Abstract
The comprehensive treatment of periodontitis stage 2 to 4 aims at the resolution of periodontal inflammation and “pocket closure”, which implies a residual probing depth of ≤4 mm and a negative BoP. However, supportive periodontal therapy (SPT) regularly leaves behind persistent periodontal pockets with 5 or more mm in residual PPD and sites that often re-colonize and re-infect. Various adjunctive options for subgingival instrumentation have been proposed to enhance the antimicrobial effects to better control the re-infection of these residual sites. The locally applied adjuncts, based on their anti-inflammatory effect, are sodium hypochlorite antiseptic cleaning gel and cross-linked hyaluronic acid (xHyA). Both recently moved into the focus of clinical research on non-surgical and surgical therapy for periodontitis. The surgical use of xHyA indicates regenerative potential, supporting periodontal regeneration. This case series retrospectively analyzes the clinical benefits of the consecutive flapless application of sodium-hypochlorite-based cleaning gel and xHyA at the SPT to achieve pocket closure, thereby reducing the need for periodontal surgery. In 29 patients, 111 sites received the treatment sequence. At 6-month re-evaluation, an overall PPD reduction exceeding 2 mm was achieved, associated with a similar CAL gain (2.02 mm); the bleeding tendency (BoP) was reduced by >60%. Pocket closure occurred in almost 25% of all the sites. Within their limits, the present data suggest that the proposed combined adjunctive treatment of residual active periodontal sites yielded significant improvement in the clinical parameters. Further studies in RCT format are required to confirm these observations.
1. Introduction
Non-surgical periodontal treatment (NSPT) results in improved probing depth, clinical attachment level, and bleeding tendency [1]. The purpose of NSPT is the resolution of periodontal inflammation and a reduction in pocket-probing depth (PPD) to 4 mm or less, resulting in pocket closure. However, residual or recurring pockets exhibiting PPD values ≥4 mm are regularly found at re-evaluation. Residual periodontal pockets facilitate the accumulation of biofilm, leading to dysbiosis within the re-colonized subgingival habitat and, thus, to persistent inflammation [2,3]. Moreover, long-term data confirm the association between residual PPD and increased risk of tooth loss [4]. Therefore, as recommended in the European Federation of Periodontology (EFP) guidelines, continuous supportive periodontal therapy (SPT) accompanied by repeated instrumentation is imperative for sustained periodontal stability [5].
In an effort to improve the outcome of non-surgical instrumentation, a variety of adjunct treatment modalities are used. In addition to systemic antibiotics, a plethora of locally administered adjunctives seek to minimize both PPD and bleeding tendency, thereby facilitating the closure of the periodontal pocket.
Most of these adjunctive treatments are based upon the antimicrobial effects delivered by either photodynamic therapy (PDT) or the use of local antibiotic chemotherapy, preferably applied as a device with sustained release kinetics [6,7,8,9,10]. Furthermore, gelatin chips sustainably releasing chlorhexidine have been described [11,12,13]. Addressing the limitations of subgingival instrumentation on pocket-closure frequency, a recent systematic review and meta-analysis evaluated the additional benefit of locally applied adjunctive therapies. Even though the authors found effects of statistical significance, the magnitude of these benefits was deduced to be rather irrelevant to clinical success in terms of pocket closure [14]. Furthermore, the microbiological analysis of samples retrieved from persistent deep pockets before and after repeated local metronidazole application revealed high counts of periodontal pathogens [9].
By contrast, a novel amino-acid-buffered sodium hypochlorite cleaning gel exhibiting antimicrobial potential was significantly effective in improving the outcome of non-surgical therapy and, thus, significantly reduced counts of Gram-negative pathogens in an artificial biofilm model [15,16].
Another strategy to improve periodontal parameters is the local administration of regenerative biologics. In an attempt to harness its well-documented regenerative properties, a recent multi-center randomized controlled trial investigated the effect of enamel-matrix derivatives (EMDs) as an adjunct to the NSPT of patients situated in SPT [17]. The authors were able to show significantly greater pocket closure for sites treated with adjunctive EMD, demonstrating biologics-based regenerative technologies as promising supplements for non-surgical therapy.
Furthermore, a review with a meta-analysis showed that the adjuvant non-surgical administration of hyaluronic acid (HA) resulted in an improvement in both clinical attachment and probing depth [18]. Currently, however, there is a lack in protocols for adjuncts to NSPT combining both antimicrobial and regenerative properties. In this retrospective case series, we propose a novel two-step approach consisting of an amino-acid-buffered sodium hypochlorite cleaning gel to assist in the decontamination of the root surface, followed by the concomitant application of a cross-linked hyaluronic acid gel (xHyA) to facilitate healing and, thus, pocket closure. We report the retrospective analysis of 6-month clinical follow-up data from patients who qualified for this therapy.
