Alkali-silica Reaction Elimination Potential of High-Performance Concrete Containing Glass Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- container waste glass = CWG,
- secondary raw material from glass jewelry production = SGJ,
- secondary raw material from glass production = SGP,
- glass from used photovoltaic panels = GPP.
- reference high-performance concrete mixture = REF HPC,
- high-performance concrete containing 50% replacement of silica flour with container waste glass = CWG HPC 50,
- high-performance concrete containing 100% replacement of silica flour with container waste glass = CWG HPC 100,
- high-performance concrete containing 50% replacement of silica flour with secondary raw material from glass jewelry production = SGJ HPC 50,
- high-performance concrete containing 100% replacement of silica flour with secondary raw material from glass jewelry production = SGJ HPC 100,
- high-performance concrete containing 50% replacement of silica flour with secondary raw material from glass production = SGP HPC 50,
- high-performance concrete containing 100% replacement of silica flour with secondary raw material from glass production = SGP HPC 100,
- high-performance concrete containing 50% replacement of silica flour with glass from used photovoltaic panels = GPP HPC 50,
- high-performance concrete containing 100% replacement of silica flour with glass from used photovoltaic panels = GPP HPC 100,
2.2. Methodology
2.2.1. ASTM Method C1260
2.2.2. TP 137
2.2.3. The Evaluation of the ASR Process
3. Results
3.1. Chemical Composition and Ecotoxicity
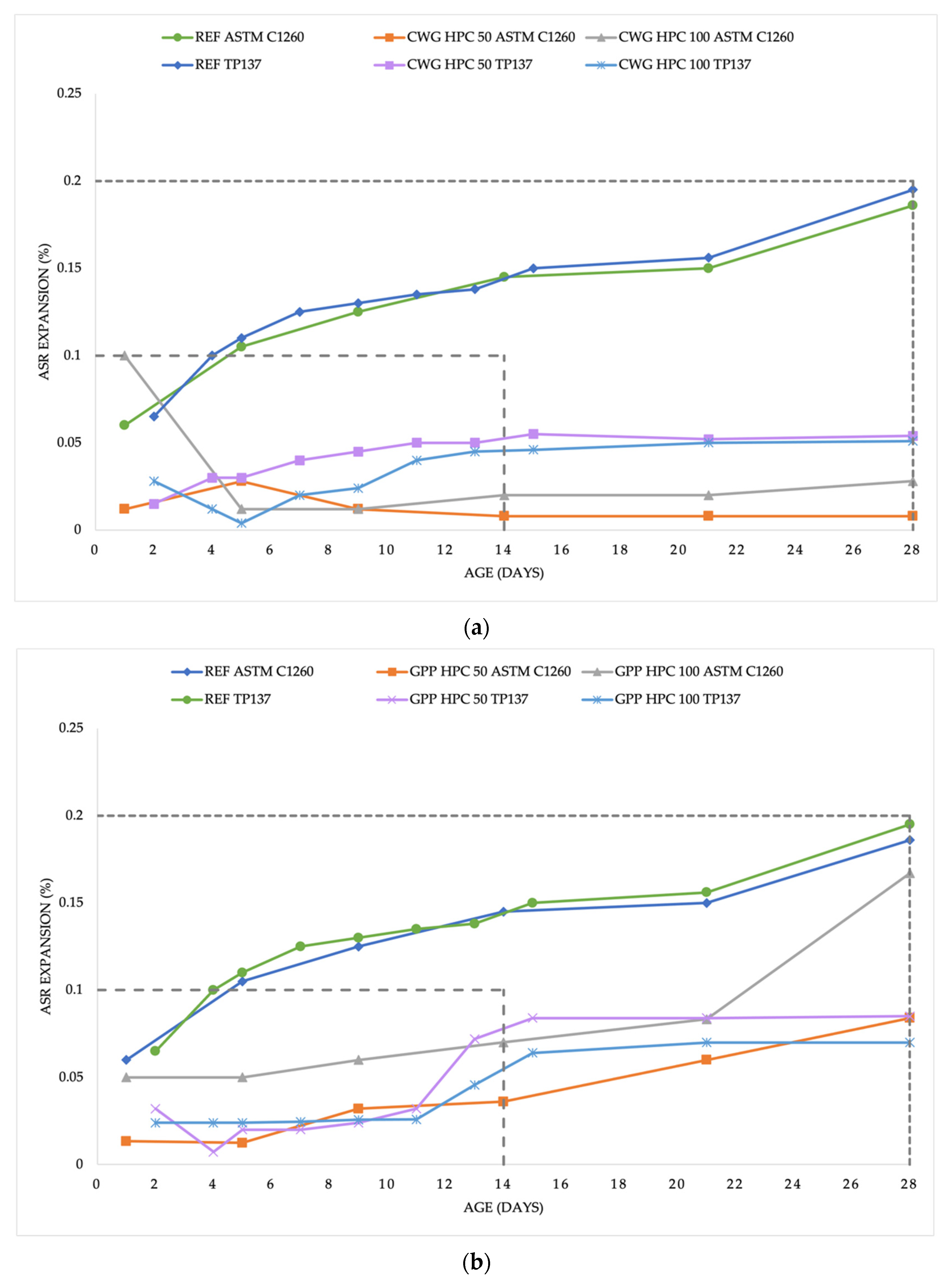
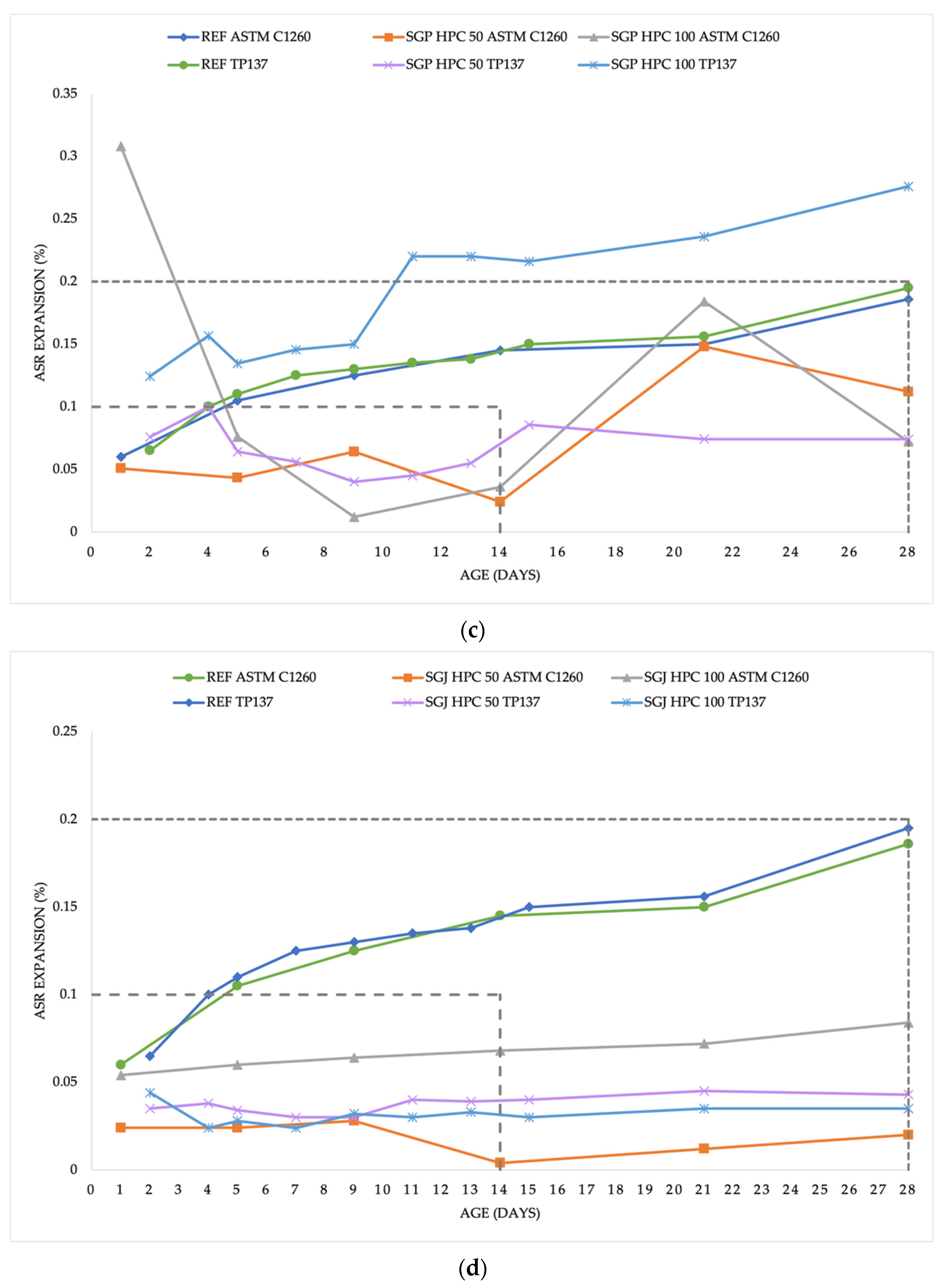
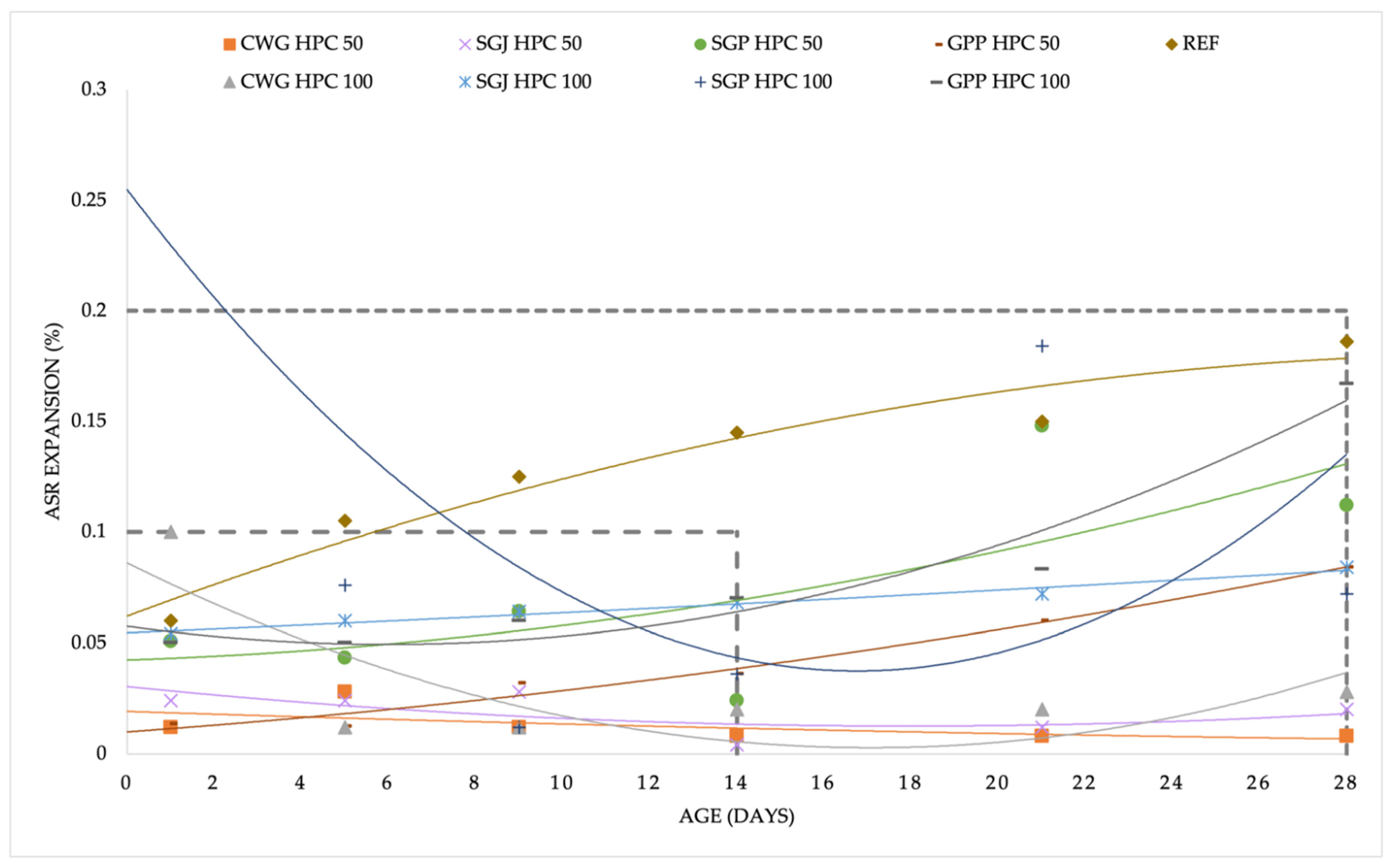
3.2. Alkali-silica Reaction Process
4. Discussion
4.1. Chemical Analysis
4.2. Alkali-silica Expansion
5. Conclusions
- The SGP leachate showed the highest content of silicon.
- The GPP leachate was classified as an ecotoxic material.
- All HPC leachates were considered environmentally safe.
- After ASTM C1260 testing, cracks appeared on one reference sample (up to 1 cm).
- No changes on the surface were observed after TP137 testing.
- GPP HPC 100 and REF HPC expansion after an extended experiment period (28 days) was between 0.1 and 0.2%. Further testing is necessary.
- SGP HPC 100 expansion after standard 14 days period was above 0.2%. Mixture may be at risk of a potential alkali-silica reaction.
- According to the standards, the conditions to exclude the occurrence of an ASR in the mixtures CWG HPC 50, CWH HPC 100, SGJ HPC 50, SGJ HPC 100, SGP HPC 50, and GPP HPC 50 were fulfilled.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariaková, D.; Jirkalová, Z.; Fořtová, K.; Pavlů, T.; Hájek, P. Potential Alkali-Silica Reaction in Recycled Concrete. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2021; p. 020029. [Google Scholar]
