Evaluation of Recycled Spent Coffee Material Treated with Animal Glue, Starch, and Red Clay as Acid Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Spent Coffee Ground and Red Clay
2.1.2. Animal Glue and Starch
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Test Methods
3. Results and Discussion
3.1. Test Result
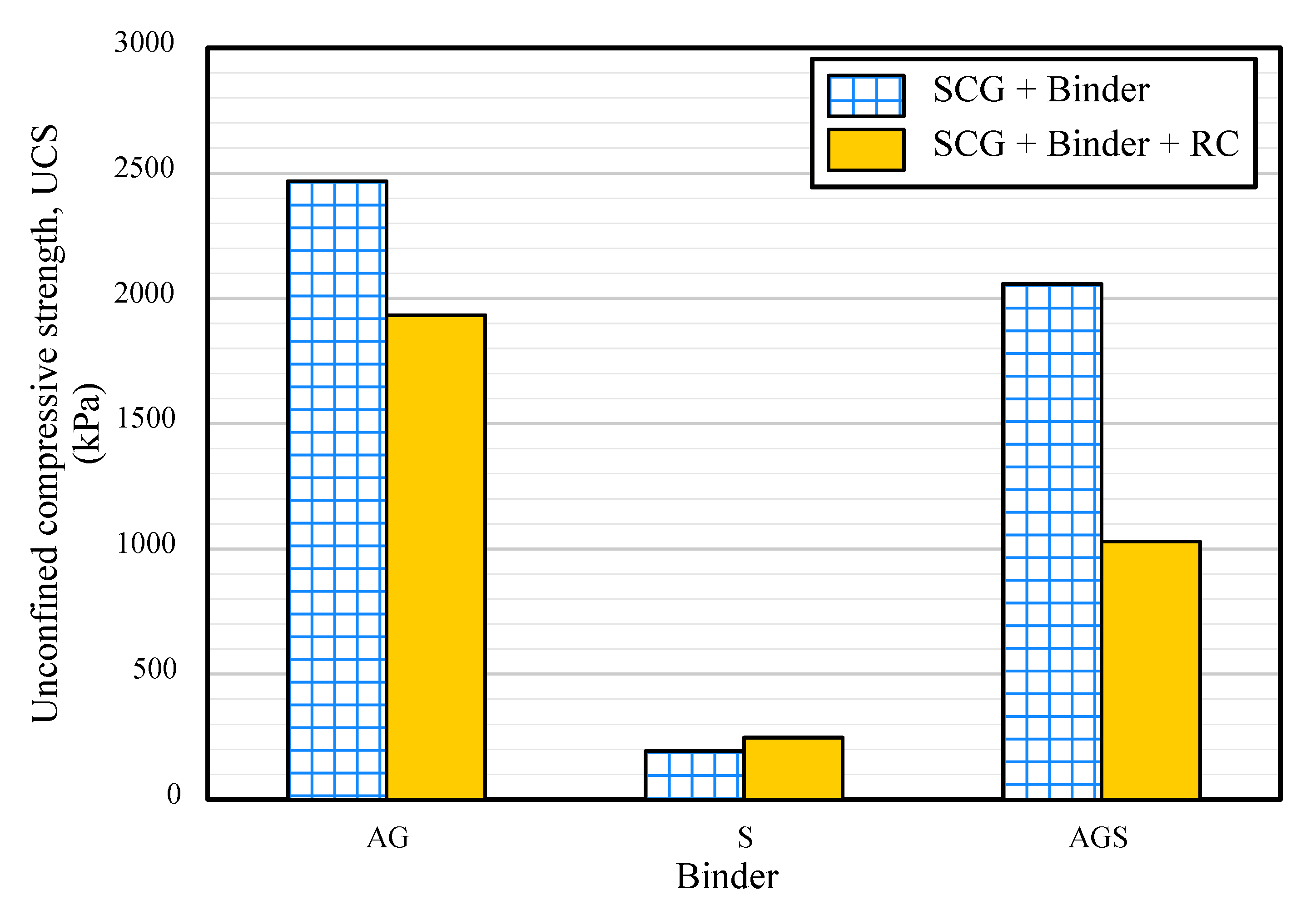
3.2. Unconfined Compressive Strength (UCS)
3.3. Slake Durability Index
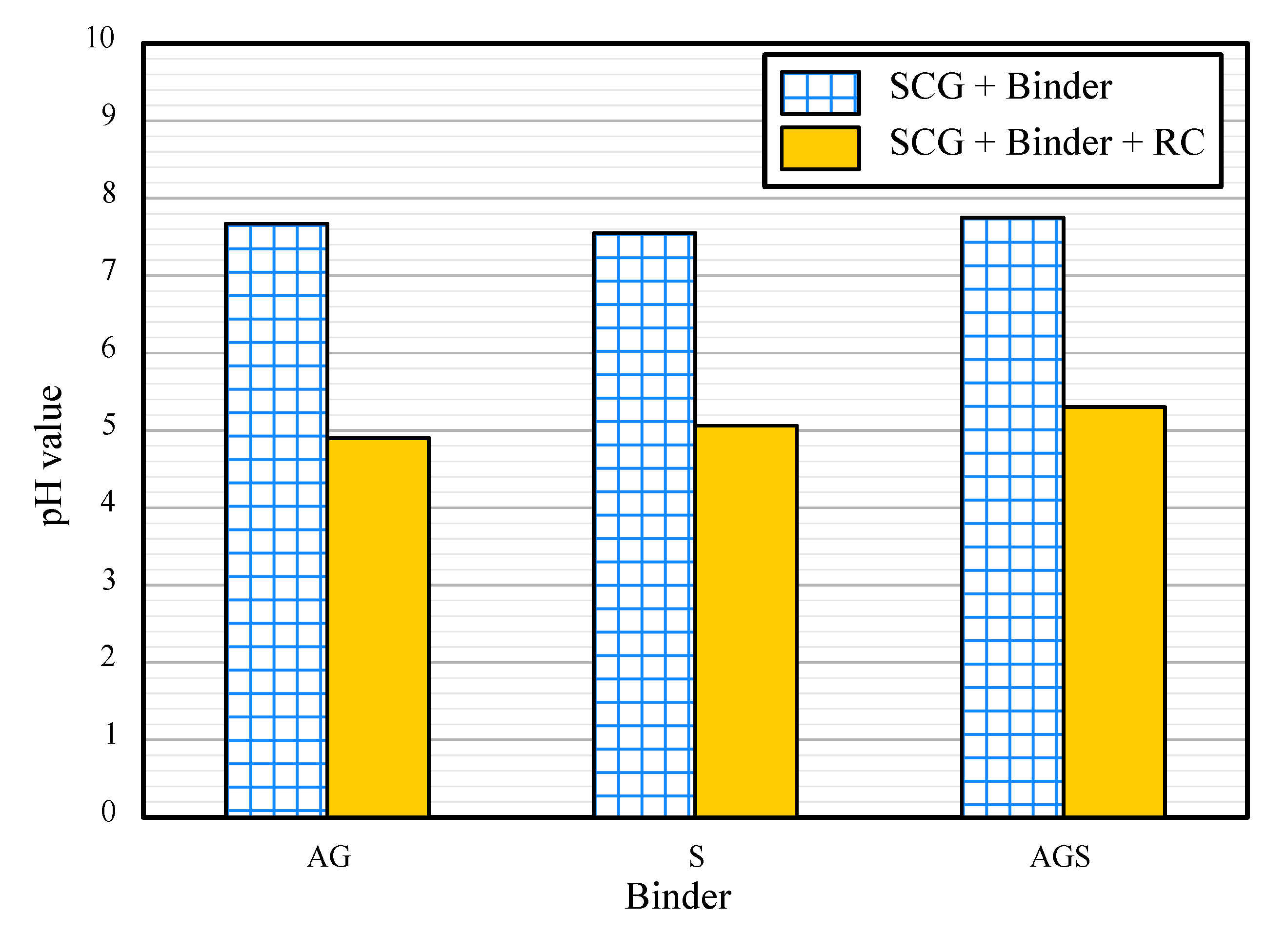
3.4. pH Value
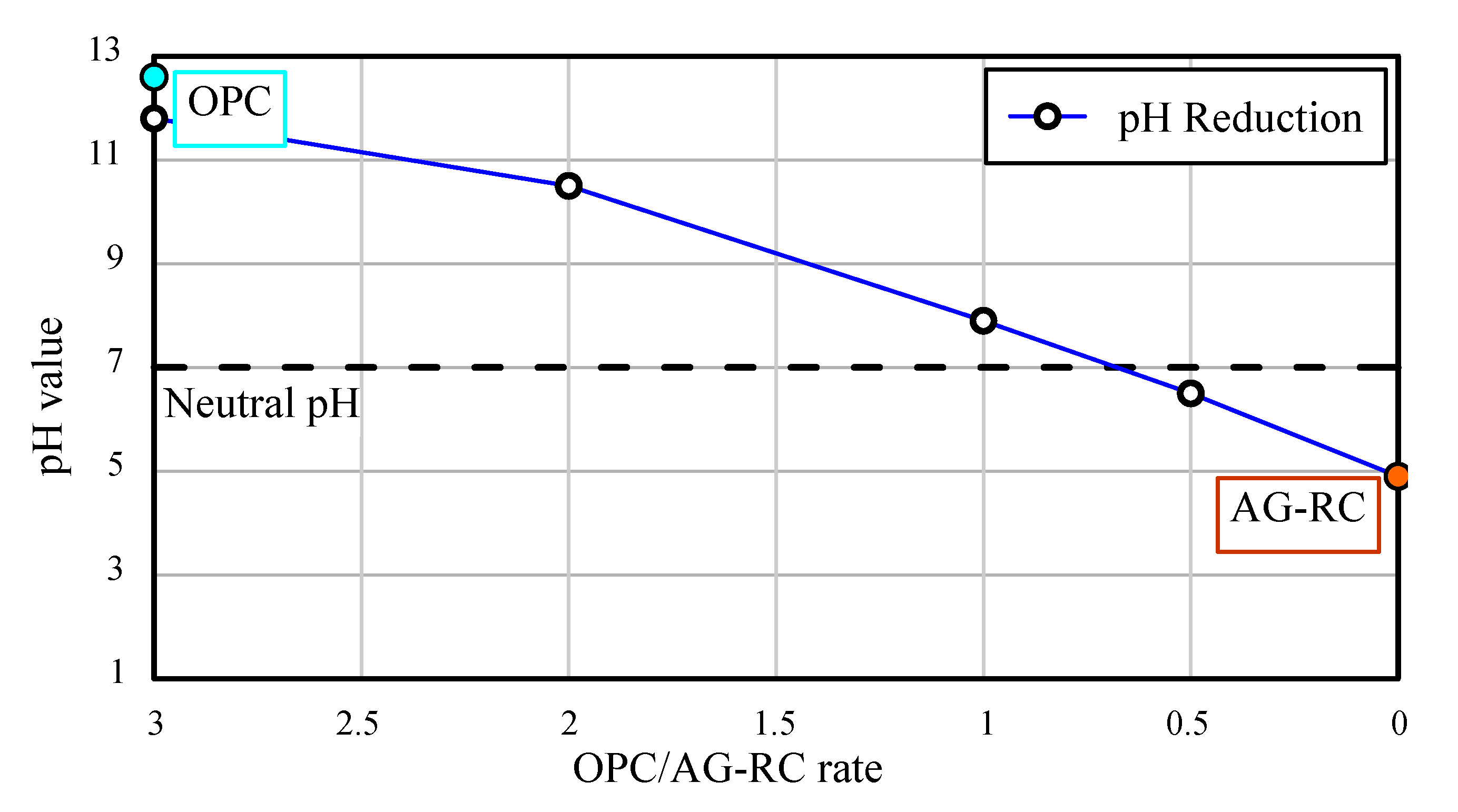
