Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications
Abstract
:1. Introduction
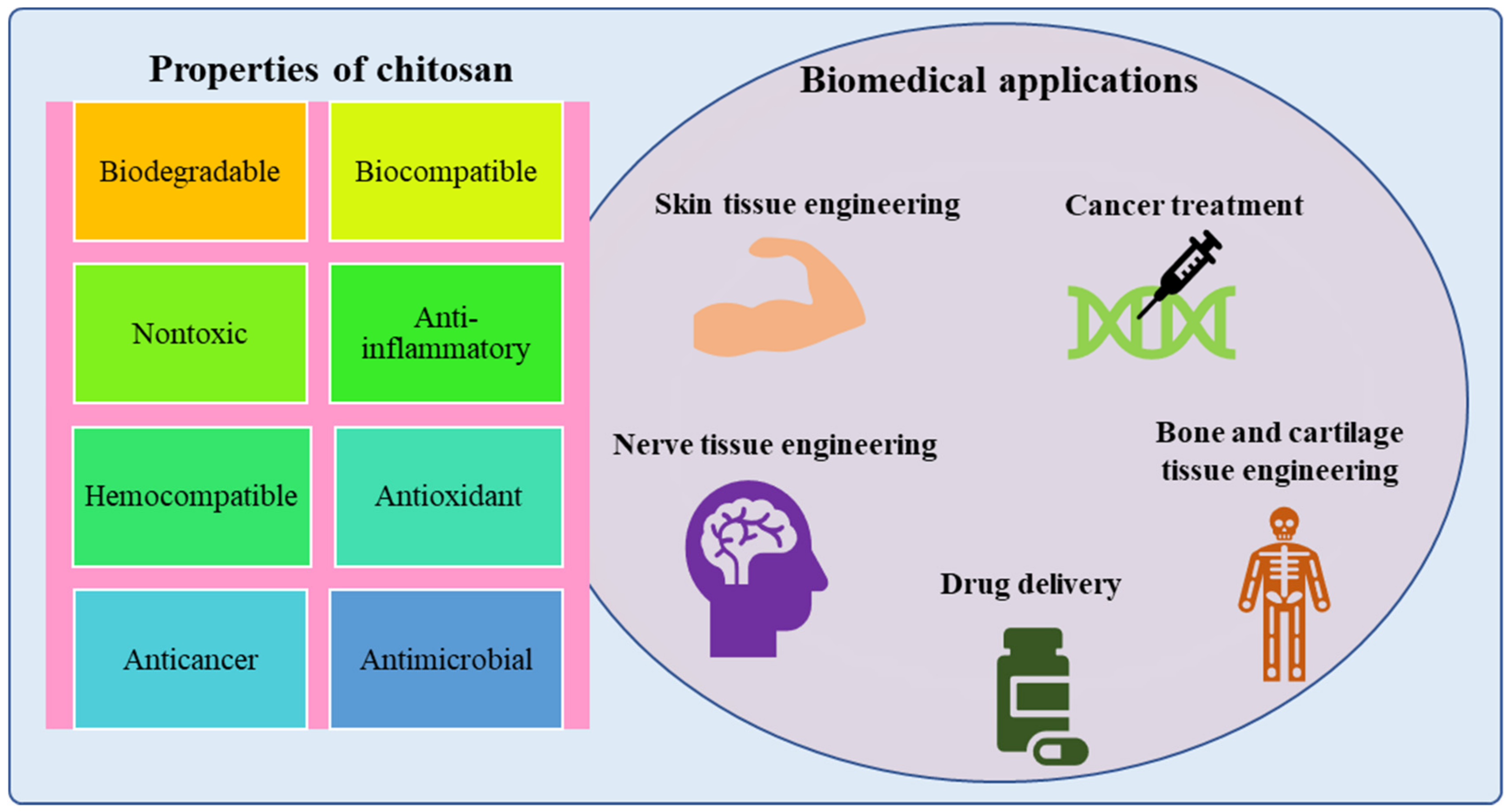
2. Properties of Chitosan for Biomedical Applications
3. Production of Chitosan Nanoparticles
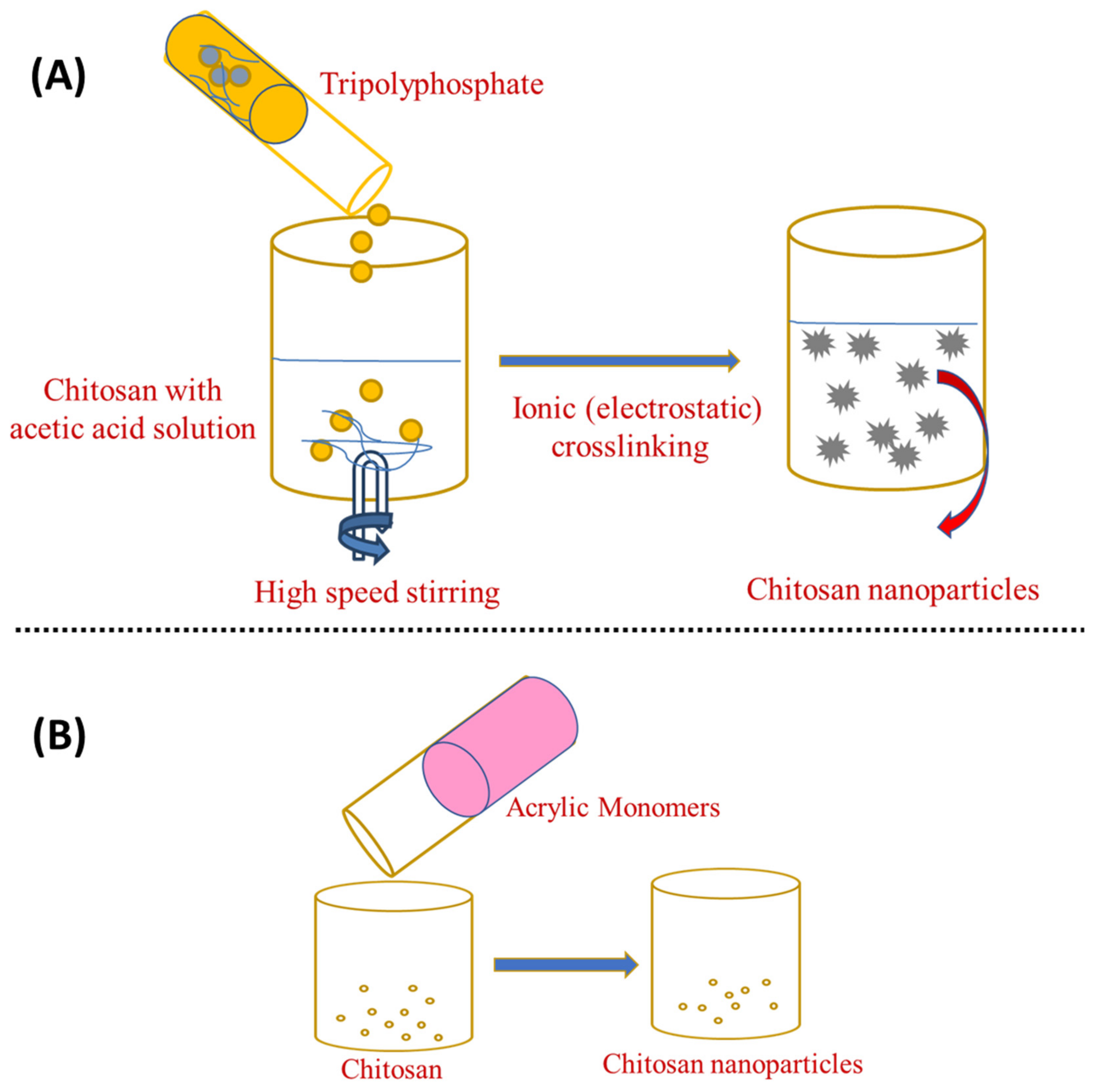
3.1. Ionotropic Gelation
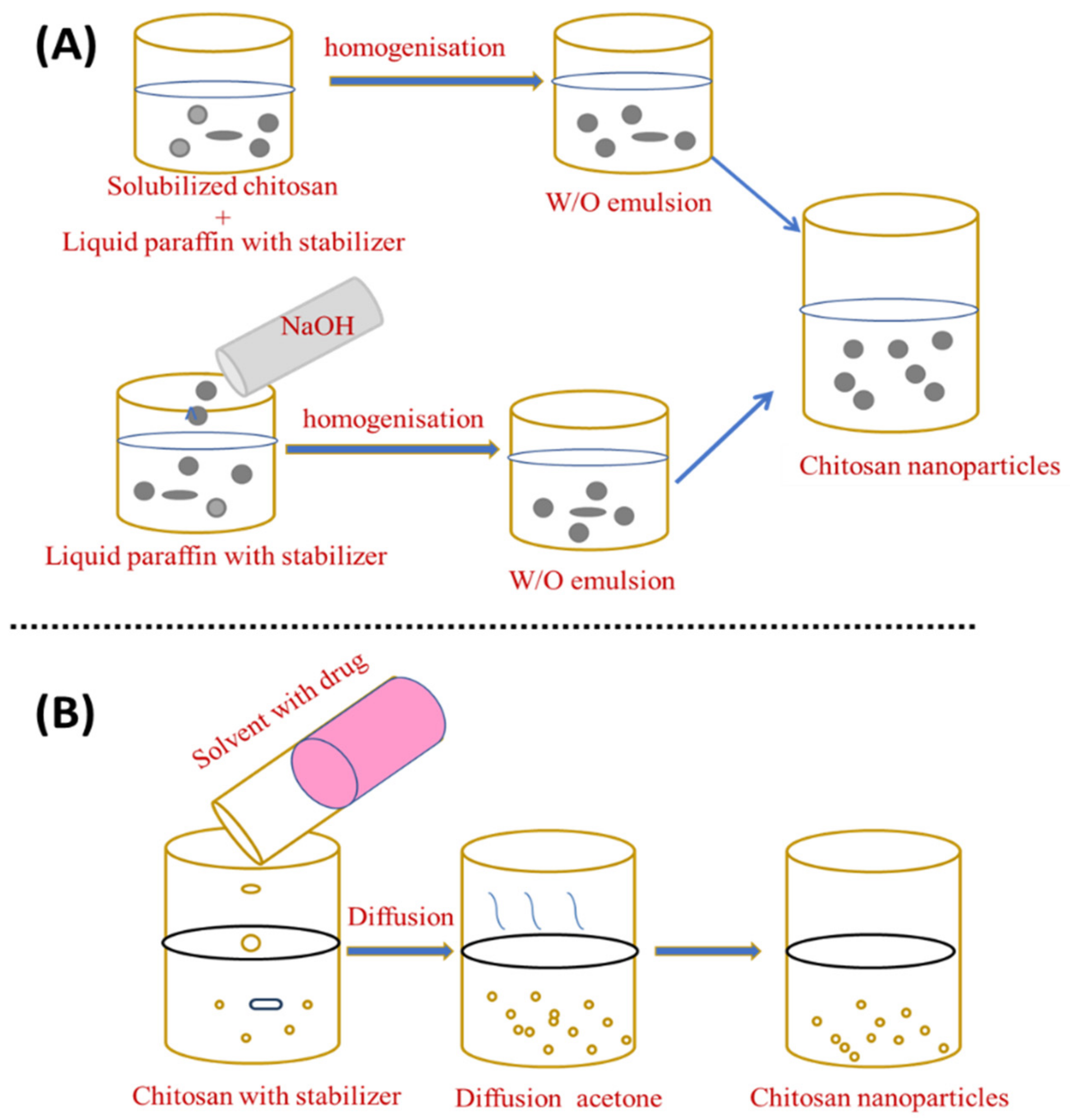
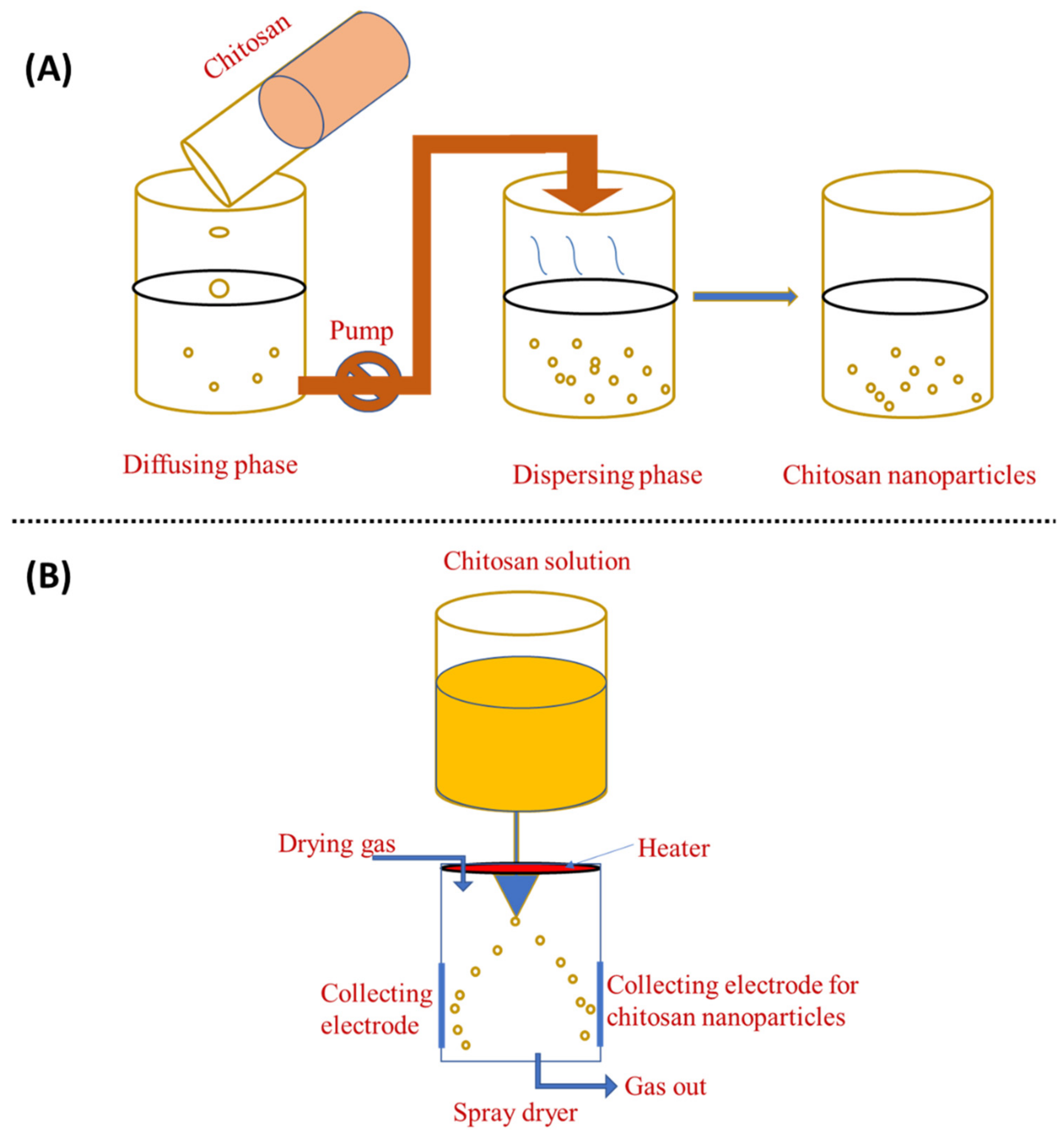
3.2. Emulsion Droplet Coalescence and Emulsion Solvent Diffusion
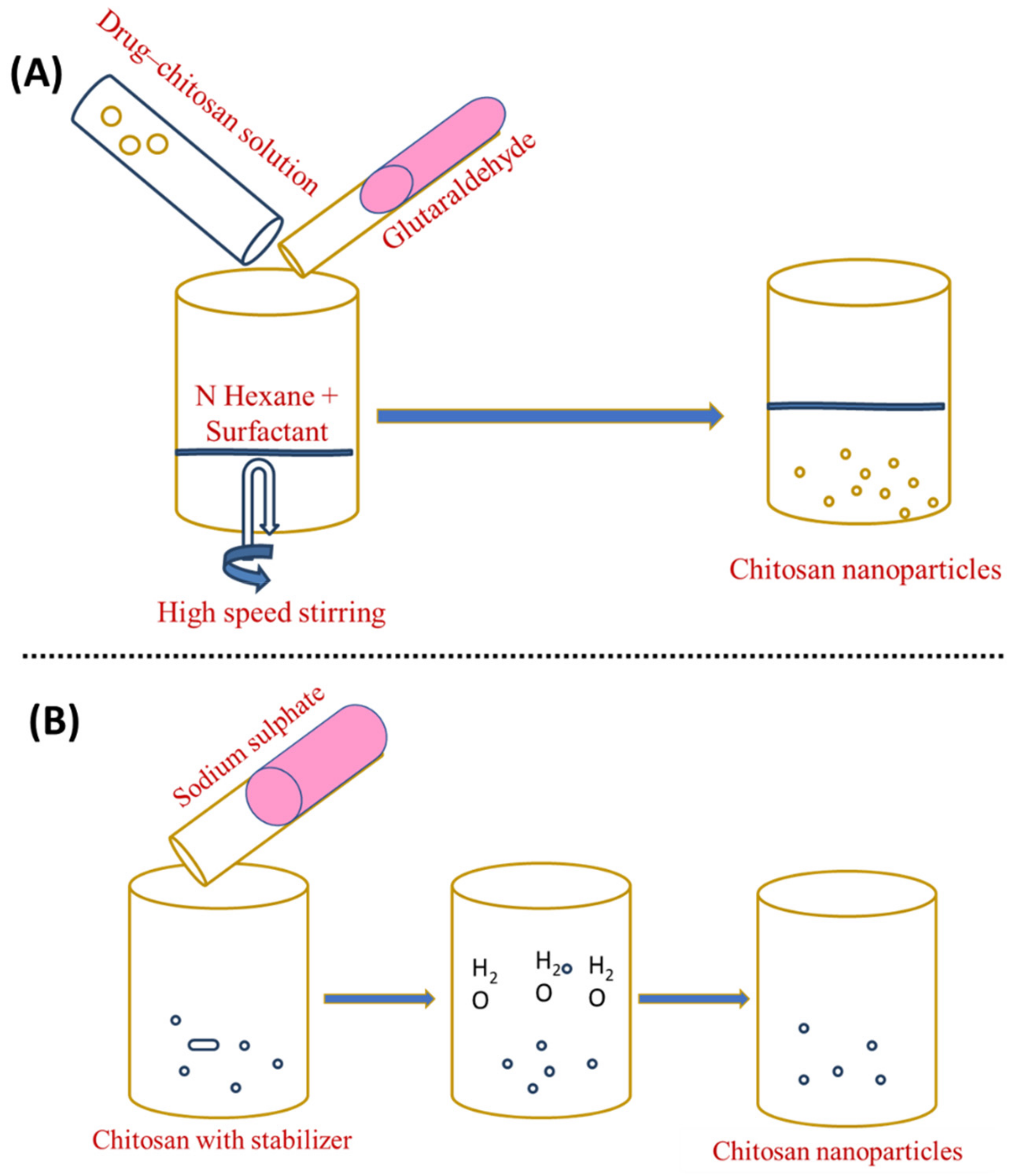
3.3. Reverse Micellar Method
3.4. Desolvation
3.5. Nano Precipitation
3.6. Spray-Drying
4. Characterisation of Chitosan Nanoparticles
5. Chitosan Nanoparticles in Biomedical Applications
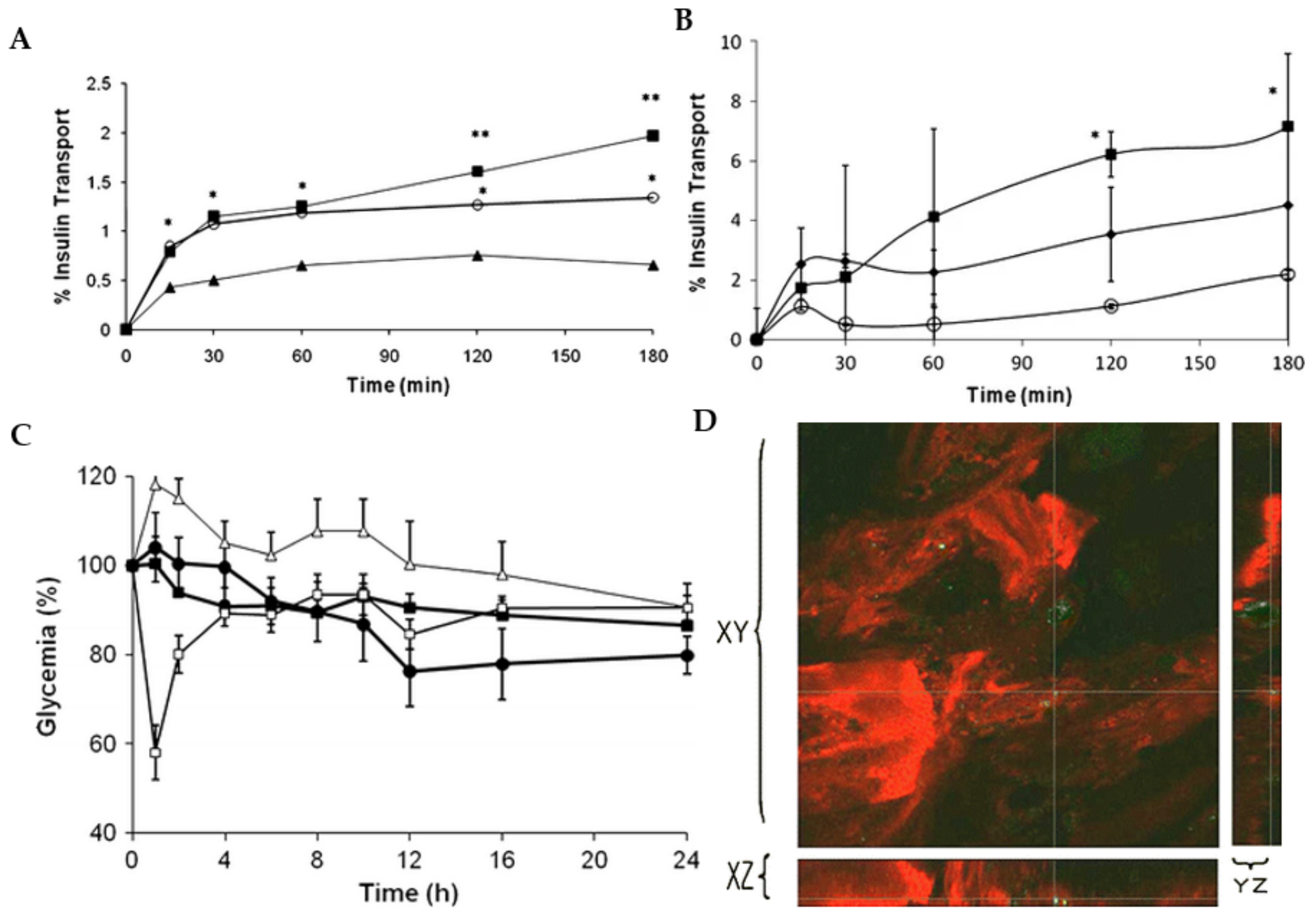
5.1. Drug Delivery
5.2. Cancer Treatment
5.3. Tissue Engineering
5.4. Antibacterial Activity
6. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vauthier, C.; Dubernet, C.; Chauvierre, C.; Brigger, I.; Couvreur, P. Drug delivery to resistant tumors: The potential of poly (alkyl cyanoacrylate) nanoparticles. J. Control. Release 2003, 93, 151–160. [Google Scholar] [CrossRef]
- Titus, D.; Samuel, E.J.J.; Roopan, S.M. Nanoparticle characterization techniques. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–319. [Google Scholar]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef]
- Whitesides, G.M. The ‘right’ size in nanobiotechnology. Nat. Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- Hatton, R.A.; Miller, A.J.; Silva, S. Carbon nanotubes: A multi-functional material for organic optoelectronics. J. Mater. Chem. 2008, 18, 1183–1192. [Google Scholar] [CrossRef]
- Doucet, D.; Retnakaran, A. Insect chitin: Metabolism, genomics and pest management. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 43, pp. 437–511. [Google Scholar]
- Cheba, B.A. Chitin and chitosan: Marine biopolymers with unique properties and versatile applications. Glob. J. Biotechnol. Biochem. 2011, 6, 149–153. [Google Scholar]
- Divya, K.; Jisha, M. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.B.; Amorim, M.; Fonte, P.; Madureira, R.; Ferreira, D.; Pintado, M.; Sarmento, B. Natural extracts into chitosan nanocarriers for rosmarinic acid drug delivery. Pharm. Biol. 2015, 53, 642–652. [Google Scholar] [CrossRef]
- Roshanravan, B.; Soltani, S.M.; Rashid, S.A.; Mahdavi, F.; Yusop, M.K. Enhancement of nitrogen release properties of urea–kaolinite fertilizer with chitosan binder. Chem. Speciat. Bioavailab. 2015, 27, 44–51. [Google Scholar] [CrossRef]
- Değirmencioğlu, N.; İrkin, R.; Değirmencioğlu, A.; Kabasakal, A. Chitosan and its application in food industry. Akad. Gida 2009, 7, 35–41. [Google Scholar]
- Perera, U.; Rajapakse, N. Chitosan nanoparticles: Preparation, characterization, and applications. In Seafood Processing By-Products; Springer: Berlin/Heidelberg, Germany, 2014; Volume 7, pp. 371–387. [Google Scholar]
- Dutta, P.K.; Dutta, J.; Tripathi, V. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Bansal, V.; Sharma, P.K.; Sharma, N.; Pal, O.P.; Malviya, R. Applications of chitosan and chitosan derivatives in drug delivery. Adv. Biol. Res. 2011, 5, 28–37. [Google Scholar]
- Scalera, F.; Gervaso, F.; Madaghiele, M.; De Benedictis, V.; Demitri, C. Preliminary assessment of chitosan nanoparticles for growth factor delivery. In Proceedings of the 2015 1st Workshop on Nanotechnology in Instrumentation and Measurement, Lecce, Italy, 24–25 July 2015; Volume 9, pp. 20–24. [Google Scholar]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef]
- Uyama, T.; Kitagawa, H.; Sugahara, K.; Kamerling, H. Comprehensive Glycoscience. Compr. Glycosci. 2007, 3, 79–104. [Google Scholar]
- Teng, W.L.; Khor, E.; Tan, T.K.; Lim, L.Y.; Tan, S.C. Concurrent production of chitin from shrimp shells and fungi. Carbohydr. Res. 2001, 332, 305–316. [Google Scholar] [CrossRef]
- Nwe, N.; Stevens, W. Production of chitin and chitosan and their applications in the medical and biological sector. Recent Res. Biomed. Asp. Chitin Chitosan 2008, 978, 161–167. [Google Scholar]
- Crestini, C.; Kovac, B.; Giovannozzi-Sermanni, G. Production and isolation of chitosan by submerged and solid-state fermentation from Lentinus edodes. Biotechnol. Bioeng. 1996, 50, 207–210. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.d.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Nwe, N.; Furuike, T.; Tamura, H. Chitin, Chitosan, Oligosaccharides and Their Derivatives, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 3–10. [Google Scholar]
