Parametric Study towards Optimization of a Short Duration Carbonation Process of Recycled Cement Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbonation Methods
2.3. Characterization Methods
3. Results and Discussion
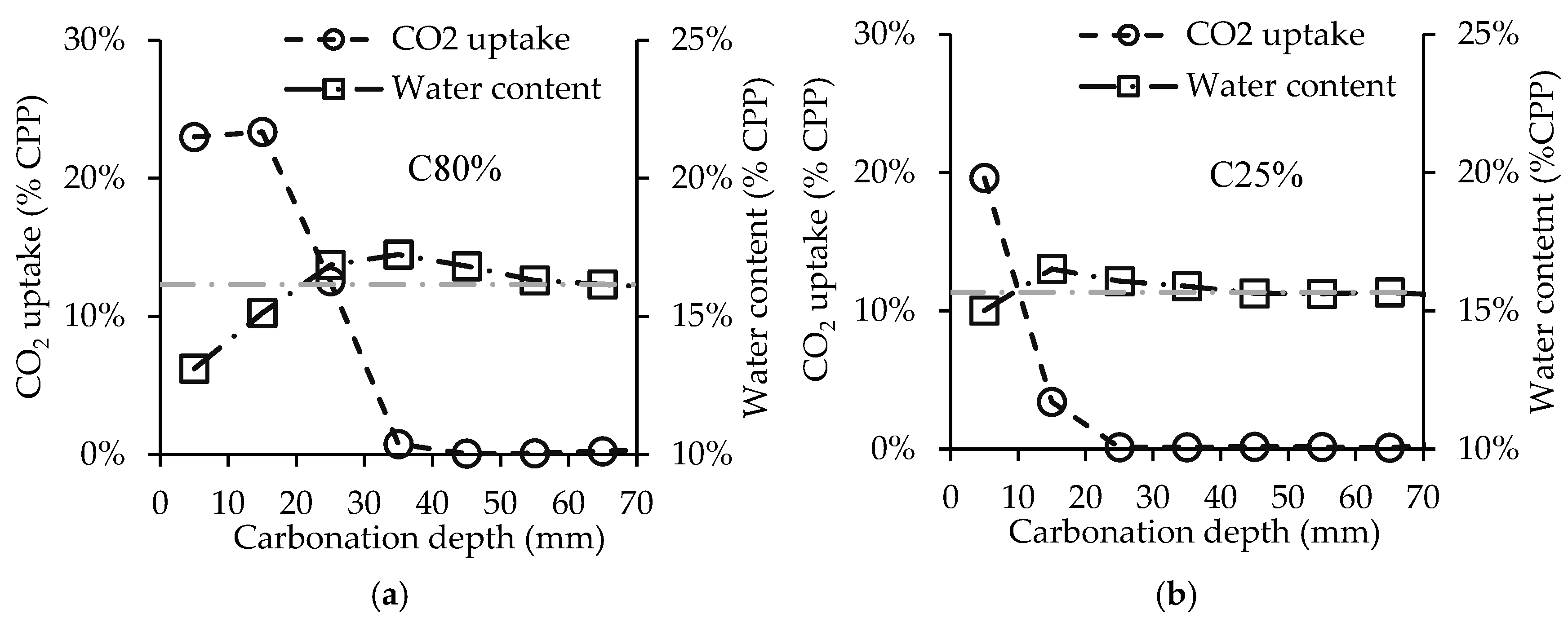
3.1. Influence of Initial Water Content
3.2. Influence of CO2 Concentration
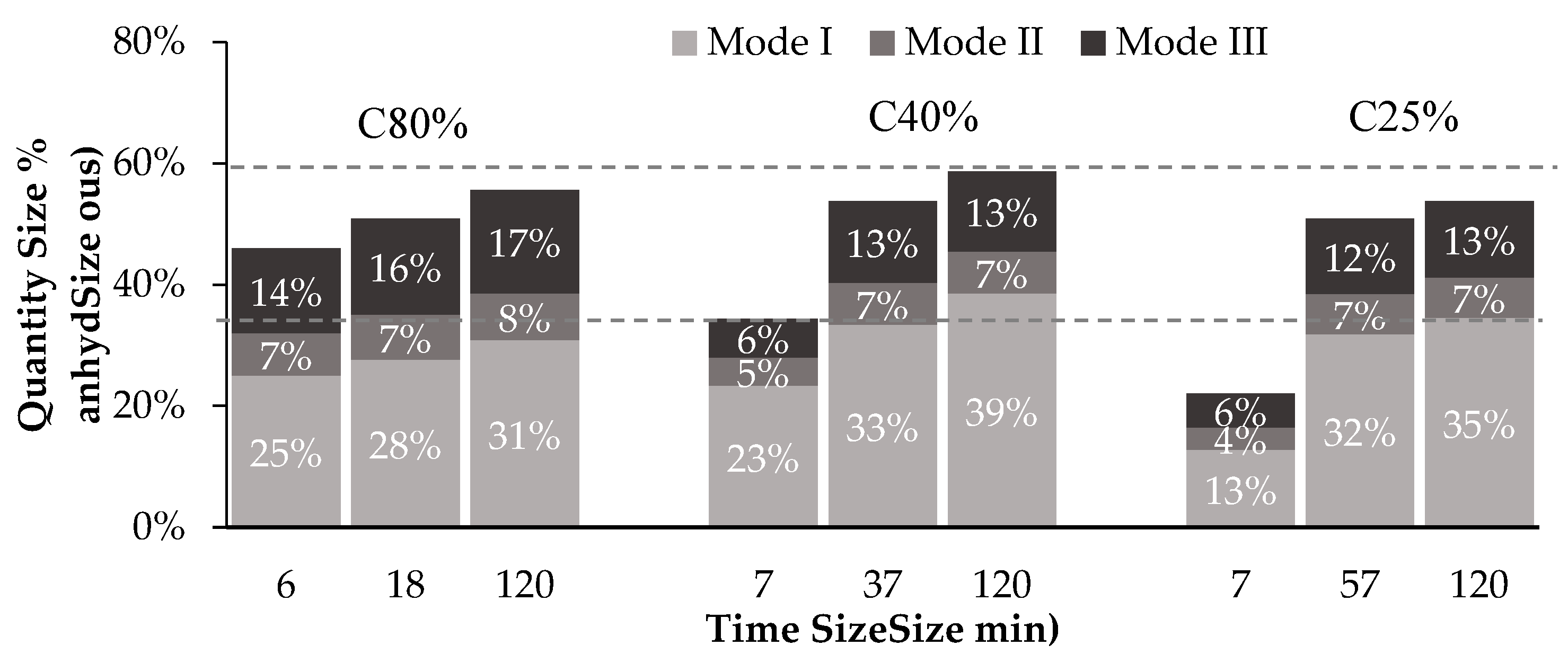
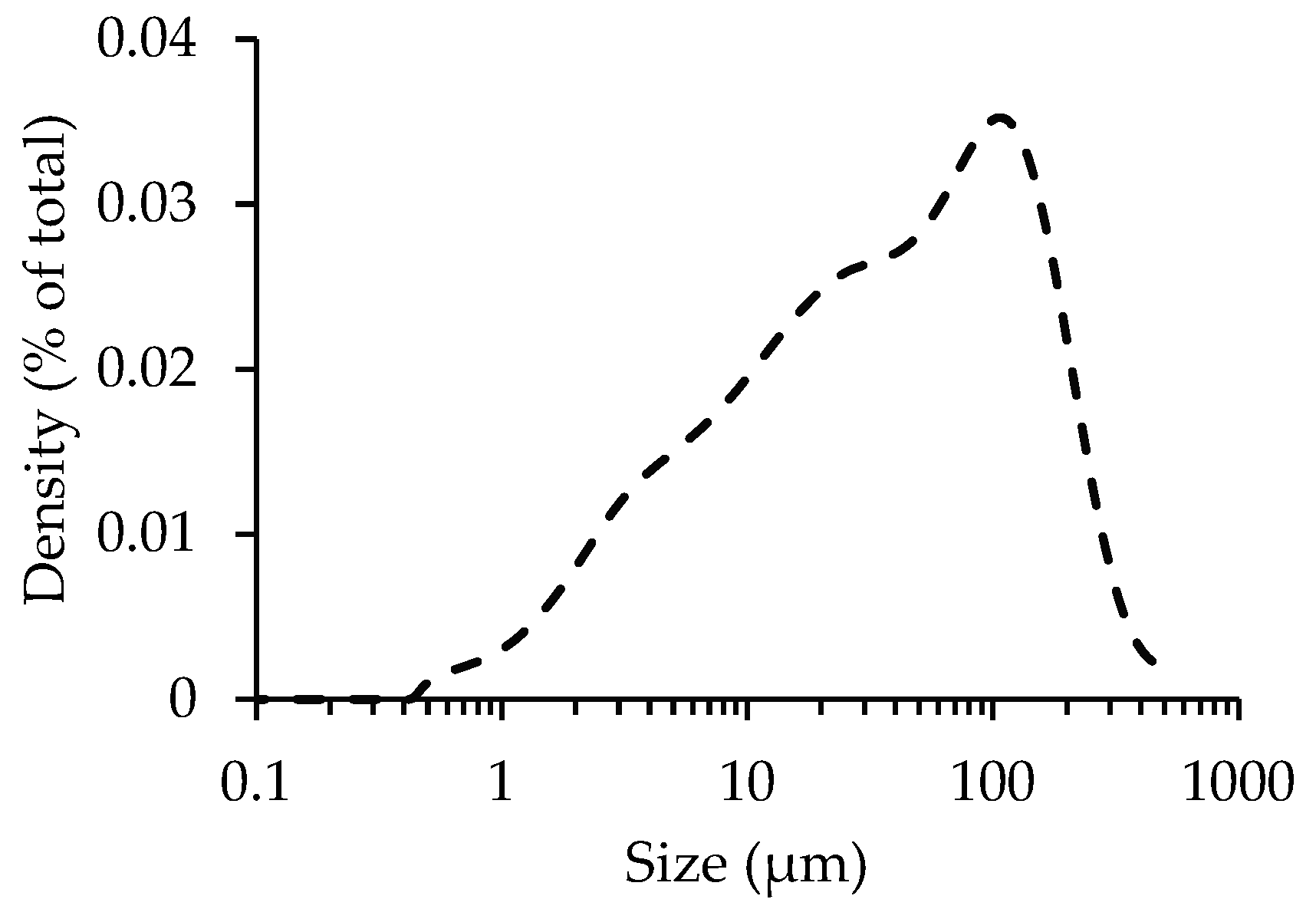
3.3. Influence of Particle Size
3.4. Influence of Powder Thickness
3.5. Influence of the Amount of CPP in Concrete Waste
4. Conclusions
- Adopting an initial water content in CPP between 16–21% provided an increase in the CO2 uptake that can reach 20%.
- After two hours, the carbonation degree of CPP was similar for the different CO2 concentrations. While the higher CO2 concentration provided more intense C-S-H carbonation, this was counteracted by the shield effect of , which prevented the complete carbonation of CH. However, the evolution of the CPP carbonation rate within the two-hour process indicates that the minimum resident time for maximum CO2 uptake decreases with increasing CO2 concentration.
- The reduction of the maximum CPP particle dimension showed a limited increase in CO2 uptake, specifically, an increase of 1.3% for a size reduction from 250 µm to 75 µm.
- The CO2 concentration had a positive impact on the carbonation depth, which may be relevant in industrial applications where the material under carbonation reaches higher quantities.
