblast behavior: A multidimensional perspective. J. Appl. Physiol. 2005, 98, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled Release Strategies for Bone, Cartilage, and Osteochondral Engineering—Part II: Challenges on the Evolution from Single to Multiple Bioactive Factor Delivery. Tissue Eng. Part B Rev. 2013, 19, 327–352. [Google Scholar] [CrossRef]
- Kuhn, L.T.; Ou, G.; Charles, L.; Hurley, M.M.; Rodner, C.M.; Gronowicz, G. Fibroblast Growth Factor-2 and Bone Morphogenetic Protein-2 Have a Synergistic Stimulatory Effect on Bone Formation in Cell Cultures from Elderly Mouse and Human Bone. J. Gerontol. Ser. A 2013, 68, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Gothard, D.; Smith, E.; Kanczler, J.; Rashidi, H.; Qutachi, O.; Henstock, J.; Rotherham, M.; El Haj, A.; Shakesheff, K.; Oreffo, R. Tissue engineered bone using select growth factors: A comprehensive review of animal studies and clinical translation studies in man. Eur. Cells Mater. 2014, 28, 166–208. [Google Scholar] [CrossRef]
- Ng, W.L.; Lee, J.M.; Yeong, W.Y.; Naing, M.W. Microvalve-based bioprinting—process, bio-inks and applications. Biomater. Sci. 2017, 5, 632–647. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Hu, C.; Xue, Z.; Wang, G.; Ding, B.; Luo, H.; Tang, L.; Kong, X.; Chen, X.; et al. MiR-17 Modulates Osteogenic Differentiation Through a Coherent Feed-Forward Loop in Mesenchymal Stem Cells Isolated from Periodontal Ligaments of Patients with Periodontitis. Stem Cells 2011, 29, 1804–1816. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.; Zhang, C. The Role of Vasculature Engineering in Dental Pulp Regeneration. J. Endod. 2017, 43, S102–S106. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.; Zhan, X.; Zhang, C.; Hargreaves, K.M.; Jin, L.; Tong, E.H. Coculture of Dental Pulp Stem Cells with Endothelial Cells Enhances Osteo-/Odontogenic and Angiogenic Potential In Vitro. J. Endod. 2012, 38, 454–463. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, H.; Zheng, W.; Tang, L.; Yang, Z.; Gao, Y.; Yang, Q.; Wang, C.; Duan, Y.; Jin, Y. Characterization of Stem Cells from Alveolar Periodontal Ligament. Tissue Eng. Part A 2011, 17, 1015–1026. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jeon, S.H.; Choung, P.-H. Efficacy of Periodontal Stem Cell Transplantation in the Treatment of Advanced Periodontitis. Cell Transplant. 2011, 20, 271–286. [Google Scholar] [CrossRef]
- Lei, M.; Li, K.; Li, B.; Gao, L.-N.; Chen, F.-M.; Jin, Y. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after In Vivo transplantation. Biomaterials 2014, 35, 6332–6343. [Google Scholar] [CrossRef]
- Tarafder, S.; Koch, A.; Jun, Y.; Chou, C.; Awadallah, M.R.; Lee, C.H. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication 2016, 8, 025003. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- D’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007, 14, 1162–1171. [Google Scholar] [CrossRef] [Green Version]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Zvonic, S.; Floyd, E.; Kassem, M.; Nuttall, M.E. Playing with bone and fat. J. Cell. Biochem. 2006, 98, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.N.; Yassin, M.A.; Suliman, S.; Lie, S.A.; Gjengedal, H.; Mustafa, K. The bone regeneration capacity of 3D-printed templates in calvarial defect models: A systematic review and meta-analysis. Acta Biomater. 2019, 91, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef]
- Ozbolat, I.T. Scaffold-Based or Scaffold-Free Bioprinting: Competing or Complementing Approaches? J. Nanotechnol. Eng. Med. 2015, 6, 024701. [Google Scholar] [CrossRef]