- Gooday, G.W. The ecology of chitin degradation. In Advances in Microbial Ecology; Springer: Berlin/Heidelberg, Germany, 1990; pp. 387–430. [Google Scholar]
- Santos, V.P.; Marques, N.S.; Maia, P.C.; Lima, M.A.B.d.; Franco, L.d.O.; Campos-Takaki, G.M.d. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Carney, B.; Slater, J.; Brück, W. Mining marine shellfish wastes for bioactive molecules: Chitin and chitosan ndash; Part A: Extraction methods. Biotechnol. J. Healthc. Nutr. Technol. 2008, 3, 871–877. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, L.; Ladavière, C. Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog. Polym. Sci. 2016, 60, 1–17. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kuno, Y.; Sugimoto, S.; Takeuchi, H.; Kawashima, Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J. Control. Release 2005, 102, 373–381. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Maté, J.I.; Gardrat, C.; Coma, V. Effect of chitosan molecular weight on the antimicrobial activity and release rate of carvacrol-enriched films. Food Hydrocoll. 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Chittchang, M.; Johnston, T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Yano, R.; Miyatake, K.; Tomohiro, I.; Shigemasa, Y.; Minami, S. Effects of chitin and chitosan on blood coagulation. Carbohydr. Polym. 2003, 53, 337–342. [Google Scholar] [CrossRef]
- Busilacchi, A.; Gigante, A.; Mattioli-Belmonte, M.; Manzotti, S.; Muzzarelli, R.A. Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr. Polym. 2013, 98, 665–676. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Rahmani Del Bakhshayesh, A.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [PubMed]
- Fini, A.; Orienti, I. The role of chitosan in drug delivery. Am. J. Drug Deliv. 2003, 1, 43–59. [Google Scholar] [CrossRef]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Watanabe, S.; Kishi, A.; Izume, M.; Ohtakara, A. Hypocholesterolemic action of chitosans with different viscosity in rats. Lipids 1988, 23, 187–191. [Google Scholar] [CrossRef]
- Rabea, E.; Badawy, M.; Steurbaut, W.; Rogge, T.; Stevens, C.; Smagghe, G.; Höfte, M. Fungicidal effect of chitosan derivatives containing an N-alkyl group on grey mould Botryti77s cinerea and rice leaf blast Pyricularia grisea. Commun. Agric. Appl. Biol. Sci. 2005, 70, 219–223. [Google Scholar]
- Sanpui, P.; Murugadoss, A.; Prasad, P.D.; Ghosh, S.S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan–Ag-nanoparticle composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef]
- Kockisch, S.; Rees, G.D.; Young, S.A.; Tsibouklis, J.; Smart, J.D. Polymeric microspheres for drug delivery to the oral cavity: An in vitro evaluation of mucoadhesive potential. J. Pharm. Sci. 2003, 92, 1614–1623. [Google Scholar] [CrossRef]
- Sinha, V.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Huo, M.; Zhang, Y.; Zhou, J.; Zou, A.; Yu, D.; Wu, Y.; Li, J.; Li, H. Synthesis and characterization of low-toxic amphiphilic chitosan derivatives and their application as micelle carrier for antitumor drug. Int. J. Pharm. 2010, 394, 162–173. [Google Scholar] [CrossRef]
- Tokoro, A.; Takewaki, N.; Suzuki, K.; Mikami, T.; Suzuki, S.; Suzuki, M. Growth-inhibitory effect of hexa-N-acetylchitohexanse and chitohexaose against Meth-A solid tumor. Chem. Pharm. Bull. 1988, 36, 784–790. [Google Scholar] [CrossRef]
- Park, P.-J.; Je, J.-Y.; Kim, S.-K. Free radical scavenging activity of chitooligosaccharides by electron spin resonance spectrometry. J. Agric. Food Chem. 2003, 51, 4624–4627. [Google Scholar] [CrossRef]
- Anisiei, A.; Oancea, F.; Marin, L. Electrospinning of chitosan-based nanofibers: From design to prospective applications. Rev. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Jose, S.; Lammers, T. Understanding the mechanism of ionic gelation for synthesis of chitosan nanoparticles using qualitative techniques. Asian J. Pharm. 2010, 4, 148–153. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117. [Google Scholar] [CrossRef]
- Colonna, C.; Conti, B.; Perugini, P.; Pavanetto, F.; Modena, T.; Dorati, R.; Genta, I. Chitosan glutamate nanoparticles for protein delivery: Development and effect on prolidase stability. J. Microencapsul. 2007, 24, 553–564. [Google Scholar] [CrossRef]
