Cation Valences and Multiferroic Properties of EuTiO3 Co-Doped with Ba and Transition Metals of Co/Ni
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
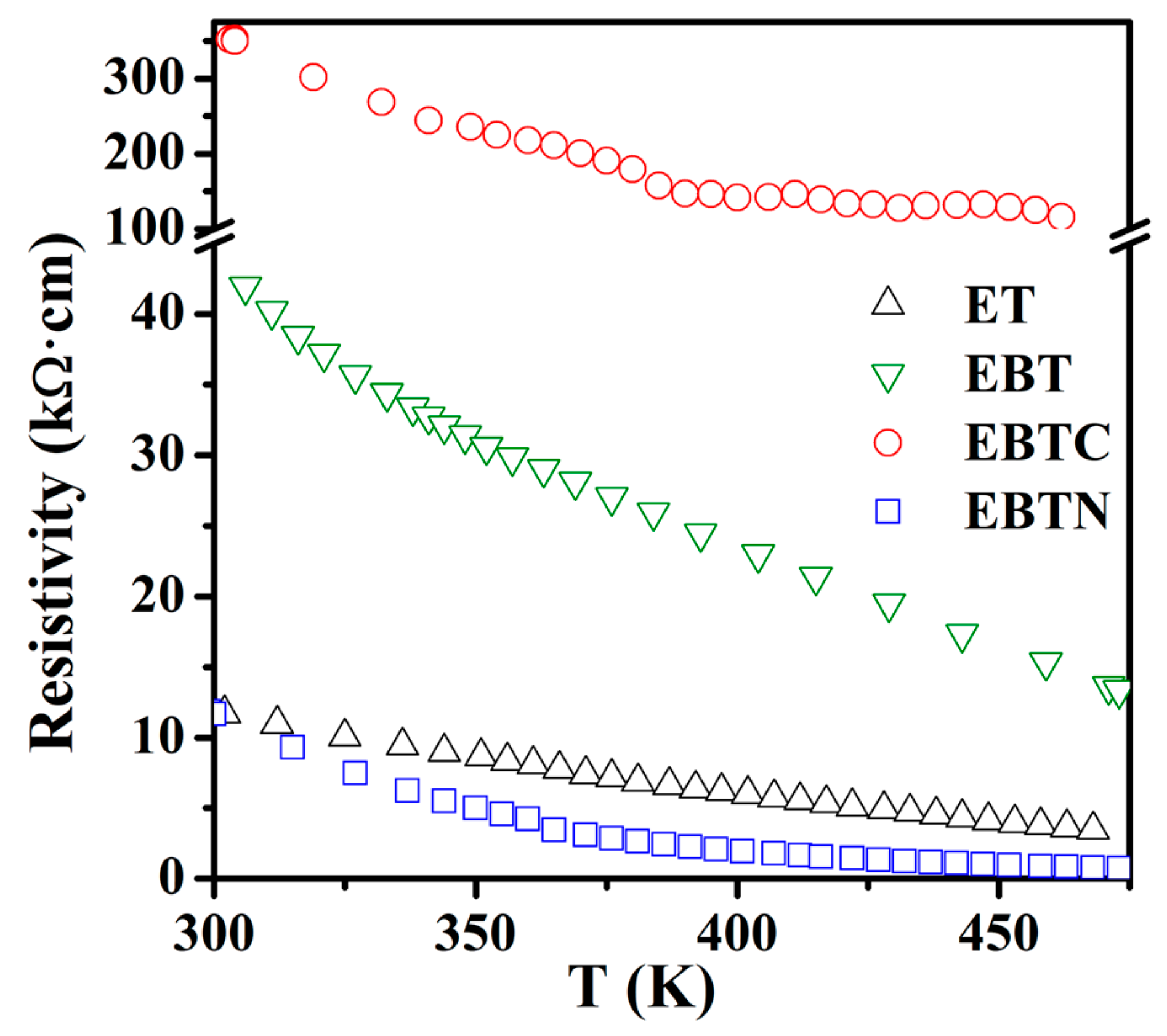
3.1. Phase Purity, Structure and Valences of Cations
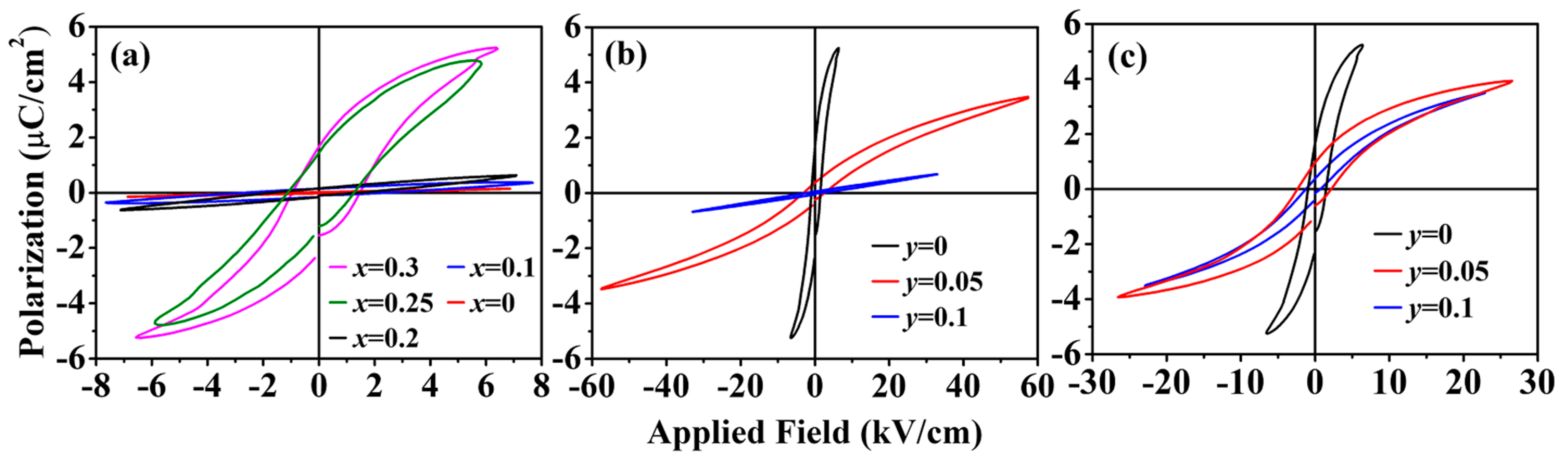
3.2. Ferroelectric Properties
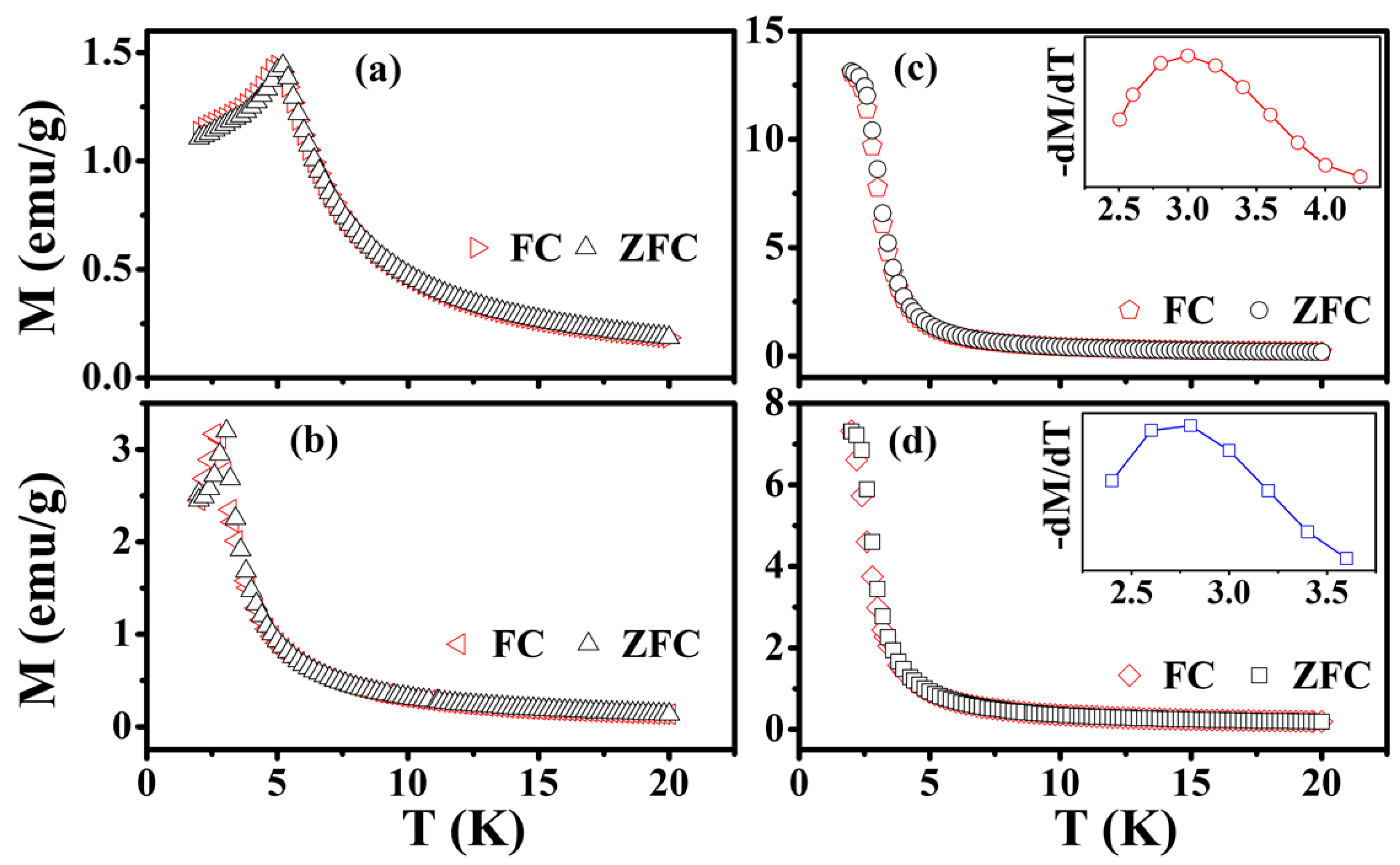
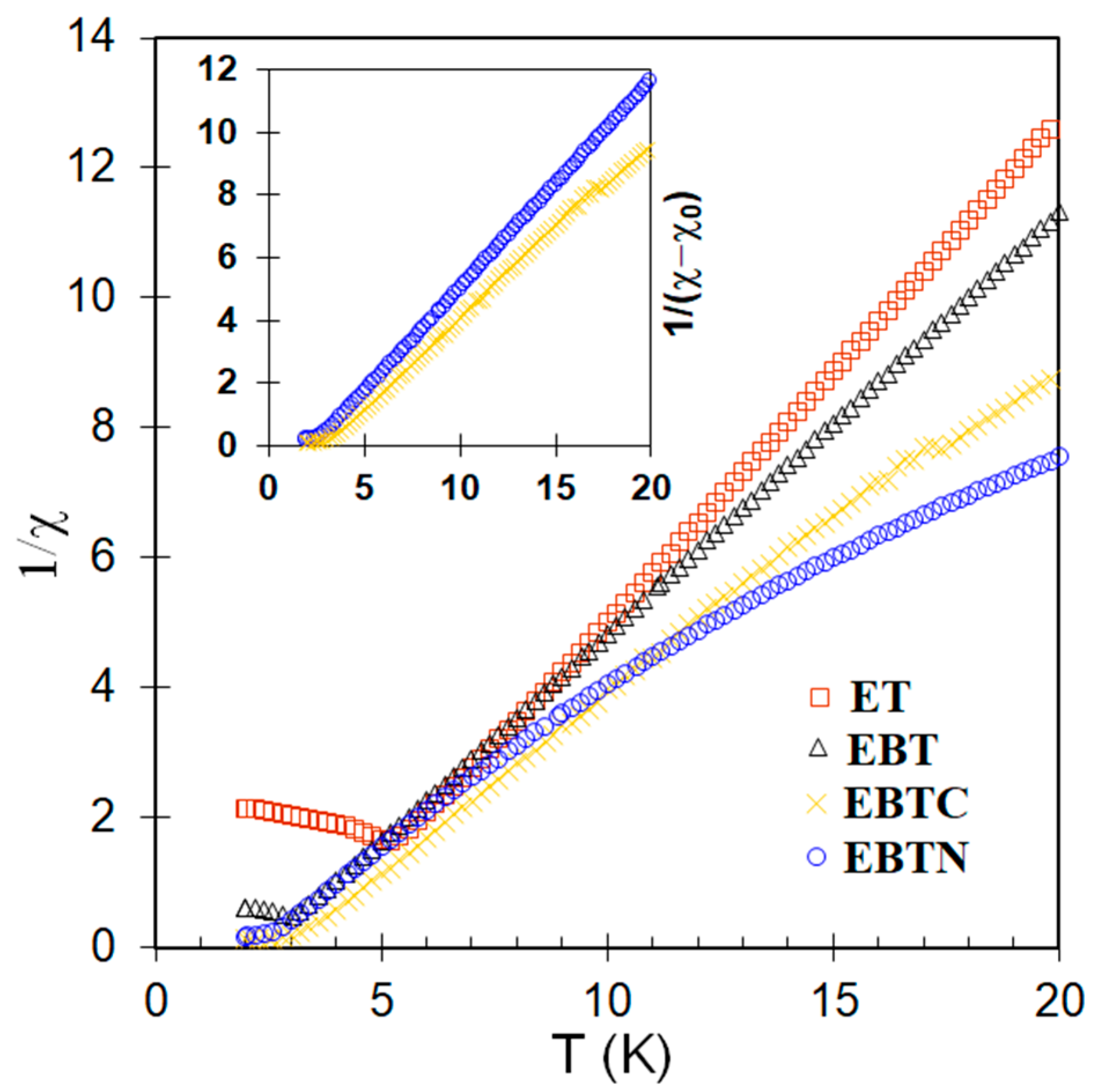
3.3. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, R.; Yang, C.; Wang, H.G.; Jiang, K.; Wu, H.; Shen, H.P.; Wang, L.; Sun, Y.; Jin, K.J.; Gao, J.; et al. Emergent multiferroism with magnetodielectric coupling in EuTiO3 created by a negative pressure control of strong spin-phonon coupling. Nat. Commun. 2022, 13, 2364. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Sterbinsky, G.; Choi, Y.; Woicik, J.; Zhu, L.; Jiang, J.; Lee, J.H.; Schlom, D.; Birol, T.; Brown, S. Multiferroic behavior in EuTiO3 films constrained by symmetry. Phys. Rev. B 2020, 101, 180409. [Google Scholar] [CrossRef]
- Lee, J.H.; Fang, L.; Vlahos, E.; Ke, X.; Jung, Y.W.; Kourkoutis, L.F.; Kim, J.W.; Ryan, P.J.; Heeg, T.; Roeckerath, M. A strong ferroelectric ferromagnet created by means of spin–lattice coupling. Nature 2010, 466, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Gregori, G.; Köhler, J.; Scott, J.; Bussmann-Holder, A. Hidden magnetism in the paramagnetic phase of EuTiO3. J. Phys. Condens. Matter 2015, 27, 496003. [Google Scholar] [CrossRef] [PubMed]
