Structural Formation of Alkali-Activated Materials Based on Thermally Treated Marl and Na2SiO3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design
2.3. Methods
3. Results and Discussion
3.1. Characterization of the Thermally Treated Marl
3.2. Microstructure of Alkali-Activated Material
| Spectra | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | Loss of Ignition |
|---|---|---|---|---|---|---|---|
| 1 | 11.46 | 0.00 | 19.49 | 54.26 | 0.00 | 2.34 | 12.46 |
| 2 | 6.29 | 0.00 | 15.72 | 57.93 | 0.00 | 9.01 | 11.05 |
| 3 | 1.14 | 0.23 | 0.29 | 2.19 | 0.00 | 41.97 | 54.19 |
| 4 | 2.23 | 0.52 | 0.91 | 16.70 | 0.18 | 39.59 | 39.87 |
| 5 | 0.85 | 0.55 | 0.56 | 6.69 | 0.00 | 42.13 | 49.22 |
| 6 | 0.82 | 0.00 | 0.56 | 4.97 | 0.00 | 39.96 | 53.69 |
3.3. Fresh, Physical and Mechanical Properties of Alkali-Activated Material
- -
- dispersion medium;
- -
- diffusion interfacial transition zone “powder particles—gel Na2SiO3”;
- -
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López, F.J.; Sugita, S.; Tagaya, M.; Kobayashi, T. Metakaolin-Based Geopolymers for Targeted Adsorbents to Heavy Metal Ion Separation. J. Mater. Sci. Chem. Eng. 2014, 2, 16–27. [Google Scholar] [CrossRef]
- Pavlíková, M.; Zemanová, L.; Záleská, M.; Pokorný, J.; Lojka, M.; Jankovský, O.; Pavlík, Z. Ternary Blended Binder for Production of a Novel Type of Lightweight Repair Mortar. Materials 2019, 12, 996. [Google Scholar] [CrossRef] [PubMed]
- Tolstoy, A.; Lesovik, V.; Fediuk, R.; Amran, M.; Gunasekaran, M.; Vatin, N.; Vasilev, Y. Production of Greener High-Strength Concrete Using Russian Quartz Sandstone Mine Waste Aggregates. Materials 2020, 13, 5575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tong, L.; Li, B.; Chen, T.; Wang, C.; Yang, G.; Zheng, Y. Eco-Friendly Geopolymer Materials: A Review of Performance Improvement, Potential Application and Sustainability Assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar] [CrossRef]
- Salamanova, M.S.; Murtazaev, S.-A.Y.; Nakhaev, M.R. Possible Alternative Solutions to Problems in the Cement Industry. Stroit. Mater. 2020, 778, 73–77. [Google Scholar] [CrossRef]
- Aliev, S.A.; Murtazayeva, R.S.-A.; Salamanova, M.S. Structure and properties of connecting alkaline activation using cement dust. Her. Dagestan State Tech. Univ. Tech. Sci. 2019, 46, 148–157. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef]