- Corradini, E.; De Moura, M.; Mattoso, L. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- LeHoux, J.; Grondin, F. Some effects of chitosan on liver function in the rat. Endocrinology 1993, 132, 1078–1084. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan-as a biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Furuike, T.; Komoto, D.; Hashimoto, H.; Tamura, H. Preparation of chitosan hydrogel and its solubility in organic acids. Int. J. Biol. Macromol. 2017, 104, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Fonte, P.; Andrade, J.C.; Seabra, V.; Sarmento, B. Chitosan-based nanoparticles as delivery systems of therapeutic proteins. In Therapeutic Proteins; Springer: Berlin/Heidelberg, Germany, 2012; pp. 471–487. [Google Scholar]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Shiraishi, S.; Imai, T.; Otagiri, M. Controlled release of indomethacin by chitosan-polyelectrolyte complex: Optimization and in vivo/in vitro evaluation. J. Control. Release 1993, 25, 217–225. [Google Scholar] [CrossRef]
- Ohya, Y.; Takei, T.; Kobayashi, H.; Ouchi, T. Release behaviour of 5-fluorouracil from chitosan-gel microspheres immobilizing 5-fluorouracil derivative coated with polysaccharides and their cell specific recognition. J. Microencapsul. 1993, 10, 1–9. [Google Scholar] [CrossRef]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef]
- Tiyaboonchai, W. Chitosan nanoparticles: A promising system for drug delivery. Naresuan Univ. J. Sci. Technol. 2013, 11, 51–66. [Google Scholar]
- Sailaja, A.K.; Amareshwar, P.; Chakravarty, P. Different techniques used for the preparation of nanoparticles using natural polymers and their application. Int. J. Pharm. Pharm. Sci. 2011, 3, 45–50. [Google Scholar]
- Kafshgari, M.H.; Khorram, M.; Khodadoost, M.; Khavari, S. Reinforcement of chitosan nanoparticles obtained by an ionic cross-linking process. Iran. Polym. J. 2011, 20, 445–456. [Google Scholar]
- Rayment, P.; Butler, M.F. Investigation of ionically crosslinked chitosan and chitosan–bovine serum albumin beads for novel gastrointestinal functionality. J. Appl. Polym. Sci. 2008, 108, 2876–2885. [Google Scholar] [CrossRef]
- Shi, L.; Chen, M.; Xinf, L.; Guo, X.; Zhao, L. Chitosan nanoparticles as drug delivery carriers for biomedical engineering. J. Chem. Soc. Pak. 2011, 33, 929–934. [Google Scholar]
- Shu, X.; Zhu, K. Chitosan/gelatin microspheres prepared by modified emulsification and ionotropic gelation. J. Microencapsul. 2001, 18, 237–245. [Google Scholar]
- Calvo, P.; Remuñan-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Patil, J.; Kamalapur, M.; Marapur, S.; Kadam, D. Ionotropic gelation and polyelectrolyte complexation: The novel techniques to design hydrogel particulate sustained, modulated drug delivery system: A review. Dig. J. Nanomater. Biostruct. 2010, 5, 241–248. [Google Scholar]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef]
- Liu, H.; Gao, C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym. Adv. Technol. 2009, 20, 613–619. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-u. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Jonassen, H.; Kjøniksen, A.-L.; Hiorth, M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Ilium, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Ichikawa, H.; Fukumori, Y. Chitosan-gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: Preparation by novel emulsion-droplet coalescence technique and characterization. Pharm. Res. 1999, 16, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y. Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with D, L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior. J. Control. Release 1993, 25, 89–98. [Google Scholar] [CrossRef]
- Karnchanajindanun, J.; Srisa-ard, M.; Baimark, Y. Genipin-cross-linked chitosan microspheres prepared by a water-in-oil emulsion solvent diffusion method for protein delivery. Carbohydr. Polym. 2011, 85, 674–680. [Google Scholar] [CrossRef]
- Brunel, F.; Véron, L.; David, L.; Domard, A.; Delair, T. A novel synthesis of chitosan nanoparticles in reverse emulsion. Langmuir 2008, 24, 11370–11377. [Google Scholar] [CrossRef]
- Pileni, M. Reverse micelles used as templates: A new understanding in nanocrystal growth. J. Exp. Nanosci. 2006, 1, 13–27. [Google Scholar] [CrossRef]
