Influence of Synthetic Limestone Sand on the Frost Resistance of Magnesium Potassium Phosphate Cement Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Test Methods
3. Results and Discussion
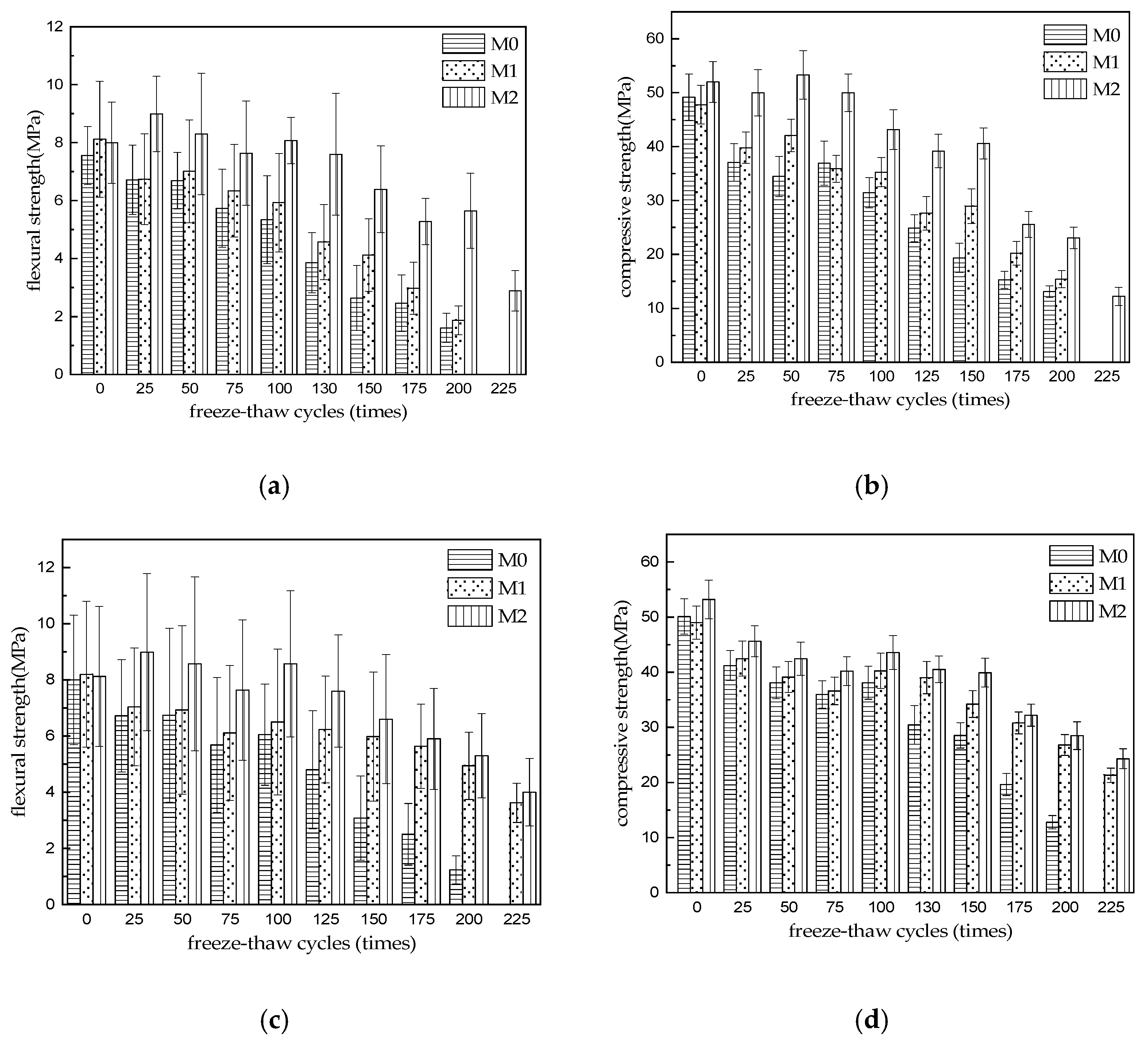
3.1. Strength Development
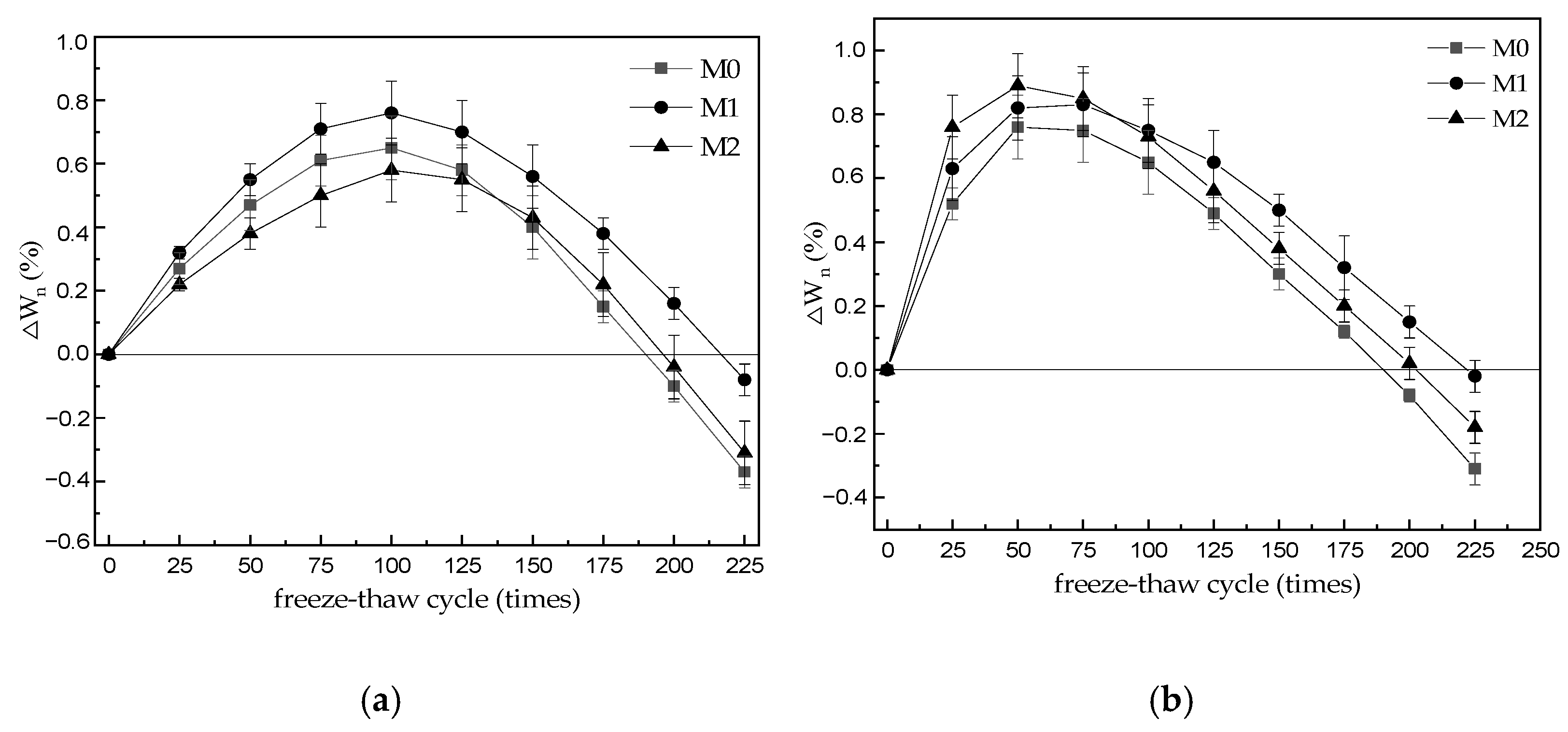
3.2. Quality Loss
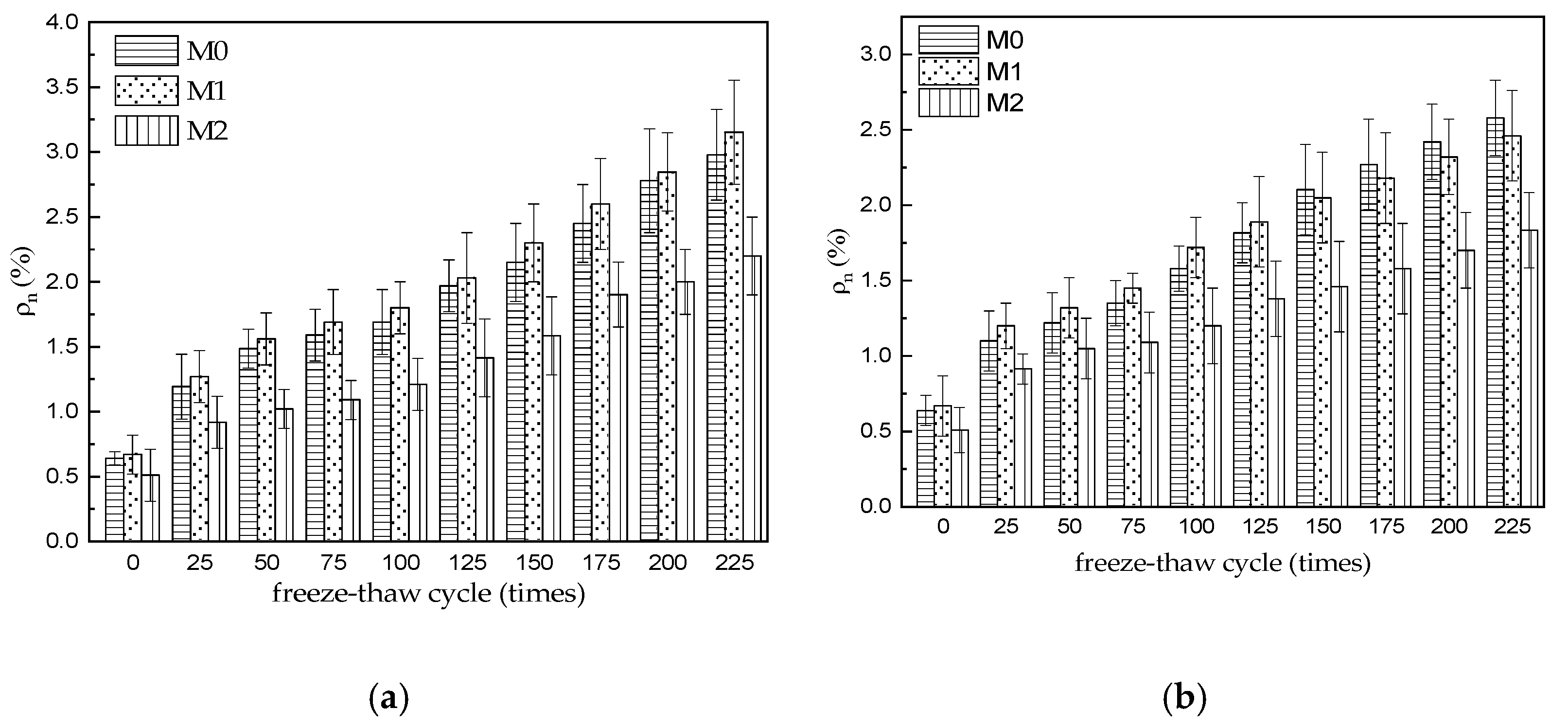
3.3. Water Absorption
3.4. Phase Analysis
3.5. TG Measurements
3.6. SEM EDS Analysis
4. Conclusions and Prospect
- (1)
- The strength of three kinds of MKPC mortar specimens decreases gradually with the increase in freeze–thaw cycles. The strength of M0 and M1 is greater than 75% of the initial strength after 75 cycles of water freezing and thawing, and the strength of M2 is greater than 75% of the initial strength after 150 cycles of water freezing and thawing. After 200 cycles of water freezing and thawing, the flexural and compressive strength of M1 and M2 specimens still reached 21.3% and 23.0%, 26.6% and 32.2% of their initial strength, respectively. After 225 cycles of water freezing and thawing, the flexural and compressive strength of M3 specimens still reached 29.3% and 22.0% of their initial strength. When the sulfate freeze–thaw cycle reaches 75 times, the strength of M0 is lower than 75% of the initial strength. When the freeze–thaw cycle reaches 125 times, the strength of M1 is still higher than 75% of the initial strength. When the freeze–thaw cycle reaches 150 times, the strength of M2 is still higher than 75% of the initial strength. The flexural strength and compressive strength of M0 were 15.4% and 