Structuring of Surface Films Formed on Magnesium in Hot Chlorobenzotriazole Vapors
Abstract
:1. Introduction
- -
- The consumption of an inhibitor is very small;
- -
- There is no waste to be disposed of;
- -
- It is efficient for items with complex geometry 1;
- -
- Its safety and environmental friendliness 2.
2. Experimental
2.1. Reagents and Materials
2.2. Samples and Electrodes
- -
- Magnesium alloy not subjected to TT or CT and exposed for 1 h in air after polishing and degreasing (preparation mode A);
- -
- Magnesium alloy after 1-h TT at 150 °C. Exposure time in air after TT—48 h (preparation mode B);
- -
- Magnesium alloy after 1-h CT at 150 °C. Exposure time in air after CT—48 h (preparation mode C);
- -
- Magnesium alloy after 1-h TT at 150 °C. Time of exposure in air after TT—432 h (preparation mode D);
- -
- Magnesium alloy after 1-h CT at 150 °C. Time of exposure in air after TT—432 h (preparation mode E).
2.3. Corrosion Tests
2.4. Voltammetry
2.5. Electrochemical Impedance Spectroscopy
2.6. X-ray Photoelectron Spectroscopy
2.7. Scanning Electron Microscopy
3. Results and Discussion
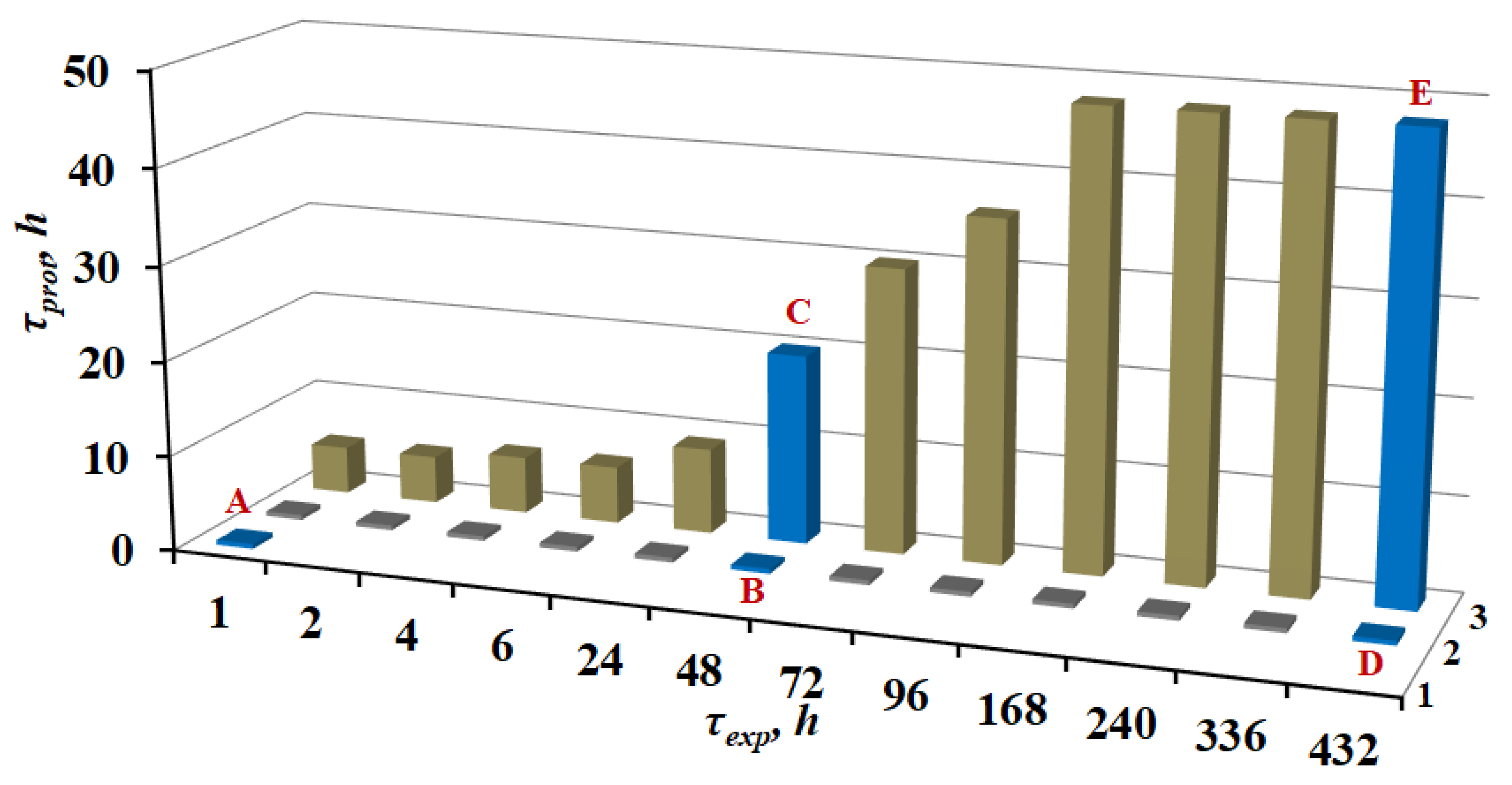
3.1. Corrosion Tests
3.2. Voltammetry
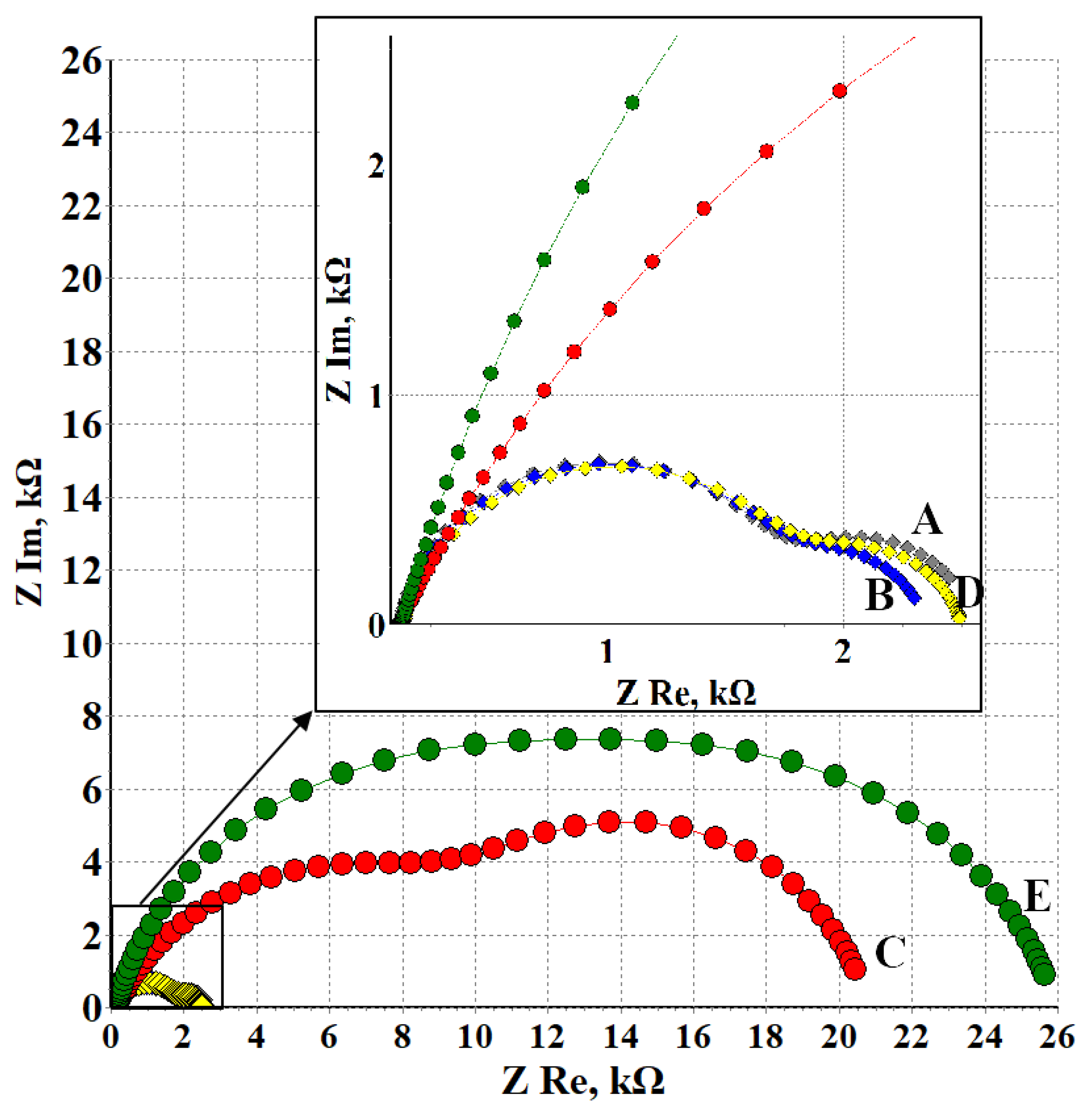
3.3. Electrochemical Impedance Spectroscopy
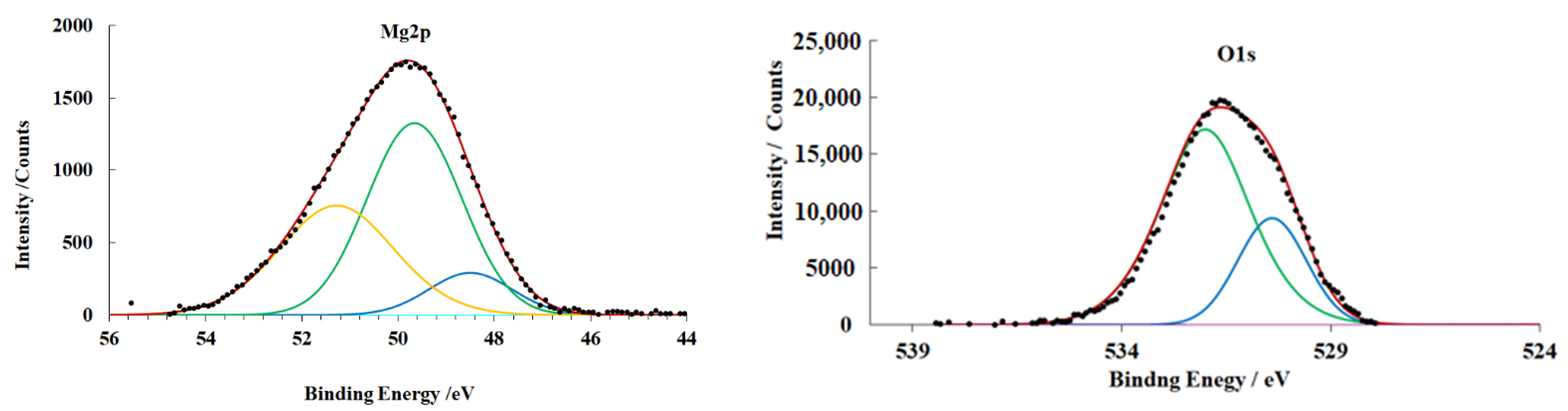
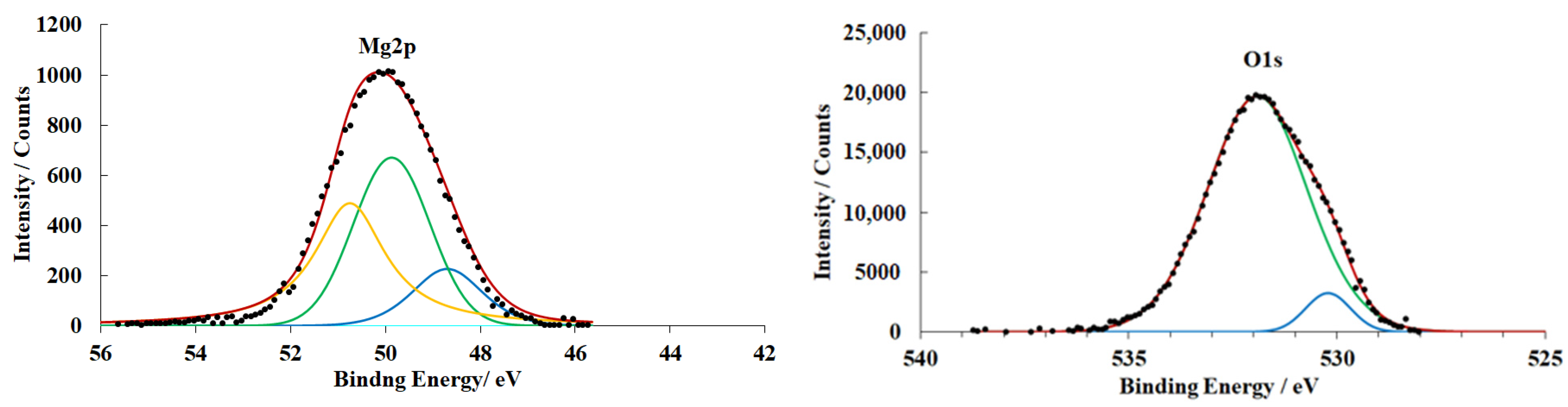
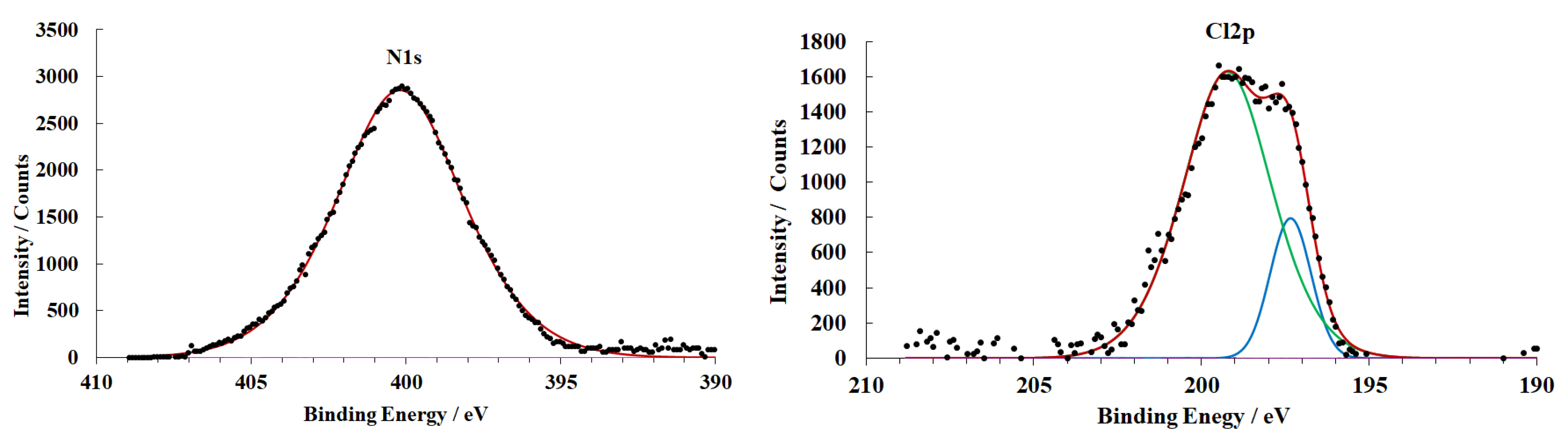
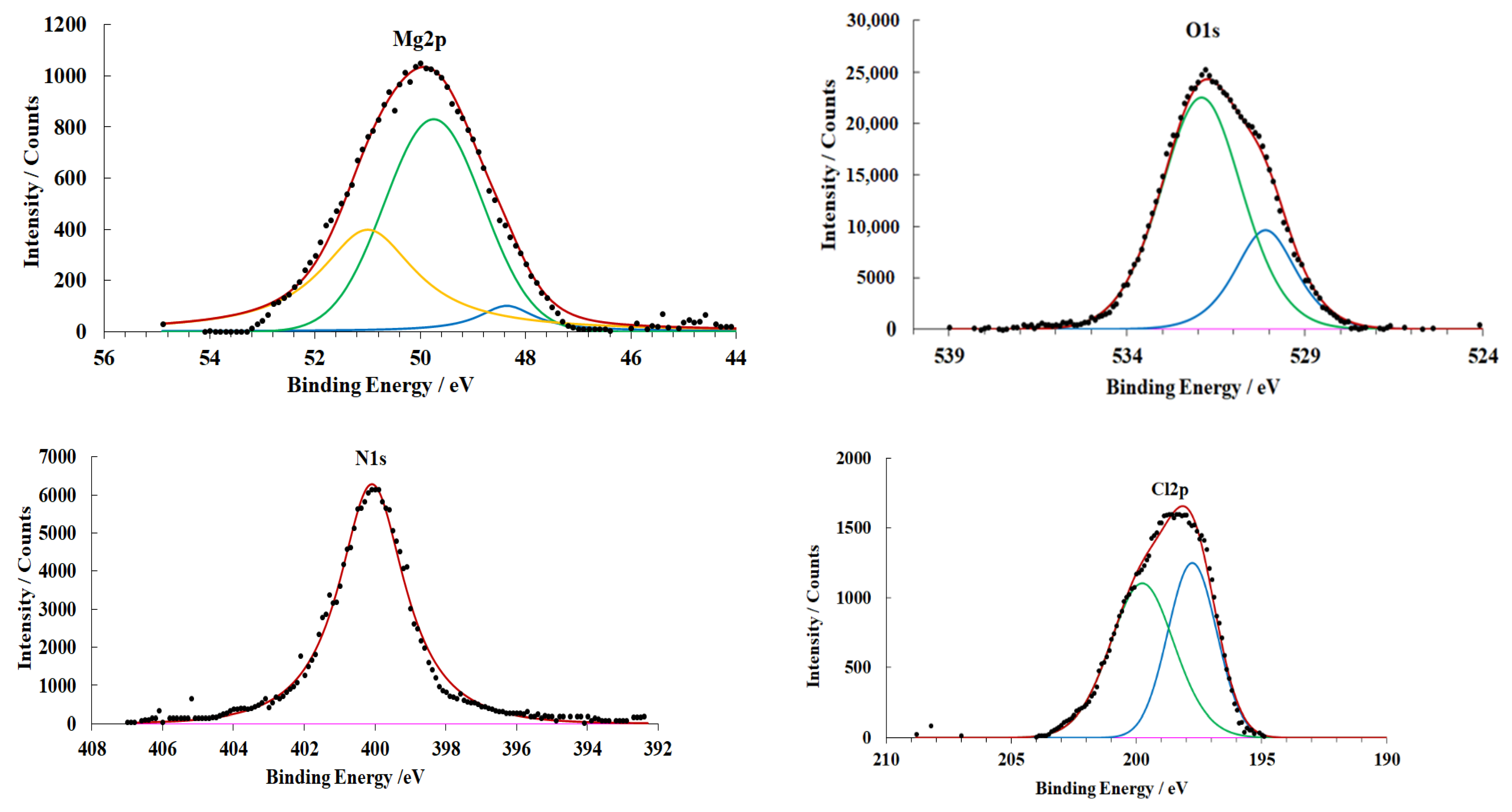
3.4. X-ray Photoelectron Spectroscopy
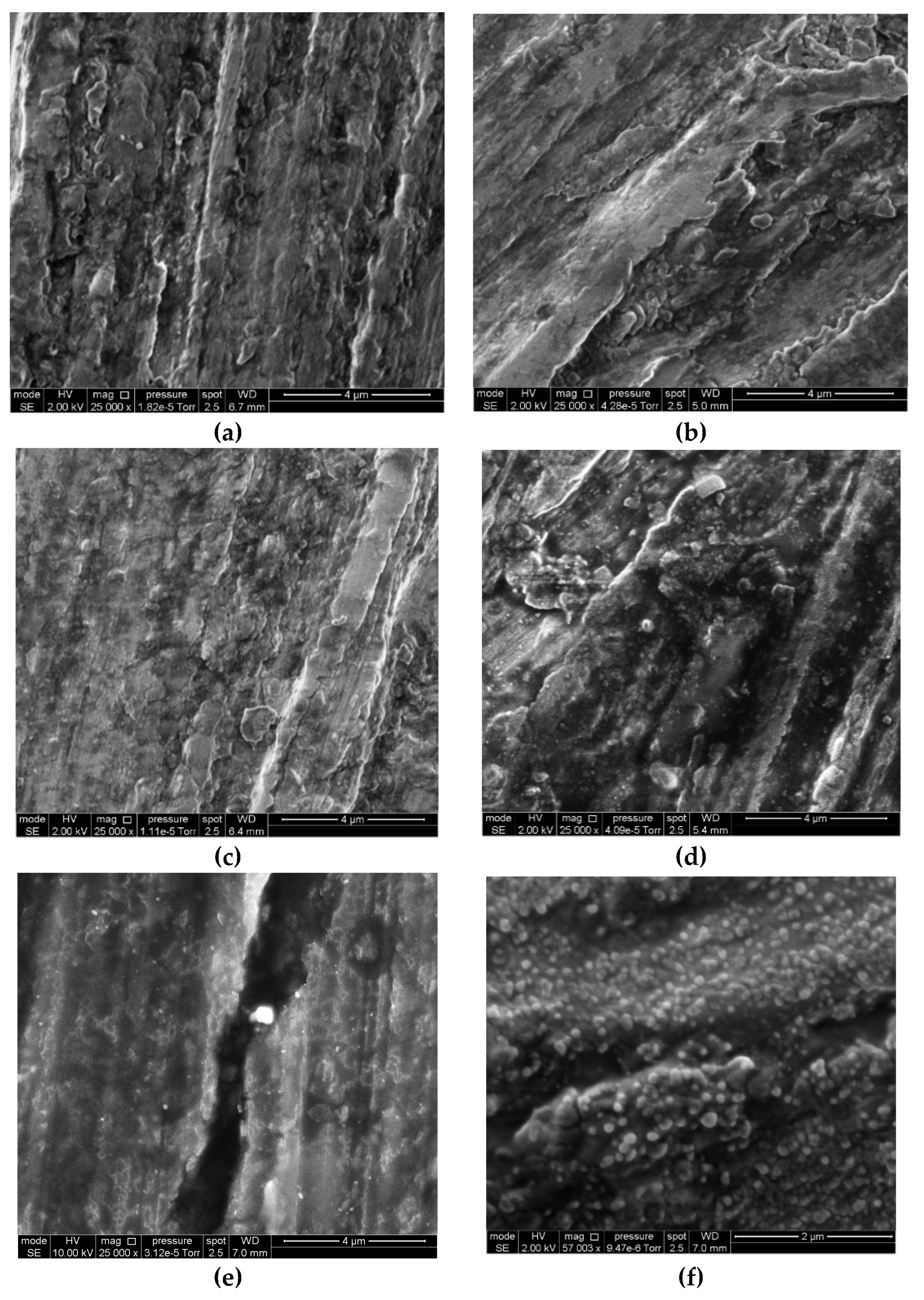
3.5. Scanning Electron Microscopy
4. Conclusions