- Mariaková, D.; Mocová, K.A.; Fořtová, K.; Pavlů, T.; Hájek, P. Waste Glass Powder Reusability in High-Performance Concrete: Leaching Behavior and Ecotoxicity. Materials 2021, 14, 4476. [Google Scholar] [CrossRef]
- Mariaková, D.; Mocová, K.A.; Fořtová, K.; Ryparová, P.; Pešta, J.; Pavlů, T. Ecotoxicity and Essential Properties of Fine-Recycled Aggregate. Materials 2021, 14, 463. [Google Scholar] [CrossRef]
- Mariaková, D.; Mocová, K.A.; Pešta, J.; Fořtová, K.; Tripathi, B.; Pavlů, T.; Hájek, P. Ecotoxicity of Concrete Containing Fine-Recycled Aggregate: Effect on Photosynthetic Pigments, Soil Enzymatic Activity and Carbonation Process. Sustainability 2022, 14, 1732. [Google Scholar] [CrossRef]
- Mariaková, D.; Vlach, T.; Pavlů, T. Glass Waste Powder Utilization in High-Performance Concrete. Acta Polytech. CTU Proc. 2019, 21, 24–27. [Google Scholar] [CrossRef]
- Mariaková, D.; Fořtová, K.; Jirkalová, Z.; Pavlů, T.; Hájek, P. High-Performance Concrete Containing Waste Vitrified Tiles. APP 2022, 33, 357–362. [Google Scholar] [CrossRef]
- Mariaková, D.; Pavlů, T. Možnosti Využití Odpadního Skla a Keramiky Do Betonu. In Proceedings of the Recyklace a Využití Stavebních Odpadů Jako Druhotných Surovin, Brno, Czech Republic, 4–5 April 2019; pp. 68–74. [Google Scholar]
- de Brito, J.; Kurda, R. The Past and Future of Sustainable Concrete: A Critical Review and New Strategies on Cement-Based Materials. J. Clean. Prod. 2021, 281, 123558. [Google Scholar] [CrossRef]