- Guguchia, Z.; Salman, Z.; Keller, H.; Roleder, K.; Köhler, J.; Bussmann-Holder, A. Complexity in the structural and magnetic properties of almost multiferroic EuTiO3. Phys. Rev. B 2016, 94, 220406. [Google Scholar] [CrossRef]
- Bussmann-Holder, A.; Köhler, J.; Roleder, K.; Guguchia, Z.; Keller, H. Unexpected magnetism at high temperature and novel magneto-dielectric-elastic coupling in EuTiO3: A critical review. Thin Solid Film. 2017, 643, 3–6. [Google Scholar] [CrossRef]
- Katsufuji, T.; Takagi, H. Coupling between magnetism and dielectric properties in quantum paraelectric EuTiO3. Phys. Rev. B 2001, 64, 054415. [Google Scholar] [CrossRef]
- Repček, D.; Kadlec, C.; Kadlec, F.; Savinov, M.; Kachlík, M.; Drahokoupil, J.; Proschek, P.; Prokleška, J.; Maca, K.; Kamba, S. Seemingly anisotropic magnetodielectric effect in isotropic EuTiO3 ceramics. Phys. Rev. B 2020, 102, 144402. [Google Scholar] [CrossRef]
- Das, R.; Prabhu, R.; Venkataramani, N.; Prasad, S.; Li, L.; Phan, M.H.; Keppens, V.; Mandrus, D.; Srikanth, H. Giant low-field magnetocaloric effect and refrigerant capacity in reduced dimensionality EuTiO3 multiferroics. J. Alloys Compd. 2021, 850, 156819. [Google Scholar] [CrossRef]
- Mo, Z.J.; Sun, Q.L.; Wang, C.H.; Wu, H.Z.; Li, L.; Meng, F.B.; Tang, C.C.; Zhao, Y.; Shen, J. Effects of Sr-doping on the giant magnetocaloric effect of EuTiO3. Ceram. Int. 2017, 43, 2083–2088. [Google Scholar] [CrossRef]
- McGuire, T.; Shafer, M.; Joenk, R.; Alperin, H.; Pickart, S. Magnetic structure of EuTiO3. J. Appl. Phys. 1966, 37, 981–982. [Google Scholar] [CrossRef]