- A fairly uniform oxide-hydroxide layer with a thickness of about 4.3 nm exists on the original surface of the magnesium alloy.
- Heat treatment results in the growth of the oxide-hydroxide layer to 6.5 nm. It is not accompanied by changes in the corrosion-electrochemical behavior of the magnesium alloy or in the surface image in the micrographs.
- Exposure of the samples to air after heat treatment is accompanied by additional oxidation of magnesium and hydroxylation of the oxide. These processes result in the thickening of the oxide-hydroxide layer to 9.4 nm. Moreover, water appears in the layer. The thickening of the film is uniform and does not result in changes in the corrosion-electrochemical behavior of the magnesium alloy or to changes in the surface appearance in the micrographs.
- Chamber treatment of the magnesium alloy is accompanied by CBTA adsorption. The oxide-hydroxide layer grows to 5.2 nm. This growth is somewhat inhibited by CBTA adsorption. CBTA adsorption slows down corrosion initiation about 10-fold and inhibits magnesium dissolution, due to inhibition of the anodic process. Inhibition occurs by a mixed blocking-activation mechanism with predominance of the latter. The appearance of the surface in the micrographs does not change upon chamber treatment of the magnesium alloy.
- Prolonged exposure of the samples after the chamber treatment is accompanied by additional oxidation of magnesium and hydroxylation of the oxide. The integral thickness of the oxide-hydroxide layer increases to 6.1 nm. However, the oxide-hydroxide layer does not grow on the entire surface, but as separate islets, whose sizes may be up to 70–200 nm. No noticeable desorption of CBTA occurs upon exposure to air. The above processes are accompanied by additional (~100-fold) inhibition of corrosion initiation and anodic dissolution of magnesium. Inhibition occurs by a mixed blocking-activation mechanism with predominance of the latter; however, the contribution of the blocking mechanism increases with exposure to air.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Kainer, K.U. Magnesium Alloys and Their Application; John Wiley & Sons Limited: Hoboken, NJ, USA, 2006. [Google Scholar]
- Sinyavsky, V.S. Features of corrosion of magnesium alloys in wide application. Technol. Light Alloy. 2011, 2, 77–85. Available online: https://cyberleninka.ru/article/n/osobennosti-korrozii-magnievyh-splavov-pri-shirokom-primenenii/viewer (accessed on 22 August 2022).