3.5. pH Reduction of the Cement Water
4. Conclusions
- ✔
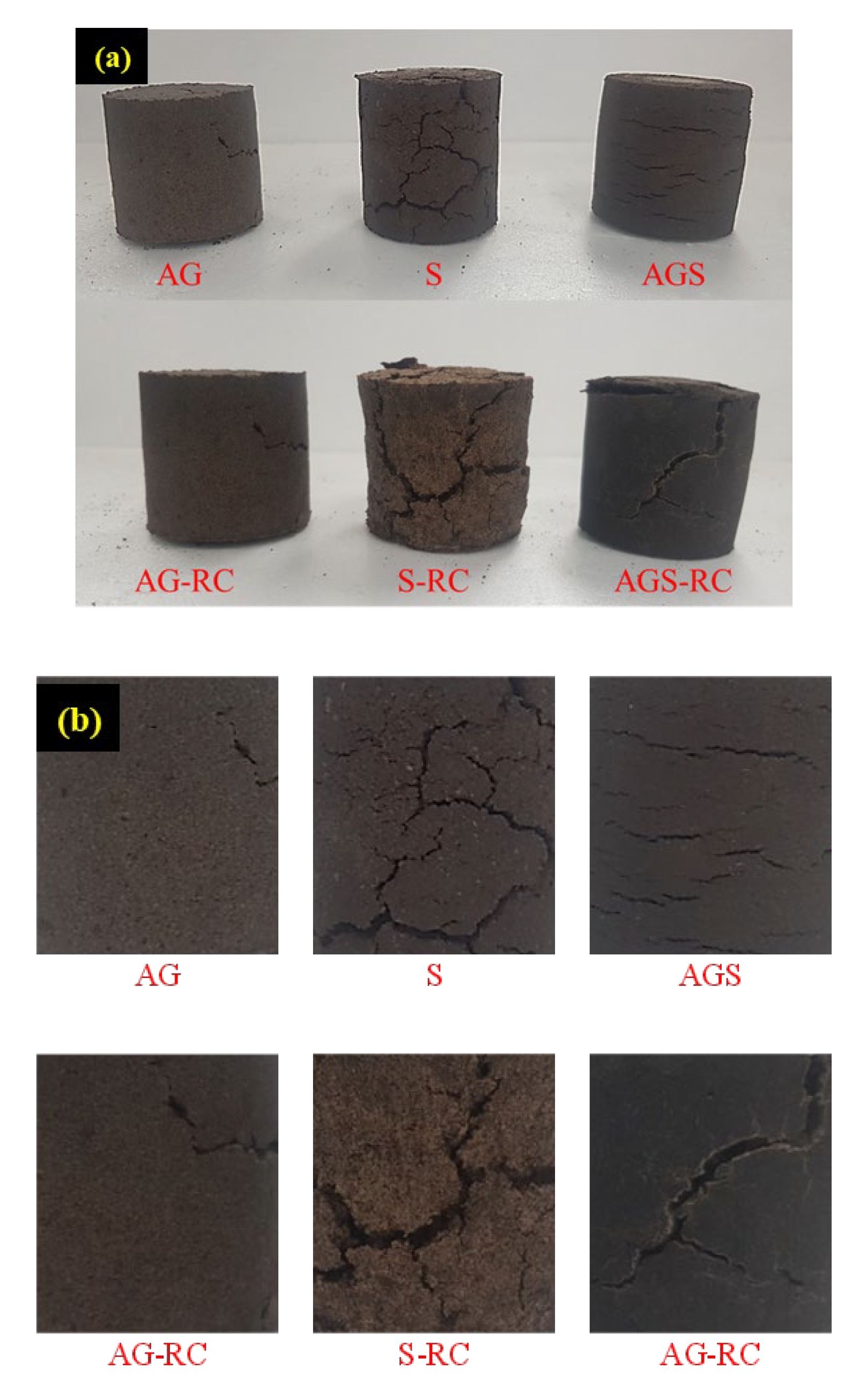
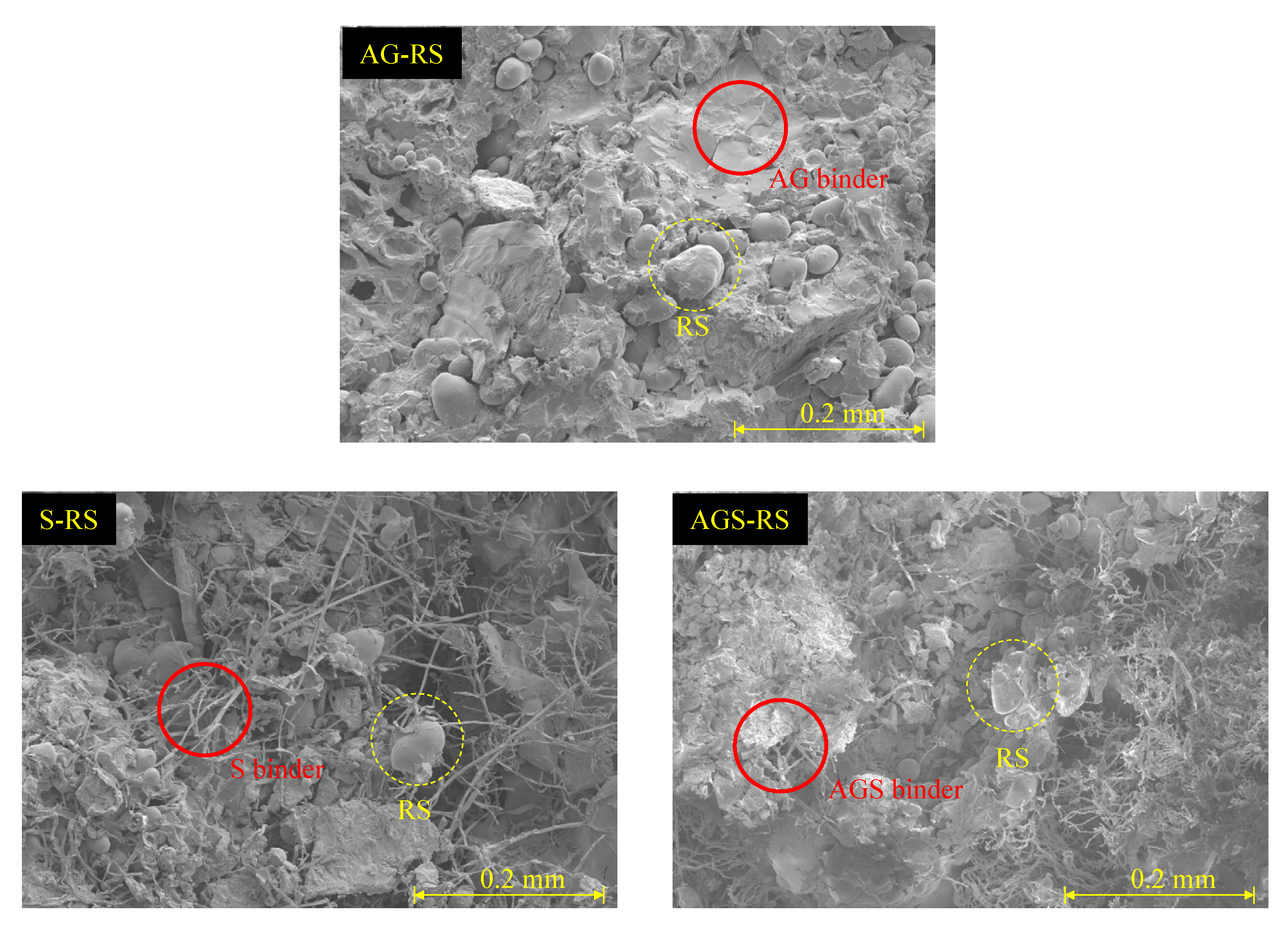
- The animal glue-treated SCG sample showed 2468 kPa of UCS, and red clay addition did not enhance the UCS. The starch binder and red clay formed numerous cracks on the surface of the sample, and the starch and red clay samples showed a poor UCS of approximately 193 kPa. It was considered that the difference in the binding structure of binders leads to the strength result.