- Fennie, C.J.; Rabe, K.M. Magnetic and electric phase control in epitaxial EuTiO3 from first principles. Phys. Rev. Lett. 2006, 97, 267602. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhou, Q.; Yang, X.; Song, Q.; Li, Z.; Qi, X.; Liu, J.M. Competition between quantum fluctuation and ferroelectric order in Eu1− xBaxTiO3. Appl. Surf. Sci. 2012, 258, 4601–4606. [Google Scholar] [CrossRef]
- Rushchanskii, K.; Kamba, S.; Goian, V.; Vaněk, P.; Savinov, M.; Prokleška, J.; Nuzhnyy, D.; Knížek, K.; Laufek, F.; Eckel, S. A multiferroic material to search for the permanent electric dipole moment of the electron. Nat. Mater. 2010, 9, 649–654. [Google Scholar] [CrossRef]
- Henderson, N.L.; Ke, X.; Schiffer, P.; Schaak, R.E. Solution precursor synthesis and magnetic properties of Eu1− xCaxTiO3. J. Solid State Chem. 2010, 183, 631–635. [Google Scholar] [CrossRef]
- Janes, D.; Bodnar, R.; Taylor, A. Europium barium titanate—A magnetic ferroelectric compound. J. Appl. Phys. 1978, 49, 1452–1454. [Google Scholar] [CrossRef]
- Hao, Z.H.; Wang, H.Y.; Zhang, Q.; Mo, Z.J. Magnetic and magnetocaloric effects of Eu0.9M0. 1TiO3 (M = Ca, Sr, Ba, La, Ce, Sm) compounds. Acta Phys. Sin. 2018, 67, 228–234. [Google Scholar]
- Katsufuji, T.; Tokura, Y. Transport and magnetic properties of a ferromagnetic metal: Eu1− xRxTiO3. Phys. Rev. B 1999, 60, R15021. [Google Scholar] [CrossRef]
- Wei, T.; Song, Q.; Zhou, Q.; Li, Z.; Qi, X.; Liu, W.; Guo, Y.; Liu, J.M. Cr-doping induced ferromagnetic behavior in antiferromagnetic EuTiO3 nanoparticles. Appl. Surf. Sci. 2011, 258, 599–603. [Google Scholar] [CrossRef]