- Amran, M.; Fediuk, R.; Abdelgader, H.S.; Murali, G.; Ozbakkaloglu, T.; Lee, Y.H.; Lee, Y.Y. Fiber-Reinforced Alkali-Activated Concrete: A Review. J. Build. Eng. 2022, 45, 103638. [Google Scholar] [CrossRef]
- Assi, L.N.; Deaver, E.; Elbatanouny, M.K.; Ziehl, P. Investigation of Early Compressive Strength of Fly Ash-Based Geopolymer Concrete. Constr. Build. Mater. 2016, 112, 807–815. [Google Scholar] [CrossRef]
- Firdous, R.; Stephan, D.; Djobo, J.N.Y. Natural Pozzolan Based Geopolymers: A Review on Mechanical, Microstructural and Durability Characteristics. Constr. Build. Mater. 2018, 190, 1251–1263. [Google Scholar] [CrossRef]
- Amran, M.; Murali, G.; Fediuk, R.; Vatin, N.; Vasilev, Y.; Abdelgader, H. Palm Oil Fuel Ash-Based Eco-Efficient Concrete: A Critical Review of the Short-Term Properties. Materials 2021, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Provis, J.L.; Zou, J.; Reid, A.; Wang, H. Toward an Indexing Approach to Evaluate Fly Ashes for Geopolymer Manufacture. Cem. Concr. Res. 2016, 85, 163–173. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.J.G.; Brouwers, H.J.H.; Kühne, H.-C. Synthesizing One-Part Geopolymers from Rice Husk Ash. Constr. Build. Mater. 2016, 124, 961–966. [Google Scholar] [CrossRef]
- Fediuk, R.; Smoliakov, A.; Stoyushko, N. Increase in Composite Binder Activity. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 156. [Google Scholar]
- Shoaei, P.; Ameri, F.; Reza Musaeei, H.; Ghasemi, T.; Cheah, C.B. Glass Powder as a Partial Precursor in Portland Cement and Alkali-Activated Slag Mortar: A Comprehensive Comparative Study. Constr. Build. Mater. 2020, 251, 118991. [Google Scholar] [CrossRef]
- Fediuk, R.S.; Lesovik, V.S.; Liseitsev, Y.L.; Timokhin, R.A.; Bituyev, A.V.; Zaiakhanov, M.Y.; Mochalov, A.V. Composite Binders for Concretes with Improved Shock Resistance. Mag. Civ. Eng. 2019, 85, 28–38. [Google Scholar] [CrossRef]
- Payá, J.; Agrela, F.; Rosales, J.; Morales, M.M.; Borrachero, M.V. Application of Alkali-Activated Industrial Waste. In New Trends in Eco-efficient and Recycled Concrete; Woodhead Publishing: Thorston, UK, 2019. [Google Scholar]
- Volodchenko, A.A.; Lesovik, V.S.; Volodchenko, A.N.; Glagolev, E.S.; Zagorodnjuk, L.H.; Pukharenko, Y.V. Composite Performance Improvement Based on Non-Conventional Natural and Technogenic Raw Materials. Int. J. Pharm. Technol. 2016. [Google Scholar] [CrossRef]