- Şanal, İ. Discussion on the Effectiveness of Cement Replacement for Carbon Dioxide (CO2) Emission Reduction in Concrete: Original Research Articles: Discussion on the Effectiveness of Cement Replacement for Carbon Dioxide (CO2) Emission Reduction. Greenh. Gases: Sci. Technol. 2018, 8, 366–378. [Google Scholar] [CrossRef]
- Aslani, F.; Ma, G.; Yim Wan, D.L.; Muselin, G. Development of High-Performance Self-Compacting Concrete Using Waste Recycled Concrete Aggregates and Rubber Granules. J. Clean. Prod. 2018, 182, 553–566. [Google Scholar] [CrossRef]
- Amin, M.; Tayeh, B.A.; Agwa, I.S. Effect of Using Mineral Admixtures and Ceramic Wastes as Coarse Aggregates on Properties of Ultrahigh-Performance Concrete. J. Clean. Prod. 2020, 273, 123073. [Google Scholar] [CrossRef]
- Kumari, S.; Agarwal, S.; Khan, S. Micro/Nano Glass Pollution as an Emerging Pollutant in near Future. J. Hazard. Mater. Adv. 2022, 6, 100063. [Google Scholar] [CrossRef]
- Dabbaghi, F.; Sadeghi-Nik, A.; Libre, N.A.; Nasrollahpour, S. Characterizing Fiber Reinforced Concrete Incorporating Zeolite and Metakaolin as Natural Pozzolans. Structures 2021, 34, 2617–2627. [Google Scholar] [CrossRef]
- Winder, C.; Carmody, M. The Dermal Toxicity of Cement. Toxicol. Ind. Health 2002, 18, 321–331. [Google Scholar] [CrossRef]
- Stanton, T.E. Influence of cement and aggregate on concrete expansion. Eng. News-Record. 1940, 124, 171–173. [Google Scholar]