2. Materials and Methods
The local ethics committee at the Witten/Herdecke University approved this retrospective evaluation of a clinical case series (S-203/2021). All the analyzed cases had been diagnosed with stage 2 to 4 periodontitis previously and had already undergone comprehensive periodontal therapy, as proposed by the EFP guidelines [5,19]. Four calibrated specialists and residents at the Department of Periodontology of Witten/Herdecke University were responsible for all treatment steps. Calibration of investigators was evaluated by analysis of variance (ANOVA), followed by Tukey’s post hoc analysis for multiple comparisons (p > 0.59 for all investigators). The decision to administer systemic antibiotics strictly complied with the EFP guidelines, following completion of initial subgingival instrumentation.
2.1. Inclusion Criteria
The proposed treatment applied to sites that exhibited persistent deep pocket depths after patients had undergone consecutive SPT re-evaluations at least twice. Sites ascribed to the treatment by protocol had never been subjected to any surgical intervention, even though patients may have received periodontal surgery at other sites. Specifically, persistent and recurrent periodontal pockets displaying ≥5 mm in PPD with positive BoP were included. The number of sites per patient assigned to the therapy was unrestricted. There was no limit to the localization of residual or recurrent pockets, and single- and multi-rooted teeth were included. In teeth with high PPD associated with furcation involvement of more than Class 1, only the change in vertical component of the defect was analyzed for this report.
2.2. Treatment Sequence
Four calibrated operators treated all patients; the operators agreed upon the treatment protocol before the first application. Following supragingival mechanical instrumentation, each site received subgingivally administrated sodium hypochlorite cleaning gel (Perisolv; Regedent AG, Zürich, Switzerland) for 30 to 45 s to support chemical disinfection and improve the scaling outcome. Subgingival instrumentation was carried out with Gracey curettes (Deppeler, American Dental Systems, Munich, Germany). The sodium hypochlorite cleaning-gel application was repeated until the instrumentation was considered sufficient (Figure 1). Sufficient instrumentation was attained when root surfaces exhibited smooth surfaces upon probing with an explorer probe (ODU 11/12 DH2, Deppeler, Rolle, Switzerland). Subsequently, 0.3 mL of the cross-linked hyaluronic acid gel (xHyA; hyaDENT BG, Regedent AG, Zürich, Switzerland) was applied into the subgingival pocket in a flapless manner until plenished. Patients were instructed to uphold daily mechanical biofilm control by means of interdental brushes and a toothbrush. Measures for oral hygiene were not adjusted in the operated area. Neither systemic antibiotics nor antiseptics for rinsing were prescribed by protocol. Within the next 7 days, a repeated subgingival xHyA application (0.3 mL) was conducted combined with the oral hygiene control. The first re-evaluation took place 5–6 months after treatment and the subsequent SPT interval was set to 3 months for a 12-month period. At the 12-month re-evaluation, a periapical radiograph taken with the parallel technique was obtained to verify the crestal bone level.
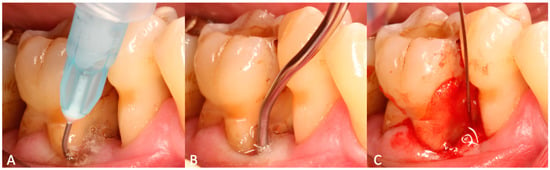
Figure 1.
Visualization of the applied treatment protocol. (A) Application of chloramine gel to the pocket for 30–45 s. (B) Scaling and root planning is performed. Chloramine gel may be applied repeatedly until non-surgical treatment is deemed sufficient. (C) Cross-linked hyaluronic acid (xHya) is applied to the pocket until plenished.
2.3. Statistical Analysis
For all obtained datasets, a descriptive data analysis was performed. Further statistical analyses included the Shapiro–Wilk, Kolmogorov–Smirnov, and D’Agostino–Pearson tests to assess data distribution. CAL gain and PPD reduction (pre–post) were both calculated by Wilcoxon signed-rank test, respectively. p-values of ≤0.05 were considered significant.