- Omar, N.I.; Baharin, B.; Lau, S.F.; Ibrahim, N.; Mohd, N.; Fauzi, A.A.; Muhammad, N.; Fernandez, N.M. The Influence of Ficus deltoidea in Preserving Alveolar Bone in Ovariectomized Rats. Veter.-Med. Int. 2020, 2020, 8862489. [Google Scholar] [CrossRef]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, S. Translational Research Challenges: Finding the right animal models. J. Investig. Med. 2012, 60, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Lorbach, O.; Baums, M.H.; Kostuj, T.; Pauly, S.; Scheibel, M.; Carr, A.; Zargar, N.; Saccomanno, M.F.; Milano, G. Advances in biology and mechanics of rotator cuff repair. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Shamsuddin, S.A.; Ramli, R.; Razali, M.; Baharin, B.; Sulaiman, S.; Hwei Ng, M.; Low, C.K.; Jabar, M.N.A.; Nordin, R.; Yahaya, N. Guided bone regeneration using autologous plasma, bone marrow cells and β-TCP/HA granules for experimental alveolar ridge reconstruction in Macaca fascicularis. J. Biomater. Tissue Eng. 2017, 7, 111–118. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Kleinschmidt, J.C. The Critical Size Defect as an Experimental Model to Test Bone Repair Materials. J. Craniofacial Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef]
- Wu, Y.; Ravnic, D.J.; Ozbolat, I.T. Intraoperative Bioprinting: Repairing Tissues and Organs in a Surgical Setting. Trends Biotechnol. 2020, 38, 594–605. [Google Scholar] [CrossRef]
- Ozbolat, I.T. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015, 33, 395–400. [Google Scholar] [CrossRef]
- Keriquel, V.; Guillemot, F.; Arnault, I.; Guillotin, B.; Miraux, S.; Amédée, J.; Fricain, J.-C.; Catros, S. In Vivo bioprinting for computer- and robotic-assisted medical intervention: Preliminary study in mice. Biofabrication 2010, 2, 014101. [Google Scholar] [CrossRef]





| Author | Cell-Laden Bioink | Other Biomaterial/ Growth Factor | Cell Types | Bioprinting Strategy | Study Design | Application |
|---|---|---|---|---|---|---|
| Lee et al., 2021 [53] | Collagen | FGF-2 | hPDLSCs | Extrusion | In vitro and in vivo | PDL regeneration |
| Wang et al., 2021 [66] | Collagen | SrCS | Human gingiva fibroblasts | Extrusion | In vitro and in vivo | Periodontal regeneration |
| Kérourédan et al., 2018 [57] | Collagen type 1 | - | SCAPs | LAB | In vitro and in vivo | Bone regeneration |
| Kérourédan et al., 2019 [58] | Collagen type 1 | VEGF | SCAPs and HUVECs | LAB | In vivo | Bone regeneration |
| Duarte Campos et al., 2020 [60] | Collagen type 1 + agarose | - | DPSCs and HUVECs | Inkjet | In vitro and ex vivo | Dental pulp regeneration |
| Keriquel et al., 2017 [56] | Collagen type 1 + nHAp | - | Mouse bone marrow stromal precursor D1 cell line | LAB | In vitro and in vivo | Bone regeneration |
| Moncal et al., 2021 [49] | Collagen + chitosan + β-glycerophosphate + nHAp | rhBMP-2 | Rat BMSCs | Extrusion | In vitro | Bone regeneration |
| Moncal et al., 2022 [50] | Collagen + chitosan + β-glycerophosphate + nHAp | PDGF and BMP-2 | Rat BMSCs | Extrusion | In vitro | Bone regeneration |
| Touya et al., 2022 [59] | Collagen type 1 + TCP (BioRoot RCS®, Septodont, Saint-Maur-des- Fossés, France) | - | SCAPs | LAB | In vitro and in vivo | Bone regeneration |
| Kim et al., 2022 [55] | Collagen type 1 or dECMs + β-TCP | - | DPSCs | Extrusion | In vitro and in vivo | Dental tissue regeneration |
| Kang et al., 2016 [41] | Gelatin + fibrinogen + HA + glycerol | PCL/TCP | hAFSCs | Extrusion | In vitro and in vivo | Alveolar bone/bone regeneration |