- ✔
- The best slake durability test index was 91% for the animal-binder-treated sample. This value can be classified as an extremely high grade. The durability index showed a trend similar to that of the UCS results. The starch binder and the red soil reduced this value. Both the treated samples had the poorest index.
- ✔
- AG, S, and AGS samples showed a pH value of approximately 7. The value decreased further with the addition of RC. The highest acid value was observed for the AG-RC samples (4.91). With a UCS of 1933 kPa, slake durability index of 89%, and acidic pH, AG-RC was the most suitable for the goal of this study.
- ✔
- The pH value of Ordinary Portland cement was 12.6, and the AG-RS sample could neutralize the cement at a 68% weight rate. In particular, if AG-RS samples are produced as aggregates and buried around underground structures or facilities, it may reduce the generation of high alkaline groundwater which occurs when groundwater contact with underground cement-based structures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Coffee Organization. Available online: https://www.ico.org/prices/new-consumption-table.pdf (accessed on 1 August 2022).
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Jones, P.T.; Geysen, D.; Tielemans, Y.; Van Passel, S.; Pontikes, Y.; Blanpain, B.; Quaghebeur, M.; Hoekstra, N. Enhanced Landfill Mining in view of multiple resource recovery: A critical review. J. Clean. Prod. 2013, 55, 45–55. [Google Scholar] [CrossRef]
- Saquing, J.M.; Saquing, C.D.; Knappe, D.R.; Barlaz, M.A. Impact of plastics on fate and transport of organic contaminants in landfills. Environ. Sci. Technol. 2010, 44, 6396–6402. [Google Scholar] [CrossRef]
- El-Fadel, M.; Findikakis, A.N.; Leckie, J.O. Environmental impacts of solid waste landfilling. J. Environ. Manag. 1997, 50, 1–25. [Google Scholar] [CrossRef]
- Vaverková, M.D. Landfill impacts on the environment. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Mello, F.V.C.; Thode Filho, S.; Carpes, R.M.; Honório, J.G.; Marques, M.R.C.; Felzenszwalb, I.; Ferraz, E.R.A. Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.Y.; Cho, H.M.; Kim, Y.U.; Lee, S.C.; Berardi, U.; Kim, S. Circular reutilization of coffee waste for sound absorbing panels: A perspective on material recycling. Environ. Res. 2020, 184, 109281. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M. Recycling coffee and tea wastes to increase plant available Fe in alkaline soils. Plant Soil 2008, 304, 249–255. [Google Scholar] [CrossRef]