- The short-term carbonation process was able to provide similar carbonation degrees of the hydrated cement paste regardless of the presence of aggregate impurities in the CPP powder.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1SP | First stopping point |
| 2SP | Second stopping point |
| 3SP | Third stopping point |
| ACC | Amorphous calcium carbonate |
| AFt | Ettringite |
| C25% | carbonation tests with a CO2 concentration of 25% |
| C40% | carbonation tests with a CO2 concentration of 40% |
| C80% | carbonation tests with a CO2 concentration of 80% |
| Calcium carbonate | |
| CCUS | Carbon capture utilization and storage |
| CH | calcium hydroxide |
| CO2c | CO2 concentration parameter |
| CPP | cement paste powder |
| CPPc | CPP originated from concrete |
| CPPp | CPP originated from a cement paste |
| C-S-H | calcium silicate hydrate |
| IFM | Ignition by furnace method |
| IWC | Initial water content parameter |
| LOI | Loss on ignition |
| LT | Layer thickness parameter |
| PS | Particle size parameter |
| PT | CPP powder type parameter |
| RH | Relative humidity |
| SCM | supplementary cementitious materials |
| SPs | Stopping points |
| TGA | Thermogravimetric analysis |
References
- Filippo, J.D.; Karpman, J.; DeShazo, J.R. The impacts of policies to reduce CO2 emission within the concrete supply chain. Cem. Concr. Compos. 2019, 101, 67–82. [Google Scholar] [CrossRef]
- UN Environment; Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Lippiatt, N.; Ling, T.-C.; Pan, S.-Y. Towards carbon-neutral construction materials: Carbonation of cement-based materials and the future perspective. J. Build. Eng. 2020, 28, 101062. [Google Scholar] [CrossRef]