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Yuwono, J.A.; Birbilis, N.; Taylor, C.D.; Williams, K.S.; Samin, A.J.; Medhekar, N.V. Aqueous electrochemistry of the magnesium surface: Thermodynamic and kinetic profiles. Corros. Sci. 2018, 147, 53–68. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2014, 60, 169–194. [Google Scholar] [CrossRef]
- Johari, N.; Alias, J.; Zanurin, A.; Mohamed, N.; Alang, N.; Zain, M. Anti-corrosive coatings of magnesium: A review. Mater. Today Proc. 2021, 48, 1842–1848. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of Corrosion-Resistant Conversion Coatings for Magnesium and Its Alloys. Corrosion 2011, 67, 1–16. [Google Scholar] [CrossRef]
- Van Phuong, N.; Gupta, M.; Moon, S. Enhanced corrosion performance of magnesium phosphate conversion coating on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 1087–1095. [Google Scholar] [CrossRef]
- Zucchi, F.; Grassi, V.; Zanotto, F. Sodium monocarboxylates as inhibitors of AZ31 alloy corrosion in a synthetic cooling water. Mater. Corros. 2009, 60, 199–205. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Zhou, S.; Wu, L. Self-assembled monolayers on magnesium alloy surfaces from carboxylate ions. Appl. Surf. Sci. 2006, 252, 3818–3827. [Google Scholar] [CrossRef]
- Dindodi, N.; Shetty, A.N. Stearate as a green corrosion inhibitor of magnesium alloy ZE41 in sulfate medium. Arab. J. Chem. 2019, 12, 1277–1289. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.; Li, W. Facile and fast fabrication of superhydrophobic surface on magnesium alloy by one-step electrodeposition method. J. Ind. Eng. Chem. 2017, 50, 50–56. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Höche, D.; Zheludkevich, M.L. Comprehensive screening of Mg corrosion inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Gao, H.; Li, Q.; Dai, Y.; Luo, F.; Zhang, H. High efficiency corrosion inhibitor 8-hydroxyquinoline and its synergistic effect with sodium dodecylbenzenesulphonate on AZ91D magnesium alloy. Corros. Sci. 2010, 52, 1603–1609. [Google Scholar] [CrossRef]
- Saji, V.S. Organic conversion coatings for magnesium and its alloys. J. Ind. Eng. Chem. 2019, 75, 20–37. [Google Scholar] [CrossRef]
- Sugimura, H.; Hozumi, A.; Kameyama, T.; Takai, O. Organosilaneself-assembledmonolayers formed at the vapour/solid interface. Surf. Interface Anal. 2002, 34, 550–554. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Gladkikh, N.A.; Maleeva, M.A.; Maksaeva, L.B.; Yurasova, T.A. The use of organosilanes to inhibit metal corrosion. A review. Int. J. Corros. Scale Inhib. 2019, 8, 882–907. [Google Scholar]
- Ishizaki, T.; Kumagai, S.; Tsunakawa, M.; Furukawa, T.; Nakamura, K. Ultrafast fabrication of superhydrophobic surfaces on engineering light metals by single-step immersion process. Mater. Lett. 2017, 193, 42–45. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, Y.; Fan, W.; Wang, Y.; Qiang, X.; Liu, Y. Fabrication and corrosion resistance of superhydrophobic magnesium alloy. Appl. Phys. A 2015, 120, 561–570. [Google Scholar] [CrossRef]
- Han, M.-H.; Go, S.-C.; Ahn, Y.-H. Fabrication of Superhydrophobic Surface on Magnesium Substrate by Chemical Etching. Bull. Korean Chem. Soc. 2012, 33, 1363–1366. [Google Scholar] [CrossRef]
- Gray-Munro, J.; Campbell, J. Mimicking the hierarchical surface topography and superhydrophobicity of the lotus leaf on magnesium alloy AZ31. Mater. Lett. 2017, 189, 271–274. [Google Scholar] [CrossRef]
- Ran, M.; Zheng, W.; Wang, H. Fabrication of superhydrophobic surfaces for corrosion protection: A review. Mater. Sci. Technol. 2019, 35, 313–326. [Google Scholar] [CrossRef]
- Yang, J.; Blawert, C.; Lamaka, S.V.; Snihirova, D.; Lu, X.; Di, S.; Zheludkevich, M.L. Corrosion protection properties of inhibitor containing hybrid PEO-epoxy coating on magnesium. Corros. Sci. 2018, 140, 99–110. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Xie, Z.-H.; Yu, G. Duplex coating combining layered double hydroxide and 8-quinolinol layers on Mg alloy for corrosion protection. Electrochim. Acta 2018, 283, 1845–1857. [Google Scholar] [CrossRef]
- Gnedenkov, A.; Sinebryukhov, S.; Mashtalyar, D.; Gnedenkov, S. Protective properties of inhibitor-containing composite coatings on a Mg alloy. Corros. Sci. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Leiva-García, R.; Connolly, B.J.; Matthews, A.; Yerokhin, A. Smart Functionalization of Ceramic-Coated AZ31 Magnesium Alloy. ACS Appl. Mater. Interfaces 2020, 12, 30833–30846. [Google Scholar] [CrossRef]
- Fan, X.-L.; Huo, Y.-F.; Li, C.-Y.; Kannan, M.B.; Chen, X.-B.; Guan, S.-K.; Zeng, R.-C.; Ma, Q.-L. Corrosion resistance of nanostructured magnesium hydroxide coating on magnesium alloy AZ31: Influence of EDTA. Rare Met. 2019, 38, 520–531. [Google Scholar] [CrossRef]
- Ogorodnikova, V.A.; Kuznetsov, Y.I.; Andreeva, N.P.; Luchkin, A.Y.; Chirkunov, A.A. Adsorption of Anions of Higher Carboxylic Acids on Magnesium from Weakly Alkaline Aqueous Solutions. Russ. J. Phys. Chem. A 2020, 94, 1104–1110. [Google Scholar] [CrossRef]
- Kuznetsov, I.; Yu, I. Organic corrosion inhibitors: Where are we now? Part I. Adsorption. Int. J. Corros. Scale Inhib. 2015, 4, 284–310. [Google Scholar]
- Antropov, L.I.; Makushin, V.F.; Panasenko, V.F. Metal Corrosion Inhibitors. Kiev. Tekhnika. 1981. Available online: https://biu.primo.exlibrisgroup.com/discovery/fulldisplay?vid=972BIU_INST:972BIU&search_scope=MyInstitution&tab=LibraryCatalog&docid=alma990024466410205776&lang=en&context=L&adaptor=Local%20Search%20Engine&query=any,contains,Understanding%C2%A0nanomaterials&offset=0 (accessed on 22 August 2022).