- Kafshgari, M.H.; Khorram, M.; Mansouri, M.; Samimi, A.; Osfouri, S. Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran. Polym. J. 2012, 21, 99–107. [Google Scholar] [CrossRef]
- Zhao, L.-M.; Shi, L.-E.; Zhang, Z.-L.; Chen, J.-M.; Shi, D.-D.; Yang, J.; Tang, Z.-X. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng. 2011, 28, 353–362. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, Z.; Yuan, H.; Wang, C.; Qi, J.; Liu, K.; Cao, R.; Zheng, H. "A protein@metal–organic framework nanocomposite for pH-triggered anticancer drug delivery". Dalton Trans. 2018, 47, 10223–10228. [Google Scholar] [CrossRef]
- Golińska, P. Biopolymer-based nanofilms: Utility and toxicity. In Biopolymer-Based Nano Films; Elsevier: Amsterdam, The Netherlands, 2021; pp. 353–385. [Google Scholar]
- Yu, L.; Wang, H.; Zhang, Y.; Zhang, B.; Liu, J. Recent advances in halloysite nanotube derived composites for water treatment. Environ. Sci. Nano 2016, 3, 28–44. [Google Scholar] [CrossRef]
- Gonzalez-Melo, C.; Garcia-Brand, A.J.; Quezada, V.; Reyes, L.H.; Muñoz-Camargo, C.; Cruz, J.C. Highly efficient synthesis of type B gelatin and low molecular weight chitosan nanoparticles: Potential applications as bioactive molecule carriers and cell-penetrating agents. Polymers 2021, 13, 4078. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Luque-Alcaraz, A.G.; Lizardi-Mendoza, J.; Goycoolea, F.; Higuera-Ciapara, I.; Argüelles-Monal, W. Preparation of chitosan nanoparticles by nanoprecipitation and their ability as a drug nanocarrier. RSC Adv. 2016, 6, 59250–59256. [Google Scholar] [CrossRef]
- Barreras-Urbina, C.G.; Ramírez-Wong, B.; López-Ahumada, G.A.; Burruel-Ibarra, S.E.; Martínez-Cruz, O.; Tapia-Hernández, J.A.; Rodriguez Felix, F. Nano-and micro-particles by nanoprecipitation: Possible application in the food and agricultural industries. Int. J. Food Prop. 2016, 19, 1912–1923. [Google Scholar] [CrossRef]
- Khan, I.U.; Serra, C.A.; Anton, N.; Vandamme, T.F. Production of nanoparticle drug delivery systems with microfluidics tools. Expert Opin. Drug Deliv. 2015, 12, 547–562. [Google Scholar] [CrossRef]
- Ngan, L.T.K.; Wang, S.-L.; Hiep, Đ.M.; Luong, P.M.; Vui, N.T.; Đinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.A.; Kıyan, H.T. Treatment of oxidative stress-induced pain and inflammation with dexketoprofen trometamol loaded different molecular weight chitosan nanoparticles: Formulation, characterization and anti-inflammatory activity by using in vivo HET-CAM assay. Microvasc. Res. 2020, 128, 103961. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; El-Helw, A.-R.M.; Ahmed, O.A.; Hosny, K.M.; Ahmed, T.A. Novel controlled release of antihypertensive drug: Preparation, in-vitro and in-vivo evaluation. Afr. J. Pharm. Pharmacol. 2013, 7, 1744–1756. [Google Scholar]
- Ahmed, T.A.; El-Say, K.M.; Mahmoud, M.F.; Samy, A.M.; Badawi, A.A. Miconazole nitrate oral disintegrating tablets: In vivo performance and stability study. Am. Assoc. Pharm. Sci. 2012, 13, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yeoh, H.H.; Lim, L.Y. Formulation pH modulates the interaction of insulin with chitosan nanoparticles. J. Pharm. Sci. 2002, 91, 1396–1404. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumari, S.; Kumaraswamy, R.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Characterization methods for chitosan-based nanomaterials. In Plant Nanobionics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–116. [Google Scholar]
- Kumar, N.V.; Basavegowda, V.R.; Murthy, A.N. Synthesis and characterization of copper-chitosan based nanofungicide and its induced defense responses in Fusarium wilt of banana. Inorg. Nano-Met. Chem. 2022, 1–9. [Google Scholar] [CrossRef]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef]
- Cover, N.F.; Lai-Yuen, S.; Parsons, A.K.; Kumar, A. Synergetic effects of doxycycline-loaded chitosan nanoparticles for improving drug delivery and efficacy. Int. J. Nanomed. 2012, 7, 2411. [Google Scholar]
- Rodrigues, S.; da Costa, A.M.R.; Grenha, A. Chitosan/carrageenan nanoparticles: Effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr. Polym. 2012, 89, 282–289. [Google Scholar] [CrossRef]