- Chatterji, S. Chemistry of Alkali–Silica Reaction and Testing of Aggregates. Cem. Concr. Compos. 2005, 27, 788–795. [Google Scholar] [CrossRef]
- ASTM C1260-07; American Society for Testing and Materials: Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). ASTM International: West Conshohocken, PA, USA, 2014.
- BS 812-123:1999; Method for Determination of Alkali-Silica Reactivity, Concrete Prism Method. British Standards Institution: London, UK, 1999.
- ČSN 72 1179; Stanovení Reaktivnosti Kameniva s Alkáliemi. Technický a Zkušební Ústav Stavební v Praze: Prague, Czech Republic, 1968.
- Dhir, R.K.; Dyer, T.D.; Tang, M.C. Alkali-Silica Reaction in Concrete Containing Glass. Mater Struct 2009, 42, 1451–1462. [Google Scholar] [CrossRef]
- Zheng, K. Pozzolanic Reaction of Glass Powder and Its Role in Controlling Alkali–Silica Reaction. Cem. Concr. Compos. 2016, 67, 30–38. [Google Scholar] [CrossRef]
- Bulteel, D.; Garcia-Diaz, E.; Vernet, C.; Zanni, H. Alkali–Silica Reaction. Cem. Concr. Res. 2002, 32, 1199–1206. [Google Scholar] [CrossRef]
- Thomas, M. The Effect of Supplementary Cementing Materials on Alkali-Silica Reaction: A Review. Cem. Concr. Res. 2011, 41, 1224–1231. [Google Scholar] [CrossRef]
- Breitenbücher, R. Alkalicko-Křemičitá Reakce—Důsledky pro Cementobetonové Kryt. Str. Autob. 2006, 205–209. [Google Scholar]
- TP 137; Vyloučení Alkalické Reakce Kameniva v Betonu Na Stavbách Pozemních Komunikací. Ministerstvo dopravy: Prague, Czech Republic, 2016.
