Distribution of Iron Nanoparticles in Arrays of Vertically Aligned Carbon Nanotubes Grown by Chemical Vapor Deposition
Abstract
1. Introduction
2. Materials and Methods
3. Results
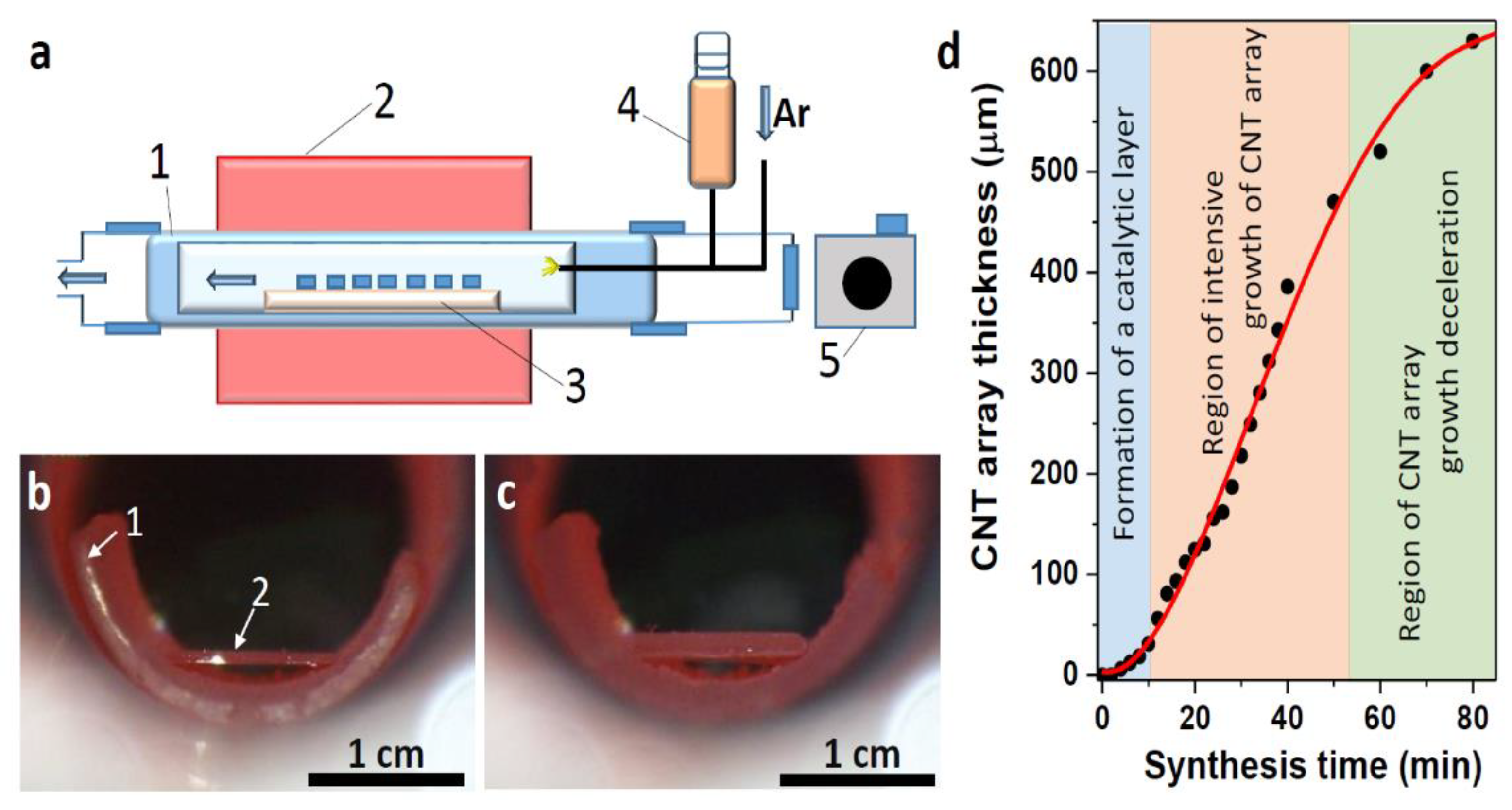
3.1. Synthesis of CNT Arrays
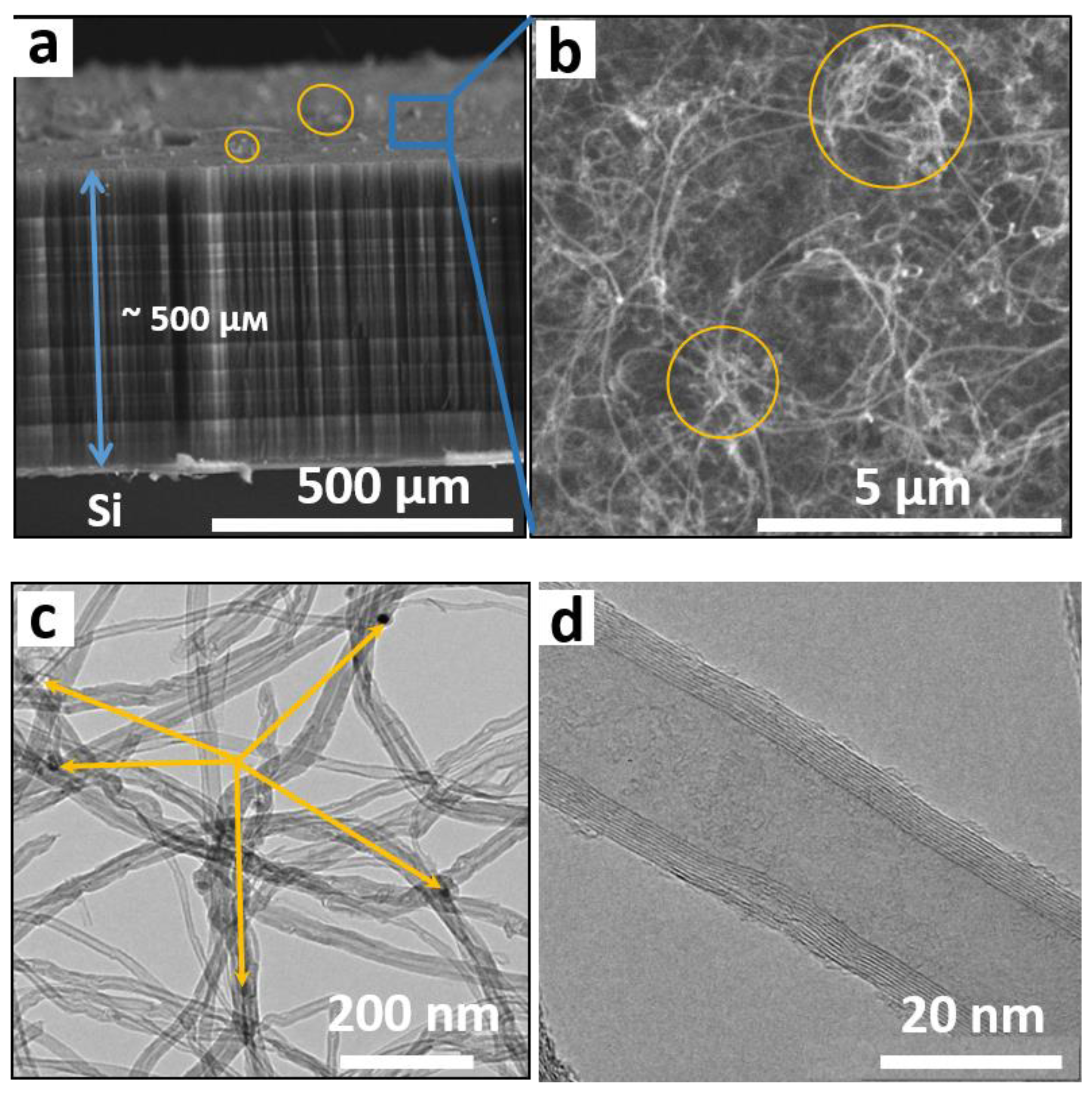
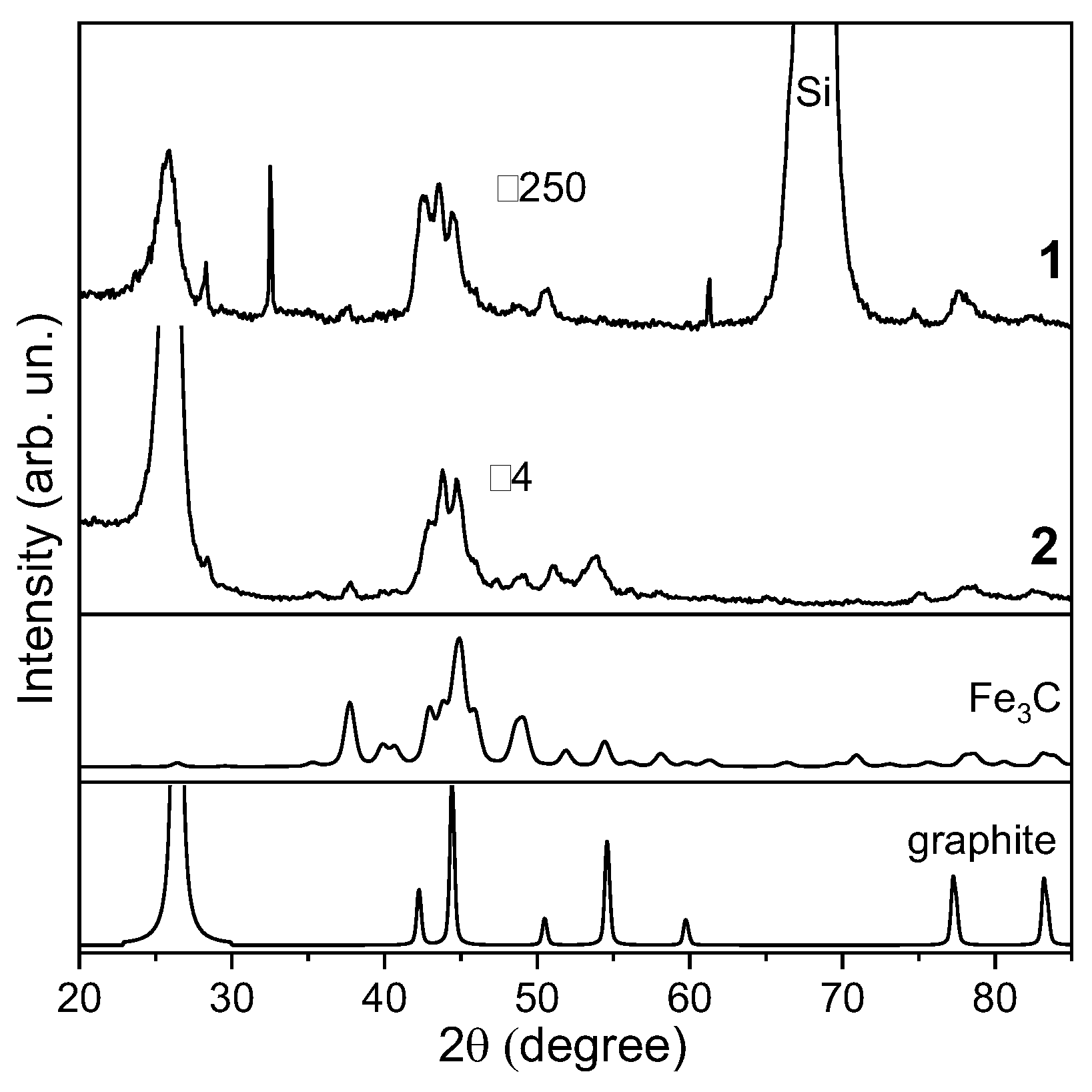
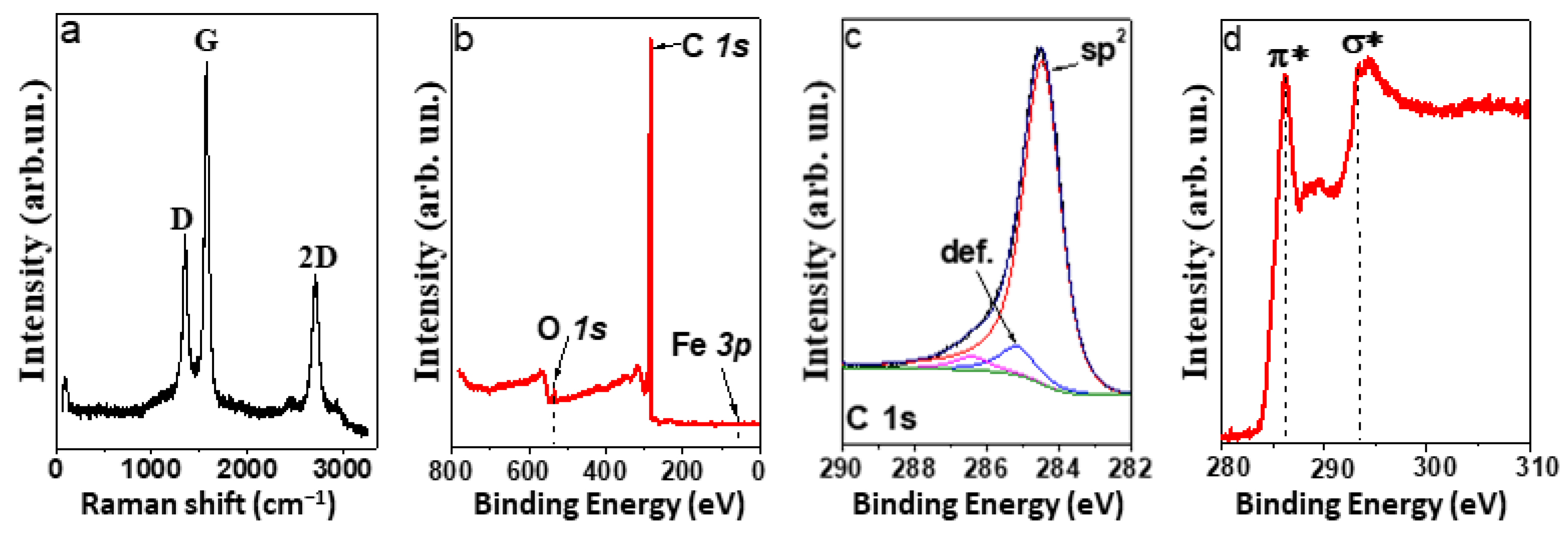
3.2. Characterization of CNT Arrays
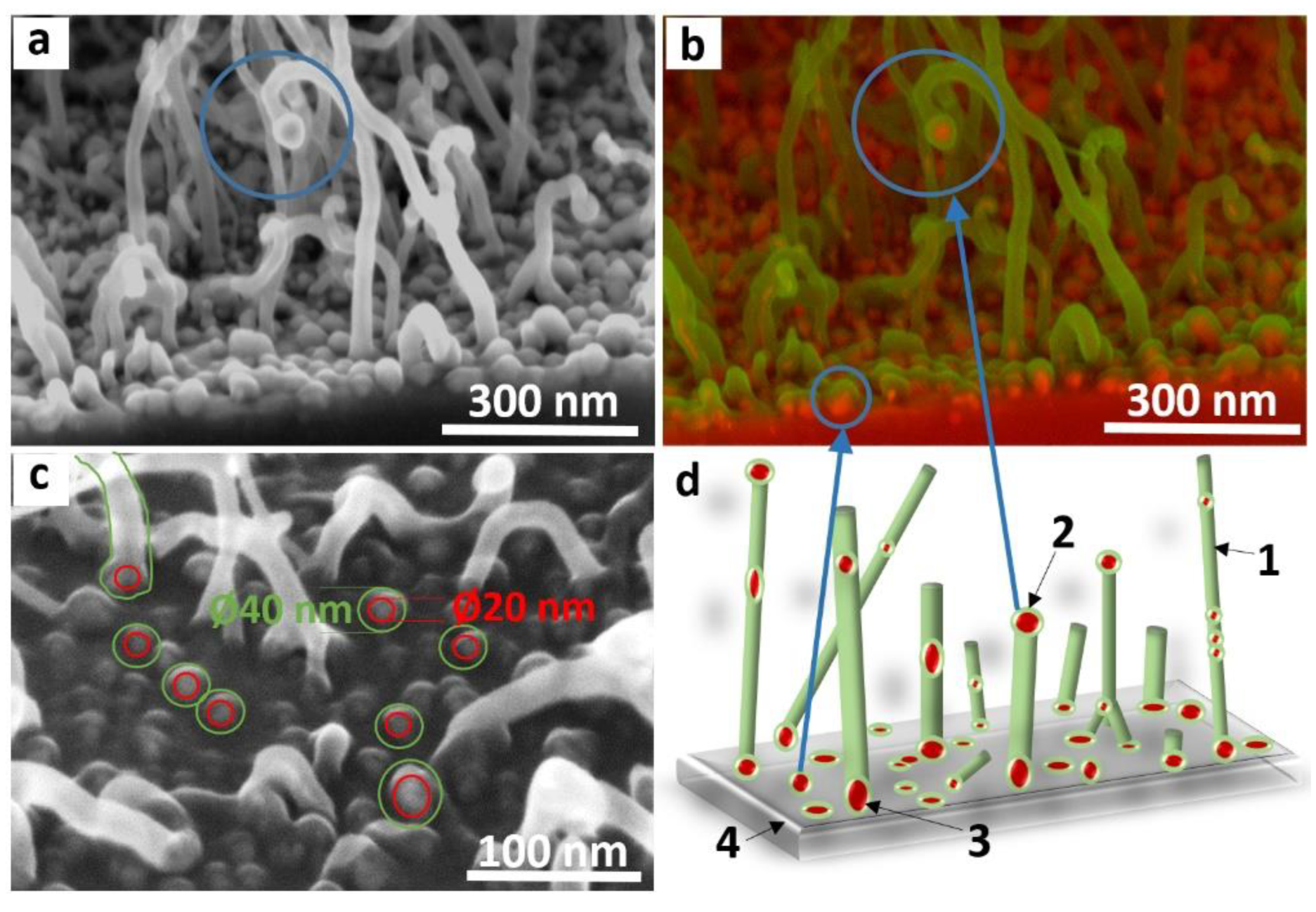
3.3. Mechanism of CNT Array Growth
3.4. Distribution of Iron Nanoparticles in MWCNT Array
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowack, B.; David, R.M.; Fissan, H.; Morris, H.; Shatkin, J.A.; Stintz, M.; Brouwer, D. Potential release scenarios for carbon nanotubes used in composites. Environ. Int. 2013, 59, 1–11. [Google Scholar] [CrossRef]
- Dong, Z.J.; Sun, B.; Zhu, H.; Yuan, G.M.; Li, B.L.; Guo, J.G.; Zhang, J. A review of aligned carbon nanotube arrays and carbon/carbon composites: Fabrication, thermal conduction properties and applications in thermal management. New Carbon Mater. 2021, 36, 873–892. [Google Scholar] [CrossRef]
- Kuznetzov, A.A.; Lee, S.B.; Zhang, M.; Baughman, R.H.; Zakhidov, A.A. Electron field emission from transparent multiwalled carbon nanotube sheets for inverted field emission displays. Carbon 2010, 48, 41–46. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Di, Y.; Lei, W.; Zhang, X.; Zhu, Z. Shadow mask field emission display with carbon nanotubes emitters. Diam. Relat. Mater. 2008, 17, 217–222. [Google Scholar] [CrossRef]
- Gorodetsky, D.V.; Kurenya, A.G.; Gusel’nikov, A.V.; Baskakova, K.I.; Smirnov, D.A.; Arkhipov, V.E.; Bulusheva, L.G.; Okotrub, A.V. Laser beam patterning of carbon nanotube arrays for the work of electron field emitters in technical vacuum. Mater. Sci. Eng. B 2020, 262, 114691. [Google Scholar] [CrossRef]
- Sugie, H.; Tanemura, M.; Filip, V.; Iwata, K.; Takahashi, K.; Okuyama, F. Carbon nanotubes as electron source in an X-ray tube. Appl. Phys. Lett. 2001, 78, 2578–2580. [Google Scholar] [CrossRef]