| Han et al., 2019 [51] | Gelatin + fibrinogen + HA + glycerol | - | DPSCs | Extrusion | In vitro | Dentin/dental pulp regeneration |
| Han et al., 2021 [52] | Demineralized dentin matrix particles + fibrinogen + gelatin | - | DPSCs | Extrusion | In vitro | Dental tissue regeneration |
| Kort-Mascort et al., 2021 [68] | Alginate + gelatin + dECMs | - | Human SCC (Cell lines: UM-SCC-12 and UM-SCC-38) | Extrusion | In vitro | Head and neck cancer in vitro model |
| Tian et al., 2021 [65] | Sodium alginate + gelatin + nHAp | - | hPDLSCs | Extrusion | In vitro | Bone regeneration |
| Park et al., 2020 [47] | Gelatin + GelMA + HA + glycerol | BMP-mimetic peptide | DPSCs | Extrusion | In vitro | Dental tissue regeneration |
| Amler et al., 2021 [62] | GelMA | - | Bone-derived MPC/Bone marrow MPC/Periosteal MPC | Stereolithography | In vitro | Bone regeneration |
| Raveendran et al., 2019 [69] | GelMA | - | hPDLSCs | Extrusion | In vitro | Periodontal regeneration |
| Kuss et al., 2017 [42] | MeHA + GelMA + HA | PCL/HAp | Porcine stromal vascular fraction from adipose tissue | Extrusion | In vitro | Alveolar bone/bone regeneration |
| Ma et al., 2015 [63] | GelMA + PEGDA | - | hPDLSCs | Inkjet | In vitro | Periodontal regeneration |
| Ma et al., 2017 [64] | GelMA + PEGDA | - | Rat PDLSCs | Inkjet | In vitro and in vivo | Alveolar bone regeneration |
| Amler et al., 2021 [61] | GelMA + PEGDA3400 | - | JHOBs and HUVECs | Stereolithography | In vitro | Alveolar bone in vitro model |
| Lin et al., 2021 [67] | Calsium silicate + GelMA | - | DPSCs | Extrusion | In vitro | Dentin regeneration |
| Chimene et al., 2020 [46] | GelMA + kCA + nSi (NICE bioink) | - | Human primary bone marrow-derived MSCs | Extrusion | In vitro | Alveolar bone regeneration |
| Athirasala et al., 2018 [43] | Alginate + dentin matrix | - | SCAPs | Extrusion | In vitro | Dentin/dental pulp regeneration |
| Walladbegi et al., 2020 [70] | Nanofibrillated cellulose + alginate (CELLINK AB, Gothenburg, Sweden) | β-TCP | hADSCs | Extrusion | In vitro | Bone regeneration |
| Dubey et al., 2020 [48] | ECM + AMP | - | DPSCs | Extrusion | In vitro | Bone regeneration |
| Dutta et al., 2021 [54] | Poloxamer-407 | - | SCAPs | Extrusion | In vitro | Dental tissue regeneration |
| Aguilar et al., 2019 [44] | - | - | Mice bone marrow stromal cells | Scaffold-free (Kenzan method) | In vitro | Bone regeneration |
| Aguilar et al., 2019 [45] | - | - | Mice bone marrow stromal cells | Scaffold-free (Kenzan method) | In vitro | Bone regeneration |
| Ono et al., 2021 [71] | - | - | Human PDL cell line 1-17 | Scaffold-free (Needle array) | In vitro | PDL regeneration |
| Author | Cell-Laden Bioink | Type of Polymer | 3D Bioprinter | 3D Bioprinting Technique | Nozzle Size | Printing Speed | Printing Pressure | Crosslinking Method | Study Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al., 2021 [53] | Collagen | Natural | 3DX Printer, T and R Biofab Co., Ltd., Siheung, Korea | Extrusion | 400 μm ~22G | - | - | Thermal | Connective tissues interface between 3D-printed implants and calvaria bone has periodontal ligament characteristics; however, FGF-2 did not play a role in periodontal regeneration |
| Wang et al., 2021 [66] | Collagen | Natural | BioScaffolder 3.1, GeSiM, Großerkmannsdorf, Germany | Extrusion | 400 μm ~22G | 1.5–2 mm/s | 10–20 kPa | Physical | Novel bilayer 3D printed SrCS with collagen bioink upregulate angiogenic- and osteogenic-related proteins and factors, and enhanced bone regeneration in vivo |