- Korikanthimath, V.S.; Hosmani, M.M. Organic recycling of coffee pulp in coffee based cropping systems. Indian Coffee 2000, 64, 4–6. [Google Scholar]
- Saeli, M.; Campisi, T. Recycling spent coffee waste for innovative thermo-plasters: Development of a prototype. In Proceedings of the ISER-International Conference on Science, Technology, Engineering and Management (ICSTEM), Riyadh, Saudi Arabia, 13 May 2022. [Google Scholar]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Spent coffee grounds as a versatile source of green energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef]
- McNutt, J. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Saberian, M.; Li, J.; Donnoli, A.; Bonderenko, E.; Oliva, P.; Gill, B.; Lockrey, S.; Siddique, R. Recycling of spent coffee grounds in construction materials: A review. J. Clean. Prod. 2021, 289, 125837. [Google Scholar] [CrossRef]
- Calvo, J.G.; Hidalgo, A.; Alonso, C.; Luco, L.F. Development of low-pH cementitious materials for HLRW repositories: Resistance against ground waters aggression. Cem. Concr. Res. 2010, 40, 1290–1297. [Google Scholar] [CrossRef]
- Slaton, N.A.; Norman, R.J.; Gilmour, J.T. Oxidation rates of commercial elemental sulfur products applied to an alkaline silt loam from Arkansas. Soil Sci. Soc. Am. J. 2001, 65, 239–243. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Jacobsen, J. Soil pH and organic matter. Nutr. Manag. Module 2009, 8, 1–12. [Google Scholar]
- Ventolà, L.; Vendrell, M.; Giraldez, P.; Merino, L. Traditional organic additives improve lime mortars: New old materials for restoration and building natural stone fabrics. Constr. Build. Mater. 2011, 25, 3313–3318. [Google Scholar] [CrossRef]
- Elert, K.; Sánchez, R.M.G.; Benavides-Reyes, C.; Ordóñez, F.L. Influence of animal glue on mineralogy, strength and weathering resistance of lime plasters. Constr. Build. Mater. 2019, 226, 625–635. [Google Scholar] [CrossRef]