- Mo, Z.; Jiang, W.; Zhao, Y.; Hao, Z.; Zheng, Z.; Zhang, W.; Li, L.; Shen, J. Low-field induced giant magnetocaloric effect in EuTi1-xNixO3 (x= 0.05, 0.1) compounds. J. Magn. Magn. Mater. 2019, 477, 258–263. [Google Scholar] [CrossRef]
- Mo, Z.J.; Sun, Q.L.; Han, S.; Zhao, Y.; Chen, X.; Li, L.; Liu, G.D.; Meng, F.B.; Shen, J. Effects of Mn-doping on the giant magnetocaloric effect of EuTiO3 compound. J. Magn. Magn. Mater. 2018, 456, 31–37. [Google Scholar] [CrossRef]
- Zhang, W.; Mo, Z.J.; Hao, Z.; Shen, J.; Luo, J.W.; Chang, R.J.; Li, L. Fe doping effect on EuTiO3: The magnetic properties and giant magnetocaloric effect. Int. J. Appl. Ceram. 2019, 16, 1606–1611. [Google Scholar] [CrossRef]
- Sun, Q.L.; Mo, Z.J.; Shen, J.; Li, Y.J.; Li, L.; Zhang, J.K.; Liu, G.D.; Tang, C.C.; Meng, F.B. Observation of giant magnetocaloric effect under low magnetic fields in EuTi1–xCoxO3. Chin. Phys. B 2017, 26, 117501. [Google Scholar] [CrossRef]
- Li, L.; Zhou, H.; Yan, J.; Mandrus, D.; Keppens, V. Research Update: Magnetic phase diagram of EuTi1− x B xO3 (B = Zr, Nb). APL Mater. 2014, 2, 110701. [Google Scholar] [CrossRef]
- Akahoshi, D.; Horie, H.; Sakai, S.; Saito, T. Ferromagnetic behavior in mixed valence europium (Eu2+/Eu3+) oxide EuTi1− x M x O3 (M = Al3+ and Ga3+). Appl. Phys. Lett. 2013, 103, 172407. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Bishop, S.R.; Tuller, H.L.; Kuru, Y.; Yildiz, B. Chemical expansion of nonstoichiometric Pr0. 1Ce0. 9O2− δ: Correlation with defect equilibrium model. J. Eur. Ceram. Soc. 2011, 31, 2351–2356. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Perry, N.H.; Bishop, S.R. Understanding chemical expansion in perovskite-structured oxides. Phys. Chem. Chem. Phys. 2015, 17, 10028–10039. [Google Scholar] [CrossRef]