- Luchkin, A.; Goncharova, O.; Andreev, N.; Arkhipushkin, I.A.; Kazanskii, L.; Kuznetsov, Y. 5-khlor-1, 2, 3-benzotriazol kak kamernyi ingibitor korrozii magnievogo splava MA8. Korroz. Mater. Zashchita 2020, 4, 27–35. [Google Scholar]
- Ishizaki, T.; Okido, M.; Masuda, Y.; Saito, N.; Sakamoto, M. Corrosion Resistant Performances of Alkanoic and Phosphonic Acids Derived Self-Assembled Monolayers on Magnesium Alloy AZ31 by Vapor-Phase Method. Langmuir 2011, 27, 6009–6017. [Google Scholar] [CrossRef] [PubMed]
- Andreev, N.N.; Goncharova, O.A.; Kuznetsov, Y.I.; Luchkin, A.Y. A method for metal protection from atmospheric corrosion. RU Patent 2649354, 2 April 2018. [Google Scholar]
- Goncharova, O.A.; Luchkin, A.Y.; Archipushkin, I.A.; Andreev, N.N.; Kuznetsov, Y.I.; Vesely, S.S. Vapor-phase protection of steel by inhibitors based on salts of higher carboxylic acids. Int. J. Corros. Scale Inhib. 2019, 3, 586–599. [Google Scholar]
- Goncharova, O.A.; Andreev, N.N.; Luchkin, A.Y.; Kuznetsov, Y.I.; Andreeva, N.P.; Vesely, S.S. Protection of copper by treatment with hot vapors of octadecylamine, 1,2,3-benzotriazole, and their mixtures. Mater. Corros. 2018, 70, 161–168. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Zhang, D.-Q.; Gao, L.-X.; Liu, Y.-Y.; Yan, H.-B.; Wei, S.-L.; Ma, T.-F. Vapor phase assembly of benzotriazole and octadecylamine complex films on aluminum alloy surface. J. Coatings Technol. Res. 2020, 18, 435–446. [Google Scholar] [CrossRef]
- Goncharova, O.A.; Luchkin, A.Y.; Kuznetsov, Y.I.; Andreev, N.N.; Andreeva, N.P.; Vesely, S.S. Octadecylamine, 1,2,3-benzotriazole and a mixture thereof as chamber inhibitors of steel corrosion. Int. J. Corros. Scale Inhib. 2018, 2, 203–212. [Google Scholar]
- Goncharova, O.A.; Kuznetsov, Y.I.; Andreev, N.N.; Luchkin, A.Y.; Andreeva, N.P.; Kuznetsov, D.S. A new corrosion inhibitor for zinc chamber treatment. Int. J. Corros. Scale Inhib. 2018, 3, 340–351. [Google Scholar]
- Luchkin, A.; Goncharova, O.; Arkhipushkin, I.; Andreev, N.; Kuznetsov, Y. The effect of oxide and adsorption layers formed in 5-Chlorobenzotriazole vapors on the corrosion resistance of copper. J. Taiwan Inst. Chem. Eng. 2020, 117, 231–241. [Google Scholar] [CrossRef]
- Luchkin, A.Y.; Goncharova, O.A.; Andreeva, N.P.; Kasatkin, V.E.; Vesely, S.S.; Kuznetsov, Y.I.; Andreev, N.N. Mutual Effects of Components of Protective Films Applied on Steel in Octadecylamine and 1,2,3-Benzotriazole Vapors. Materials 2021, 14, 7181. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, H.; Gao, L.; Zhang, D.; Zhao, Y.; Sun, M.; Dai, Y. Surface modification for Al alloy by volatile corrosion inhibitors from cyclohexylamine, octanoic acid, and their reaction product. Mater. Corros. 2021, 72, 1468–1477. [Google Scholar] [CrossRef]
- GOST 14957. Interstate Standard. Wrought Magnesium Alloys. Grades. Available online: https://auremo.biz/gosts/gost-14957-76.html (accessed on 22 August 2022).
