Adsorption Properties of Essential Oils on Polylactic Acid Microparticles of Different Sizes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microparticles Properties
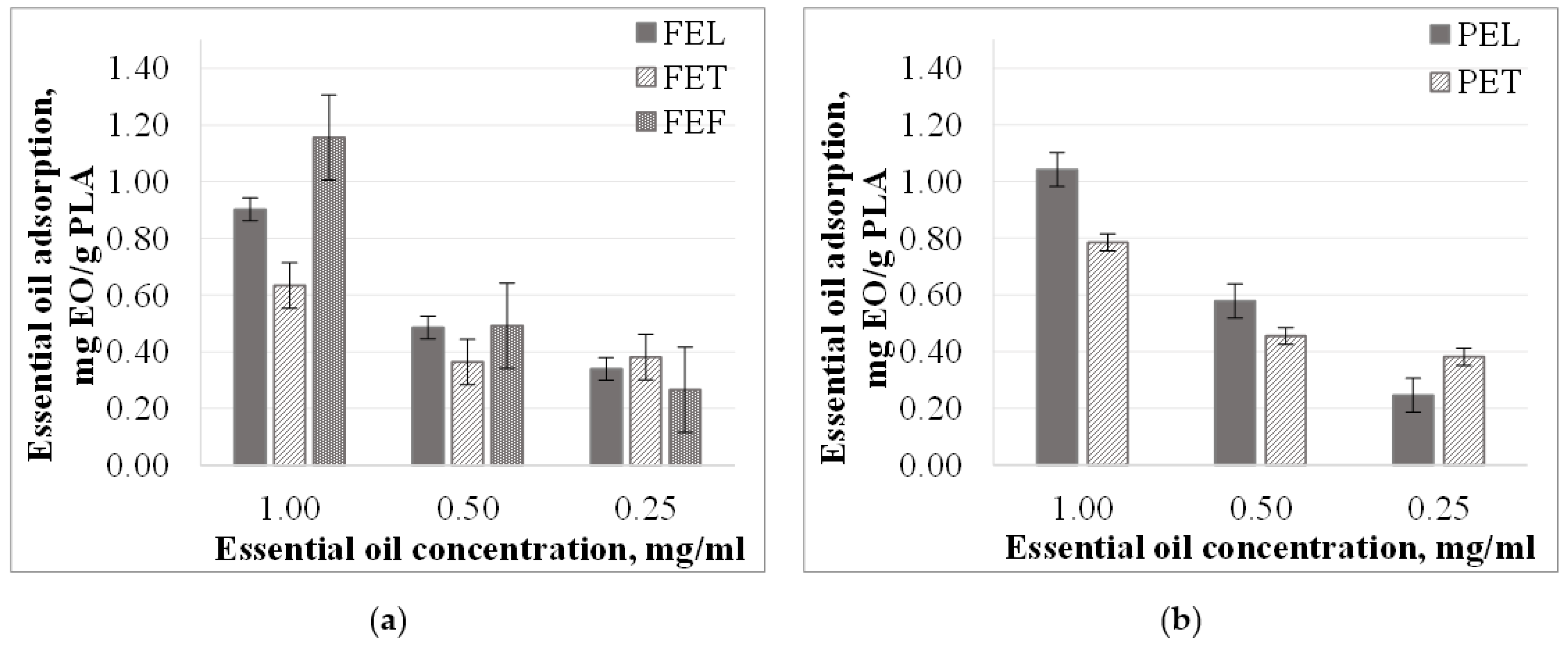
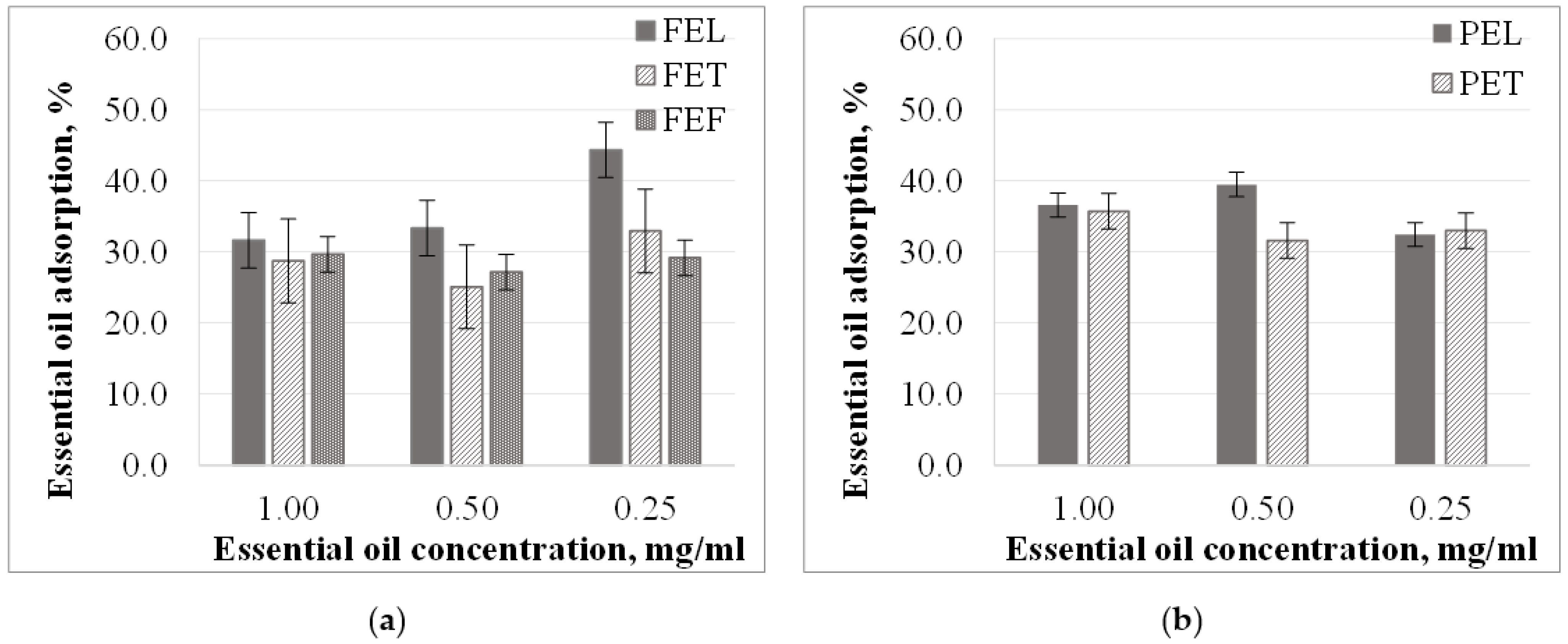
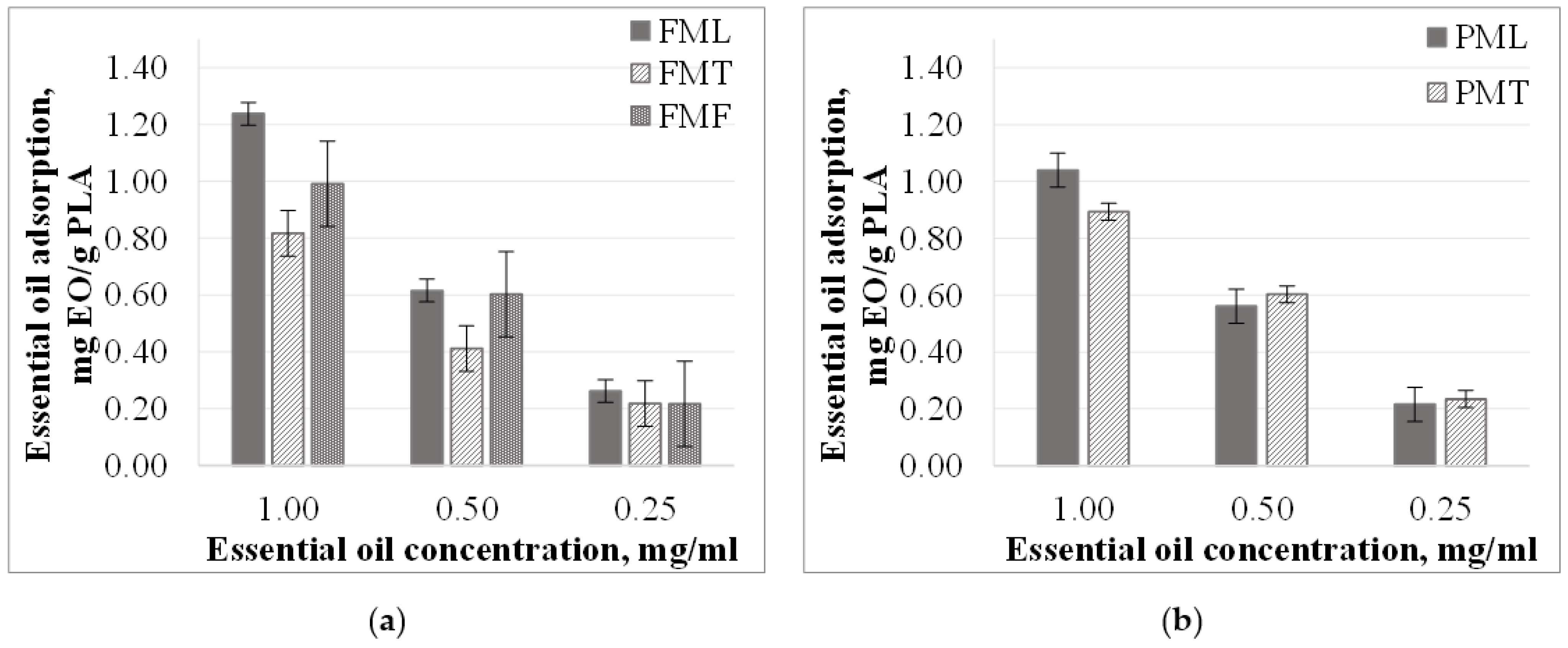
3.2. Microparticles Essential Oil Adsorption from Different Concentration Solutions
3.2.1. Essential Oil Adsorption from Ethanol Solutions
3.2.2. Essential Oil Adsorption from Methanol Solutions
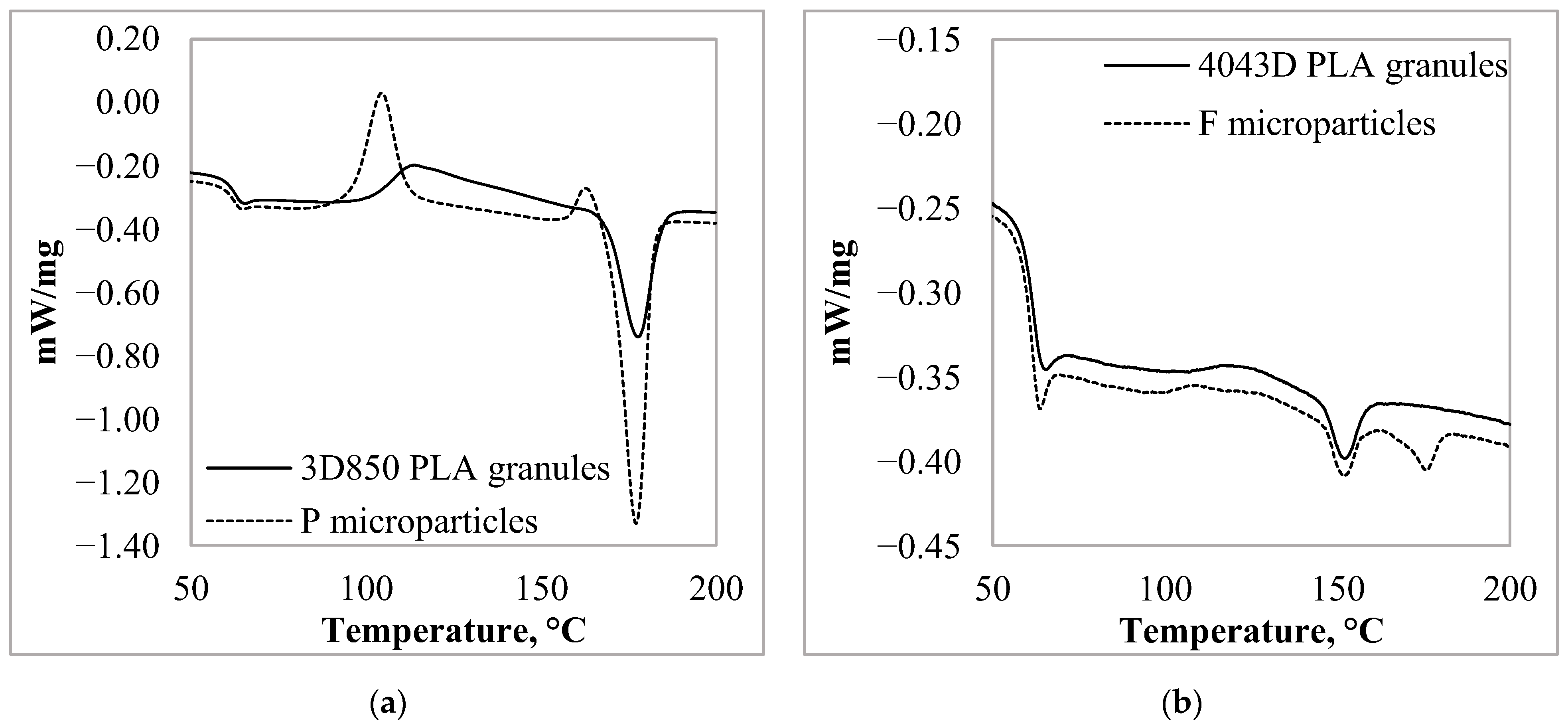
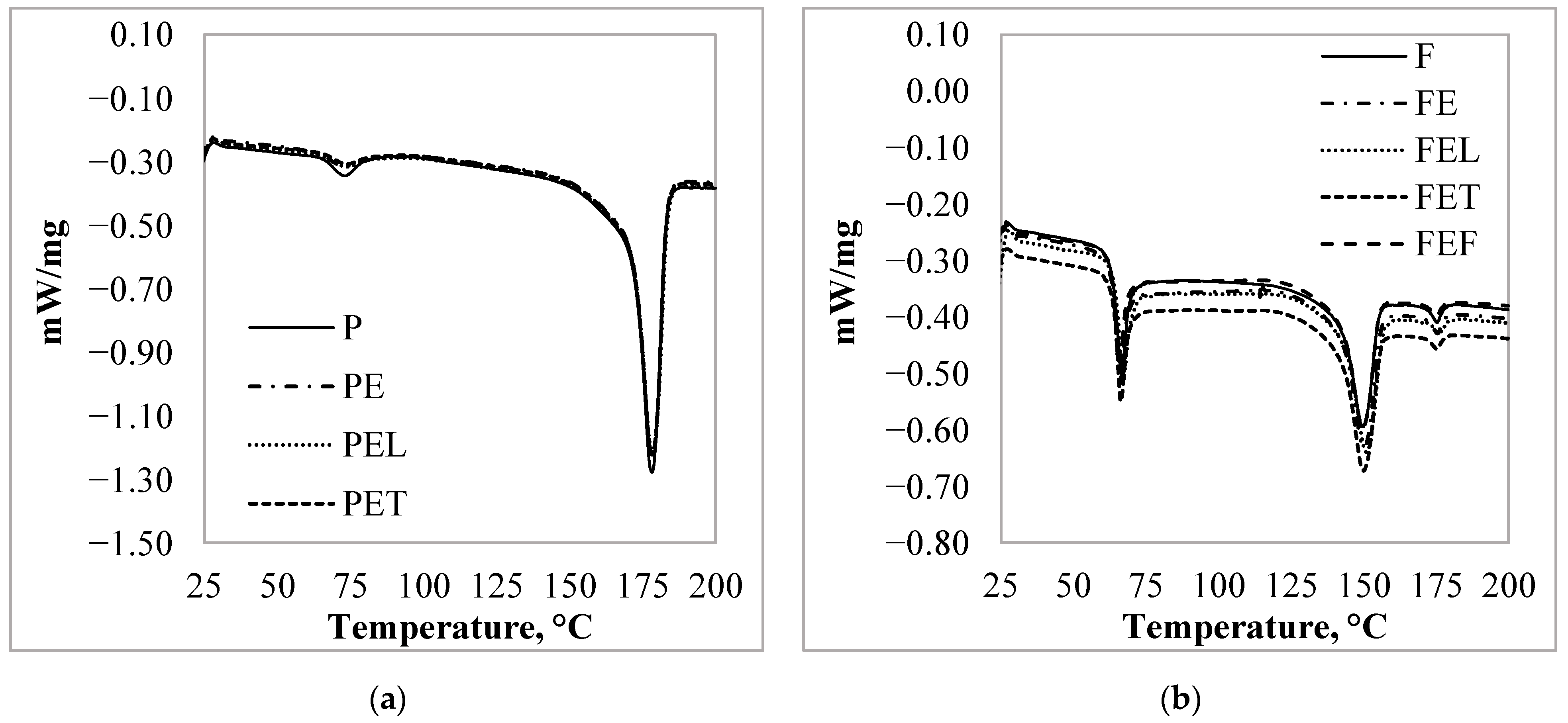
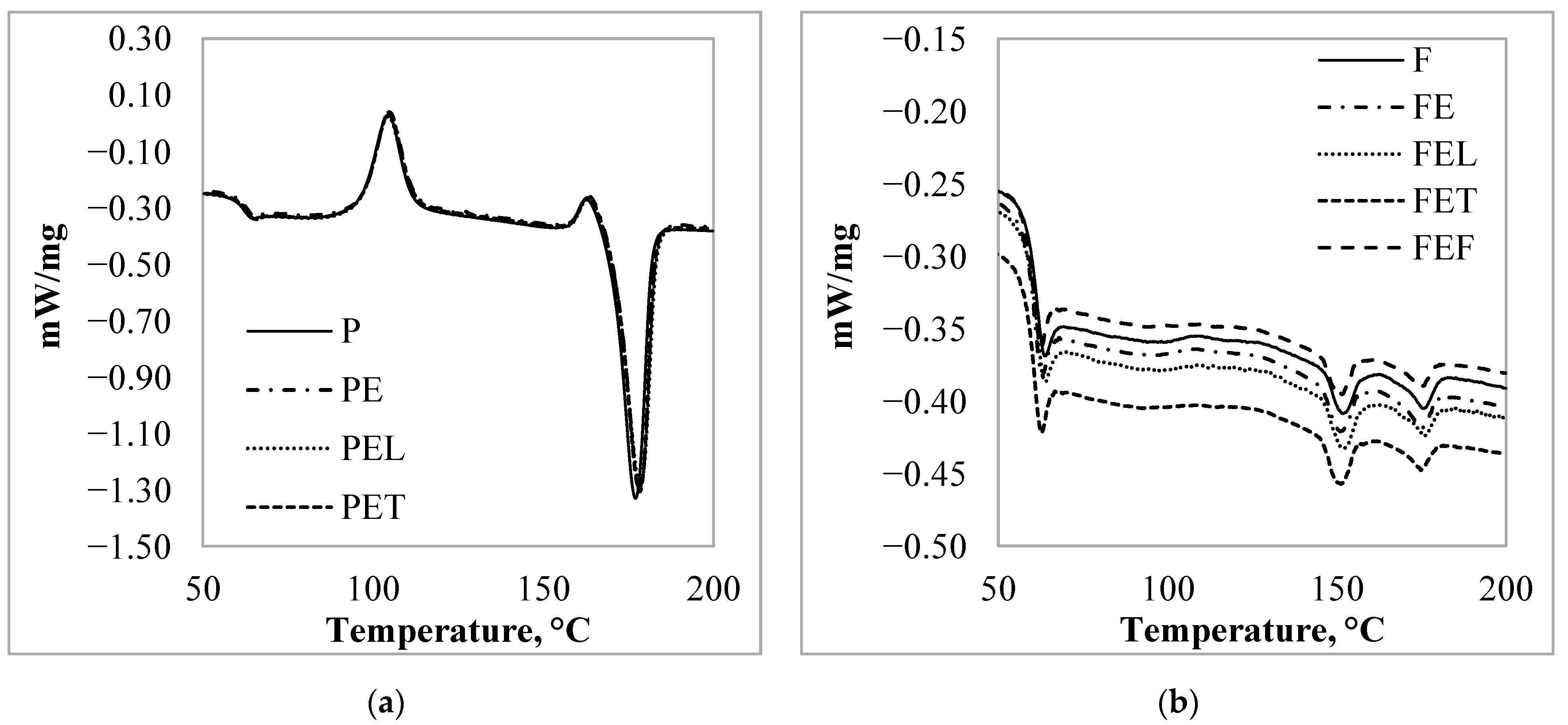
3.3. Thermal Characteristic of the PLA Particles
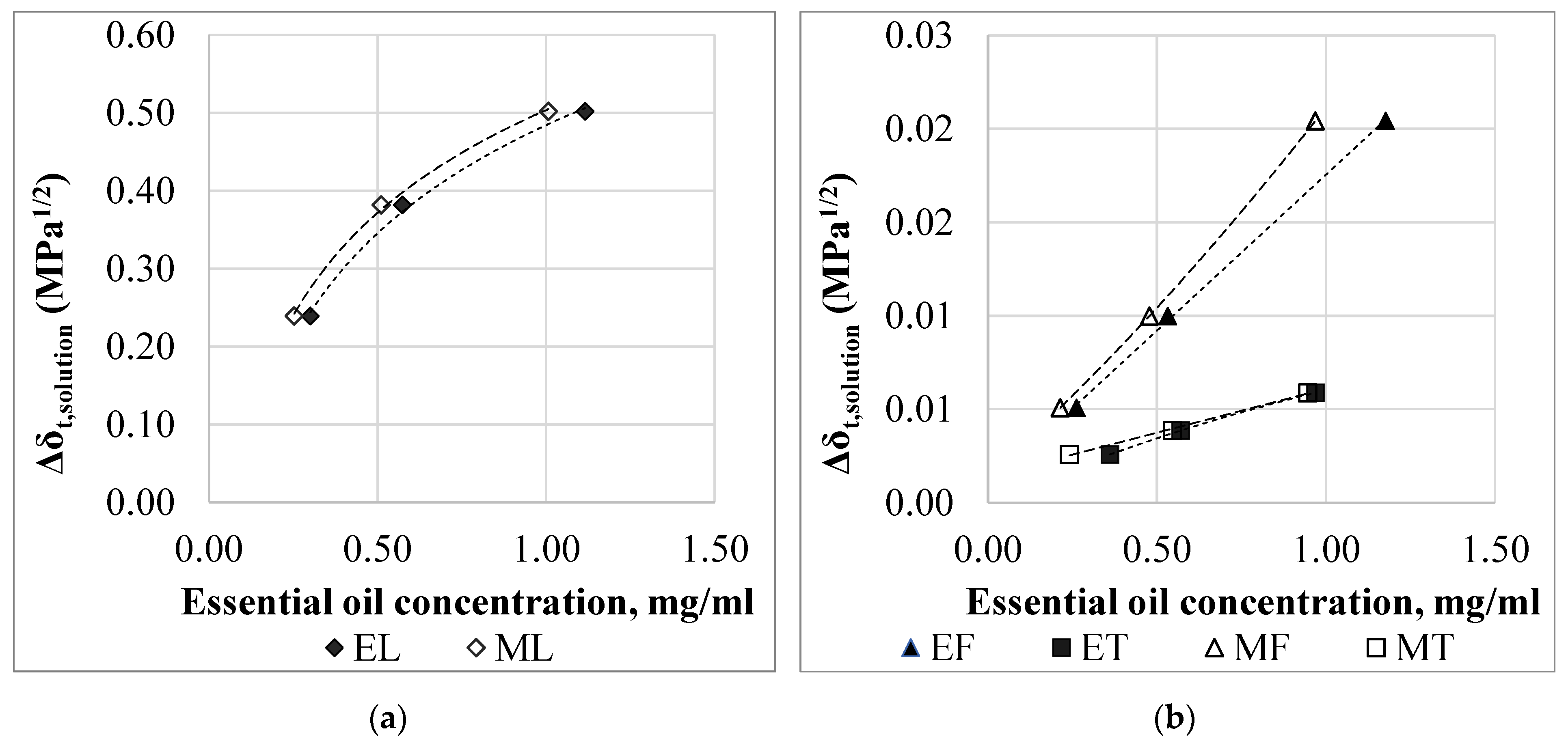
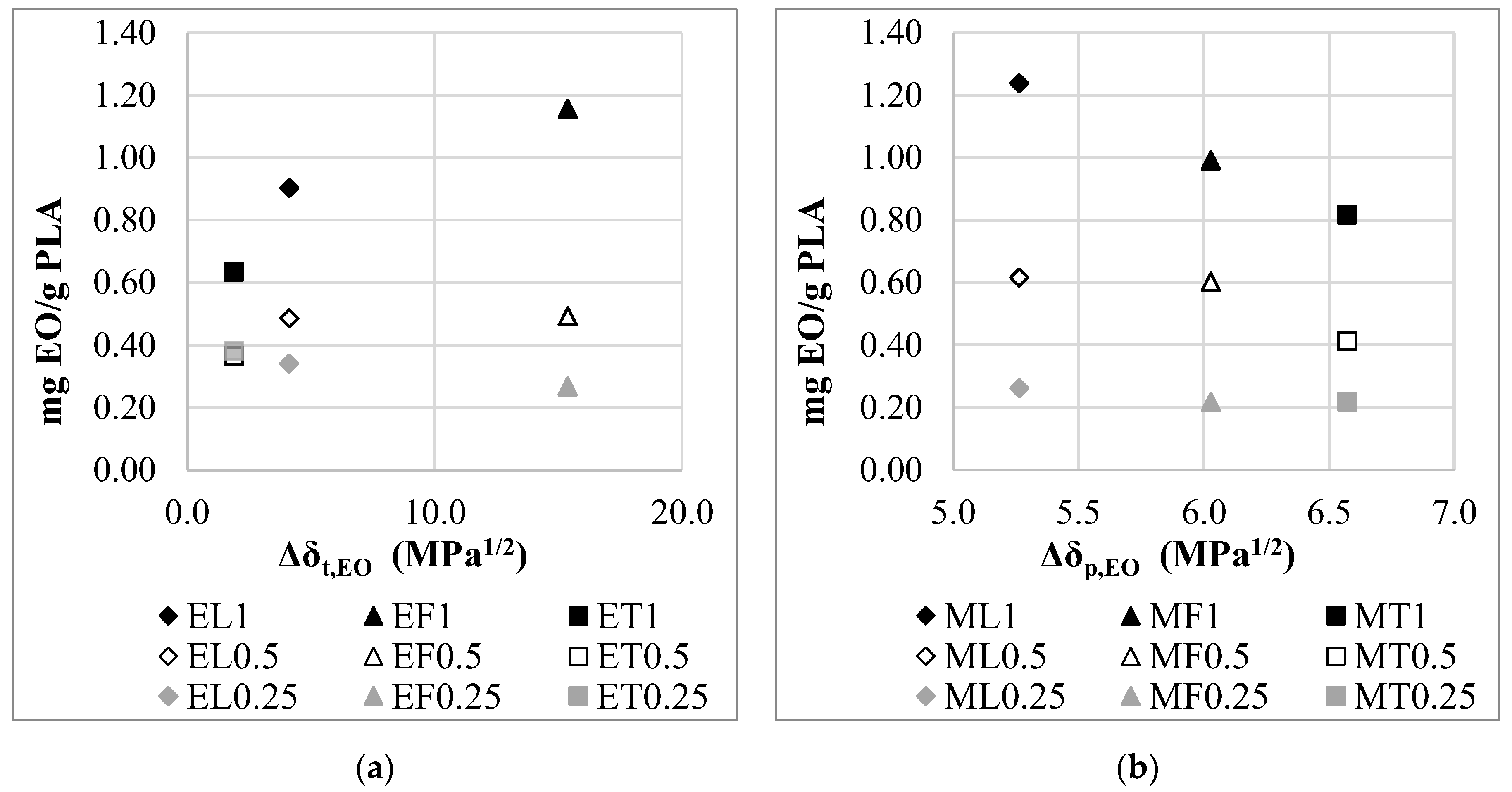
3.4. Correlation of the Adsorption Properties with the Hansen Solubility Parameter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farah, S.; Anderson, D.D.; Lander, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.R. Poly(lacticacid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, N.; Singh, R.; Ahuja, I.P.S.; Kumar, R.; Singh, D.; Ramniwas, S.; Verma, A.K.; Mittal, D. 