- Casettari, L.; Castagnino, E.; Stolnik, S.; Lewis, A.; Howdle, S.M.; Illum, L. Surface characterisation of bioadhesive PLGA/chitosan microparticles produced by supercritical fluid technology. Pharm. Res. 2011, 28, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.-S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.-W.; Kim, I.-S. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Uthaman, S.; Lee, S.J.; Cherukula, K.; Cho, C.-S.; Park, I.-K. Polysaccharide-coated magnetic nanoparticles for imaging and gene therapy. Biomed. Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Orienti, I.; Aiedeh, K.; Gianasi, E.; Bertasi, V.; Zecchi, V. Indomethacin loaded chitosan microspheres. Correlation between the erosion process and release kinetics. J. Microencapsul. 1996, 13, 463–472. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Kang, K.-H.; Je, J.-Y.; Pham, H.N.-D.; Byun, H.-G.; Kim, S.-K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Aruna, U.; Rajalakshmi, R.; Muzib, Y.I.; Vinesha, V.; Sushma, M.; Vandana, K.; Kumar, N.V. Role of chitosan nanoparticles in cancer therapy. Int. J. Innov. Pharm. Sci. Res. 2013, 4, 318–324. [Google Scholar]
- Chua, B.Y.; Al Kobaisi, M.; Zeng, W.; Mainwaring, D.; Jackson, D.C. Chitosan microparticles and nanoparticles as biocompatible delivery vehicles for peptide and protein-based immunocontraceptive vaccines. Mol. Pharm. 2012, 9, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, C.; Xia, X.; Liu, Y. Self-assembled lecithin/chitosan nanoparticles for oral insulin delivery: Preparation and functional evaluation. Int. J. Nanomed. 2016, 11, 761–769. [Google Scholar] [CrossRef]
- Huang, W.-C.; Wang, W.; Xue, C.; Mao, X. Effective enzyme immobilization onto a magnetic chitin nanofiber composite. ACS Sustain. Chem. Eng. 2018, 6, 8118–8124. [Google Scholar] [CrossRef]
- Min, K.H.; Park, K.; Kim, Y.-S.; Bae, S.M.; Lee, S.; Jo, H.G.; Park, R.-W.; Kim, I.-S.; Jeong, S.Y.; Kim, K. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Control. Release 2008, 127, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Belgamwar, A.; Khan, S.; Yeole, P. Intranasal chitosan-g-HPβCD nanoparticles of efavirenz for the CNS targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Vy Phan, T.T.; Kim, H.; Lee, K.D. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Adebisi, A.O.; Laity, P.R.; Conway, B.R. Formulation and evaluation of floating mucoadhesive alginate beads for targeting H elicobacter pylori. J. Pharm. Pharmacol. 2015, 67, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, J.; Wang, J.; Yin, Z.; Zhu, Y.; Liu, W. Development of timolol-loaded galactosylated chitosan nanoparticles and evaluation of their potential for ocular drug delivery. AAPS Pharm. Sci. Technol. 2017, 18, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Jeevithan, E.; Chelliah, R.; Kathiresan, K.; Wen-Hui, W.; Oh, D.-H.; Wang, M.-H. Zinc-chitosan nanoparticles induced apoptosis in human acute T-lymphocyte leukemia through activation of tumor necrosis factor receptor CD95 and apoptosis-related genes. Int. J. Biol. Macromol. 2018, 119, 1144–1153. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Chen, M.; Chen, X.; Zheng, N. Palladium-based nanomaterials for cancer imaging and therapy. Theranostics 2020, 10, 10057–10074. [Google Scholar] [CrossRef]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M. Chitosan-based nanoparticles against viral infections. Front. Cell. Infect. Microbiol. 2021, 11, 643–653. [Google Scholar] [CrossRef]
- Verma, A.; Dubey, J.; Hegde, R.R.; Rastogi, V.; Pandit, J. Helicobacter pylori: Past, current and future treatment strategies with gastroretentive drug delivery systems. J. Drug Target 2016, 24, 897–915. [Google Scholar] [CrossRef]
- Morabito, F.; Voso, M.T.; Hohaus, S.; Gentile, M.; Vigna, E.; Recchia, A.G.; Iovino, L.; Benedetti, E.; Lo-Coco, F.; Galimberti, S. Panobinostat for the treatment of acute myelogenous leukemia. Expert Opin. Investig. Drugs 2016, 25, 1117–1131. [Google Scholar] [CrossRef]
- Arca, H.Ç.; Günbeyaz, M.; Şenel, S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev. Vaccines 2009, 8, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Kaur, R.; Rajput, R.; Nag, P.; Kumar, S.; Singh, M. Synthesis, characterization and evaluation of antioxidant properties of catechin hydrate nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 39, 398–407. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar]