25.5% of the initial strength after 200 cycles of sulfate freezing and thawing, but M0 was destroyed after 225 cycles, and the flexural strength and compressive strength of M1 and M2 were 44.1% and 49.3%, 43.5% and 45.7% of the initial strength, respectively. The results show that the resistance to freezing and thawing of M3 specimens made of common river sand and limestone sand is obviously higher than that of MKPC mortar (M0 and M1) made of single river sand and limestone sand. The resistance of M0 to sulfate freezing and thawing is lower than that of water freezing and thawing, but the resistance of M1 and M2 containing limestone sand is obviously higher than that of M0 mortar containing river sand alone.
- (2)
- With the increase in freeze–thaw cycles, the mass change rate of M0–M2 specimen increases at first and then decreases gradually. When water is frozen and thawed 225 times, the mass loss rates of M0, M1, and M2 specimens are 0.37%, 0.31%, and 0.08%, respectively. When the sulfate solution is frozen and thawed 225 times, the mass loss rates of M0, M1, and M2 specimens are 0.31%, 0.18%, and 0.02%, respectively, which is slightly lower than that of the water frozen and thawed for 225 times. The results show that the mass loss of MKPC mortar is still far lower than the 5% damage standard when it is frozen and thawed 225 times in water and 5% Na2SO4 solution.
- (3)