3D printed scaffolds for tissue engineering applications: Mechanical, morphological, thermal, in-vitro and in-vivo investigations. CIRP J. Manuf. Sci. Technol. 2012, 32, 205–216. [Google Scholar] [CrossRef]
- Kamarudin, S.H.; Abdullah, L.C.; Aung, M.M.; Ratnam, C.T.; Jusoh, E.R. A study of mechanical and morphological properties of PLA based biocomposites prepared with EJO vegetable oil based plasticiser and kenaf fibres. Mater. Res. Express 2018, 5, 085314. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Jiang, L.; Chuan, Y.; Yuan, M.; Chen, H. Characterization of Active Packaging Films Made from Poly(Lactic Acid)/Poly(Trimethylene Carbonate) Incorporated with Oregano Essential Oil. Molecules 2016, 21, 695. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Mulla, M.Z.; Arfat, Y.A. Thermo-mechanical, structural characterization and antibacterial performance of solvent casted polylactide/cinnamon oil composite films. Food Control 2016, 69, 196–204. [Google Scholar] [CrossRef]
- Yuyue, Q.; Wenhui, L.; Dong, L.; Minglong, Y.; Lin, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N. A comparative study of oregano (Origanum vulgare L.) essential oil-based polycaprolactone nanocapsules/microspheres: Preparation, physicochemical characterization, and storage stability. Ind. Crops Prod. 2019, 140, 111669. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Tawab, H.M. Green pesticides: Essential oils as biopesticides in insect-pest management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymericcarriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef]
- Martins, I.M.; Rodrigues, S.N.; Barreiro, M.F.; Rodrigues, A.E. Release of Thyme Oil from Polylactide Microcapsules. Ind. Eng. Chem. Res. 2011, 50, 13752–13761. [Google Scholar] [CrossRef]
- Martins, I.M.; Rodrigues, S.N.; Barreiro, M.F.; Rodrigues, A.E. Microencapsulation of thyme oil by coacervation. J. Microencapsul. 2009, 26, 667–675. [Google Scholar] [CrossRef]