- Mansour, G.; Zoumaki, M.; Tsongas, K.; Tzetzis, D. Starch-sandstone materials in the construction industry. Results Eng. 2020, 8, 100182. [Google Scholar] [CrossRef]
- Kulshreshtha, Y.; Schlangen, E.; Jonkers, H.M.; Vardon, P.J.; Van Paassen, L.A. CoRncrete: A corn starch based building material. Constr. Build. Mater. 2017, 154, 411–423. [Google Scholar] [CrossRef]
- İkizler, S.B.; Aytekın, M.; Türker, E.; Yavuz, H.İ. Effect of Fibers on Swelling Characteristics of Bentonite. In Proceedings of the 2nd International Conference on New Developments in Soil Mechanics and Geotechnical Engineering, Lefkoşa, Cyprus (Kktc), 28–30 May 2009; pp. 328–335. Available online: https://www.researchgate.net/publication/233898015_Effect_of_fibers_on_swelling_characteristics_of_bentonite (accessed on 10 July 2022).
- Tangboriboon, N.; Samattai, S.; Kamonsawas, J.; Sirivat, A. Processing of kaolinite and alumina loaded in natural rubber composite foams. Mater. Manuf. Processes 2015, 30, 595–604. [Google Scholar] [CrossRef]
- ASTM D 2166; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. Annual Book of ASTM Standards: Montgomery County, MD, USA, 2000.
- KS F 2314; Standard Test Method for Unconfined Compression Test of Soils. Korean Standards Association: Seoul, Korea, 2018.
- ASTM D 4644; Standard Test Method for Slake Durability of Shales and Other Similar Weak Rocks. Annual Book of ASTM Standards: Montgomery County, MD, USA, 2008.
- Sumary, D.P.; Kyobe, J.W.; Jomanga, K.E.; Gomezulu, E. Indicative Investigation of Amount Caffeine in Some Commercial Teas and Coffees Found in the Dodoma Markets. Tanzan. J. Nat. Appl. Sci. 2012, 2, 323–327. [Google Scholar]
- Khatua, P.K.; Dubey, R.K.; Shahoo, S.C.; Kalawate, A. Environment Friendly, Exterior Grade Resin Adhesive from Phenol-Animal Glue Formaldehyde (PGF). Int. J. Polym. Sci. 2015, 1, 1–7. [Google Scholar]
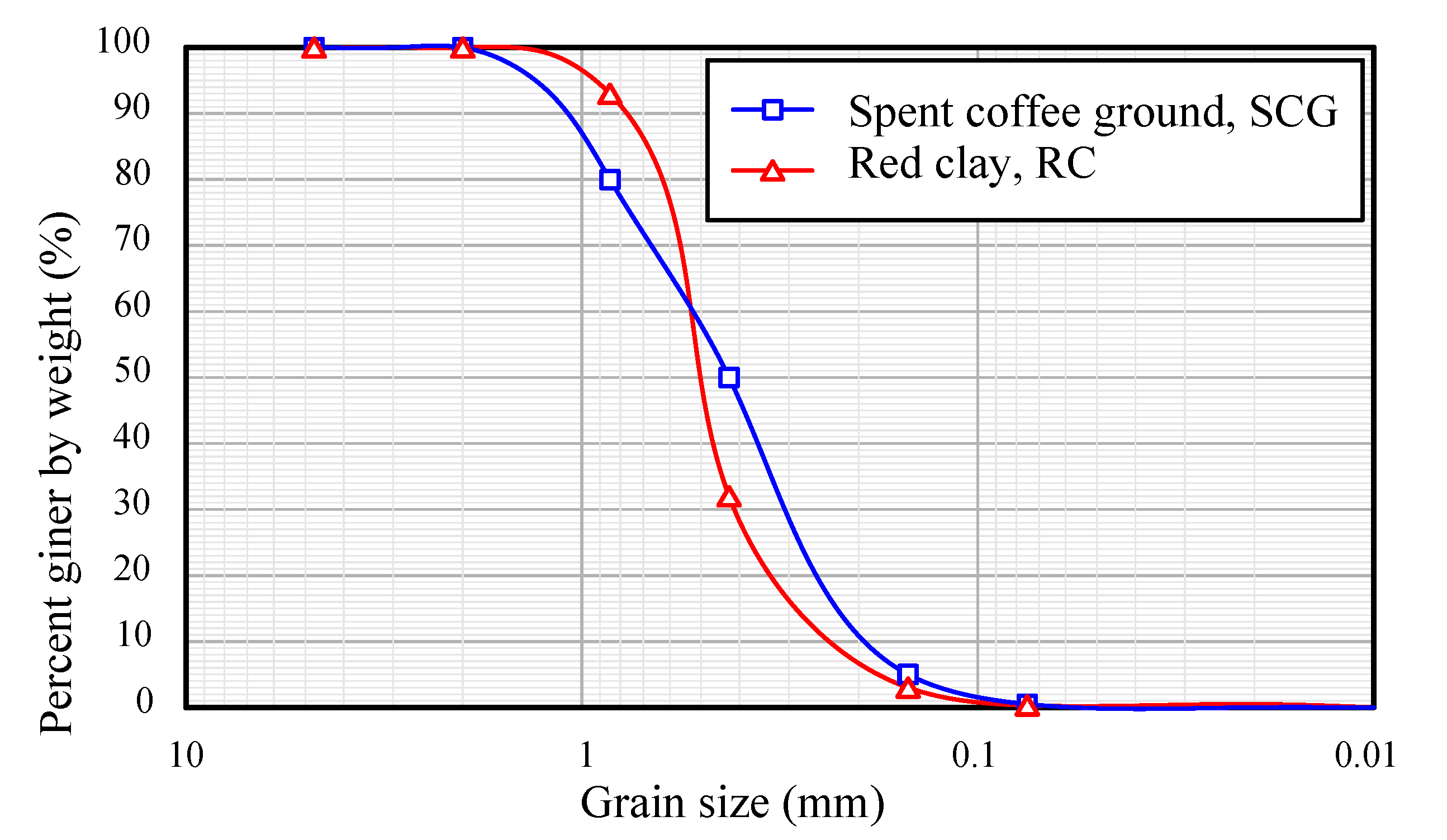
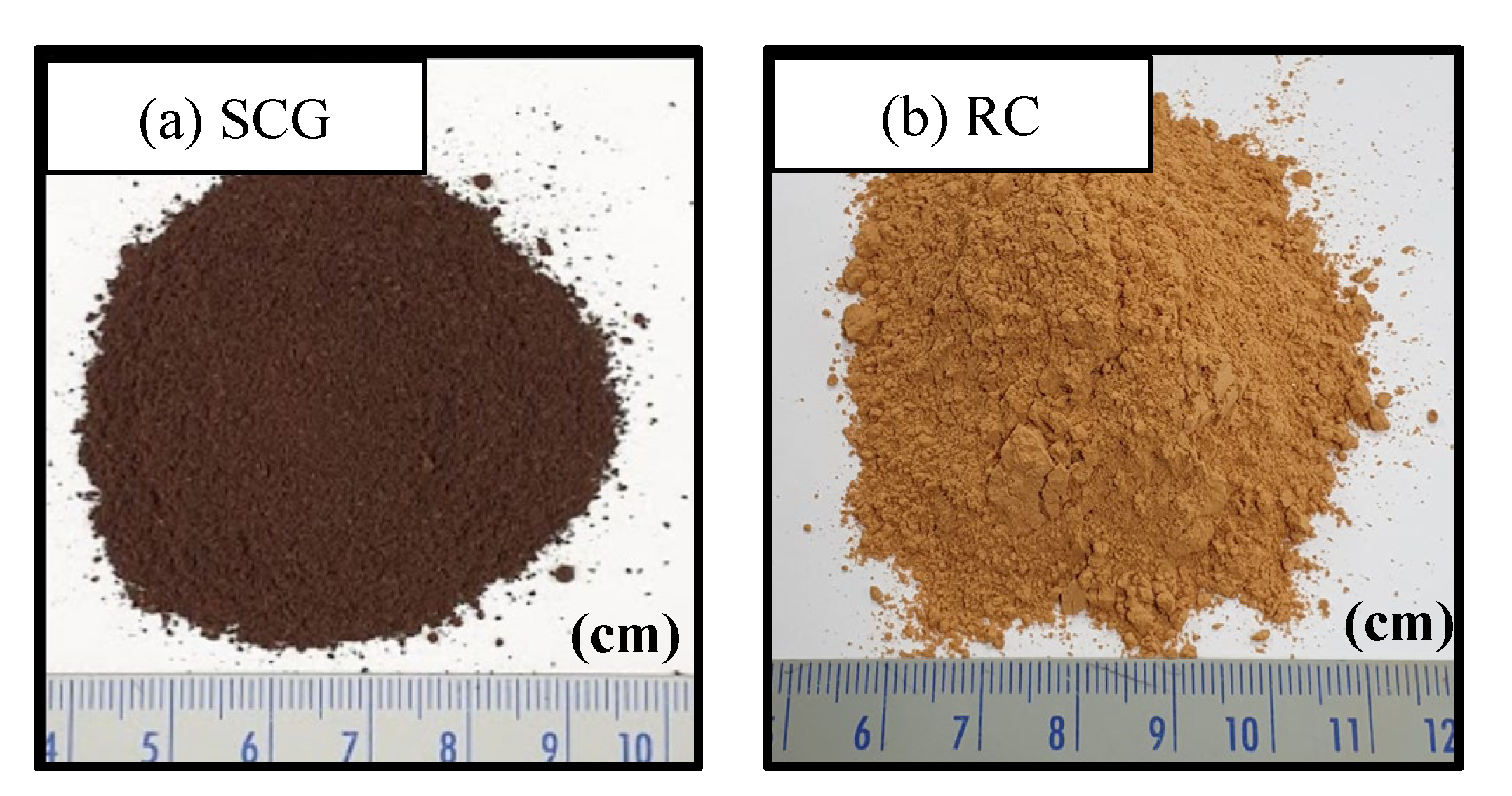



| Materials | Pressed Density (t/m3) | Coefficient of Uniformity, Cu | Coefficient of Curvature, Cc |
|---|---|---|---|
| Spent coffee ground, SCG | 118 | 3.00 | 0.81 |
| Red clay, RC | 235 | 2.82 | 1.29 |