- Barbieri, S.; Buttini, F.; Rossi, A.; Bettini, R.; Colombo, P.; Ponchel, G.; Sonvico, F.; Colombo, G. Ex vivo permeation of tamoxifen and its 4-OH metabolite through rat intestine from lecithin/chitosan nanoparticles. Int. J. Pharm. 2015, 491, 99–104. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Pearce, J. Nanoparticle Theranostics Targeting CLEC14A on Tumour Vasculature; University of Birmingham: Birmingham, UK, 2020. [Google Scholar]
- Diop, M.; Auberval, N.; Viciglio, A.; Langlois, A.; Bietiger, W.; Mura, C.; Peronet, C.; Bekel, A.; David, D.J.; Zhao, M. Design, characterisation, and bioefficiency of insulin–chitosan nanoparticles after stabilisation by freeze-drying or cross-linking. Int. J. Pharm. 2015, 491, 402–408. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Sangeetha, D.; Gomathi, T. Sunitinib loaded chitosan nanoparticles formulation and its evaluation. Int. J. Biol. Macromol. 2016, 82, 952–958. [Google Scholar] [CrossRef]
- Van der Lubben, I.; Verhoef, J.; Borchard, G.; Junginger, H. Chitosan for mucosal vaccination. Adv. Drug Deliv. Rev. 2001, 52, 139–144. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Ho, P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2018, 13, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Shahnaz, G.; Vetter, A.; Barthelmes, J.; Rahmat, D.; Laffleur, F.; Iqbal, J.; Perera, G.; Schlocker, W.; Dünnhaput, S.; Augustijns, P. Thiolated chitosan nanoparticles for the nasal administration of leuprolide: Bioavailability and pharmacokinetic characterization. Int. J. Pharm. 2012, 428, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Rytting, E.; Nguyen, J.; Wang, X.; Kissel, T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2008, 5, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Ferro, V. Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Nanoscale 2016, 8, 14341–14358. [Google Scholar] [CrossRef] [PubMed]
- Rawal, T.; Parmar, R.; Tyagi, R.K.; Butani, S. Rifampicin loaded chitosan nanoparticle dry powder presents an improved therapeutic approach for alveolar tuberculosis. Colloids Surf. B Biointerfaces 2017, 154, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad, S.; Gilani, K.; Moazeni, E.; Ghazi-Khansari, M.; Najafabadi, A.R.; Mohajel, N. Development of chitosan-based nanoparticles for pulmonary delivery of itraconazole as dry powder formulation. Powder Technol. 2012, 222, 65–70. [Google Scholar] [CrossRef]
- Fonte, P.; Andrade, F.; Araújo, F.; Andrade, C.; das Neves, J.; Sarmento, B. Chitosan-coated solid lipid nanoparticles for insulin delivery. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 508, pp. 295–314. [Google Scholar]
- Fonte, P.; Nogueira, T.; Gehm, C.; Ferreira, D.; Sarmento, B. Chitosan-coated solid lipid nanoparticles enhance the oral absorption of insulin. Drug Deliv. Transl. Res. 2011, 1, 299–308. [Google Scholar] [CrossRef]
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef]
- Cross, D.; Burmester, J.K. Gene therapy for cancer treatment: Past, present and future. Clin. Med. Res. 2006, 4, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L.-S. Delivery of siRNA therapeutics: Barriers and carriers. Am. Assoc. Pharm. Sci. 2010, 12, 492–503. [Google Scholar] [CrossRef]
- Wei, M.; Good, D. Novel Gene Therapy Approaches; BoD–Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Klibanov, A. Non-viral gene therapy: Polycation-mediated DNA delivery. Appl. Microbiol. Biotechnol. 2003, 62, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kedmi, R.; Ben-Arie, N.; Peer, D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials 2010, 31, 6867–6875. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, N. Cationic polymer optimization for efficient gene delivery. Mini Rev. Med. Chem. 2010, 10, 108–125. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Yousefpour, P.; Atyabi, F.; Vasheghani-Farahani, E.; Movahedi, A.-A.M.; Dinarvand, R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumab. Int. J. Nanomed. 2011, 6, 1977–1990. [Google Scholar]
- Trickler, W.; Nagvekar, A.; Dash, A. A novel nanoparticle formulation for sustained paclitaxel delivery. Am. Assoc. Pharm. Sci. 2008, 9, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, Y.-S.; Kim, S.; Park, J.H.; Kim, K.; Choi, K.; Chung, H.; Jeong, S.Y.; Park, R.-W.; Kim, I.-S. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J. Control. Release 2006, 111, 228–234. [Google Scholar] [CrossRef]
- Ghaz-Jahanian, M.A.; Abbaspour-Aghdam, F.; Anarjan, N.; Berenjian, A.; Jafarizadeh-Malmiri, H. Application of chitosan-based nanocarriers in tumor-targeted drug delivery. Mol. Biotechnol. 2015, 57, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138. [Google Scholar] [CrossRef]