| Kérourédan et al., 2018 [57] | Collagen type 1 | Natural | LAB workstation (U1026, Inserm, Bordeaux, France) | LAB | - | - | - | - | Potential use of magnetic resonance imaging and bioprinted micron superparamagnetic iron oxide-labeled cells to track cell patterns in vitro and calvarium defect model in mouse |
| Kérourédan et al., 2019 [58] | Collagen type 1 | Natural | LAB workstation (U1026, Inserm, Bordeaux, France) | LAB | - | - | - | - | In situ printing of HUVECs enhance vascularization and bone regeneration in calvarial defects |
| Duarte Campos et al., 2020 [60] | Collagen type 1 + agarose | Natural | Hand-held bioprinter (DropGun, BlackDrop Biodrucker GmbH, Aachen, Germany) | Inkjet | 300 μm ~23G | - | 25–250 kPa | Thermal | Handheld in situ bioprinting of cell-loaded collagen-based bioinks demonstrated successful vasculogenesis |
| Keriquel et al., 2017 [56] | Collagen type 1 + nHAp | Natural | LAB workstation (U1026, Inserm, Bordeaux, France) | LAB | - | 250 μm/s | - | - | 3D printed disk form of nHAp-collagen and D1 cells (bone marrow stromal precursor cells) showed the formation of mature bone in a calvarial defect model |
| Moncal et al., 2021 [49] | Collagen + chitosan + β-glycerophosphate + nHAp | Natural | In-house developed MultiArm Bioprinter, Iowa City, IA, USA | Extrusion | 22G~410 μm | 400 mm/min | 80–140 kPa | Thermal and physical | Hybrid intra-operative bioprinting induced bone regeneration with nearly 80% regenerated critical size calvarial bone defect |
| Moncal et al., 2022 [50] | Collagen + chitosan + β-glycerophosphate + nHAp | Natural | In-house developed MultiArm Bioprinter, Iowa City, IA, USA | Extrusion | 22G~410 μm | 400 mm/min | 80–140 kPa | Thermal and physical | Bioprinted bone constructs with the controlled co-delivery release of growth factors resulted in bone regeneration in critical-sized calvarial defects |
| Touya et al., 2022 [59] | Collagen type 1 + TCP (BioRoot RCS®, Septodont, France) | Natural | LAB workstation (U1026, Inserm, Bordeaux, France) | LAB | - | - | - | - | TCP-based ink demonstrated positive significance upon cell motility, and early osteogenic differentiation in vitro. However, the bioink was not successful in regenerating critical size cranial bone defects in vivo |
| Kim et al., 2022 [55] | Collagen type 1 or dECMs + β-TCP | Natural | DTR3–2210 T-SG; DASA Robot, Bucheon, Korea | Extrusion | 250 μm ~25G | 10 mm/s | 17–22 kPa | Genipin | The hDPSC-laden bone-derived dECM biocomposite enhanced both osteogenic and odontogenic differentiation in vitro and in vivo |
| Kang et al., 2016 [41] | Gelatin + fibrinogen + HA + glycerol | Natural | Integrated tissue–organ printing system | Extrusion | 300 μm ~23 G | - | 50–80 kPa | Thrombin | 3D tissue construct provides a favorable microenvironment for osteogenic differentiation of hAFSCs in vitro and showed the formation of mature, vascularized bone tissues in the calvarial bone defect model |
| Han et al., 2019 [51] | Gelatin + fibrinogen + HA + glycerol | Natural | Integrated tissue–organ printing system | Extrusion | 250 μm ~25G | 50–90 mm/min | - | Thrombin | Fibrin-based cell-laden bioink demonstrated spatial regulation of DPSC differentiation for the construction of 3D dentin–pulp complexes |
| Han et al., 2021 [52] | Demineralized dentin matrix particles + fibrinogen + gelatin | Natural | Homemade 3D bioprinter, Ulsan, Korea | Extrusion | 300 μm ~23G | 50 mm/min | 200 kPa | Thrombin | DDMp bioink can be used to fabricate 3D cellular dental constructs and showed significantly improvement in odontogenic differentiation of DPSCs |