- The initial water absorption of M0–M2 specimens is 0.64%, 0.51%, and 0.67%, respectively (before freezing and thawing), which indicates that the initial open porosity of M2 specimens is obviously lower than that of M0 and M1 specimens, which should be attributed to the decrease in water–cement ratio of MKPC mortar with the same fluidity due to the reasonable mix of two aggregates. With the increase in freeze–thaw cycles, the water absorption of the three MKPC mortar specimens gradually increased. After 225 water freeze–thaw cycles, the water absorption was 2.98%, 3.15%, and 2.20%, respectively. After 225 sulfate freeze–thaw cycles, the water absorption of M0–M2 specimens is 2.58%, 2.46%, and 1.83% respectively, which is lower than that of the corresponding specimen after 225 sulfate freeze–thaw cycles in water.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, L.; Liu, C. Durability of Concrete and Its Protective Repair; China Construction Industry Press: Beijing, China, 1990. [Google Scholar]
- Liang, Y.; Yuan, Y. Mechanism of concrete destruction under sodium sulfate and magnesium sulfate solution. J. Chin. Ceram. Soc. 2007, 35, 504–508. (In Chinese) [Google Scholar]
- Tonelli, M.; Martini, F.; Calucci, L.; Fratini, E.; Geppi, M.; Ridi, F.; Borsacchi, S.; Baglioni, P. Structural characterization of magnesium silicate hydrate: Towards the design of eco-sustainable cements. Dalton Trans. 2016, 45, 3294–3304. [Google Scholar] [CrossRef] [PubMed]
- Martini, F.; Tonelli, M.; Geppi, M.; Ridi, F.; Borsacchi, S.; Calucci, L. Hydration of MgO/SiO2 and Portland cement mixtures: A structural investigation of the hydrated phases by means of X-ray diffraction and solid-state NMR spectroscopy. Cem. Concr. Res. 2017, 102, 60–67. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, M.; Zhu, J. Damage evolution in cement mortar due to erosion of sulphate. Corros. Sci. 2008, 50, 2478–2483. [Google Scholar] [CrossRef]