- Calado, L.M.; Taryba, M.G.; Carmezim, M.J.; Montemor, M.F. Self-healing ceria-modified coating for corrosion protection of AZ31 magnesium alloy. Corros. Sci. 2018, 142, 12–21. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B. 1972, 5, 4709–4713. [Google Scholar] [CrossRef]
- Mohai, M. XPS MultiQuant: Multimodel XPS quantification software. Surf. Interface Anal. 2004, 36, 828–832. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Cumpson, P.J.; Seah, M.P. Elastic Scattering Corrections in AES and XPS. II. Estimating Attenuation Lengths and Conditions Required for their Valid Use in Overlayer/Substrate Experiments. Surf. Interface Anal. 1997, 25, 430–446. [Google Scholar] [CrossRef]
- Goncharova, O.A.; Luchkin, A.Y.; Andreeva, N.P.; Kasatkin, V.E.; Andreev, N.N.; Kuznetsov, Y.I. Steel protection with cham-ber inhibitor IFKhAN-131. Korroz. Mater. Zashchita 2021, 9, 18–33. [Google Scholar]
- Kazansky, L.P. DFT & PM3 calculated charges on atoms in heterocyclic molecules and XPS. Corros. Sci. 2016, 112, 724–727. [Google Scholar]
- Kazanskii, L.P.; Selyaninov, I.A. XPES of 1,2,3-benzotriazole nanolayers formed on iron surface. Prot. Met. Phys. Chem. Surfaces 2010, 46, 797–804. [Google Scholar] [CrossRef]




| Fe | Si | Mn | Ni | Ce | Al | Cu | Be | Mg | Zn | Impurities |
|---|---|---|---|---|---|---|---|---|---|---|
| <0.05 | <0.1 | 1.3–2.2 | <0.007 | 0.15–0.35 | <0.1 | <0.05 | <0.002 | 96.84–98.55 | <0.3 | 0.3 |
| Preparation Modes | Rct Ohm∙cm2 | CPEdl (S sn/cm2) | ndl | Rsl Ohm∙cm2 | CPEsl (S sn/cm2) | nsl | Rr Ohm∙cm2 | Z, % |
|---|---|---|---|---|---|---|---|---|
| A | 746 | 5.9 × 10−4 | 0.85 | 1693 | 8.7 × 10−6 | 0.88 | 119 | |
| B | 499 | 5.8 × 10−4 | 0.86 | 1724 | 9.7 × 10−6 | 0.86 | 123 | −9.71 |
| C | 8704 | 1.17 × 10−5 | 0.90 | 12,029 | 1.78 × 10−6 | 0.77 | 118 | 88.24 |
| D | 615 | 5.8 × 10−4 | 0.83 | 1755 | 9.6 × 10−6 | 0.84 | 131 | −2.91 |
| E | 8991 | 9.2 × 10−6 | 0.81 | 16,832 | 1.01 × 10−6 | 0.86 | 123 | 90.55 |
| Preparation Conditions | γsl | γct | γct/γsl |
|---|---|---|---|
| C | 7.0 | 17.4 | 2.43 |
| E | 9.8 | 18.0 | 1.83 |
| Sample Preparation Mode | Mg, % | MgO, % | MgOxOHy, % | OHL Thickness, nm |
|---|---|---|---|---|
| A | 30.4 | 46.2 | 23.4 | 4.3 |
| B | 6.2 | 28.6 | 65.2 | 6.5 |
| C | 16.1 | 34.3 | 49.6 | 5.2 |
| D | - | 21.8 | 78.2 | 9.4 |
| E | 11.4 | 32.4 | 56.2 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goncharova, O.A.; Luchkin, A.Y.; Senchikhin, I.N.; Makarychev, Y.B.; Luchkina, V.A.; Dement’eva, O.V.; Vesely, S.S.; Andreev, N.N. Structuring of Surface Films Formed on Magnesium in Hot Chlorobenzotriazole Vapors. Materials 2022, 15, 6625. https://doi.org/10.3390/ma15196625
Goncharova OA, Luchkin AY, Senchikhin IN, Makarychev YB, Luchkina VA, Dement’eva OV, Vesely SS, Andreev NN. Structuring of Surface Films Formed on Magnesium in Hot Chlorobenzotriazole Vapors. Materials. 2022; 15(19):6625. https://doi.org/10.3390/ma15196625
Chicago/Turabian StyleGoncharova, Olga A., Andrey Yu Luchkin, Ivan N. Senchikhin, Yury B. Makarychev, Victoriya A. Luchkina, Olga V. Dement’eva, Sergey S. Vesely, and Nickolay N. Andreev. 2022. "Structuring of Surface Films Formed on Magnesium in Hot Chlorobenzotriazole Vapors" Materials 15, no. 19: 6625. https://doi.org/10.3390/ma15196625
APA StyleGoncharova, O. A., Luchkin, A. Y., Senchikhin, I. N., Makarychev, Y. B., Luchkina, V. A., Dement’eva, O. V., Vesely, S. S., & Andreev, N. N. (2022). Structuring of Surface Films Formed on Magnesium in Hot Chlorobenzotriazole Vapors. Materials, 15(19), 6625. https://doi.org/10.3390/ma15196625