- Petrovsky, N.; Aguilar, J.C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Cooper, P.D. Carbohydrate-based immune adjuvants. Expert Rev. Vaccines 2011, 10, 523–537. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar]
- Powell, B.S.; Andrianov, A.K.; Fusco, P.C. Polyionic vaccine adjuvants: Another look at aluminum salts and polyelectrolytes. Clin. Exp. Vaccine Res. 2015, 4, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Zaharoff, D.A.; Hance, K.W.; Rogers, C.J.; Schlom, J.; Greiner, J. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J. Immunother. 2010, 33, 697. [Google Scholar] [CrossRef]
- Wen, Z.-S.; Xu, Y.-L.; Zou, X.-T.; Xu, Z.-R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Leena, R.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Bakhshayesh, D.; Rahmani, A.; Asadi, N.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An overview of advanced biocompatible and biomimetic materials for creation of replacement structures in the musculoskeletal systems: Focusing on cartilage tissue engineering. J. Biol. Eng. 2019, 13, 1–21. [Google Scholar] [CrossRef]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef]
- Rajabian, A.; Rameshrad, M.; Hosseinzadeh, H. Therapeutic potential of Panax ginseng and its constituents, ginsenosides and gintonin, in neurological and neurodegenerative disorders: A patent review. Expert Opin. Ther. Pat. 2019, 29, 55–72. [Google Scholar] [CrossRef]
- Amani, H.; Kazerooni, H.; Hassanpoor, H.; Akbarzadeh, A.; Pazoki-Toroudi, H. Tailoring synthetic polymeric biomaterials towards nerve tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3524–3539. [Google Scholar] [CrossRef]
- Entekhabi, E.; Nazarpak, M.H.; Moztarzadeh, F.; Sadeghi, A. Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater. Sci. Eng. C 2016, 69, 380–387. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.-W.; Li, J.-G.; Sun, X.-D. Preparation and mechanical property of a novel 3D porous magnesium scaffold for bone tissue engineering. Mater. Sci. Eng. C 2014, 42, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Titorencu, I.; Georgiana Albu, M.; Nemecz, M.; Jinga, V.V. Natural polymer-cell bioconstructs for bone tissue engineering. Curr. Stem Cell Res. Ther. 2017, 12, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Venkatesan, J.; Kim, S.-K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef] [PubMed]
- Tseomashko, N.E.; Rai, M.; Vasil’kov, A.Y. New hybrid materials for wound cover dressings. In Biopolymer-Based Nano Films; Elsevier: Amsterdam, The Netherlands, 2021; pp. 203–245. [Google Scholar]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Ying, R.; Wang, H.; Sun, R.; Chen, K. Preparation and properties of a highly dispersed nano-hydroxyapatite colloid used as a reinforcing filler for chitosan. Mater. Sci. Eng. C 2020, 110, 110689. [Google Scholar] [CrossRef] [PubMed]
- Gaihre, B.; Jayasuriya, A.C. Comparative investigation of porous nano-hydroxyapaptite/chitosan, nano-zirconia/chitosan and novel nano-calcium zirconate/chitosan composite scaffolds for their potential applications in bone regeneration. Mater. Sci. Eng. C 2018, 91, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.K.; Elçin, A.E.; Elçin, Y.M. Strontium-modified chitosan/montmorillonite composites as bone tissue engineering scaffold. Mater. Sci. Eng. C 2018, 89, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chen, Y.-K.; Lu, M.; Lou, K.-L.; Yu, J. Photo-crosslinked keratin/chitosan membranes as potential wound dressing materials. Polymers 2018, 10, 987. [Google Scholar] [CrossRef]
- Lu, H.-T.; Lu, T.-W.; Chen, C.-H.; Mi, F.-L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef]
- Nettles, D.L.; Elder, S.H.; Gilbert, J.A. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002, 8, 1009–1016. [Google Scholar] [CrossRef]
- Marlovits, S.; Zeller, P.; Singer, P.; Resinger, C.; Vécsei, V. Cartilage repair: Generations of autologous chondrocyte transplantation. Eur. J. Radiol. 2006, 57, 24–31. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Cucchiarini, M.; Madry, H. Hydrogels for precision meniscus tissue engineering: A comprehensive review. Connect. Tissue Res. 2017, 58, 317–328. [Google Scholar] [CrossRef]