- Gao, X.X.; Michaud, P.; Joussein, E.; Rossignol, S. Behavior of Metakaolin-Based Potassium Geopolymers in Acidic Solutions. J. Non-Cryst. Solids 2013, 380, 95–102. [Google Scholar] [CrossRef]
- Fediuk, R. Reducing Permeability of Fiber Concrete Using Composite Binders. Spec. Top. Rev. Porous Media Int. J. 2018, 9, 79–89. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Wang, T.; Zheng, Y.; Qiu, L.; Sun, S. Compressive Strength and Anti-Chloride Ion Penetration Assessment of Geopolymer Mortar Merging PVA Fiber and Nano-SiO2 Using RBF–BP Composite Neural Network. Nanotechnol. Rev. 2022, 11, 1181–1192. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, P.; Wu, J.; Jing, Y.; Zhang, D.; Zhang, T. Comprehensive Review of the Properties of Fly Ash-Based Geopolymer with Additive of Nano-SiO2. Nanotechnol. Rev. 2022, 11, 1478–1498. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Guo, J.; Zhang, H.; Wang, T. Effect of Municipal Solid Waste Incineration Ash on Microstructure and Hydration Mechanism of Geopolymer Composites. Buildings 2022, 12, 723. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of Alkali-Activated Materials: Progress and Perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Semenov, P.; Uzunian, A.; Davidenko, A.; Al, E. First Study of Radiation Hardness of Lead Tungstate Crystals at Low Temperatures. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 582, 575–580. [Google Scholar] [CrossRef]
- Kadhim, A.; Sadique, M.; Al-Mufti, R.; Hashim, K. Long-Term Performance of Novel High-Calcium One-Part Alkali-Activated Cement Developed from Thermally Activated Lime Kiln Dust. J. Build. Eng. 2020, 32, 101766. [Google Scholar] [CrossRef]
- Abid, S.R.; Murali, G.; Amran, M.; Vatin, N.; Fediuk, R.; Karelina, M. Evaluation of Mode II Fracture Toughness of Hybrid Fibrous Geopolymer Composites. Materials 2021, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.; Bahadory, M.; Tovia, F.; Rostami, H. Properties of Alkali-Activated Fly Ash: High Performance to Lightweight. Int. J. Sustain. Eng. 2010, 3, 211–218. [Google Scholar] [CrossRef]
- Vasilyeva, N.; Fedorova, E.; Kolesnikov, A. Big Data as a Tool for Building a Predictive Model of Mill Roll Wear. Symmetry 2021, 13, 859. [Google Scholar] [CrossRef]