- Kwon, E.; Ahn, J.; Cho, B.; Park, D. A study on development of recycled cement made from waste cementitious powder. Constr. Build. Mater. 2015, 83, 174–180. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 emissions from OPC and recycled cement production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Ling, T.-C.; Cheng, X.; Mo, K.H. Effect of particle size and CO2 treatment of waste cement powder on properties of cement paste. Can. J. Civ. Eng. 2020, 48, 522–531. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Zhang, J.; Wang, J. Effects of carbonated hardened cement paste powder on hydration and microstructure of Portland cement. Constr. Build. Mater. 2018, 186, 699–708. [Google Scholar] [CrossRef]
- Wu, H.; Liang, C.; Xiao, J.; Ma, Z. Properties and CO2-curing enhancement of cement-based materials containing various sources of waste hardened cement paste powder. J. Build. Eng. 2021, 44, 102677. [Google Scholar] [CrossRef]
- Skocek, J.; Zajac, M.; Haha, M.B. Carbon Capture and Utilization by mineralization of cement pastes derived from recycled concrete. Nat. Res. 2020, 10, 5614. [Google Scholar] [CrossRef]
- International Energy Agency. Cement Sustainability Initiative, Technology Roadmap: Low-Carbon Transition in the Cement Industry. 2018. Available online: https://www.iea.org/reports/technology-roadmap-low-carbon-transition-in-the-cement-industry (accessed on 1 June 2022).