- Parmee, R.J.; Collins, C.M.; Milne, W.I.; Cole, M.T. X-ray generation using carbon nanotubes. Nano Converg. 2015, 2, 1–27. [Google Scholar] [CrossRef]
- Yue, G.Z.; Qiu, Q.; Gao, B.; Cheng, Y.; Zhang, J.; Shimoda, H.; Zhou, O. Generation of continuous and pulsed diagnostic imaging X-ray radiation using a carbon-nanotube-based field-emission cathode. Appl. Phys. Lett. 2002, 81, 355–357. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, J.-P.; Noh, Y.-R.; Jo, K.-C.; Lee, S.-Y.; Choi, H.-Y.; Kim, J.-U. X-ray images obtained from cold cathodes using carbon nanotubes coated with gallium-doped zinc oxide thin films. Thin Solid Films. 2010, 519, 1743–1748. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Chao, K.J.; Stevens, R.M.; Delzeit, L.; Cassell, A.; Han, J.; Meyyappan, M. Carbon nanotube tip probes: Stability and lateral resolution in scanning probe microscopy and application to surface science in semiconductors. Nanotechnology 2001, 12, 363–367. [Google Scholar] [CrossRef]
- Tovee, P.D.; Pumarol, M.E.; Rosamond, M.C.; Jones, R.; Petty, M.C.; Zezeb, D.A.; Kolosov, O.V. Nanoscale resolution scanning thermal microscopy using carbon nanotube tipped thermal probes. Phys. Chem. Chem. Phys. 2014, 16, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Peng, A.; Wang, Z.Y.; Zhang, H.; Shi, Z.; Gu, Z.; Ding, B. Growth of aligned carbon nanotube arrays on metallic substrate and its application to supercapacitors. Solid State Commun. 2008, 146, 380–383. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.; Yang, Y. Carbon nanotube arrays and their composites for electrochemical capacitors and lithium-ion batteries. Energy Environ. Sci. 2009, 2, 932–943. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Arkhipov, V.E.; Fedorovskaya, E.O.; Zhang, S.; Kurenya, A.G.; Kanygin, M.A.; Asanov, I.P.; Tsygankova, A.R.; Chen, X.; Song, H.; et al. Fabrication of free-stanfing aligned multiwalled carbon nanotube array for Li-ion batteries. J. Power Sour. 2016, 311, 42–48. [Google Scholar] [CrossRef]