- Sudarshan, N.; Hoover, D.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Tuzlakoglu, K.; Reis, R. Formation of bone-like apatite layer on chitosan fiber mesh scaffolds by a biomimetic spraying process. J. Mater. Sci. Mater. Med. 2007, 18, 1279–1286. [Google Scholar] [CrossRef]
- Tsai, G.-J.; Su, W.-H. Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Ali, S.W.; Purwar, R.; Rajendran, S. Ecofriendly antimicrobial finishing of textiles using bioactive agents based on natural products. Indian J. Fibre Text. Res. 2009, 34, 295–304. [Google Scholar]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef]
- Hipalaswins, W.M.; Balakumaran, M.; Jagadeeswari, S. Synthesis, characterization and antibacterial activity of chitosan nanoparticles and its impact on seed germination. J. Acad. Ind. Res. 2016, 5, 65. [Google Scholar]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Corona-Rangel, M. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. Lebensm.-Wiss. Und-Technol. 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Mofazzal, J.M.A.; Al Musawi, S.; Pirestani, M.; Fasihi, R.M.; Ahmadi, K.; Rajayi, H.; Mohammad, H.Z.; Kamali, M.; Mirnejad, R. Curcumin-loaded chitosan tripolyphosphate nanoparticles as a safe, natural and effective antibiotic inhibits the infection of Staphylococcus aureus and Pseudomonas aeruginosa in vivo. Iran. J. Biotechnol. 2014, 12, e1012. [Google Scholar]
- Pilon, L.; Spricigo, P.C.; Miranda, M.; de Moura, M.R.; Assis, O.B.G.; Mattoso, L.H.C.; Ferreira, M.D. Chitosan nanoparticle coatings reduce microbial growth on fresh-cut apples while not affecting quality attributes. Int. J. Food Sci. Technol. 2015, 50, 440–448. [Google Scholar] [CrossRef] [Green Version]
| Chitosan Nanoparticles | Biomedical Application | Findings | References |
|---|---|---|---|
| Ch-Au particles | Biomedical sensors | Immobilisation of enzymes | [119] |
| Ch–montmorillonite nanocomposites | Biomedical sensors | Used for anionic detection in aqueous samples | [120] |
| Ch-RNAi complexes | Gene therapy | Transfection of CHO-K1, HEK293, H1299, HepG2 cells | [121] |
| Ch-grafted polyethylene glycol methacrylate | Ophthalmic diseases | No cytotoxicity, hemocompatible | [122] |
| Graphene/AuNP/Ch electrode | Glucose biosensor | High electrocatalytic activity toward hydrogen peroxide and oxygen | [123] |
| Insulin-loaded lecithin/ChNP | Drug delivery system | Increased bioavailability, release, and enhanced therapeutic properties | [124] |
| Chitin nanofiber composite | Therapeutic enzyme immobilisation | Separation of immobilised chymotrypsin is easy and recycled | [125] |
| Modified glycol ChNP-encapsulated camptothecin | Cancer therapy | Efficient drug delivery system | [126] |
| Palladium NP chitosan oligosaccharide with RGD peptide | Breast cancer therapy by enhancing photothermal effects | Enhanced imaging and tumour therapy | [127] |
| Saquinavir-loaded ChNP | Anti-HIV system | Strains of HIV—NL4-3 and indie-C1 responded to the delivery system | [128] |
| Sodium alginate with Ch and olive oil-coated beads | Helicobacter pylori infections | Controlled release of active clarithromycin | [129] |
| Timolol maleate-galactosylated ChNP | Ocular delivery of timolol maleate | Enhanced penetration and retention | [125] |
| Zinc-ChNP | Acute lymphoblastic leukaemia | Induced apoptosis in human acute T-lymphocyte leukaemia | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.u.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. https://doi.org/10.3390/ma15196521
Bashir SM, Ahmed Rather G, Patrício A, Haq Z, Sheikh AA, Shah MZuH, Singh H, Khan AA, Imtiyaz S, Ahmad SB, et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials. 2022; 15(19):6521. https://doi.org/10.3390/ma15196521
Chicago/Turabian StyleBashir, Showkeen Muzamil, Gulzar Ahmed Rather, Ana Patrício, Zulfiqar Haq, Amir Amin Sheikh, Mohd Zahoor ul Haq Shah, Hemant Singh, Azmat Alam Khan, Sofi Imtiyaz, Sheikh Bilal Ahmad, and et al. 2022. "Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications" Materials 15, no. 19: 6521. https://doi.org/10.3390/ma15196521
APA StyleBashir, S. M., Ahmed Rather, G., Patrício, A., Haq, Z., Sheikh, A. A., Shah, M. Z. u. H., Singh, H., Khan, A. A., Imtiyaz, S., Ahmad, S. B., Nabi, S., Rakhshan, R., Hassan, S., & Fonte, P. (2022). Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials, 15(19), 6521. https://doi.org/10.3390/ma15196521