- Zhao, R.; Li, W.; Chen, L.; Meng, Q.; Yang, J.; Wang, H.; Wang, Y.; Tang, R.; Yang, H. Conduction mechanisms of epitaxial EuTiO3 thin films. Appl. Phys. Lett. 2012, 101, 102901. [Google Scholar] [CrossRef]
- Fujita, K.; Wakasugi, N.; Murai, S.; Zong, Y.; Tanaka, K. High-quality antiferromagnetic EuTiO3 epitaxial thin films on SrTiO3 prepared by pulsed laser deposition and postannealing. Appl. Phys. Lett. 2009, 94, 062512. [Google Scholar] [CrossRef]
- Li, W.; Gu, J.; He, Q.; Zhang, K.H.; Wang, C.; Jin, K.; Wang, Y.; Acosta, M.; Wang, H.; Borisevich, A.Y. Oxygen-vacancy-mediated dielectric property in perovskite Eu0.5Ba0.5TiO3-δ epitaxial thin films. Appl. Phys. Lett. 2018, 112, 182906. [Google Scholar] [CrossRef] [Green Version]
- Mariscal, A.; Quesada, A.; Martin-Luengo, A.T.; García, M.A.; Bonanni, A.; Fernández, J.; Serna, R. Europium monoxide nanocrystalline thin films with high near-infrared transparency. Appl. Surf. Sci. 2018, 456, 980–984. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M. XPS studies of solvated metal atom dispersed (SMAD) catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. J. Am. Chem. Soc. 1991, 113, 855–861. [Google Scholar] [CrossRef]
- Alex, C.; Sarma, S.C.; Peter, S.C.; John, N.S. Competing effect of Co3+ reducibility and oxygen-deficient defects toward high oxygen evolution activity in Co3O4 systems in alkaline medium. ACS Appl. Energy Mater. 2020, 3, 5439–5447. [Google Scholar] [CrossRef]