- Martins, I.M.; Rodrigues, S.N.; Barreiro, M.F.; Rodrigues, A.E. Polylactide-Based Thyme Oil Microcapsules Production: Evaluation of Surfactants. Ind. Eng. Chem. Res. 2011, 50, 898–904. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zanid, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef]
- Martins, I.M.; Rodrigues, S.N.; Barreiro, M.F.; Rodrigues, A.E. Release Studies of Thymol and p Cymene from Polylactide Microcapsules. Ind. Eng. Chem. Res. 2012, 51, 11565–11571. [Google Scholar] [CrossRef]
- Biswal, A.K.; Vashisht, I.; Khan, A.; Sharma, S. Synthesis, characterization and antibacterial activity of thymol-loaded polylactic acid microparticles entrapped with essential oils of varying viscosity. J. Mater. Sci. 2019, 54, 9745–9758. [Google Scholar] [CrossRef]
- Dusankova, M.; Pummerova, M.; Sedlarik, V. Microspheres of essential oil in polylactic acid and poly(methyl methacrylate) matrices and ther blends. J. Microencapsul. 2019, 36, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. Sorption of ethyl acetate and d-limonene in poly(lactide) polymers. J. Sci. Food Agric. 2006, 86, 648–656. [Google Scholar] [CrossRef]