- Ruffini, G.; Dunne, S.; Farrés, E.; Marco-Pallarés, J.; Ray, C.; Mendoza, E.; Grau, C. A dry electrophysiology electrode using CNT arrays. Sens. Actuators A Phys. 2006, 132, 34–41. [Google Scholar] [CrossRef]
- Fedorovskaya, E.O.; Apartsin, E.K.; Novopashina, D.S.; Venyaminova, A.G.; Kurenya, A.G.; Bulusheva, L.G.; Okotrub, A.V. RNA-modified carbon nanotube arrays regognizing RNA via electrochemical capacitance response. Mater. Des. 2016, 100, 67–72. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, Q.; Wang, Z.; Zhang, Y.; Wu, J.; Fan, K.; Sun, L.; Zhu, M.; Ng, Z.K.; Teo, E.H.T.; et al. Real-time THz beam profiling and monitoring via flexible vertically aligned carbo nanotube arrays. Adv. Opt. Mater. 2022, 2201363. [Google Scholar] [CrossRef]
- Karakashov, B.; Mayne-L’Hermite, M.; Pinault, M. Conducting interface for efficient growth of vertically aligned carbon nanotubes: Towards nano-engineered carbon composite. Nanomaterials 2022, 12, 2300. [Google Scholar] [CrossRef]
- Huang, S.; Du, X.; Ma, M.; Xiong, L. Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials. Nanotechnol. Rev. 2021, 10, 1592–1623. [Google Scholar] [CrossRef]
- Kohls, A.; Maurer Ditty, M.; Dehghandehnavi, F.; Zheng, S.Y. Vertically aligned carbon nanotubes as a unique material for biomedical applications. ACS Appl. Mater. Interfaces 2022, 14, 6287–6306. [Google Scholar] [CrossRef]
- Cho, W.; Schulz, M.; Shanov, V. Growth and characterization of vertically aligned centimeter long CNT arrays. Carbon 2014, 72, 264–273. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, B.; Zhang, M. Optimizing reaction condition for synthesizing spinnable carbon nanotube arrays by chemical vapor deposition. J. Mater. Sci. 2013, 48, 7749–7756. [Google Scholar] [CrossRef]
- Hart, A.J.; Slocum, A.H. Rapid growth and flow-mediated nucleation of millimeter-scale aligned carbon nanotube structures from a thin-film catalyst. J. Phys. Chem. B 2006, 1, 8250–8257. [Google Scholar] [CrossRef]