| Kort-Mascort et al., 2021 [68] | Alginate + gelatin + dECMs | Natural | BioScaffolder 3.1, GeSiM, Großerkmannsdorf, Germany | Extrusion | 22G ~400 μm | 10 ± 2 mm/s | 45 ± 10 kPa | Calcium chloride | Cell-laden dECM-based bioink demonstrated tumor spheroids development by squamous cell carcinoma cells with high cell viability and proliferation |
| Tian et al., 2021 [65] | Sodium alginate + gelatin + nHAp | Natural | 3D Bioplotter (EnvisionTEC GmbH, Gladbeck, Germany) | Extrusion | 400 μm ~22G | 6 mm/s | 200 kPa | Calcium chloride | The hPDLSCs-laden bioink demonstrated good biocompatibility, stimulation of cell survival, proliferation and osteoblast |
| Park et al., 2020 [47] | Gelatin + GelMA + HA + glycerol | Natural | Integrated tissue–organ printing system | Extrusion | 330 μm ~23G | 150 mm/min | 130–160 kPa | Photopolymerization | Novel BMP-GelMA bioink showed high viability, proliferation and odontogenic differentiation of hDPSC |
| Amler et al., 2021 [62] | GelMA | Natural | Cellbricks GmbH, Berlin, Germany | Stereolithography | - | - | - | Photopolymerization | Periosteum-derived cells showed higher mineralization of print matrix and superior osteogenic potential for 3D bone constructs |
| Raveendran et al., 2019 [69] | GelMA | Natural | BioScaffolder 3.1, GeSiM, Großerkmannsdorf, Germany | Extrusion | ~220 μm 25G | 10–12 mm/s | 135 kPa | Photopolymerization | The best 3D bioprinting outcome of the periodontal ligament was obtained using 12.5% GelMA concentration with 0.05% LAP extruded through a 25G needle at 135kPa and crosslinking with UV-irradiation |
| Kuss et al., 2017 [42] | MeHA + GelMA + HA | Natural | 3D Bioplotter (EnvisionTEC GmbH, Gladbeck, Germany) | Extrusion | ~400 μm 22G | 1.8–2.2 mm/s | - | Photopolymerization | Short-term hypoxia (up to 7 days) promoted microvessel formation of SVFC-laden constructs without significantly affecting the cell viability compared to long-term hypoxia (more than 14 days) |
| Ma et al., 2015 [63] | GelMA + PEGDA | Natural and synthetic | Customer-designed pressure-assisted valve-based bioprinting system | Inkjet | 150 μm ~30G | - | 40–60 kPa | Photopolymerization | Volume ratios of GelMA to PEG bioink have an impact on cell viability and spreading of hPDLSCs. The increasing ratio of PEG leads to a decrease in hPDLSCs viability and spreading area |
| Ma et al., 2017 [64] | GelMA + PEGDA | Natural and synthetic | Customer-designed pressure-assisted valve-based bioprinting system | Inkjet | 150 μm ~30G | - | 50 kPa | Photopolymerization | An increase in the volume ratio of 3D GelMA-PEGDA in vitro resulted in an increase in cell proliferation, spreading and osteogenic differentiation of PDLSCs. New bone formation was observed in the alveolar defect treated with 3D bioprinted PDLSC hydrogel in a rat model |
| Amler et al., 2021 [61] | GelMA + PEGDA3400 | Natural and synthetic | Cellbricks GmbH, Berlin, Germany | Stereolithography | - | - | - | Photopolymerization | 3D bioprinted constructs containing primary JHOBs with vasculature-like channel structures comprising endothelial cells demonstrated the survival of both cells and mineralization of the bone matrix |
| Lin et al., 2021 [67] | Calsium silicate + GelMA | Natural | BioX, CELLINK, Gothenburg, Sweden | Extrusion | 30G~150 μm | 20 mm/s | 180 kPa | Photopolymerization | Calcium silicate/GelMA scaffolds enhanced mechanical properties and odontogenesis of hDPSCs |