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2020, 105, 103446. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, Z.; Wu, H.; Wang, W. The utilization of eco-friendly recycled powder from concrete and brick waste in new concrete: A critical review. Cem. Concr. Compos. 2020, 114, 103807. [Google Scholar] [CrossRef]
- Sáez, P.V.; Osmani, M. A diagnosis of construction and demolition waste generation and recovery practice in the European Union. J. Clean. Prod. 2019, 241, 188400. [Google Scholar]
- Gálvez-Martos, J.-L.; Styles, D.; Schoenberger, H.; Zeschmar-Lahl, B. Construction and demolition waste best management practice in Europe. Resources, Conservation & Recycling 2018, 136, 166–178. [Google Scholar]
- Oikonomou, N.D. Recycled concrete aggregates. Cem. Concr. Compos. 2005, 27, 315–318. [Google Scholar] [CrossRef]
- Mostashari-Rad, F.; Ghasemi-Mobtaker, H.; Taki, M.; Ghahderijani, M.; Saber, Z.; Chau, K.-W.; Nabavi-Pelesaraei, A. Data supporting midpoint-weighting life cycle assessment and energy forms of cumulative exergy demand for horticultural crops. Data Brief 2020, 33, 106490. [Google Scholar] [CrossRef] [PubMed]
- Carriço, A.; Bogas, J.A.; Hu, S.; Real, S.; Pereira, M.F.C. Novel separation process for obtaining recycled cement and high-quality recycled sand from waste hardened concrete. J. Clean. Prod. 2021, 309, 127375. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. A maturity approach to estimate compressive strength developmentof CO2-cured concrete blocks. Cem. Concr. Compos. 2018, 85, 153–160. [Google Scholar] [CrossRef]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Huang, H.; Yin, S. Effects of Ca/Si ratio, aluminum and magnesium on the carbonation behavior of calcium silicate hydrate. Materials 2019, 12, 1268. [Google Scholar] [CrossRef]
- Sevelsted, T.F.; Skibsted, J. Carbonation of C–S–H and C–A–S–H samples studied by 13C, 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2015, 71, 56–65. [Google Scholar] [CrossRef]
- Wen, Y.; Xiang, L.; Jin, Y. Synthesis of plate-like calcium carbonate via carbonation route. Mater. Lett. 2003, 57, 2565–2571. [Google Scholar] [CrossRef]
- Silva, A.; Nogueira, R.; Bogas, A.; Abrantes, J.; Wawrzynczak, D.; Sciubidlo, A.; Majchrzak-Kuceba, I. Valorisation of recycled cement paste: Feasibility of a short duration carbonation process. Materials 2022, 15, 6001. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Shi, C.; Poon, C.S. Methods for the assessment of carbon dioxide absorbed by cementitious materials. In Carbon Dioxide Sequestration in Cementitious Construction Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 103–126. [Google Scholar]