3. Results
This retrospective analysis included 29 patients with 111 treated teeth/sites, ranging from 1 to 17 per patient. The mean age was 54.6 years, and 69% were female (20:9; 69% vs. 31%). All patients were normo-glycemic and 7% (n = 2) were smokers. Table 1 discloses the demographics, habits, and health condition of the participants. All of them participated in the SPT program offered by the Department of Periodontology.

Table 1.
Patient demographics and mean clinical parameters before (pre) and after (post = 6 months) the treatment. CAL = clinical attachment level, PPD = probing pocket depth, BOP = bleeding on probing, * = Wilcoxon signed-rank test.
The mean PPD at baseline was 7.19 (±1.89) mm, and the CAL loss was 7.96 (±2.2) mm; 97.6% of all sites presented with positive BoP. Consecutive six-month re-evaluation revealed an overall PPD reduction of 2.04 mm and a clinical attachment level gain of 2.02 mm, indicating that no further progression in gingival recession occurred. The BoP frequency decreased to 40.1%. Stratified by furcation involvement (12 teeth), the mean CAL gain was 1.5 mm (p = 0.0195), whereas the treatment of single-rooted teeth resulted in a 2.04 mm (p < 0.001) CAL gain (Table 2 and Table 3, Figure 2). Both measurements yielded statistically significant differences compared to the baseline values. In terms of pocket closure, 25 out of 99 (25.25%) sites in the single-rooted teeth exhibited pocket closure, with a PPD < 4 mm and a negative BoP.

Table 2.
Descriptive statistics of PPD and CAL development in furcation-involved sites after combined chloramine and xHya treatment. Pre = baseline, Post = 6 months post treatment, * = Wilcoxon signed-rank test.

Table 3.
Descriptive statistics of PPD and CAL development in sites without furcation involvement after combined chloramine and xHya treatment. Pre = baseline, Post = 6 months post treatment, * = Wilcoxon signed-rank test.
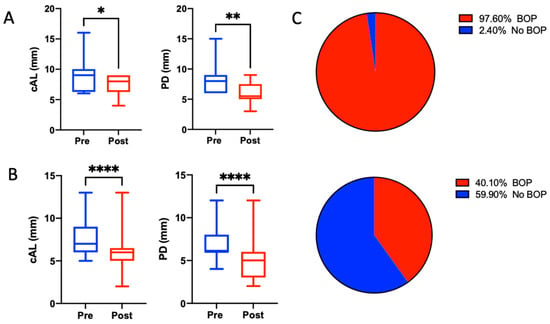
Figure 2.
Boxplots for clinical parameters before and after the treatment sequence of non-furcation-involved (A) and furcation-involved (B) sites. Whiskers represent minimum and maximum values. * p < 0.05, ** p < 0.01, **** p < 0.0001. (C) Amount of sites exhibiting bleeding on probing before (upper) and after (lower) the treatment.
4. Discussion
This retrospective case series shows that the combination of an antiseptic adjunctive cleaning gel and xHyA applied subgingivally for the treatment of persistently deep periodontal pockets at SPT visit yielded clinically relevant improvements in PPD reduction, CAL gain, and BoP frequency. The follow-up of the reported cases revealed statistically significant improvement in all three of these parameters. The overall CAL gain exceeded 2 mm on average in sites previously classified as non-responding and persistent. Although a minor number of treated sites exhibited complete pocket closure after three to six months, the two-component flapless adjunctive treatment considerably reduced the need for periodontal surgery. Sites ascribed to surgical step3 therapy according to the EFP guidelines clinically improved to such an extent that the periodontal surgery became redundant. To the best of our knowledge, this is the first report of the combined use of antiseptic and biologic approaches in flapless periodontal treatment. As each site received both adjunctive materials administered at one visit, we must emphasize that a discussion of the individual contributions to the results appeared unnecessary.
Recent in vitro, pre-clinical, and clinical studies investigated either the sodium hypochlorite cleaning gel or the xHyA application in a separate manner. The antimicrobial effects of the sodium hypochlorite cleaning gel became evident [16,20]. Cell-based experiments also disclosed the high level of cytocompatibility of its compounds [20,21]. However, the benefits of adjunctive sodium hypochlorite cleaning gel for NSPT remain controversial. Sodium hypochlorite gel failed to affect the clinical outcome of ultrasonic or manual subgingival instrumentation in SPT treatment. Nevertheless, its use was associated with significantly reduced recolonization of the sites by T. denticola and T. forsythia [22]. By contrast, the adjunctive benefit of sodium hypochlorite gel formulation for minimally invasive non-surgical therapy (MINST) was positively evaluated by a recent RCT [15]. The authors compared the outcome of step-2 therapy after delivering it to untreated stage-3 and -4 periodontitis patients in both study arms. Moreover, in an RCT study from a Scandinavian research group, diabetic foot ulcers resolved significantly quicker under treatment with this cleaning gel formulation than those in the control group [23].