| Chimene et al., 2020 [46] | GelMA + kCA + nSi (NICE bioink) | Natural | Modified ANET A8 3D printer, Shenzhen, China | Extrusion | 400 μm ~22G | 15 mm/s | - | Photopolymerization | 3D NICE cell-laden bioink demonstrated the ability to form osteo-related mineralized ECM without the growth factor |
| Athirasala et al., 2018 [43] | Alginate + dentin matrix | Natural | Hyrel 3D, Norcross, GA, USA | Extrusion | Coaxial: 26–19G | - | - | Calcium chloride | Cell-laden alginate and dentin matrix enhances odontogenic differentiation of SCAPs |
| Walladbegi et al., 2020 [70] | Nanofibrillated cellulose + alginate (CELLINK AB, Gothenburg, Sweden) | Natural | Inkredible, CELLINK AB, Gothenburg, Sweden | Extrusion | Coaxial: 22–16G | - | 75 kPa and 85 kPa | Calcium chloride | A coaxial needle enables the printing of a stable scaffold with viable hADSCs |
| Dubey et al., 2020 [48] | ECM + AMP | Natural | 3DDiscovery, regenHU, Villaz-St-Pierre, Switzerland | Extrusion | - | 15–20 mm/s | 30–50 kPa | Physical | ECM/AMP-bioprinted constructs demonstrated osteogenic differentiation of DPSCs without the need for chemical inducers |
| Dutta et al., 2021 [54] | Poloxamer-407 | Synthetic | CELLINK BIO-X 3D printer, Gothenburg, Sweden | Extrusion | 27G | 5 mm/s | 35 kPa | Photopolymerization | 3D bioprinted poloxamer hydrogels with low voltage–frequency electromagnetic fields stimulation (5V-1 Hz, 0.62 mT) enhance the SCAPs viability and osteogenic potential |
| Aguilar et al., 2019 [44] | - | - | Regenova Bio 3D Printer, Cyfuse K.K, Tokyo, Japan | Scaffold-free (Kenzan method) | - | - | - | - | Centrifugation cell method generated tighter BMSC spheroid formation with the optimal technique of 40k cells aggregate under 150-300G |
| Aguilar et al., 2019 [45] | - | - | Regenova Bio 3D Printer, Cyfuse K.K, Tokyo, Japan | Scaffold-free (Kenzan method) | - | - | - | - | Optimization of scaffold-free bioprinting resulted in a reduction in print times, the use of bioprinting nozzles and fabrication of more robust constructs |
| Ono et al., 2021 [71] | - | - | Regenova Bio 3D Printer, Cyfuse K.K, Tokyo, Japan | Scaffold-free (Needle array) | 240 μm ~26G | - | - | - | 3D bioprinted tubular structures and hydroxyapatite core materials exhibited high cell viability, collagen fibers and strongly expressed factors associated with periodontal ligament tissues |
| Author | Cell Type | Cell Densities | Max Cell Viability (%) | 3D Bioprinting Technique | Targeted Tissue |
|---|---|---|---|---|---|
| Han et al., 2019 [51] | DPSCs | 3 × 106 cells/mL | >90 | Extrusion | Dentin/dental pulp |
| Park et al., 2020 [47] | DPSCs | - | >90 | Extrusion | Dental tissue |
| Dubey et al., 2020 [48] | DPSCs | 1 × 106 cells/mL | >90 | Extrusion | Bone |
| Han et al., 2021 [52] | DPSCs | 3 × 106 cells/mL | >95 | Extrusion | Dental tissue |
| Lin et al., 2021 [67] | DPSCs | 5 × 106 cells/mL | - | Extrusion | Dentin/pulp |
| Kim et al., 2022 [55] | DPSCs | 1 × 107 cells/mL | >95 | Extrusion | Dental tissue |
| Duarte Campos et al., 2020 [60] | DPSCs and HUVECs | 3 × 106 cells/mL (both type of cells) | - | Inkjet | Dental pulp |
| Ma et al. 2015 [63] | hPDLSCs | 1 × 106 cells/mL | 82.4 ± 4.7 | Inkjet | Periodontal ligament |
| Raveendran et al., 2019 [69] | hPDLSCs | 2.0 × 106 cells/mL | >70 | Extrusion | Periodontal ligament |
| Lee et al., 2021 [53] | hPDLSCs | 1 × 107 cells/mL | - | Extrusion | Periodontal ligament |
| Tian et al., 2021 [65] | hPDLSCs | - | - | Extrusion | Bone |
| Ma et al., 2017 [64] | Rat PDLSCs | 1 × 106 cells/mL | ~90 | Inkjet | Bone |
| Athirasala et al., 2018 [43] | SCAPs | 0.8 × 106 cells/mL | >90% | Extrusion | Dentin/dental pulp |
| Kérourédan et al., 2018 [57] | SCAPs | 7 × 107 cells/mL | LAB | Bone | |