- Mariaková, D. Glass Powder Waste Utilization in High Performance Concrete. Ph.D. Thesis, CTU in Prague, Prague, Czech Republic, 2018. [Google Scholar]
- EN12457-4:2002; Characterization of Waste—Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 4: One Stage Batch Test at a Liquid to Solid Ratio of 10 I/Kg for Materials with Particle Size below 10 Mm (without or with Size Reduction). CEN: Brussels, Belgium, 2002.
- DAfStb—Richtlinie: Vorbeugende Massnahmen Gegen Schädigende Alkalireaktion Im Beton (Alkali–Richtlinie). Ausgabe Februar 2007—Berichtigung: April 2010. Available online: https://www.stb-pruefinstitut.de/sites/default/files/download/2011_04_13%20Vortrag%202_AKR.pdf. (accessed on 13 October 2011).
- Mariaková, D.; Jirkalová, Z.; Řepka, J.; Vlach, T.; Hájek, P. Využití odpadního skla z fotovoltaických panelů ve vysokohodnotném betonu. In Proceedings of the Sborník Příspěvků 16. KONFERENCE SPECIÁLNÍ BETONY; SKURKON: Žár nad Sázavou, Česká Republika; pp. 25–30.
- McCarthy, M.J.; Dhir, R.K.; Halliday, J.E.; Wibowo, A. Role of PFA Quality and Conditioning in Minimising Alkali–Silica Reaction in Concrete. Mag. Concr. Res. 2006, 58, 49–61. [Google Scholar] [CrossRef]
- Lindgård, J.; Rodum, E.; Pedersen, B. Alkali-Silica Reactions in Concrete—Relationship between Water Content and Observed Damage on Structures; ACI International: Farmington Hills, MI, USA, 2006; ISBN 978-0-87031-207-6. [Google Scholar]
- Relling, R.H. Coastal Concrete Bridges: Moisture State, Chloride Permeability and Aging Effects. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 1999. [Google Scholar]
- Pan, J.W.; Feng, Y.T.; Wang, J.T.; Sun, Q.C.; Zhang, C.H.; Owen, D.R.J. Modeling of Alkali-Silica Reaction in Concrete: A Review. Front. Struct. Civ. Eng. 2012, 6, 1–18. [Google Scholar] [CrossRef]
- Dent Glasser, L.S.; Kataoka, N. On the Role of Calcium in the Alkali-Aggregate Reaction. Cem. Concr. Res. 1982, 12, 321–331. [Google Scholar] [CrossRef]
- Zhuang, Y.; Qian, C.; Xu, W. Calculation of Alkali Silica Reaction (ASR) Induced Expansion before Cracking of Concrete. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2013, 28, 110–116. [Google Scholar] [CrossRef]
- Qian, C.; Zhuang, Y.; Huang, H. Numerical Calculation of Expansion Induced by Alkali Silica Reaction. Constr. Build. Mater. 2016, 103, 117–122. [Google Scholar] [CrossRef]



| Content [kg] | REF HPC | CWG HPC 50 | CWG HPC 100 | SGJ HPC 50 | SGJ HPC 100 | SGP HPC 50 | SGP HPC 100 | GPP HPC 50 | GPP HPC 100 |
|---|---|---|---|---|---|---|---|---|---|
| Cement I 42.5 R | 680 | 680 | 680 | 680 | 680 | 680 | 680 | 650 | 650 |
| Fine sand 1/6 | 576 | 576 | 576 | 576 | 576 | 576 | 576 | 600 | 600 |