- Sohn, J.I.; Choi, C.J.; Lee, S.; Seong, T.Y. Effects of Fe film thickness and pretreatments on the growth behaviours of carbon nanotubes on Fe-doped (001) Si substrates. Jpn. J. Appl. Phys. 2002, 41, 4731. [Google Scholar] [CrossRef]
- Chopra, N.; Hinds, B. Catalytic size control of multiwalled carbon nanotube diameter in xylene chemical vapor deposition process. Inorganica Chim. Acta 2004, 357, 3920–3926. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, G.A.; Zhen, R.; Zhao, Y.; Liang, C. Effects of the restructuring of Fe catalyst films on chemical vapor deposition of carbon nanotubes. Surf. Coat. Technol. 2008, 202, 3157–3163. [Google Scholar] [CrossRef]
- Kim, C.D.; Kang, J.T.; Ryu, H.W.; Lee, I.S.; Park, J.H.; Lee, C.S.; Lee, H.R. Wall-controlled growth of carbon nanotubes using temperature treatment. Jpn. J. Appl. Phys. 2008, 47, 4803. [Google Scholar] [CrossRef]
- Cho, W.; Schulz, M.; Shanov, V. Growth termination mechanism of vertically aligned centimeter long carbon nanotube arrays. Carbon 2014, 69, 609–620. [Google Scholar] [CrossRef]
- Park, J.B.; Choi, G.S.; Cho, Y.S.; Hong, S.Y.; Kim, D.; Choi, S.Y.; Cho, K.I. Characterization of Fe-catalyzed carbon nanotubes grown by thermal chemical vapor deposition. J. Cryst. Growth. 2002, 244, 211–217. [Google Scholar] [CrossRef]
- Pinault, M.; Mayne-L’Hermite, M.; Reynaud, C.; Pichot, V.; Launois, P.; Ballutaud, D. Growth of multiwalled carbon nanotubes during the initial stages of aerosol-assisted CCVD. Carbon 2005, 43, 2968–2976. [Google Scholar] [CrossRef]
- Pinault, M.; Pichot, V.; Khodja, H.; Launois, P.; Reynaud, C.; Mayne-L’Hermite, M. Evidence of sequential lift in growth of aligned multiwalled carbon nanotube multilayers. Nano Lett. 2005, 5, 2394–2398. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Kanygin, M.A.; Kurenya, A.G.; Kudashov, A.G.; Bulusheva, L.G.; Molodtsov, S.L. NEXAFS detection of graphitic layers formed in the process of carbon nanotube arrays synthesis. Nucl. Instrum. Methods Phys. Res. A 2009, 603, 115–118. [Google Scholar] [CrossRef]