- Vayer, M.; Vital, A.; Sinturel, C. New insights into polymer-solvent affinity in thin films. Eur. Polym. J. 2017, 93, 132–139. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sato, S.; Gondo, D.; Wada, T.; Kanehashi, S.; Nagai, K. Effects of Various Liquid Organic Solvents on Solvent-Induced Crystallization of Amorphous Poly(lactic acid) Film. J. Appl. Polym. Sci. 2013, 129, 1607–1617. [Google Scholar] [CrossRef]
- Singh, B.; Singh, P.; Sutherland, A.J.; Pal, K. Control of shape and size of poly (lactic acid) microspheres based on surfactant and polymer concentration. Mater. Lett. 2017, 195, 48–51. [Google Scholar] [CrossRef]
- Zhang, H.; Xiang, S.; Luan, Q.; Bao, Y.; Deng, Q.; Zheng, M.; Liu, S.; Song, J.; Tang, H.; Huang, F. Development of poly (lactic acid) microspheres and their potential application in Pickering emulsions stabilization. Int. J. Biol. 2017, 108, 105–111. [Google Scholar] [CrossRef]
- Shi, X.D.; Sun, P.J.; Gan, Z.H. Preparation of Porous Polylactide Microspheres and Their Application in Tissue Engineering. Chin. J. Polym. Sci. 2018, 36, 712–719. [Google Scholar] [CrossRef]