- Danish, A.; Mosaberpanah, M.A.; Salim, M.U. Past and Present Techniques of Self-Healing in Cementitious Materials: A Critical Review on Efficiency of Implemented Treatments. J. Mater. Res. Technol. 2020, 9, 6883–6899. [Google Scholar] [CrossRef]
- Danish, A.; Mosaberpanah, M.A. Formation Mechanism and Applications of Cenospheres: A Review. J. Mater. Sci. 2020, 55, 4539–4557. [Google Scholar] [CrossRef]
- Mohd Ariffin, M.A.; Hussin, M.W.; Rafique Bhutta, M.A. Mix Design and Compressive Strength of Geopolymer Concrete Containing Blended Ash from Agro-Industrial Wastes. Adv. Mater. Res. 2011, 339, 452–457. [Google Scholar] [CrossRef]
- Fediuk, R.; Pak, A.; Kuzmin, D. Fine-Grained Concrete of Composite Binder. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Call, B.M. Guide for the Use of Silica Fume in Concrete; ACI–American Concrete Institute–Committee: Farmington Hills, MI, USA, 2000. [Google Scholar]
- Villa, C.; Pecina, E.T.; Torres, R.; Gómez, L. Geopolymer Synthesis Using Alkaline Activation of Natural Zeolite. Constr. Build. Mater. 2010, 24, 2084–2090. [Google Scholar] [CrossRef]
- Shubbar, A.A.; Sadique, M.; Nasr, M.S.; Al-Khafaji, Z.S.; Hashim, K.S. The Impact of Grinding Time on Properties of Cement Mortar Incorporated High Volume Waste Paper Sludge Ash. Karbala Int. J. Mod. Sci. 2020, 6, 7. [Google Scholar] [CrossRef]
- Kiran Kumar, N.L.N.; Gopala Krishna Sastry, K.V.S. Effects of Nano Silica on the Strengths of Geopolymer Concrete Cured at Ambient Temperature. Int. J. Civ. Eng. Technol. 2017, 8, 437–444. [Google Scholar]
- Jeyaseela, J.; Vishnuram, B.G. Study on Workability and Durability Characteristics of Self-Compacting Geopolymer Concrete Composites. Int. J. Adv. Technol. Eng. Sci. 2015, 3, 1246–1256. [Google Scholar]
- Haddaji, Y.; Majdoubi, H.; Mansouri, S.; Tamraoui, Y.; El bouchti, M.; Manoun, B.; Oumam, M.; Hannache, H. Effect of Synthetic Fibers on the Properties of Geopolymers Based on Non-Heat Treated Phosphate Mine Tailing. Mater. Chem. Phys. 2021, 260, 124147. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.; Hu, S.; Wang, J.; Wang, T. High-Temperature Behavior of Polyvinyl Alcohol Fiber-Reinforced Metakaolin/Fly Ash-Based Geopolymer Mortar. Compos. Part B Eng. 2022, 244, 110171. [Google Scholar] [CrossRef]