Hyaluronic acid (HA) is a glycosaminoglycan heteropolysaccharide and, in its native form, it is both a light-molecular-weight (LMWHA) and a high-molecular-weight long polymer (HMWHA) [24]. HA is an important natural component of the extracellular matrix and is almost ubiquitously present in mammalian tissues, including the periodontium [25]. Several studies confirmed bacteriostatic [26,27], fungostatic [28], anti-inflammatory [29], anti-edematous [30], osteoinductive [29,31,32,33], and pro-angiogenic [34] properties of HA. In animal studies on skin wounds, HA promoted enhanced connective-tissue elasticity and healing, improved re-epithelialization, and appeared to increase microvascular density [34,35]. HA sufficiently improved wound healing in extraoral wounds, skin ulcers, and intraoral injuries [36,37,38].
The potential of xHyA to promote periodontal regeneration became a subject in a recent series of histological evaluations in dogs’ mandibles. The histomorphometric assessments revealed that xHyA-treated intraosseous and furcation sites formed significantly greater areas of new cementum and periodontal ligament fibers on previously exposed root surfaces. Similar observations were made from the same treatment sequence applied in gingival recessions [39,40,41].
The clinical results mediated by xHyA indicated a substantial benefit, which was corroborated by both a recent RCT study and a case series [42,43]. Beyond the positive effects of xHyA unfolded in the surgical context, its adjunctive use in NSPT yielded inconsistent outcomes in clinical studies [44,45,46].
In our retrospective analysis, we found a significant probing-depth reduction accompanied by a significant gain in clinical attachment (Figure 2). Moreover, the needlessness of root conditioning and drying the wound area increased the ease of handling and delivered strong arguments in favor of xHyA as an adjunct to flapless subgingival instrumentation, as well as accounting for its hygroscopic/wound-stabilizing and regenerative properties. In addition, compliance with the second visit scheduled for repeated xHyA application was high in all the patients. With respect to the proposed protocol, the sodium hypochlorite cleaning-gel application may offer further advantages to NSPT by means of improving the mechanical biofilm removal, thus enhancing the effects of the xHyA. Therefore, we consider the proposed protocol highly beneficial for NSPT. However, the presented results require further confirmation by randomized controlled clinical trials, which may also account for the exposure time and application frequency of the hypochlorite gel.
Author Contributions
D.D., A.F. contributed to conception, design, data acquisition, and interpretation, performed all the statistical analyses, and drafted and critically revised the manuscript. P.L.; R.M.J. contributed to data acquisition and critically revised the manuscript. H.B. contributed to the conception, design, and data acquisition and critically revised the manuscript. A.S. critically proofread and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. D.D. was supported by an internal Ph.D. scholarship of the Witten/Herdecke University.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local Ethics Committee of Witten/Herdecke University (S-203/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available because they were derived from patients.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 155–175. [Google Scholar] [CrossRef]
- Kebschull, M.; Chapple, I. Evidence-based, personalised and minimally invasive treatment for periodontitis patients—the new EFP S3-level clinical treatment guidelines. Br. Dent. J. 2020, 229, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Matuliene, G.; Pjetursson, B.E.; Salvi, G.E.; Schmidlin, K.; Brägger, U.; Zwahlen, M.; Lang, N.P. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J. Clin. Periodontol. 2008, 35, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; On behalf of the EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 176–198. [Google Scholar] [CrossRef]
- Cosgarea, R.; Eick, S.; Batori-Andronescu, I.; Jepsen, S.; Arweiler, N.B.; Rößler, R.; Conrad, T.; Ramseier, C.A.; Sculean, A. Clinical and Microbiological Evaluation of Local Doxycycline and Antimicrobial Photodynamic Therapy during Supportive Periodontal Therapy: A Randomized Clinical Trial. Antibiotics 2021, 10, 277. [Google Scholar] [CrossRef]