| Dutta et al., 2021 [54] | SCAPs | 2.5 × 104 cells/mL | - | Extrusion | Dental tissue |
| Touya et al., 2022 [59] | SCAPs | 2 × 103 cells/mL | - | LAB | Bone |
| Kérourédan et al., 2019 [58] | SCAPs and HUVECs | 7 × 107 cells/mL | - | LAB | Bone |
| Wang et al., 2021 [66] | Human gingiva fibroblasts | 5 × 105 cells/mL | - | Extrusion | Periodontal ligament/Bone |
| Ono et al., 2021 [71] | Human PDL cell line 1–17 | 2.5 × 104 cells/mL | - | Scaffold-free (Kenzan method) | Periodontal ligament |
| Kort-Mascort et al., 2021 [68] | Human SCC (Cell lines: UM-SCC-12 and UM-SCC-38) | 1 × 106 cells/mL | >95 | Extrusion | Dental tissue |
| Chimene et al., 2020 [46] | Human primary bone marrow-derived MSCs | - | - | Extrusion | Bone |
| Amler et al., 2021 [62] | Bone-derived MPC/Bone marrow MPC/Periosteal MPC | 20 × 106 cells/mL | - | Stereolithography | Bone |
| Moncal et al., 2021 [49] | Rat BMSCs | 5 × 106 cells/mL | >95 | Extrusion | Bone |
| Moncal et al., 2022 [50] | Rat BMSCs | 8 × 105 cells/mL | >95 | Extrusion | Bone |
| Aguilar et al., 2019 [44] | Mice bone marrow stromal cells | - | - | Scaffold-free (Kenzan method) | Bone |
| Aguilar et al., 2019 [45] | Mice bone marrow stromal cells | - | - | Scaffold-free (Kenzan method) | Bone |
| Keriquel et al., 2017 [56] | Mouse bone marrow stromal precursor D1 cell line | 120 × 106 cells/mL | - | LAB | Bone |
| Amler et al., 2021 [61] | JHOBs and HUVECs | 20 × 106 cells/mL | - | Stereolithography | Bone |
| Walladbegi et al., 2020 [70] | hADSCs | 4 × 106 cells/mL | ~80 | Extrusion | Bone |
| Kuss et al., 2017 [42] | Porcine stromal vascular fraction from adipose tissue | 4 × 106 cells/mL | - | Extrusion | Bone |
| Kang et al., 2016 [41] | hAFSCs | 5 × 106 cells/mL | 91 ± 2 | Extrusion | Bone |
| Author | Animal Model | Sex | Age | Weight | Defect Area | Defect Size | In Situ Printing | Time of Sacrifice |
|---|---|---|---|---|---|---|---|---|
| Keriquel et al., 2017 [56] | Balb/c mice | Female | 12 weeks | 19–20 g | Calvarium | 3.3 mm diameter | Yes | 8 weeks |
| Kérourédan et al., 2018 [57] | NOG mice | Female | 10 weeks | 25–26 g | Calvarium | 3.3 mm diameter | Yes | - |
| Kérourédan et al., 2019 [58] | NSG mice | Female | 10 weeks | 25–26 g | Calvarium | 3.3 mm diameter | Yes | 4 or 8 weeks |
| Touya et al., 2022 [59] | NSG mice | Female | 8 weeks | - | Calvarium | 3.3 mm diameter | Yes | 4 weeks or 8 weeks |
| Kang et al., 2016 [41] | Sprague Dawley rats | - | - | 250–300 g | Calvarium | 8 mm diameter, 1.2 mm depth | No | 20 weeks |
| Lee et al., 2021 [53] | Athymic rats | Male | 9 weeks | - | Calvarium | 8 mm diameter, 1.5 mm depth | No | 6 weeks |
| Wang et al., 2021 [66] | New Zealand white rabbit | Female | - | 2 kg | Calvarium | 7 mm diameter, 8 mm depth | No | 12 weeks |
| Ma et al., 2017 [64] | Sprague Dawley rats | - | 33 months | 230–250 g | Alveolar bone | 4 mm length × 3 mm width × 2 mm height | No | 3 and 6 weeks |
| Kim et al., 2022 [55] | Athymic nude mice | - | - | - | Dorsal subcutaneous | - | No | 8 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials 2022, 15, 6398. https://doi.org/10.3390/ma15186398
Mohd N, Razali M, Ghazali MJ, Abu Kasim NH. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials. 2022; 15(18):6398. https://doi.org/10.3390/ma15186398
Chicago/Turabian StyleMohd, Nurulhuda, Masfueh Razali, Mariyam Jameelah Ghazali, and Noor Hayaty Abu Kasim. 2022. "Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review" Materials 15, no. 18: 6398. https://doi.org/10.3390/ma15186398