- Lassalle, V.; Ferreira, M.L. PLA nano-and microparticles for drug delivery: An overview of the methods of preparation. Macromol. Biosci. 2007, 7, 767–783. [Google Scholar] [CrossRef]
- Chen, J.L.; Chiang, C.H.; Yeh, M.K. The mechanism of PLA microparticle formation by waterin-oil-in-water solvent evaporation method. J. Microencapsul. 2002, 19, 333–346. [Google Scholar] [CrossRef]
- NatureWorks, Ingeo Biopolymer 3D850 Technical Data Sheet. Available online: https://www.natureworksllc.com/~/media/Files/NatureWorks/Technical-Documents/Technical-Data-Sheets/TechnicalDataSheet_3D850_monofilament_pdf.pdf (accessed on 1 August 2022).
- NatureWorks, Ingeo Biopolymer 4043D Technical Data Sheet. Available online: https://www.natureworksllc.com/~/media/Technical_Resources/Technical_Data_Sheets/TechnicalDataSheet_4043D_films_pdf.pdf (accessed on 1 August 2022).
- Van Krevelen, D.W.; Nijenhuis, K.T. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009; pp. 201–225. [Google Scholar]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.; Maspoch, M. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Saiter, A.; Delpouve, N.; Dargent, E.; Saiter, J. Cooperative rearranging region size determination by temperature modulated DSC in semi-crystalline poly(L-lactide acid). Eur. Polym. J. 2007, 43, 4675–4682. [Google Scholar] [CrossRef]
- Kamarudin, S.H.; Abdullah, L.C.; Aung, M.M.; Ratnam, C.T. Thermal and structural analysis of epoxidized jatropha oil and alkaline treated kenaf fiber reinforced poly (Lactic acid) biocomposites. Polymers 2020, 12, 2604. [Google Scholar] [CrossRef] [PubMed]
- Vay, K.; Scheler, S.; Frieß, W. Application of Hansen solubility parameters for understanding and prediction of drug distribution in microspheres. Int. J. Pharm. 2011, 416, 202–209. [Google Scholar] [CrossRef] [PubMed]


| Particles Type | Solvents | Essential Oils | Solution Concentration | ||||
|---|---|---|---|---|---|---|---|
| P | from 3D850 PLA | E | Ethanol | L | Lemongrass essential oil | 0.25 | 0.25 mg/mL |
| F | from 4043D PLA | M | Methanol | T | Thyme essential oil | 0.5 | 0.5 mg/mL |
| F | Fennel essential oil | 1 | 1 mg/mL | ||||