- Christodoulides, N.; Nikolidakis, D.; Chondros, P.; Becker, J.; Schwarz, F.; Rössler, R.; Sculean, A. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: A randomized, controlled clinical trial. J. Periodontol. 2008, 79, 1638–1644. [Google Scholar] [CrossRef]
- Riep, B.; Purucker, P.; Bernimoulin, J.P. Repeated local metronidazole-therapy as adjunct to scaling and root planing in maintenance patients. J. Clin. Periodontol. 1999, 26, 710–715. [Google Scholar]
- Rudhart, A.; Purucker, P.; Kage, A.; Hopfenmüller, W.; Bernimoulin, J.P. Local metronidazole application in maintenance patients. Clinical and microbiological evaluation. J. Periodontol. 1998, 69, 1148–1154. [Google Scholar]
- Jeffcoat, M.K.; Palcanis, K.G.; Weatherford, T.W.; Reese, M.; Geurs, N.C.; Flashner, M. Use of a biodegradable chlorhexidine chip in the treatment of adult periodontitis: Clinical and radiographic findings. J. Periodontol. 2000, 71, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kasaj, A.; Chiriachide, A.; Willershausen, B. The adjunctive use of a controlled-release chlorhexidine chip following treatment with a new ultrasonic device in supportive periodontal therapy: A prospective, controlled clinical study. Int. J. Dent. Hyg. 2007, 5, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Mombelli, A.; Mayfield, L.; Rutar, A.; Suvan, J.; Garrett, S.; Lang, N.P. Local antimicrobial therapy after initial periodontal treatment. J. Clin. Periodontol. 2002, 29, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Matesanz, P.; Martín, C.; Oud, V.; Feres, M.; Teughels, W. Adjunctive effect of locally delivered antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 239–256. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Ramaglia, L.; Isola, G.; Blasi, A.; Salvi, G.E.; Sculean, A. Changes in clinical parameters following adjunctive local sodium hypochlorite gel in minimally invasive nonsurgical therapy (MINST) of periodontal pockets: A 6-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5331–5340. [Google Scholar] [CrossRef]
- Jurczyk, K.; Nietzsche, S.; Ender, C.; Sculean, A.; Eick, S. In-vitro activity of sodium-hypochlorite gel on bacteria associated with periodontitis. Clin. Oral Investig. 2016, 20, 2165–2173. [Google Scholar] [CrossRef]
- Jentsch, H.F.; Roccuzzo, M.; Pilloni, A.; Kasaj, A.; Fimmers, R.; Jepsen, S. Flapless application of enamel matrix derivative in periodontal retreatment: A multicentre randomized feasibility trial. J. Clin. Periodontol. 2021, 48, 659–667. [Google Scholar] [CrossRef]
- Eliezer, M.; Imber, J.C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef]
- Mueller, A.; Fujioka-Kobayashi, M.; Mueller, H.D.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. Effect of hyaluronic acid on morphological changes to dentin surfaces and subsequent effect on periodontal ligament cell survival, attachment, and spreading. Clin. Oral Investig. 2017, 21, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Megally, A.; Zekeridou, A.; Cancela, J.; Giannopoulou, C.; Mombelli, A. Short ultrasonic debridement with adjunctive low-concentrated hypochlorite/amino acid gel during periodontal maintenance: Randomized clinical trial of 12 months. Clin. Oral Investig. 2020, 24, 201–209. [Google Scholar] [CrossRef]
- Bergqvist, K.; Almhöjd, U.; Herrmann, I.; Eliasson, B. The role of chloramines in treatment of diabetic foot ulcers: An exploratory multicentre randomised controlled trial. Clin. Diabetes Endocrinol. 2016, 2, 6. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Trimmel, B.; Gede, N.; Hegyi, P.; Szakács, Z.; Mezey, G.A.; Varga, E.; Kivovics, M.; Hanák, L.; Rumbus, Z.; Szabó, G. Relative performance of various biomaterials used for maxillary sinus augmentation: A Bayesian network meta-analysis. Clin. Oral. Implants Res. 2021, 32, 135–153. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, X.; Qin, L.; Zhai, M.; Yuan, J.; Chen, J.; Li, D. Effect of hyaluronic acid in bone formation and its applications in dentistry. J. Biomed. Mater. Res. Part A 2016, 104, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.A.; Dragoo, J.L.; Samimi, B.; Bruckner, D.A.; Bernard, G.W.; Hedrick, M.; Benhaim, P. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem. Biophys. Res. Commun. 