- Lyubutin, I.S.; Anosova, O.A.; Frolov, K.V.; Sulyanov, S.N.; Okotrub, A.V.; Kudashov, A.G.; Bulusheva, L.G. Iron nanoparticles in aligned arrays of pure and nitrogen-doped carbon nanotubes. Carbon 2012, 50, 2628–2634. [Google Scholar] [CrossRef]
- Vermisoglou, E.C.; Karanikolos, G.N.; Pilatos, G.; Devlin, E.; Romanos, G.E.; Veziri, C.U.; Kanellopoulos, N.K. Aligned carbon nanotubes with ferromagnetic behavior. Adv. Mat. 2010, 22, 473–477. [Google Scholar] [CrossRef]
- Prischepa, S.; Danilyuk, A. Anisotropic temperature-dependent interaction of ferromagnetic nanoparticles embedded inside CNT. Int. J. Nanosci. 2019, 18, 1940015. [Google Scholar] [CrossRef]
- Gao, C.; Tao, F.; Lin, W.; Xu, Z.; Xue, Z. Ordered arrays of magnetic metal nanotubes and nanowires encapsulated with carbon tubes. J. Nanosci. Nanotechnol. 2008, 8, 4494–4499. [Google Scholar] [CrossRef]
- Ray, S.C.; Bhattacharyya, S.; Wu, S.L.; Ling, D.C.; Pong, W.F.; Giorcelli, M.; Tagliaferro, A. High coercivity magnetic multi-wall carbon nanotubes for low-dimensional high-density magnetic recording media. Diam. Relat. Mater. 2010, 19, 553–556. [Google Scholar] [CrossRef]
- Lange, C.M.; Shen, T.C. Fabrication of height-modulated carbon nanotube forests: Morphologies and prospects for broadband absorption. Carbon Trends 2021, 4, 100070. [Google Scholar] [CrossRef]
- Bellucci, S.; Micciulla, F.; Shunin, Y.; Zhukovskii, Y.; Gopejenko, V.; Burlutskaya, N.; Capobianchi, A. Memory nanodevices based on carbon nanotube-FePt interconnects: Electromagnetic simulations and magnetically stimulated nanotube growth. J. Mat. Sci. Eng. B 2015, 5, 120–134. [Google Scholar] [CrossRef][Green Version]
- Hsu, W.H.; Lin, C.W.; Chen, Y.H.; Wu, S.R.; Tsai, H.Y. Study on carbon nanotube/shape memory polymer composites and their applications in wireless worm actuator. J. Mech. 2021, 37, 636–650. [Google Scholar] [CrossRef]