| Clay | Color | Component (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | K2O | Fe2O3 | TiO2 | MgO | CaO | L.O.I | ||
| Red clay | Red | 48.63 | 25.74 | 4.93 | 16.91 | 0.74 | 0.62 | 2.13 | 0.30 |
| Bentonite | Gray | 61.28 | 17.79 | 1.24 | 3.01 | - | 2.10 | 4.54 | 10.04 |
| Kaolinite | White | 46.70 | 36.70 | 1.25 | 1.09 | 0.05 | 0.05 | 0.01 | 14.30 |
| ID | Clay Material | Binder | Binder Ratio | Red Clay Ratio | Curing Time |
|---|---|---|---|---|---|
| AG | SCG | Animal glue | 15 % | - | 7 days (148 h) |
| S | Starch | ||||
| AGS | Animal glue and Starch | ||||
| AG-RC | SCG and Red clay | Animal glue | 20% | ||
| S-RC | Starch | ||||
| AGS-RC | Animal glue and Starch |
| ID | Height (mm) | Diameter (mm) | Total Unit Weight (kN/m3) | Water Content (%) | UCS (kPa) | Slake Durability Index, Id | pH Value |
|---|---|---|---|---|---|---|---|
| AG | 4.95 | 4.97 | 11.81 | 0.84 | 2468 | 91 | 7.67 |
| S | 4.51 | 4.62 | 9.21 | 0.54 | 247 | 85 | 7.55 |
| AGS | 4.83 | 4.79 | 10.72 | 0.61 | 2058 | 57 | 7.75 |
| AG-RC | 4.97 | 4.99 | 14.36 | 0.94 | 1933 | 88 | 4.9 |
| S-RC | 4.73 | 4.71 | 13.10 | 0.64 | 193 | 57 | 5.06 |
| AGS-RC | 4.95 | 4.96 | 12.31 | 0.51 | 1029 | 49 | 5.3 |
| AG-RC (g) | OPC (g) | Water (L) | OPC/AG-RC Rate * | pH Value |
|---|---|---|---|---|
| 0 | 100 | 1 | - | 12.6 |
| 100 | 0 | 0 | 4.9 | |
| 50 | 0.5 | 6.5 | ||
| 100 | 1.0 | 7.9 | ||
| 200 | 2.0 | 10.5 | ||
| 300 | 3.0 | 11.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-S.; Woo, S.-W.; Lee, J.-S.; Yun, Y.-M.; Lee, D.-E. Evaluation of Recycled Spent Coffee Material Treated with Animal Glue, Starch, and Red Clay as Acid Materials. Materials 2022, 15, 6622. https://doi.org/10.3390/ma15196622
Park S-S, Woo S-W, Lee J-S, Yun Y-M, Lee D-E. Evaluation of Recycled Spent Coffee Material Treated with Animal Glue, Starch, and Red Clay as Acid Materials. Materials. 2022; 15(19):6622. https://doi.org/10.3390/ma15196622
Chicago/Turabian StylePark, Sung-Sik, Seung-Wook Woo, Jung-Shin Lee, Young-Mook Yun, and Dong-Eun Lee. 2022. "Evaluation of Recycled Spent Coffee Material Treated with Animal Glue, Starch, and Red Clay as Acid Materials" Materials 15, no. 19: 6622. https://doi.org/10.3390/ma15196622