- Seehra, S.S.; Gupta, S.; Kumar, S. Rapid setting magnesium phosphate cement for quick repair of concrete pavements characterisation and durability aspects. Cem. Concr. Res. 1993, 23, 254–266. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, B.; Wu, X. Characteristics and durability test of magnesium phosphate cement-based material for rapid repair of concrete. Mater. Struct. 2000, 33, 229–234. [Google Scholar] [CrossRef]
- Jiang, H.; Liang, B.; Zhang, L. Investigation on MPB with super early strength for repair of concrete. J. Constr. Mater. 2001, 4, 196–198. (In Chinese) [Google Scholar]
- Yang, Q.; Zhang, S.; Wu, X. Deicer-scaling resistance of phosphate cement-based binder for rapid repair of concrete. Cem. Concr. Res. 2002, 32, 165–168. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Hosphate Ceramics: Twenty-First Century Materials with Diverse Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zhu, D.; Li, Z.; Xing, F. Chemical durability investigation of magnesium phosphosilicate cement. Key Eng. Mater. 2006, 302–303, 275–281. [Google Scholar]
- Li, Y.; Shi, T.; Li, J. Effects of fly ash and quartz sand on water-resistance and salt-resistance of magnesium phosphate cement. Constr. Build. Mater. 2016, 105, 384–390. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Q.; Wu, Q.; Li, X.; Sun, Z. The effect of seawater curing on properties of magnesium potassium phosphate cement. Constr. Build. Mater. 2017, 141, 470–478. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Zheng, S. Experimental research on seawater erosion resistance of magnesium potassium phosphate cement pastes. Constr. Build. Mater. 2018, 183, 534–543. [Google Scholar]