- Zhang, P.; Kang, L.; Zheng, Y.; Zhang, T.; Zhang, B. Influence of SiO2/Na2O Molar Ratio on Mechanical Properties and Durability of Metakaolin-Fly Ash Blend Alkali-Activated Sustainable Mortar Incorporating Manufactured Sand. J. Mater. Res. Technol. 2022, 18, 3553–3563. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Wang, T. Influencing Factors Analysis and Optimized Prediction Model for Rheology and Flowability of Nano-SiO2 and PVA Fiber Reinforced Alkali-Activated Composites. J. Clean. Prod. 2022, 366, 132988. [Google Scholar] [CrossRef]







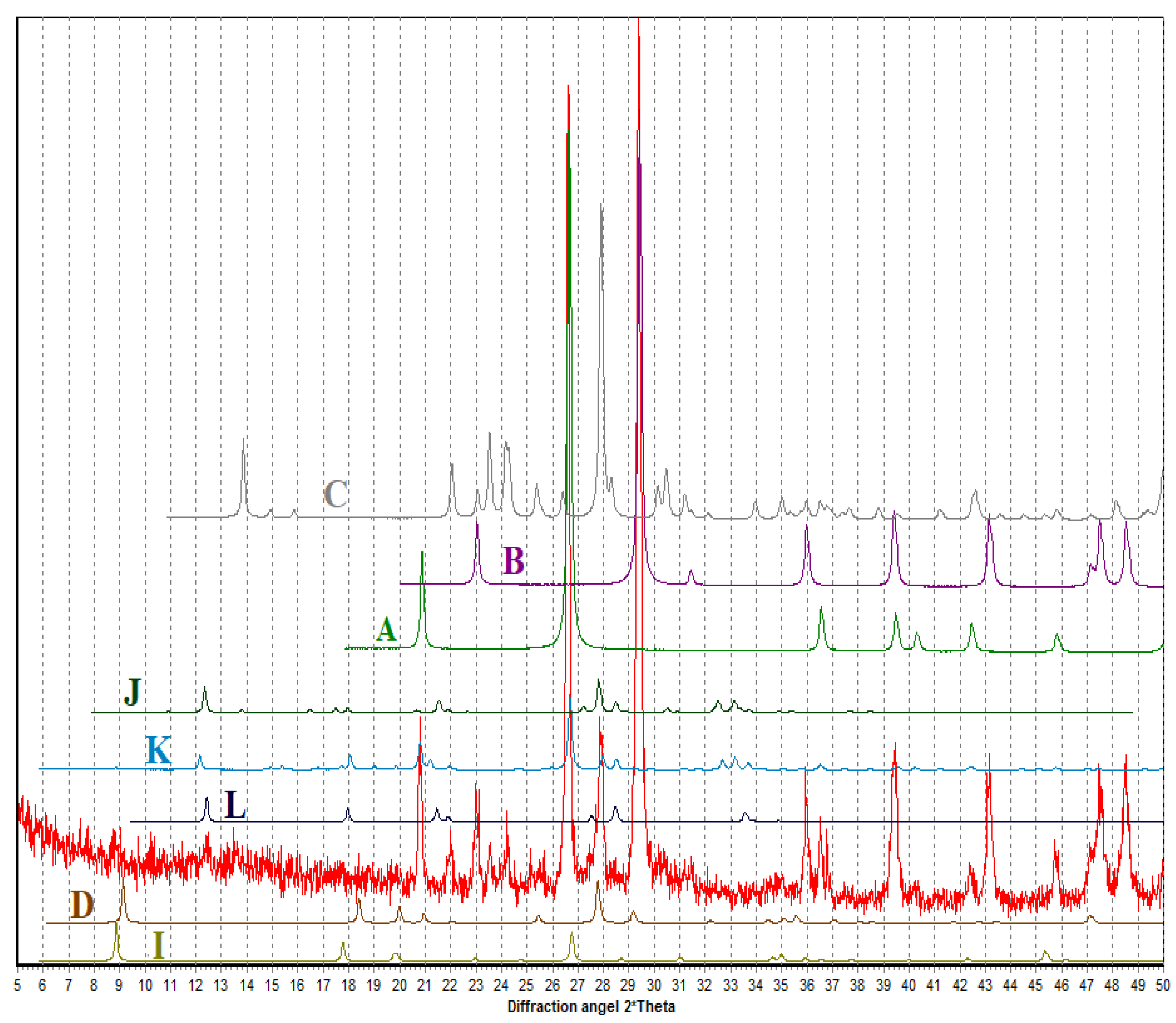
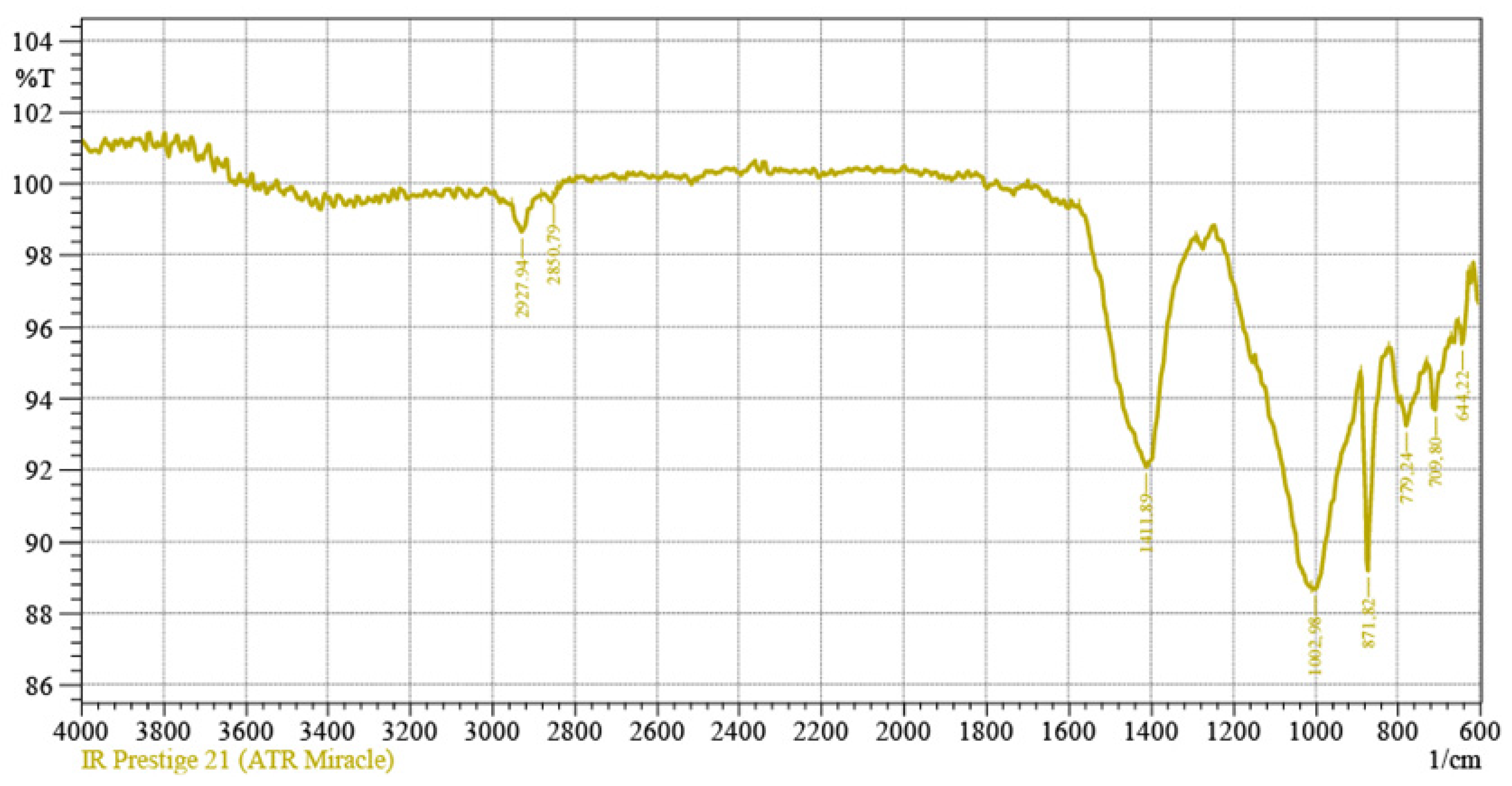
| Marl | CaO | SiO2 | Al2O3 | Fe2O3 | Na2O | MgO | CO2 | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|
| Initial | 66.61 | 12.29 | 2.23 | 1.16 | 0.38 | 0.25 | 17.08 | - |
| Treated | 61.53 | 12.11 | 2.07 | 1.12 | 0.29 | 0.10 | - | 22.78 |
| Mix ID | Components, kg per 1 m3 | ||||||
|---|---|---|---|---|---|---|---|
| Marl | Marl Thermally Treated at 700 °C | Na2SiO3 | H2O | Na2SiF6 | NaOH | Sand | |
| M1 | 700 | - | 280 | - | - | 70 | 1040 |
| M2 | 700 | - | 280 | - | 16.8 | 70 | 1040 |
| TM1 | - | 700 | 280 | - | - | 70 | 1040 |
| TM2 | - | 700 | - | 280 | - | 70 | 1040 |
| Properties | TM1 | TM2 | M1 | M2 |
|---|---|---|---|---|
| Normal density of AAM, % | 56.0 | 40.0 | 51.0 | 52.0 |
| Setting time | 00–26 | 01–37 | 01–07 | 00–55 |
| Start/end, hours-min | 00–32 | 06–29 | 02–29 | 01–43 |
| Average density, g/cm3 | 1.90 | 1.80 | 2.00 | 2.01 |
| Water absorption, wt.% | 10.2 | 11.4 | 11.9 | 11.7 |
| Strength, MPa: | ||||
| Flexural | 4.7 | 0.2 | 1.0 | 1.1 |
| Compressive | 42.6 | 6.7 | 9.0 | 9.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mintsaev, M.; Murtazaev, S.-A.; Salamanova, M.; Bataev, D.; Saidumov, M.; Murtazaev, I.; Fediuk, R. Structural Formation of Alkali-Activated Materials Based on Thermally Treated Marl and Na2SiO3. Materials 2022, 15, 6576. https://doi.org/10.3390/ma15196576
Mintsaev M, Murtazaev S-A, Salamanova M, Bataev D, Saidumov M, Murtazaev I, Fediuk R. Structural Formation of Alkali-Activated Materials Based on Thermally Treated Marl and Na2SiO3. Materials. 2022; 15(19):6576. https://doi.org/10.3390/ma15196576
Chicago/Turabian StyleMintsaev, Magomed, Sayd-Alvi Murtazaev, Madina Salamanova, Dena Bataev, Magomed Saidumov, Imran Murtazaev, and Roman Fediuk. 2022. "Structural Formation of Alkali-Activated Materials Based on Thermally Treated Marl and Na2SiO3" Materials 15, no. 19: 6576. https://doi.org/10.3390/ma15196576