- Liu, Z.; Meng, W. Fundamental understanding of carbonation curing and durability of carbonation-cured cement-based composites: A review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Galan, A.; Glasser, F.P.; Baza, D.; Andrade, C. Assessment of the protective effect of carbonation on portlandite crystals. Cem. Concr. Res. 2015, 74, 68–77. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Elucidating the accelerated carbonation products of calcium silicates using multi-technique approach. J. CO2 Util. 2018, 23, 61–74. [Google Scholar] [CrossRef]
- Wang, D.; Fang, Y.; Zhang, Y.; Chang, J. Changes in mineral composition, growth of calcite crystal, and promotion of physico-chemical properties induced by carbonation of β-C2S. J. CO2 Util. 2019, 34, 149–162. [Google Scholar] [CrossRef]
- Kaddah, F.; Ranaivomanana, H.; Amiri, O.; Roziere, E. Accelerated carbonation of recycled concrete aggregates: Investigation on the microstructure and transport properties at cement paste and mortar scales. J. CO2 Util. 2022, 57, 101885. [Google Scholar] [CrossRef]
- Auroy, M.; Poyet, S.; Bescop, P.L.; Torrenti, J.-M.; Charpentier, T.; Moskura, M.; Bourbon, X. Comparison between natural and accelerated carbonation (3% CO2): Impact on mineralogy, microstructure, water retention and cracking. Cem. Concr. Res. 2018, 109, 64–80. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Lin, S.; Tang, L.; Dong, Z.; Xing, F.; Dong, B.; Hong, S. Changes in chemical phases and microscopic characteristics of fly ash blended cement pastes in different CO2 concentrations. Constr. Build. Mater. 2020, 257, 119598. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.-Q.; Tang, L.-P.; Dong, Z.-J. XRD and 29Si MAS NMR study on carbonated cement paste under accelerated carbonation using different concentration of CO2. Mater. Today Commun. 2019, 19, 464–470. [Google Scholar] [CrossRef]
- Vogler, N.; Drabetzki, P.; Lindemann, M.; Kuhne, H.-C. Description of the concrete carbonation process with adjusted depth resolved thermogravimetric analysis. J. Therm. Anal. Calorim. 2021, 147, 6167–6180. [Google Scholar] [CrossRef]
- Wang, D.; Noguchi, T.; Nozaki, T. Increasing efficiency of carbon dioxide sequestration through high temperature carbonation of cement-based materials. J. Clean. Prod. 2019, 238, 117980. [Google Scholar] [CrossRef]
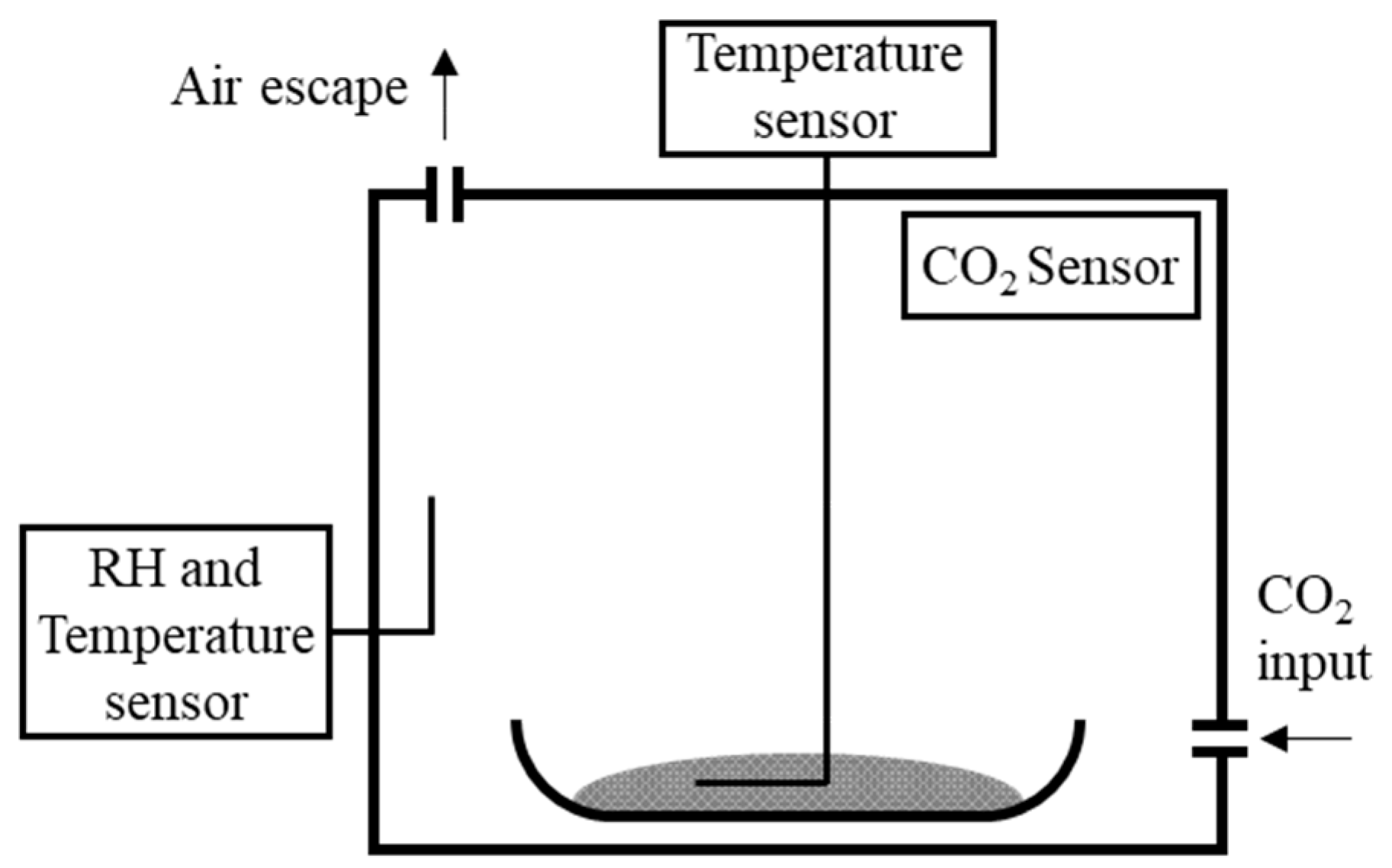
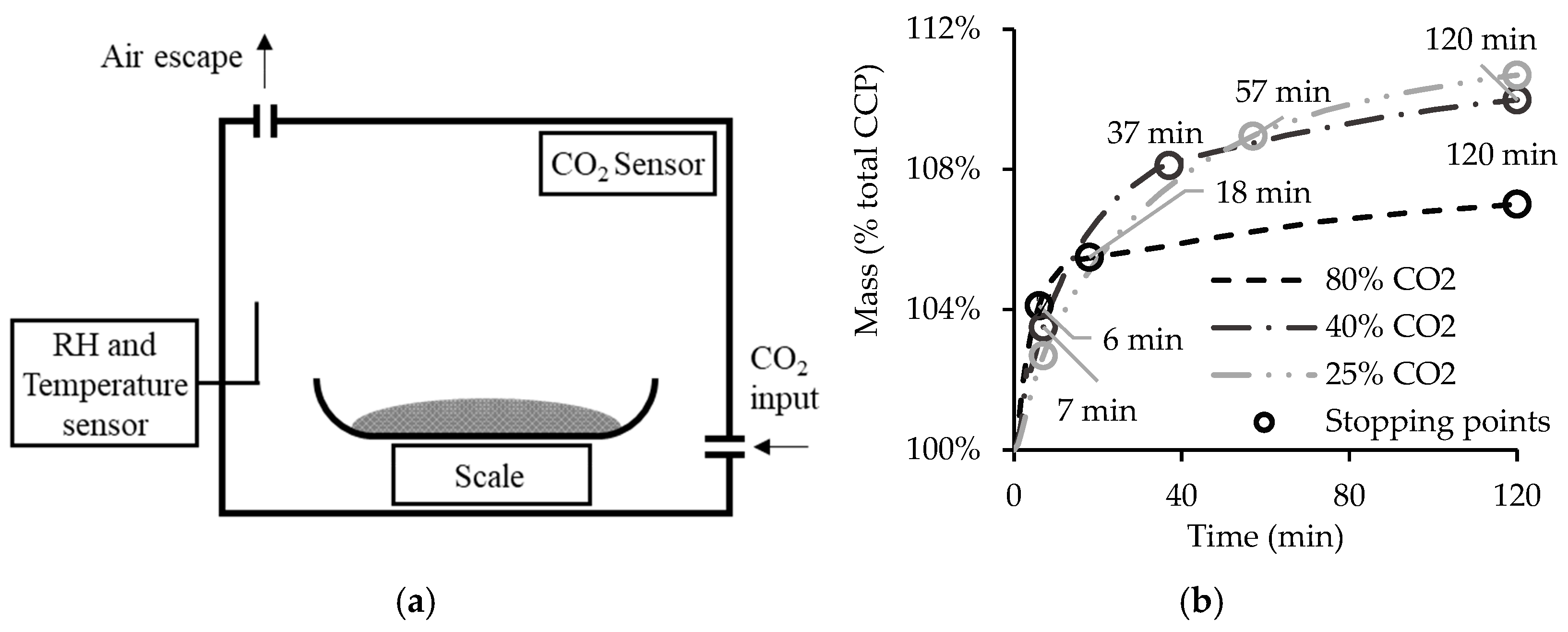
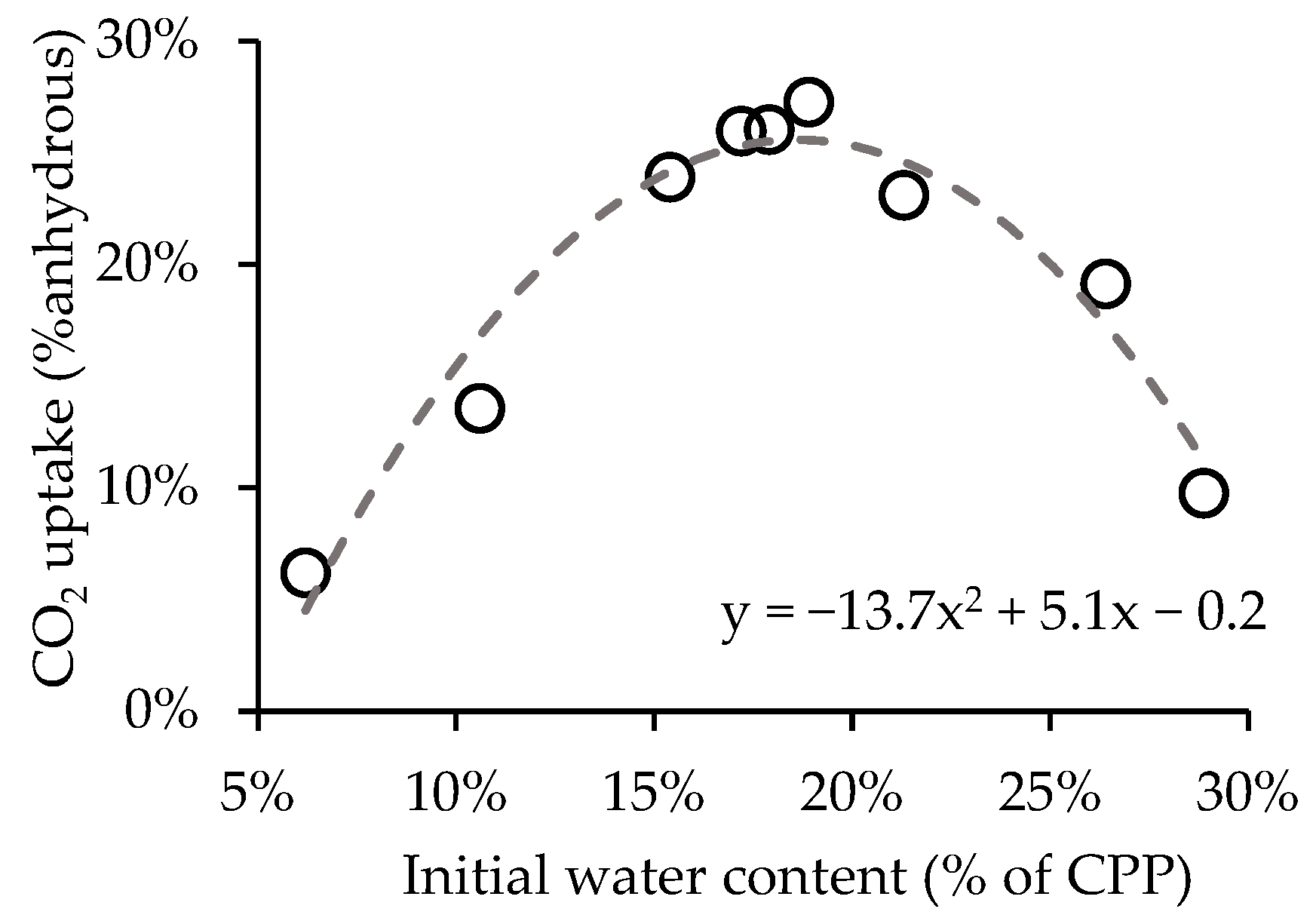


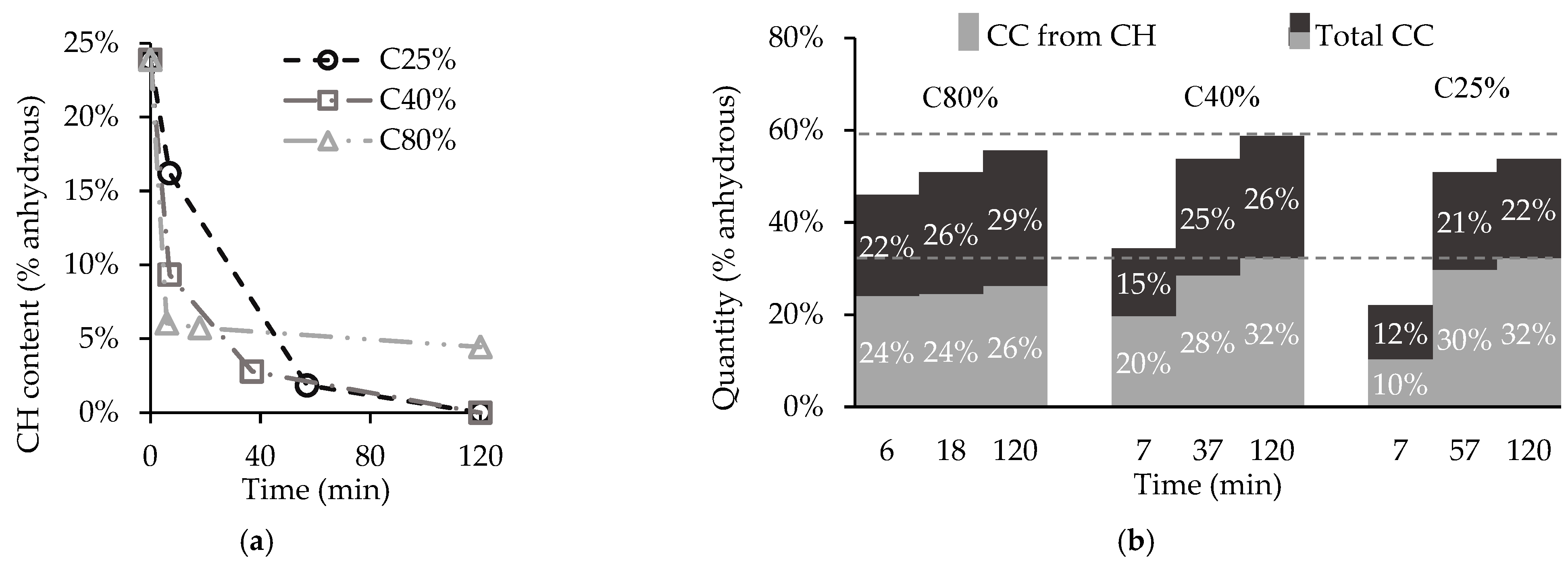


| Aggregate Type | w/c | OPC | Coarse Aggregate | Fine Aggregate | Water |
|---|---|---|---|---|---|
| Limestone gravel | 0.55 | 360 kg/m3 | 406 L/m3 | 260 L/m3 | 198 L/m3 |
| Testing Conditions Applied in Each Section | Testing Parameter Analysed in Each Section | ||||
|---|---|---|---|---|---|