- Rashed, A.O.; Merenda, A.; Kondo, T.; Lima, M.; Razal, J.; Kong, L.; Dumée, L.F. Carbon nanotube membranes-Strategies and challenges towards scalable manufacturing and practical separation applications. Sep. Purif. Technol. 2021, 257, 117929. [Google Scholar] [CrossRef]
- Nitodas, S.F.; Das, M.; Shah, R. Applications of polymeric membranes with carbon nanotubes: A review. Membranes 2022, 12, 454. [Google Scholar] [CrossRef]
- Singh, C.; Shaffer, M.S.P.; Windle, A. Production of controlled architectures of aligned carbon nanotubes by an injection chemical vapor deposition method. Carbon. 2003, 41, 359–368. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Bulusheva, L.G.; Kudashov, A.G.; Belavin, V.V.; Komogortsev, S.V. Arrays of carbon nanotubes aligned perpendicular to the substrate surface: Anisotropy of structure and properties. Nanotechnol. Russ. 2008, 3, 191–200. [Google Scholar] [CrossRef]
- Charon, E.; Pinault, M.; Mayne-L’Hermite, M.; Reynaud, C. One-step synthesis of highly pure and well-crystallized vertically aligned carbon nanotubes. Carbon 2021, 173, 758–768. [Google Scholar] [CrossRef]
- Combrisson, A.; Charon, E.; Pinault, M.; Reynaud, C.; Mayne-L’Hermite, M. Critical role of the acetylene content and Fe/C ratio on the thickness and density of vertically aligned carbon nanotubes grown at low temperature by a one-step catalytic chemical vapor deposition process. Nanomaterials 2022, 12, 2338. [Google Scholar] [CrossRef]
- Meysami, S.S.; Koós, A.A.; Grobert, N. Aerosol-assisted chemical vapour deposition synthesis of multi-wall carbon nanotubes: II. An analytical study. Carbon 2013, 58, 159–169. [Google Scholar] [CrossRef]
- Komogortsev, S.V.; Iskhakov, R.S.; Denisova, E.A.; Balaev, A.D.; Myagkov, V.G.; Bulina, N.V.; Kudashov, A.G.; Okotrub, A.V. Magnetic anisotropy in the films of oriented carbon nanotubes filled with iron nanoparticles. Tech. Phys. Lett. 2005, 31, 454–456. [Google Scholar] [CrossRef]