- Venezia, A.; Loxton, C. Low pressure oxidation of Ni3Al alloys at elevated temperatures as studied by X-ray photoelectron spectroscopy and Auger spectroscopy. Surf. Sci. 1988, 194, 136–148. [Google Scholar] [CrossRef]
- Nasir, M.; Khan, M.; Rini, E.; Agbo, S.A.; Sen, S. Exploring the role of Fe substitution on electronic, structural, and magnetic properties of La2NiMnO6 double perovskites. Appl. Phys. A 2021, 127, 208. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Barroso, N.; Galetti, A.E.; Arrúa, L.A.; Cornaglia, L.; Abello, M.C. XPS study of the surface properties and Ni particle size determination of Ni-supported catalysts. Surf. Interface Anal. 2014, 46, 521–529. [Google Scholar] [CrossRef]
- Ettema, A.R.; Haas, C. An X-ray photoemission spectroscopy study of interlayer charge transfer in some misfit layer compounds. J. Condens. Matter Phys. 1993, 5, 3817. [Google Scholar] [CrossRef]
- Greczynski, G.; Jensen, J.; Greene, J.E.; Petrov, I.; Hultman, L. X-ray photoelectron spectroscopy analyses of the electronic structure of polycrystalline Ti1-xAlxN thin films with 0 ≤ x ≤ 0.96. Surf. Sci. Spectra 2014, 21, 35–49. [Google Scholar] [CrossRef]
- Ederer, C.; Harris, T.; Kováčik, R. Mechanism of ferroelectric instabilities in non-d0 perovskites: LaCrO3 versus CaMnO3. Phys. Rev. B 2011, 83, 054110. [Google Scholar] [CrossRef]
- Rubi, K.; Kumar, P.; Maheswar Repaka, D.; Chen, R.; Wang, J.S.; Mahendiran, R. Giant magnetocaloric effect in magnetoelectric Eu1-xBaxTiO3. Appl. Phys. Lett. 2014, 104, 032407. [Google Scholar] [CrossRef] [Green Version]