- Yang, J.; Wang, L.; Jin, C.; Sheng, D. Effect of fly ash on the corrosion resistance of magnesium potassium phosphate cement paste in sulfate solution. Constr. Build. Mater. 2020, 237, 117–639. [Google Scholar]
- Chong, L.; Yang, J.; Xu, Z.Z.; Xu, X. Freezing and thawing resistance of MKPC paste under different corrosion solutions. Constr. Build. Mater. 2019, 212, 663–674. [Google Scholar] [CrossRef]
- Jianming, Y.; Tao, L.; Xuancheng, X. Effect of fine aggregates on properties of magnesium potassium phosphate cement mortar. J. Mater. Civ. Eng. 2017, 29, 06017012. [Google Scholar] [CrossRef]
- Jinbiao, L. Experimental study on high powdered limestone mechanism sand in C60 pump concrete. China Build. Mater. Technol. 2018, 27, 97–99. [Google Scholar]
- Yang, J.; Shi, C.; Chang, Y.; Yang, N. Hydration hardening characteristics of potassium magnesium phosphate cement paste containing composite retarders. Build. Mater. Sci. J. 2013, 16, 43–49. (In Chinese) [Google Scholar]
- JGJ 52-2006; Standard of the Ministry of Building Materials of China: Standard of Sand and Stone Quality and Inspection Method for Ordinary Concrete. Ministry of Construction of China: Beijing, China, 2006.
- GB/T50082-2009; Standard for Test Methods for Long-Term Performance and Durability of Ordinary Concrete. Ministry of Housing and Urban-Rural Development of China: Beijing, China, 2019.