2004, 321, 472–478. [Google Scholar] [CrossRef]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A. Bacteriostatic effects of hyaluronic acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, Y.Y.; Chang, J.Y.; Kho, H.S. Influences of hyaluronic acid on the anticandidal activities of lysozyme and the peroxidase system. Oral Dis. 2011, 17, 577–583. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, C. Stimulation of osteoinduction in bone wound healing by high-molecular hyaluronic acid. Bone 1995, 16, 9–15. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R. Hyaluronic Acid: A boon in periodontal therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- de Brito Bezerra, B.; Mendes Brazão, M.A.; de Campos, M.L.G.; Casati, M.Z.; Sallum, E.A.; Sallum, A.W. Association of hyaluronic acid with a collagen scaffold may improve bone healing in critical-size bone defects. Clin. Oral Implants Res. 2012, 23, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Ariyoshi, W.; Iwanaga, K.; Okinaga, T.; Habu, M.; Yoshioka, I.; Tominaga, K.; Nishihara, T. Mechanism involved in enhancement of osteoblast differentiation by hyaluronic acid. Biochem. Biophys. Res. Commun. 2011, 405, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.M.; Silva, G.A.; Lima, M.F.; Calliari, M.V.; Almeida, A.P.; Alves, J.B.; Ferreira, A.J. Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch. Oral Biol. 2008, 53, 1155–1162. [Google Scholar] [CrossRef]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Correction to: Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral Investig. 2018, 22, 2961–2962. [Google Scholar] [CrossRef]
- Yıldırım, S.; Özener, H.Ö.; Doğan, B.; Kuru, B. Effect of topically applied hyaluronic acid on pain and palatal epithelial wound healing: An examiner-masked, randomized, controlled clinical trial. J. Periodontol. 2018, 89, 36–45. [Google Scholar] [CrossRef]
- Humbert, P.; Mikosinki, J.; Benchikhi, H.; Allaert, F.A. Efficacy and safety of a gauze pad containing hyaluronic acid in treatment of leg ulcers of venous or mixed origin: A double-blind, randomised, controlled trial. Int. Wound J. 2013, 10, 159–166. [Google Scholar] [CrossRef]
- Juhasz, I.; Zoltan, P.; Erdei, I. Treatment of partial thickness burns with Zn-hyaluronan: Lessons of a clinical pilot study. Ann. Burn. Fire Disasters 2012, 25, 82–85. [Google Scholar]
- Shirakata, Y.; Nakamura, T.; Kawakami, Y.; Imafuji, T.; Shinohara, Y.; Noguchi, K.; Sculean, A. Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a cross-linked hyaluronic acid gel. An experimental study in dogs. J. Clin. Periodontol. 2021, 48, 570–580. [Google Scholar] [CrossRef]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Shinohara, Y.; Iwata, M.; Setoguchi, F.; Noguchi, K.; Sculean, A. Cross-linked hyaluronic acid-gel with or without a collagen matrix in the treatment of class III furcation defects: A histologic and histomorphometric study in dogs. J. Clin. Periodontol. 2022. ahead of print. [Google Scholar] [CrossRef]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Kawakami, Y.; Shinohara, Y.; Noguchi, K.; Pilloni, A.; Sculean, A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: A preclinical study in dogs. Quintessence Int. 2021, 308–316. [Google Scholar] [CrossRef]
- Pilloni, A.; Rojas, M.A.; Marini, L.; Russo, P.; Shirakata, Y.; Sculean, A.; Iacono, R. Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either hyaluronic acid or an enamel matrix derivative: A 24-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5095–5107. [Google Scholar] [CrossRef]
- Božić, D.; Ćatović, I.; Badovinac, A.; Musić, L.; Par, M.; Sculean, A. Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral. Materials 2021, 14, 6795. [Google Scholar] [CrossRef]
- Pilloni, A.; Zeza, B.; Kuis, D.; Vrazic, D.; Domic, T.; Olszewska-Czyz, I.; Popova, C.; Kotsilkov, K.; Firkova, E.; Dermendzieva, Y.; et al. Treatment of Residual Periodontal Pockets Using a Hyaluronic Acid-Based Gel: A 12 Month Multicenter Randomized Triple-Blinded Clinical Trial. Antibiotics 2021, 10, 924. [Google Scholar] [CrossRef]
- Olszewska-Czyz, I.; Kralik, K.; Prpic, J. Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis. Biomolecules 2021, 11, 1491. [Google Scholar] [CrossRef]
- Rajan, P.; Baramappa, R.; Rao, N.M.; Pavaluri, A.K.; Indeevar, P.; Rahaman, S.M.U. Hyaluronic Acid as an adjunct to scaling and root planing in chronic periodontitis. A randomized clinical trail. J. Clin. Diagn. Res. JCDR 2014, 8, ZC11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).