- Takikawa, Y.; Ebisu, S.; Nagata, S. Van Vleck paramagnetism of the trivalent Eu ions. J. Phys. Chem. Solids 2010, 71, 1592–1598. [Google Scholar] [CrossRef]
- Bustingorry, S.; Pomiro, F.; Aurelio, G.; Curiale, J. Second-order magnetic critical points at finite magnetic fields: Revisiting Arrott plots. Phys. Rev. B 2016, 93, 224429. [Google Scholar] [CrossRef]
- Banerjee, S.K. On a generalised approach to first and second order magnetic transitions. Phys. Lett. 1964, 12, 16–17. [Google Scholar] [CrossRef]
- Mo, Z.J.; Hao, Z.H.; Shen, J.; Li, L.; Wu, J.F.; Hu, F.X.; Sun, J.R.; Shen, B.G. Observation of giant magnetocaloric effect in EuTi1-xCrxO3. J. Alloy. Compd. 2015, 649, 674–678. [Google Scholar] [CrossRef]
- Akamatsu, H.; Kumagai, Y.; Oba, F.; Fujita, K.; Murakami, H.; Tanaka, K.; Tanaka, I. Antiferromagnetic superexchange via 3 d states of titanium in EuTiO3 as seen from hybrid Hartree-Fock density functional calculations. Phys. Rev. B 2011, 83, 214421. [Google Scholar] [CrossRef]
- Chien, C.L.; DeBenedetti, S.; Barros, F.D.S. Magnetic properties of EuTiO3, Eu2TiO4, and Eu3Ti2O7. Phys. Rev. B 1974, 10, 3913. [Google Scholar] [CrossRef]
- Kornienko, A.A.; Dunina, E.B.; Fomicheva, L.A. Determination of odd-symmetry crystal-field parameters from optical spectra. Opt. Spectrosc. 2014, 116, 683–690. [Google Scholar] [CrossRef]
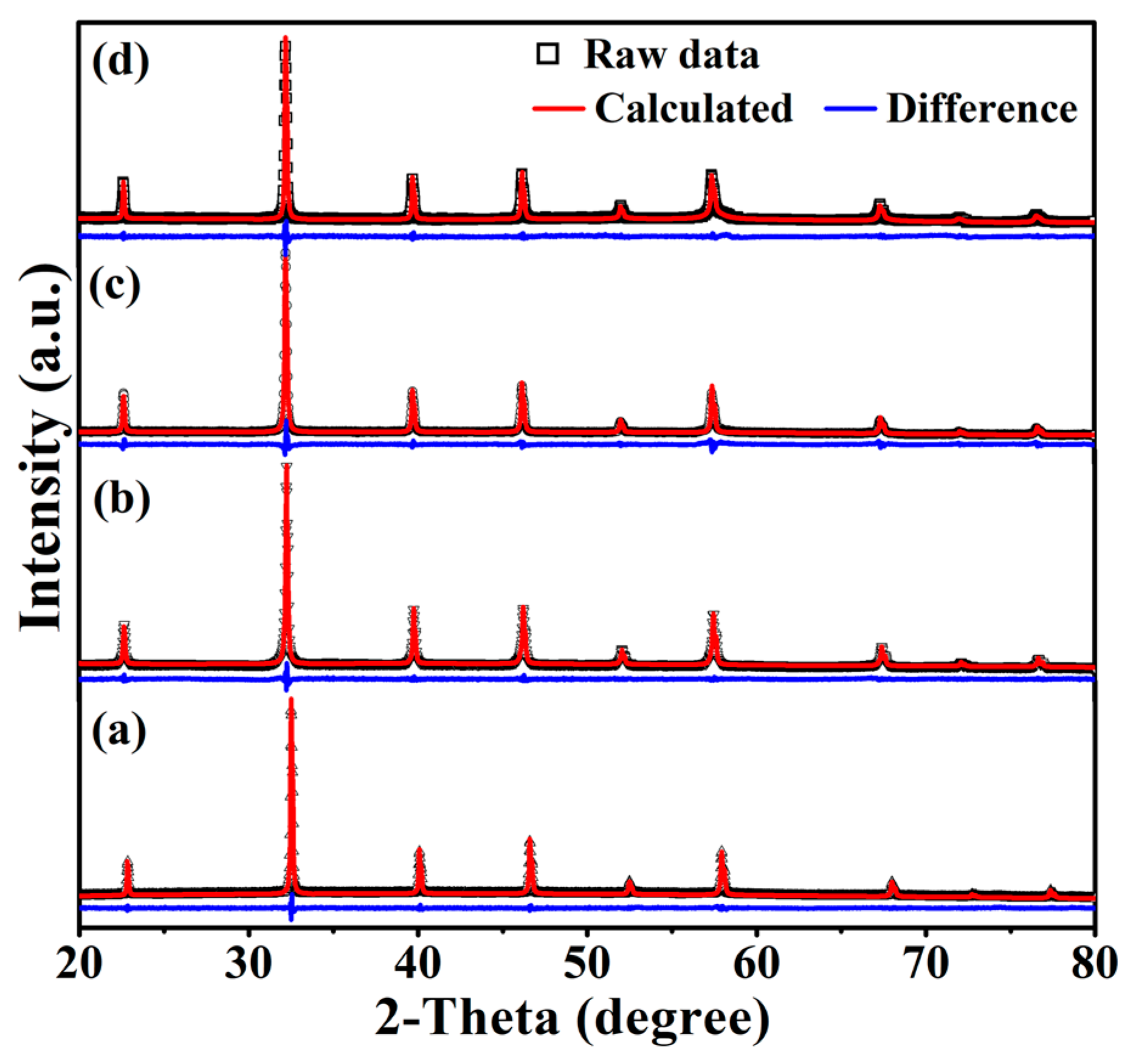




| Sample | a (Å) | Rwp (%) | Rp (%) | GOF | |
|---|---|---|---|---|---|
| EuTiO3 | 3.8976(3) | 3.82 | 3.00 | 1.19 | |