| Material | δd, (J/cm)1/2 | δp, (J/cm)1/2 | δh, (J/cm)1/2 | δt, (J/cm)1/2 | RED |
|---|---|---|---|---|---|
| PLA | 18.6 | 9.9 | 6.0 | 21.9 | - |
| Lemongrass essential oil | 16.4 | 4.6 | 5.1 | 17.8 | 0.65 |
| Thyme essential oil | 21.3 | 3.2 | 9.2 | 23.8 | 0.89 |
| Fennel essential oil | 24.3 | 3.9 | 28.0 | 37.3 | 2.39 |
| Ethanol | 15.1 | 8.4 | 18.3 | 25.2 | 1.33 |
| Methanol | 14.5 | 11.5 | 21.4 | 28.3 | 1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virág, L.; Bocsi, R.; Pethő, D. Adsorption Properties of Essential Oils on Polylactic Acid Microparticles of Different Sizes. Materials 2022, 15, 6602. https://doi.org/10.3390/ma15196602
Virág L, Bocsi R, Pethő D. Adsorption Properties of Essential Oils on Polylactic Acid Microparticles of Different Sizes. Materials. 2022; 15(19):6602. https://doi.org/10.3390/ma15196602
Chicago/Turabian StyleVirág, Lilla, Róbert Bocsi, and Dóra Pethő. 2022. "Adsorption Properties of Essential Oils on Polylactic Acid Microparticles of Different Sizes" Materials 15, no. 19: 6602. https://doi.org/10.3390/ma15196602