- JGJ/T70-2009; Standard for Test Methods of Basic Properties of Building Mortar. State Administration for Market Regulation: Beijing, China, 2009.
- Zhao, X.; Wei, J.; Huang, Y. The comparison of evaluation indexes for durability degradation of concrete in frezzing–thawing cycles. J. Huazhong Univ. Sci. Technol. 2003, 31, 103106. (In Chinese) [Google Scholar]
- ASTM C1585-2013; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2013.
- Quanbing, Y. One of machine mades on the deicer frost scaling of concrete (II): Degree of saturation and ice-formation pressure during freezing–thawing cycles. J. Constr. Mater. 2012, 15, 741–745. (In Chinese) [Google Scholar]
- Sotiriadis, K.; Nikolopoulou, E.; Tsivilis, S. Sulfate resistance of limestone cement concrete exposed to combined chloride and sulfate environment at low temperature. Cem. Concr. Compos. 2012, 34, 903–910. [Google Scholar] [CrossRef]




| Category | Fineness Modulus | Clay Content (%) | Bulk Density (kg/m3) | Gradation |
|---|---|---|---|---|
| Common river sand | 2.53 | 0.80 | 1450 | JGJ52-2006, Zone II [20] |
| Limestone Sand | 3.65 | 0.00 | 1460 | JGJ52-2006, Zone II [20] |
| Code | Fine Aggregate Variety | Ww/WMKPC | Fluidity (mm) | Flexural Strength (MPa) 3d 28d | Compressive Strength (MPa) 3d 28d | ||
|---|---|---|---|---|---|---|---|
| M0 | 100%Rs | 0.20 | 140 | 6.16 | 8.02 | 35.1 | 51.6 |
| M1 | 100%Ls | 0.20 | 141 | 6.46 | 8.32 | 35.2 | 50.3 |
| M2 | 34%Rs + 66%Ls | 0.18 | 138 | 7.32 | 8.25 | 39.3 | 54.8 |
| Element Name | O | Mg | S | P | Cl | K | Ca | Na | |
|---|---|---|---|---|---|---|---|---|---|
| Percentage of atoms (%) | A | 71.63 | 9.74 | 9.52 | 9.11 | ||||
| B | 69.17 | 10.83 | 9.65 | 0.14 | 9.18 | 1.03 | |||
| C | 69.21 | 8.84 | 1.18 | 9.08 | 0.24 | 9.30 | 2.15 | ||
| D | 72.30 | 10.36 | 8.12 | 9.22 | |||||
| E | 73.52 | 8.43 | 6.95 | 6.07 | 2.11 | 2.92 | |||
| F | 66.35 | 7.47 | 2.56 | 8.85 | 0.35 | 9.55 | 2.86 | 2.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Hou, Y.; Mei, J.; Yang, J.; Gan, T. Influence of Synthetic Limestone Sand on the Frost Resistance of Magnesium Potassium Phosphate Cement Mortar. Materials 2022, 15, 6517. https://doi.org/10.3390/ma15196517
Wu Q, Hou Y, Mei J, Yang J, Gan T. Influence of Synthetic Limestone Sand on the Frost Resistance of Magnesium Potassium Phosphate Cement Mortar. Materials. 2022; 15(19):6517. https://doi.org/10.3390/ma15196517
Chicago/Turabian StyleWu, Qianqian, Yuying Hou, Jiangtao Mei, Jianming Yang, and Tao Gan. 2022. "Influence of Synthetic Limestone Sand on the Frost Resistance of Magnesium Potassium Phosphate Cement Mortar" Materials 15, no. 19: 6517. https://doi.org/10.3390/ma15196517
APA StyleWu, Q., Hou, Y., Mei, J., Yang, J., & Gan, T. (2022). Influence of Synthetic Limestone Sand on the Frost Resistance of Magnesium Potassium Phosphate Cement Mortar. Materials, 15(19), 6517. https://doi.org/10.3390/ma15196517