| Coarse sand 6/12 | 384 | 384 | 384 | 384 | 384 | 384 | 384 | 600 | 600 |
| Microsilica | 175 | 175 | 175 | 175 | 175 | 175 | 175 | - | - |
| SF | 325 | 162.5 | - | 162.5 | - | 162.5 | - | 120 | - |
| CWG | - | 162.5 | 325 | - | - | - | - | - | - |
| SGJ | - | - | - | 162.5 | 325 | - | - | - | - |
| SGP | - | - | - | - | - | 162.5 | 325 | - | - |
| GPP | - | - | - | - | - | - | - | 120 | 240 |
| Plastificators | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 30 | 30 |
| Water | 171 | 171 | 171 | 171 | 171 | 171 | 171 | 180 | 180 |
| Element [mg/L] | SF | CWG | SGJ | SGP | GPP | REF HPC | CWG HPC 100 | SGJ HPC 100 | SGP HPC 100 | GPP HPC 100 |
|---|---|---|---|---|---|---|---|---|---|---|
| Si | 24.0 | 14.8 | 8.2 | 48.7 | 14.7 | 3.0 | 2.5 | 2.3 | 3.6 | 5.2 |
| Na | 0.7 | 128.3 | 194.7 | 50.6 | 87.1 | 2.2 | 3.0 | 6.2 | 1.2 | 3.6 |
| K | 1.9 | 4.5 | 110.5 | 32.7 | 0.7 | 18.6 | 15.6 | 10.8 | 18.7 | 8.8 |
| Ca | 3.4 | 5.7 | 3.0 | 1.5 | 6.1 | 20.6 | 26.5 | 20.4 | 20.3 | 54.2 |
| Al | ˂0.8 | 3.0 | 7.9 | ˂0.8 | 27.9 | ˂0.8 | ˂0.8 | ˂0.8 | ˂0.8 | ˂0.8 |
| pH | 6.8 | 10.5 | 10.9 | 11.1 | 10.3 | 11.0 | 11.1 | 11.0 | 10.9 | 11.4 |
| Ecotoxicity | S | S | S | S | E | S | S | S | S | S |
| Type | Material | SiO2 | Al2O3 | Na2O | CaO | Fe2O3 | K2O | Sum |
|---|---|---|---|---|---|---|---|---|
| Reference | SF | 99.68 | 0.17 | <0.005 | 0.03 | 0.03 | <0.005 | 99.9 |
| Glass | CWG | 68.94 | 2.06 | 14.50 | 10.50 | 0.37 | 0.75 | 97.1 |
| SGJ | 65.68 | 1.38 | 12.27 | 5.83 | 0.15 | 8.60 | 93.9 | |
| SGP | 63.58 | 0.51 | 16.61 | 2.64 | 0.08 | 7.02 | 90.4 | |
| GPP | 60.35 | 19.89 | 10.36 | 5.47 | 0.77 | <0.005 | 96.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariaková, D.; Mocová, K.A.; Fořtová, K.; Pavlů, T.; Hájek, P. Alkali-silica Reaction Elimination Potential of High-Performance Concrete Containing Glass Powder. Materials 2022, 15, 6574. https://doi.org/10.3390/ma15196574
Mariaková D, Mocová KA, Fořtová K, Pavlů T, Hájek P. Alkali-silica Reaction Elimination Potential of High-Performance Concrete Containing Glass Powder. Materials. 2022; 15(19):6574. https://doi.org/10.3390/ma15196574
Chicago/Turabian StyleMariaková, Diana, Klára Anna Mocová, Kristina Fořtová, Tereza Pavlů, and Petr Hájek. 2022. "Alkali-silica Reaction Elimination Potential of High-Performance Concrete Containing Glass Powder" Materials 15, no. 19: 6574. https://doi.org/10.3390/ma15196574
APA StyleMariaková, D., Mocová, K. A., Fořtová, K., Pavlů, T., & Hájek, P. (2022). Alkali-silica Reaction Elimination Potential of High-Performance Concrete Containing Glass Powder. Materials, 15(19), 6574. https://doi.org/10.3390/ma15196574