| 3.1. IWC | 3.2. CO2c | 3.3. PS | 3.4. LT | 3.5. PT | |
| Initial water content (IWC) | ≈0% to 30% | 15–20% | 15–20% | 15–20% | 15–20% |
| CO2 content (CO2c) | 80% | 25%, 40%, 80% | 80%, 25% | 80%, 25% | 80%, 25% |
| Particle size (PS) | 250 µm | 250 µm | 250, 75 µm | 250 µm | 250 µm |
| Layer thickness (LT) | 5 mm | 5 mm | 5 mm | 0–70 mm | 5 mm |
| CPP powder type (PT) | CPPp | CPPp | CPPp | CPPp | CPPp, CPPc |
| Testing techniques | IFM | TGA | IFM | IFM | IFM |
| Stopping points | 3SPµ | 1SP, 2SP, 3SP | 2SP, 3SP | 3SP | 2SP, 3SP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Nogueira, R.; Bogas, A.; Wawrzyńczak, D.; Ściubidło, A.; Majchrzak-Kucęba, I. Parametric Study towards Optimization of a Short Duration Carbonation Process of Recycled Cement Paste. Materials 2022, 15, 6513. https://doi.org/10.3390/ma15196513
Silva A, Nogueira R, Bogas A, Wawrzyńczak D, Ściubidło A, Majchrzak-Kucęba I. Parametric Study towards Optimization of a Short Duration Carbonation Process of Recycled Cement Paste. Materials. 2022; 15(19):6513. https://doi.org/10.3390/ma15196513
Chicago/Turabian StyleSilva, André, Rita Nogueira, Alexandre Bogas, Dariusz Wawrzyńczak, Aleksandra Ściubidło, and Izabela Majchrzak-Kucęba. 2022. "Parametric Study towards Optimization of a Short Duration Carbonation Process of Recycled Cement Paste" Materials 15, no. 19: 6513. https://doi.org/10.3390/ma15196513