- Zagainova, V.S.; Makarova, T.L.; Okotrub, A.V.; Kurenya, A.G.; Komogortsev, S.V.; Bulusheva, L.G. Magnetic properties of carbon nanotubes with low content of Fe. Fuller. Nanotub. Carbon Nanostruct. 2010, 18, 569–573. [Google Scholar] [CrossRef]
- Odunmbaku, O.; Boi, F.S.; Guo, J.; Lan, M.; He, Y.; Yu, T.; Xiang, G. Chlorine-assisted synthesis of Fe3C-filled mm-long vertically aligned arrays of multiwall carbon nanotubes. Mater. Res. Express 2018, 6, 015040. [Google Scholar] [CrossRef]
- Gusel’nikov, A.V.; Safronov, A.V.; Kurenya, A.G.; Arkhipov, V.E.; Bolgarin, S.G.; Ivanov, A.E.; Okotrub, A.V. A laboratory CVD reactor for the synthesis of vertically oriented carbon nanotube arrays. Instrum. Exp. Tech. 2018, 61, 604–609. [Google Scholar] [CrossRef]
- Gusel’nikov, A.V.; Safronov, A.V.; Kurenya, A.G.; Arkhipov, V.E.; Bolgarin, S.G.; Ivanov, A.E.; Okotrub, A.V. The automation of a CVD-reactor for the synthesis of vertically oriented carbon nanotube arrays. Instrum. Exp. Tech. 2018, 61, 482–485. [Google Scholar] [CrossRef]
- Kurenya, A.G.; Gorodetsky, D.V.; Arkhipov, V.E.; Okotrub, A.V. Evaluation of the optimal carrier gas flow rate for the carbon nanotubes growth. Tech. Phys. Lett. 2012, 39, 258–261. [Google Scholar] [CrossRef]
- Sharma, P.; Pavelyev, V.; Kumar, S.; Mishra, P.; Islam, S.S.; Tripathi, N. Analysis on the synthesis of vertically aligned carbon nanotubes: Growth mechanism and techniques. J. Mater. Sci. Mater. Electron. 2020, 31, 4399–4443. [Google Scholar] [CrossRef]
- Das, R.; Bee Abd Hamid, S.; Eaqub Ali, M.; Ramakrishna, S.; Yongzhi, W. Carbon nanotubes characterization by X-ray powder diffraction—A review. Curr. Nanosci. 2015, 11, 23–35. [Google Scholar] [CrossRef]
- Ma, Y.; Skytt, P.; Wassdahl, N.; Glans, P.; Guo, J.; Nordgren, J. Core excitons and vibronic coupling in diamond and graphite. Phys. Rev. Lett. 1993, 71, 3728. [Google Scholar] [CrossRef]