| Eu0.7Ba0.3TiO3 | 3.9340(2) | 5.00 | 3.95 | 1.21 | |
| Eu0.7Ba0.3Ti1−xCoxO3 | x = 0.05 | 3.93105(3) | 4.89 | 5.15 | 1.93 |
| x = 0.1 | 3.93325(18) | 5.21 | 4.06 | 1.54 | |
| Eu0.7Ba0.3Ti1−xNixO3 | x = 0.05 | 3.92930(6) | 5.51 | 4.26 | 1.11 |
| x = 0.1 | 3.93075(4) | 5.28 | 4.16 | 1.27 | |
| Sample | Pr (μC/cm2) | EC (kV/cm) | |
|---|---|---|---|
| Eu1−xBaxTiO3 | x = 0 | 0.111 | 4.77 |
| x = 0.1 | 0.133 | 2.25 | |
| x = 0.2 | 0.158 | 1.53 | |
| x = 0.25 | 1.51 | 1.19 | |
| x = 0.3 | 2.01 | 1.21 | |
| Eu0.7Ba0.3Ti1−yCoyO3 | y = 0.05 | 0.408 | 2.85 |
| y = 0.1 | 0.0528 | 1.94 | |
| Eu0.7Ba0.3Ti1−yNiyO3 | y = 0.05 | 1.07 | 2.27 |
| y = 0.1 | 0.415 | 0.957 | |
| Sample | χ0 | θ (K) | TC (K) | TN (K) |
|---|---|---|---|---|
| EuTiO3 | 0 | 3.50 | -- | 5.20 |
| Eu0.7Ba0.3TiO3 | 0 | 2.55 | -- | 3.05 |
| Eu0.7Ba0.3Ti0.95Co0.05O3 | 8.52 × 10−3 | 3.03 | 2.98 | -- |
| Eu0.7Ba0.3Ti0.90Ni0.10O3 | 4.62 × 10−2 | 2.48 | 2.72 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-C.; Qi, X. Cation Valences and Multiferroic Properties of EuTiO3 Co-Doped with Ba and Transition Metals of Co/Ni. Materials 2022, 15, 6652. https://doi.org/10.3390/ma15196652
Lin T-C, Qi X. Cation Valences and Multiferroic Properties of EuTiO3 Co-Doped with Ba and Transition Metals of Co/Ni. Materials. 2022; 15(19):6652. https://doi.org/10.3390/ma15196652
Chicago/Turabian StyleLin, Tzu-Chiao, and Xiaoding Qi. 2022. "Cation Valences and Multiferroic Properties of EuTiO3 Co-Doped with Ba and Transition Metals of Co/Ni" Materials 15, no. 19: 6652. https://doi.org/10.3390/ma15196652