- Kaiser, A.L.; Lidston, D.L.; Peterson, S.C.; Acauan, L.H.; Steiner, S.A.; de Villoria, R.G.; Wardle, B.L. Substrate adhesion evolves non-monotonically with processing time in millimeter-scale aligned carbon nanotube arrays. Nanoscale 2021, 13, 261–271. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Meysami, S.S.; Koós, A.A.; Dillon, F.; Dutta, M.; Grobert, N. Aerosol-assisted chemical vapour deposition synthesis of multi-wall carbon nanotubes: III. Towards upscaling. Carbon 2015, 88, 148–156. [Google Scholar] [CrossRef]
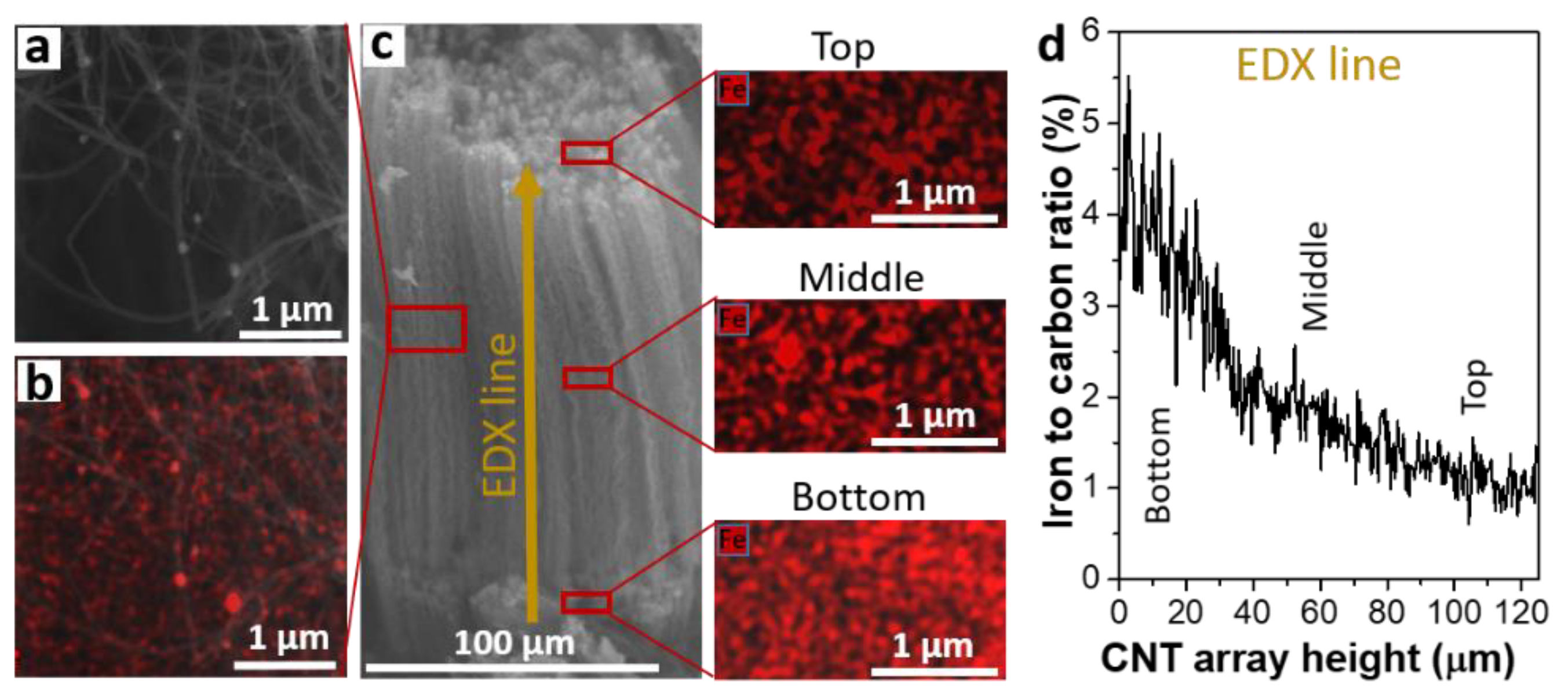
| Regions | Carbon | Silicon | Iron | Oxygen |
|---|---|---|---|---|
| Top | 90 | 2 | 4 | 4 |
| Middle | 88 | 5 | 5 | 2 |
| Bottom | 82 | 10 | 6 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okotrub, A.V.; Gorodetskiy, D.V.; Gusel’nikov, A.V.; Kondranova, A.M.; Bulusheva, L.G.; Korabovska, M.; Meija, R.; Erts, D. Distribution of Iron Nanoparticles in Arrays of Vertically Aligned Carbon Nanotubes Grown by Chemical Vapor Deposition. Materials 2022, 15, 6639. https://doi.org/10.3390/ma15196639
Okotrub AV, Gorodetskiy DV, Gusel’nikov AV, Kondranova AM, Bulusheva LG, Korabovska M, Meija R, Erts D. Distribution of Iron Nanoparticles in Arrays of Vertically Aligned Carbon Nanotubes Grown by Chemical Vapor Deposition. Materials. 2022; 15(19):6639. https://doi.org/10.3390/ma15196639
Chicago/Turabian StyleOkotrub, Alexander V., Dmitriy V. Gorodetskiy, Artem V. Gusel’nikov, Anastasiya M. Kondranova, Lyubov G. Bulusheva, Mariya Korabovska, Raimonds Meija, and Donats Erts. 2022. "Distribution of Iron Nanoparticles in Arrays of Vertically Aligned Carbon Nanotubes Grown by Chemical Vapor Deposition" Materials 15, no. 19: 6639. https://doi.org/10.3390/ma15196639
APA StyleOkotrub, A. V., Gorodetskiy, D. V., Gusel’nikov, A. V., Kondranova, A. M., Bulusheva, L. G., Korabovska, M., Meija, R., & Erts, D. (2022). Distribution of Iron Nanoparticles in Arrays of Vertically Aligned Carbon Nanotubes Grown by Chemical Vapor Deposition. Materials, 15(19), 6639. https://doi.org/10.3390/ma15196639







